Evaluation of Mass Trapping Devices for the Control of the European Cherry Fruit Fly [Rhagoletis cerasi (L.)]
Abstract
:1. Introduction
2. Materials and Methods
2.1. Comparison of Traps and Attractants
- Folded yellow sticky trap: “Econex Cromática Amarilla” (20 × 25 cm) roof shape like, exposing its glued surface on the underside (Econex, Murcia, Spain). The baits were hung, with a wire, in the center of the trap underside. Approximate price: 0.5 €/trap. Two different baits were combined with this trap:
- Econex Rhagoletis cerasi 90 days: Food attractant dispenser of unknown composition (Econex, Murcia, Spain). Approximate duration: 90 days. Price: 4.8 €/dispenser.
- Homemade ammonium acetate dispenser: Two 3 mm hole perforated polythylene Ziploc® bag (6 × 4 cm), filled in with 15 g of ammonium acetate (Labkem, Barcelona, Spain). Approximate duration: 60 days. Price: 0.3 €/dispenser.
- Decis® Trap Cerasi: A ready-to-use dry trap with a transparent top part internally impregnated with deltamethrin as a killing agent (Bayer CropScience International, Monheim am Rhein, Germany). The attractant is placed in the lower part, which is hemispherical, orange in color and bearing four lateral holes. Appoximated price: 3.5 €/trap. Two different baits were combined with this trap:
- Econex Rhagoletis cerasi 90 days.
- Homemade ammonium acetate dispenser.
- Cera Trap® bottle trap: 1.5 L plastic bottle with four 8 mm diameter equidistant holes in the upper third of the bottle and baited with its own attractant Cera Trap® (hydrolysed proteins 5.5%) (Bioibérica, Barcelona, Spain). Approximate duration: 90 days. Price: 3.5 €/trap.
- Magnet® MED: Attract-and-kill device consisting of a deltamethrin impregnated paper envelope that incorporates two membrane dispensers with trimethylamine and ammonium acetate as attractants (Suterra Europe, Valencia, Spain). Approximate duration: 6 months. Price 5.2 €/trap.In order to evaluate and compare the effectiveness of this trap, its outer shell was covered with insect glue. In this way, the attracted insects remained stuck in the trap and were counted.
- Easy trap®: Made up of two rectangular plastic halves (one yellow and the other transparent), each bearing one of the two 12 mm holes, placed one in front of the other (Sorygar, Madrid, Spain). Price: 1.5 €/trap. This trap was tested with three different attractants:
- Biocebo®: Liquid attractant (hydrolyzed protein 30%) (Bioibérica, Barcelona, Spain), at 9%. Price: 4 €/l.
- Econex Rhagoletis cerasi 90 days.
- Homemade ammonium acetate dispenser.
Econex Rhagoletis cerasi 90 days and homemade ammonium acetate dispensers were placed inside the trap, hanging down from the upper end by means of a wire. The trap contained some water with a few drops of detergent to prevent scape of incoming flies. - Tephri-trap ecological®: A yellow cylindrical trap with a funnel at the botton and four lateral holes (Sorygar, Madrid, Spain). The inlet holes are provided with nets (6 mm mesh size) to prevent the entry of larger beneficial insects. Price: 2.5 €/trap. Two baits were evaluated with this trap:
- Econex Rhagoletis cerasi 90 days.
- Homemade ammonium acetate dispenser.
In both cases, baits were hung inside the trap, which was filled with soapy water to trap incoming flies. - Olipe trap: 1.5 L PET bottle with five 6 mm diameter lateral holes, set and equidistant around the middle of the bottle height. Price: 0.5 €/trap. Inside the trap, a homemade ammonium acetate dispenser was hung and some water with a few drops of detergent was put in order to retain captured flies.
- Econex Bottle Trap: 1 L PET bottle with four 12 mm diameter lateral holes provided with yellow and truncated-cone shaped inserts helping flies enter while making it difficult find an a way out (Econex, Murcia, Spain). Price: 2.5 €/trap. Two baits were evaluated with this trap:
- Econex Diammonium Phosphate RC: Diammonium phosphate dosed in 25 g sachets. The content of each sachet works in solution with 250 mL of water. Price: 0.6 €/sachet.
- Ceratinex®: Food attractant (45% Torula yeast, 45% Borax) in the form of water-soluble tablets (to solve 4 or 5 tablets with 500 mL of water) (Econex, Murcia, Spain). Price: 0.4 €/tablet.
2.2. Evaluation of Mass Trapping Efficacy
2.3. Statistical Analysis
3. Results
3.1. Comparison of Traps and Attractants
3.2. Evaluation of Mass Trapping Efficacy
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Daniel, C.; Grunder, J. Integrated management of European cherry fruit fly Rhagoletis cerasi (L.): Situation in Switzerland and Europe. Insects 2012, 3, 956–988. [Google Scholar] [CrossRef] [PubMed]
- Barringer, L. First record of the European cherry fruit fly, Rhagoletis cerasi (Linnaeus) (Diptera: Tephritidae), in North America. Insecta Mundi 2018, 0622, 1–4. [Google Scholar]
- Wakie, T.T.; Yee, W.L.; Neven, L.G. Assessing the risk of establishment of Rhagoletis cerasi (Diptera: Tephritidae) in the United States and globally. J. Econ. Entomol. 2018, 111, 1275–1284. [Google Scholar] [CrossRef] [PubMed]
- Boller, E.; Prokopy, R.J. Bionomics and management of Rhagoletis. Annu. Rev. Entomol. 1976, 21, 223–246. [Google Scholar] [CrossRef]
- Fimiani, P. Multilarval infestations by Rhagoletis cerasi L. (Diptera: Trypetidae) in cherry fruits. In Fruit Flies of Economic Importance; Cavalloro, R., Ed.; Balkema: Rotterdam, The Netherlands, 1983; pp. 52–59. [Google Scholar]
- Ioannou, C.S.; Papanastasiou, S.A.; Zarpas, K.D.; Miranda, M.A.; Sciarretta, A.; Nestel, D.; Papadopoulos, N.T. Development and field testing of a spatial decision support system to control populations of the European cherry fruit fly, Rhagoletis cerasi, in Commercial Orchards. Agronomy 2019, 9, 568. [Google Scholar] [CrossRef]
- Boller, E.F.; Avilla, J.; Joerg, E.; Malavolta, C.; Wijnands, F.G.; Esbjerg, P. Integrated Production. Principles and Technical Guidelines, 3rd ed.; OILB/WPRS Bull; IOBC: Wädenswil, Switzerland, 2004; Volume 27. [Google Scholar]
- Martín Gil, A.; Lozano, C.M.; Cruz, J.I. (Eds.) Guía de Gestión Integrada de Plagas: Frutales de Hueso: Albaricoque, Melocotón, Nectarina, Ciruelo y Cerezo; Ministerio de Agricultura y Pesca, Alimentación y Medio Ambiente: Madrid, Spain, 2005; pp. 83–85. Available online: https://www.mapa.gob.es/es/agricultura/temas/sanidad-vegetal/guiafrutalesdehuesoweb_tcm30-57949.pdf (accessed on 20 June 2022).
- Daniel, C.; Baker, B. Dispersal of Rhagoletis cerasi in commercial cherry orchards: Efficacy of soil covering nets for Cherry Fruit Fly control. Insects 2013, 4, 168–176. [Google Scholar] [CrossRef]
- Daniel, C.; Wyss, E. Susceptibility of different life stages of the European Cherry Fruit Fly, Rhagoletis cerasi, to entomopathogenic fungi. J. Appl. Entomol. 2009, 133, 473–483. [Google Scholar] [CrossRef]
- Daniel, C.; Wyss, E. Field applications of Beauveria bassiana to control the European Cherry Fruit Fly Rhagoletis cerasi. J. Appl. Entomol. 2010, 134, 675–681. [Google Scholar] [CrossRef]
- Köppler, K.; Peters, A.; Vogt, H. Initial Results in the Application of Entomopathogenic Nematodes against the European Cherry Fruit Fly Rhagoletis cerasi L. (Diptera: Tephritidae). IOBC/WPRS Bull. 2005, 28, 13–18. [Google Scholar]
- Kuske, S.; Daniel, C.; Wyss, E.; Sarraquigne, J.; Jermini, M.; Conedera, M.; Grunder, J. Biocontrol potential of entomopathogenic nematodes against nut and orchard pests. IOBC/WPRS Bull. 2005, 28, 163–167. [Google Scholar]
- Herz, A.; Köppler, K.; Vogt, H. Kann der Einsatz Entomopathogener Nematoden zur Nachhaltigen Bekämpfung der Kirschfruchtfliege Beitragen? 9. Wissenschaftstagung Ökologischer Landbau; Zikeli, S., Claupein, W., Dabbert, S., Kaufmann, B., Müller, T., Valle Zárate, A., Eds.; Verlag Dr. Köster: Berlin, Germany, 2007; Available online: https://orgprints.org/13795/ (accessed on 22 June 2022).
- Köppler, K.; Vogt, H.; Storch, V. Bait sprays to control the European Cherry Fruit Fly Rhagoletis cerasi. IOBC/WPRS Bull. 2008, 37, 59–66. [Google Scholar]
- Caruso, S.; Tommasini, M.G.; Barbari, G.; Ioriatti, C.; Altindisli, F.Ö.; Børve, J.; Escudero-Colomar, L.A.; Lucchi, A.; Molinari, F. Investigation on adulticide bait (Spintor-Fly®) to control the cherry fruit fly in Emilia-Romagna (North Italy). Trials 2010–2012. IOBC/wprs Bull. 2013, 91, 37–42. [Google Scholar]
- Registro de Productos Fitosanitarios. MAPA (Spanish Ministry of Agriculture, Fisheries and Food). Available online: https://www.mapa.gob.es/es/agricultura/temas/sanidad-vegetal/productos-fitosanitarios/registro/menu.asp (accessed on 22 August 2022).
- Prokopy, R.J.; Boller, E. Response of European Cherry Fruit Flies to colored rectangles. J. Econ. Entomol. 1971, 64, 1444–1447. [Google Scholar] [CrossRef]
- Agee, H.R.; Boller, E.; Remund, U.; Davis, J.C.; Chambers, D.L. Spectral sensitivities and visual attractant studies on the Mediterranean Fruit Fly, Ceratitis capitata (Wiedemann), Olive Fly, Dacus oleae (Gmelin), and the European Cherry Fruit Fly, Rhagoletis cerasi (L.) (Diptera, Tephritidae). J. Appl. Entomol. 1982, 93, 403–412. [Google Scholar] [CrossRef]
- Katsoyannos, B.I.; Papadopoulos, N.T.; Stavridis, D. Evaluation of trap types and food attractants for Rhagoletis cerasi (Diptera: Tephritidae). J. Econ. Entomol. 2000, 93, 1005–1010. [Google Scholar] [CrossRef]
- Daniel, C.; Mathis, S.; Feichtinger, G. A new visual trap for Rhagoletis cerasi (L.) (Diptera: Tephritidae). Insects 2014, 5, 564–576. [Google Scholar] [CrossRef]
- De Maeyer, L.; Companys, V.; Ricci, M.; Hyzy, N.; Izquierdo Casas, J.; Abts, W.; Engel, C.; Belien, T.; Clymans, R.; Shirring, A. Mass trapping with Decis™ Trap to manage fly control of Rhagoletis cerasi and Drosophila suzukii in IPM cherry orchards. Acta Hortic. 2020, 1286, 219–226. [Google Scholar] [CrossRef]
- Grodner, J.; Świech, K.; Rozpara, E.; Danelski, W. Food attractant to control the population of Rhagoletis cerasi L. (Diptera: Tephritidae) and its use in organic sweet cherry orchard in Poland. J. Res. Appl. Agric. Eng. 2016, 61, 167–172. [Google Scholar]
- Katsoyannos, B.I. Female attraction to males in Rhagoletis cerasi. Entomol. Soc. Am. 1976, 5, 474–476. [Google Scholar]
- Sarles, L.; Verhaeghe, A.; Francis, F.; Verheggen, F.J. Semiochemicals of Rhagoletis fruit flies: Potential for integrated pest management. Crop Prot. 2015, 78, 114–118. [Google Scholar] [CrossRef]
- Florian, T.; Macavei, L.I.; Hulujan, I.B.; Vasian, I.; Totos, S.; Gorgan, M.; Florian, V. Testing of semio-chemical products in monitoring and control of Rhagoletis cerasi L. AgroLife Sci. J. 2018, 7, 61–68. [Google Scholar]
- Macavei, L.I.; Oltean, I.; Vasian, I.; Florian, T.; Varga, M.I.; Băeţan, R.; Maistrello, L. Potential for attractive semiochemical lures in Rhagoletis cerasi (L.) management: A field study. J. Entomol. Res. Soc. 2018, 20, 1–9. [Google Scholar]
- Howse, P.E.; Stevens, I.D.R.; Jones, O.T. Mass trapping. In Insect Pheromones and Their Use in Pest Management; Howse, P.E., Stevens, I.D.R., Jones, O.T., Eds.; Springer: Dordrecht, The Netherlands, 1998; pp. 280–299. [Google Scholar] [CrossRef]
- Navarro-Llopis, V.; Sanchis Cabanes, J.; Vacas, S. Dispositivos de atracción y muerte para el control de Ceratitis capitata, ¿cómo afectan a las moscas atraídas los mosqueros y las láminas impregnadas de insecticida? Levante Agric. 2019, 445, 33–36. [Google Scholar]
- Thomas, D.B. Nontarget insects captured in fruit fly (Diptera: Tephritidae) surveillance traps. J. Econ. Entomol. 2003, 96, 1732–1737. [Google Scholar] [CrossRef]
- Porcel, M.; Ruano, F.; Sanllorente, O.; Caballero, J.A.; Campos, M. Incidence of the OLIPE masstrapping on olive non-target arthropods. Span. J. Agric. Res. 2009, 3, 660–664. [Google Scholar] [CrossRef]
- Seris, E.; Cobo, A.; Pascual, S.; Cobos, G.; Ros, P.; Castillo, E.; Sánchez-Ramos, I.; Marcotegui, A.; González-Núñez, M. Capture of natural enemies by different devices used in mass-trapping of Bactrocera oleae (Rossi). IOBC/WPRS Bull. 2010, 59, 33. [Google Scholar]
- Tschorsnig, H.-P.; Seris, E.; Cobo, A.; Cobos, G.; Pascual, S.; Ros, J.; González-Núñez, M. Tachinidae (Diptera) collected in traps used for mass-trapping of Bactrocera oleae (Rossi) (Diptera: Tephritidae) in olive groves in Central Spain. Span. J. Agric. Res. 2011, 9, 1298–1306. [Google Scholar] [CrossRef]
- Navarro-Llopis, V.; Primo Millo, J.; Vacas González, S. Efficacy of attract-and-kill devices for the control of Ceratitis capitata. Pest Manag. Sci. 2012, 69, 478–482. [Google Scholar] [CrossRef] [PubMed]
- Böckmann, E.; Köppler, K.; Hummel, E.; Vogt, H. Bait spray for control of European cherry fruit fly: An appraisal based on semi-field and field studies. Pest. Manag. Sci. 2014, 70, 502–509. [Google Scholar] [CrossRef]
- European Comision (EC). Commission Regulation (EC) No 889/2008 of 5 September 2008 Laying Down Detailed Rules for the Implementation of Council Regulation (EC) No 834/2007 on Organic Production and Labelling of Organic Products with Regard to Organic Production, Labelling and Control (Consolidated Text 01.01.2021); European Commission: Brussels, Belgium, 2021; Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A02008R0889-20210101#B-2 (accessed on 1 August 2022).
- Williams, T.; Valle, J.; Viñuela, E. Is the naturally derived insecticide spinosad® compatible with insect natural enemies? Biocontrol Sci. Technol. 2003, 13, 459–475. [Google Scholar] [CrossRef]
- Kakani, E.G.; Zygouridis, N.E.; Tsoumani, K.T.; Seraphides, N.; Zalom, F.G.; Mathiopoulos, K.D. Spinosad resistance development in wild olive fruit fly Bactrocera oleae (Diptera: Tephritidae) populations in California. Pest. Manag. Sci. 2010, 66, 447–453. [Google Scholar] [CrossRef]
- Guillem-Amat, A.; Sánchez, L.; López-Errasquín, E.; Ureña, E.; Hernández-Crespo, P.; Ortego, F. Field detection and predicted evolution of spinosad resistance in Ceratitis capitata. Pest. Manag. Sci. 2020, 76, 3702–3710. [Google Scholar] [CrossRef]
- Hsu, J.-C.; Chou, M.-Y.; Mau, R.F.; Maeda, C.; Shikano, I.; Manoukis, N.C.; Vargas, R.I. Spinosad resistance in field populations of melon fly, Zeugodacus cucurbitae (Coquillett), in Hawaii. Pest. Manag. Sci. 2021, 77, 5439–5444. [Google Scholar] [CrossRef]

| Trials (Dates 1) | Devices 2 | N 3 |
|---|---|---|
| 2014 (29/05–28/07) | Cera Trap® bottle trap Magnet® MED Easy trap® + Biocebo® Folded yellow sticky trap + Econex Rhagoletis cerasi 90 days Folded yellow sticky trap + Homemade AmAc. dispenser | 6 |
| 2015 (29/05–27/07) | Cera Trap® bottle trap Easy trap® + Biocebo® Decis® trap + Econex Rhagoletis cerasi 90 days Decis® trap + Homemade AmAc. dispenser Folded yellow sticky trap + Econex Rhagoletis cerasi 90 days Folded yellow sticky trap + Homemade AmAc. dispenser | 4 |
| 2016 (03/06–29/07) | Cera Trap® bottle trap Olipe trap + Homemade AmAc. dispenser Easy trap® + Econex Rhagoletis cerasi 90 days Easy trap® + Homemade AmAc. dispenser Tephri-trap ecological® + Econex Rhagoletis cerasi 90 days Tephri-trap ecological® + Homemade AmAc. dispenser Folded yellow sticky trap + Econex Rhagoletis cerasi 90 days Folded yellow sticky trap + Homemade AmAc. dispenser | 5 |
| 2017 (26/05–07/07) | Econex Bottle Trap + Econex Diammonium Phosphate RC Econex Bottle Trap + Ceratinex® Folded yellow sticky trap + Homemade AmAc. dispenser | 6 |
| Trial | Location (Coordinates) Altitude 1 | Dates 2 | Trap + Attractant 3 | Number of Trees (Area) |
|---|---|---|---|---|
| Rozas | Lagunilla (Salamanca) (40°20′00″ N 5°57′06″ W) 916 m a.s.l. | 2017 (26/05–07/07) | Folded yellow sticky trap + Homemade AmAc. dispenser | 450 (0.7 ha) |
| Cruz vieja | Lagunilla (Salamanca) (40°19′50″ N 5°57′04″ W) 923 m a.s.l. | 2017 (26/05–07/07) | Folded yellow sticky trap + Homemade AmAc. dispenser | 320 (0.6 ha) |
| Cañadilla | El Torno (Cáceres) (40°07′16″ N 5°56′19″ W) 449 m a.s.l. | 2021 (6/05–08/07) | Folded yellow sticky trap + Econex Rhagoletis cerasi 90 days | 112 (0.4 ha) |
| Barrachina | La Almunia (Zaragoza) (41°27′07″ N 1°23′59″ W) 455 m a.s.l. | 2021 (13/05–18/06) | Folded yellow sticky trap + Econex Rhagoletis cerasi 90 days | 106 (0.4 ha) |
| Trials | Temperature and Rain 1 | Devices | Flies/Trap 2 ( ± SE) | % ♀ |
|---|---|---|---|---|
| 2014 | 20.5 °C 28 mm | Cera Trap® bottle trap Magnet® MED Easy trap® + Biocebo® Folded yellow sticky trap + Econex Rhagoletis cerasi 90 days Folded yellow sticky trap + Homemade AmAc. dispenser | 0.67 ± 0.42 a 0 a 0.67 ± 0.33 a 4.00 ± 1.63 b 4.50 ± 1.09 b | 75.0 ± 25.0 a - 50.0 ± 35.4 a 55.1 ± 16.2 a 52.4 ± 13.2 a |
| 2015 | 24.1 °C 17 mm | Cera Trap® bottle trap Easy trap® + Biocebo® Decis® trap + Econex Rhagoletis cerasi 90 days Decis® trap + Homemade AmAc. dispenser Folded yellow sticky trap + Econex Rhagoletis cerasi 90 days Folded yellow sticky trap + Homemade AmAc. dispenser | 2.00 ± 0.41 a 0 a 2.00 ± 0.82 a 1.25 ± 0.48 a 26.25 ± 10.52 b 13.00 ± 2.16 b | 45.8 ± 20.8 a - 58.3 ± 22.1 a 66.7 ± 16.7 a 52.5 ± 4.2 a 49.8 ± 5.9 a |
| 2016 | 23.6 °C 35 mm | Cera Trap® bottle trap Olipe trap + Homemade AmAc. dispenser Easy trap® + Econex Rhagoletis cerasi 90 days Easy trap® + Homemade AmAc. dispenser Tephri-trap ecological® + Econex Rhagoletis cerasi 90 days Tephri-trap ecological® + Homemade AmAc. dispenser Folded yellow sticky trap + Econex Rhagoletis cerasi 90 days Folded yellow sticky trap + Homemade AmAc. dispenser | 0 a 1.80 ± 0.73 ab 1.30 ± 0.93 ab 5.40 ± 2.84 bc 4.60 ± 1.89 bc 5.40 ± 1.36 bc 10.60 ± 3.20 c 15.60 ± 3.75 c | - 44.4 ± 17.2 a 66.7 ± 29.1 a 40.7 ± 15.2 a 52.6 ± 20.5 a 54.5 ± 6.7 a 49.1 ± 4.4 a 47.4 ± 4.6 a |
| 2017 | 21.2 °C 94 mm | Econex Bottle Trap + Econex Diammonium Phosphate RC Econex Bottle Trap + Ceratinex® Folded yellow sticky trap + Homemade AmAc. dispenser | 0.50 ± 0.34 a 0.67 ± 0.49 a 13.17 ± 3.24 b | 41.7 ± 0.73 a 25.0 ± 0.73 a 33.3 ± 0.73 a |
| Devices | Price 1 (€/Device) | Handling Costs (€/Device) 2 | Total Cost 4 (€/ha) | ||
|---|---|---|---|---|---|
| Preparation | Placement and Removal | Maintenance 3 | |||
| Folded yellow sticky trap + Econex Rhagoletis cerasi 90 days | 5.3 | 0.1 | 0.15 | - | 2775 |
| Folded yellow sticky trap + Homemade AmAc. dispenser | 0.8 | 0.2 | 0.15 | - | 575 |
| Decis® trap + Econex Rhagoletis cerasi 90 days | 8.3 | 0.05 | 0.15 | - | 4250 |
| Decis® trap + Homemade AmAc. dispenser | 3.8 | 0.15 | 0.15 | - | 2050 |
| Cera Trap® bottle trap | 3.5 | - | 0.15 | - | 1825 |
| Magnet® MED | 5.2 | - | 0.15 | - | 2675 |
| Easy trap® + Econex Rhagoletis cerasi 90 days | 6.3 | 0.05 | 0.15 | 0.1 | 3300 |
| Easy trap® + Homemade AmAc. dispenser | 1.8 | 0.15 | 0.15 | 0.1 | 1100 |
| Easy trap® + Biocebo® | 2.5 | 0.07 | 0.15 | 0.1 | 1410 |
| Tephri-trap ecological® + Econex Rhagoletis cerasi 90 days | 7.3 | 0.05 | 0.15 | 0.1 | 3800 |
| Tephri-trap ecological® + Homemade AmAc. dispenser | 2.8 | 0.15 | 0.15 | 0.1 | 1600 |
| Olipe trap + Homemade AmAc. dispenser | 0.8 | 0.15 | 0.15 | 0.1 | 600 |
| Econex Bottle Trap + Econex Diammonium Phosphate RC | 3.1 | 0.07 | 0.15 | 0.1 | 1710 |
| Econex Bottle Trap + Ceratinex® | 4.1 | 0.07 | 0.15 | 0.1 | 2210 |
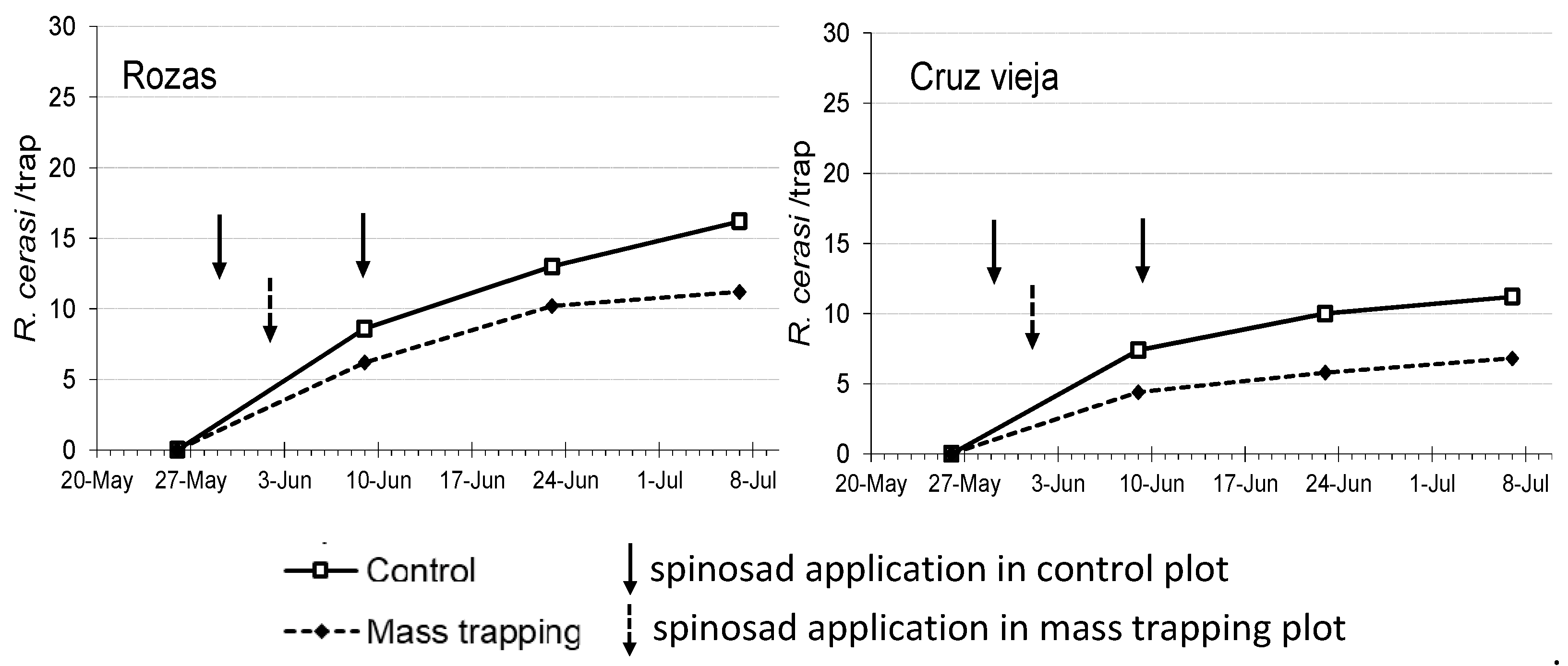
| Trial | Treatment | Captures (Flies/Trap) 1 ± SE | Spinosad Applications per Year | Damaged Cherries (%) ± SE (n) 2 |
|---|---|---|---|---|
| Rozas | Mass trapping Control | 11.20 ± 2.44 16.20 ± 2.76 | 1 2 | 0.99 ± 0.46 (421) 2.03 ± 0.61 (432) |
| Cruz vieja | Mass trapping Control | 6.80 ± 1.50 11.20 ± 1.60 | 1 2 | 0.79 ± 0.43 (383) 1.98 ± 0.56 (403) |
| Cañadilla | Mass trapping Control | 0.20 ± 0.20 0.60 ± 0.24 | 0 0 | 0.74 ± 0.40 (415) 1.83 ± 0.70 (391) |
| Barrachina | Mass trapping Control | 0.00 ± 0.00 0.20 ± 0.20 | 0 0 | 0.20 ± 0.20 (494) 0.47 ± 0.32 (443) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Núñez, M.; Cobos, G.; Sánchez-Ramos, I. Evaluation of Mass Trapping Devices for the Control of the European Cherry Fruit Fly [Rhagoletis cerasi (L.)]. Horticulturae 2022, 8, 869. https://doi.org/10.3390/horticulturae8100869
González-Núñez M, Cobos G, Sánchez-Ramos I. Evaluation of Mass Trapping Devices for the Control of the European Cherry Fruit Fly [Rhagoletis cerasi (L.)]. Horticulturae. 2022; 8(10):869. https://doi.org/10.3390/horticulturae8100869
Chicago/Turabian StyleGonzález-Núñez, Manuel, Guillermo Cobos, and Ismael Sánchez-Ramos. 2022. "Evaluation of Mass Trapping Devices for the Control of the European Cherry Fruit Fly [Rhagoletis cerasi (L.)]" Horticulturae 8, no. 10: 869. https://doi.org/10.3390/horticulturae8100869
APA StyleGonzález-Núñez, M., Cobos, G., & Sánchez-Ramos, I. (2022). Evaluation of Mass Trapping Devices for the Control of the European Cherry Fruit Fly [Rhagoletis cerasi (L.)]. Horticulturae, 8(10), 869. https://doi.org/10.3390/horticulturae8100869








