Abstract
Saffron is the world’s most coveted spicy plant that has medicinal value. Currently, due to diverse types of difficulties in growing this plant outdoor, the tendency to produce it indoor has been increased. Optimized indoor conditions for growing saffron plants is not fully determined so far. This study was conducted to investigate the interactive effects of two plant growth regulators (PGRs), including gibberellic acid (GA3) and γ-aminobutyric acid (GABA) and four light recipes, including white, monochromatic blue, monochromatic red, and a combination of 50% red and 50% blue on the flower yield and phytochemical components (such as crocin, picrocrocin and safranal) in stigmas of indoor-grown saffron. The results showed that exogenous GABA application and combined red and blue LED lights enhanced the performance of saffron flowers in terms of the number of flowers (up to 1.97 per corm) as well as the fresh and dry weight of flowers and stigmas. In saffron, the concentration of three major secondary metabolites is of great importance since it determines its commercial, pharmaceutical quality. GABA induced saffron’s chemical ingredients toward the phytochemicals safranal (up to 5.03%) and picrocrocin (up to 15.8%), while GA3 induced them toward the carotenoid pigment crocin (up to 25.1%). In conclusion, the application of GABA with a combination of red and blue lights enhanced the production of high-quality stigmas and positively affected the yield of flowers in saffron plants.
1. Introduction
Saffron is the world’s most coveted, lucrative spice, which is mainly derived from the stigmas of the saffron (Crocus sativus L.) flower. It is used for flavoring, fragrance, dyes and medicines. Saffron’s taste and iodoform-like or hay-like fragrance result from the phytochemicals such as picrocrocin and safranal [,]. Likewise, it contains a carotenoid pigment, crocin, which imparts a rich golden-yellow hue to dishes and textiles. The global yield of saffron under open field conditions is estimated at 3.4 kg ha−1 (418 t y−1 of production in 121,338 ha) []. While some doubts remain on its origin [], it is believed that saffron originated from Iran []. Iran currently produces 90% of the world’s saffron [,].
Saffron is still produced traditionally in arid and semi-arid regions []. Producing saffron outdoors faces many difficulties such as limited yield due to the negative impacts of climate change, most importantly increased temperatures. Modern technologies using indoor culture methods have not been widely used for the production of saffron, despite the antiquity of its cultivation []. Controlled-environment agriculture (CEA), which includes indoor agriculture (IA) and vertical farming, is a technology-based approach that recently attracted the attention of saffron production. The CEA aims to provide protection from the outdoor constraints and to maintain proper growing conditions throughout the development of the crop. Production takes place within an enclosed growing structure such as a greenhouse, vertical farm, or indoor grow room []. The positive effects of light emitting diode (LED) lamps on saffron cultivation have been shown in a LED-equipped controlled-environment room []. CEA optimizes the use of resources such as water, energy, land and space, capital investment and labor [], and it paves the way toward creating the optimal growing conditions for saffron production. CEA aims to optimize plant growth mainly through using soilless farming techniques such as hydroponics and aeroponics techniques []. Fogponics is an advanced form of aeroponics that uses some specific mechanisms (for example ultrasonic, compressed air, or heating elements) to form a suspension of much smaller particles of nutrient enriched water (5–30 μm), or even as a vapor [,]. The ultrasonic fog generator capable of vibrating at supersonic frequencies and produced micro droplets of water nutrient make it easier and faster for the plant to absorb via the roots.
Aghhavani-Shajari and collaborators compared the flowering of saffron between an open field and a controlled environment []. They reported that, in the first and the second flowering seasons, the percentage of flowering corms was higher under the soilless production system (39 and 65%, respectively) than the traditional production in soil (6 and 56%, respectively). Even though saffron flowers can be simply produced through indoor farming methods, the production of high-quality saffron through indoor farming methods is still challenging. Flower initiation and emergence in saffron are sensitive to environmental conditions, such as humidity, temperature and light []. Light is a key factor affecting phytochemical synthesis and accumulation in plants []. Over the past few years, light quality (i.e., the spectral specificity of light) has been remarkably considered as an important environmental factor in improving plant growth and quality in the indoor production of plants. Recent development in artificial lighting technology increases its use in indoor cultivation systems []. Current applications of vertical farming coupled with other advanced technologies, such as specialized LED lights, have resulted in over 10 times higher crop yields than traditional farming methods []. Numerous studies have investigated the growth and physiology of various plant species under red and blue lights [,,,]. LEDs with specified wavebands of light can be an easy tool to understand how the light spectrum influences plant growth and physiology. Red and blue spectra are the most effective lights in plant growth and development, especially for saffron production [].
A large number of related chemical compounds are synthesized by humans and are used to regulate the growth and development of cultivated plants. These manmade compounds are called plant growth regulators (PGRs) that are used in many different techniques. PGRs play important roles in plant growth and development [] owing to their important regulating role in plant physiology and biochemistry []. PGRs are one of the factors influencing essential oil production []. A four-carbon non-protein amino acid, γ-aminobutyric acid (GABA), is a significant component of the free amino acid pool in most prokaryotic and eukaryotic organisms []. GABA is also found in plants [,]. It has been shown that GABA controls a broad range of biological and physiological processes (such as C and N metabolisms, plant-microbe interactions, signal transduction and stress-derived responses) during plant growth and development []. Evidence also suggests a role in cell signaling in plants [,]. Gibberellic acid (GA3) is a hormone found in plants and fungi []. It is also produced industrially using microorganisms []. Since GA3 regulates growth, applications of very low concentrations (less than 250 ppm, according to Keshtkar and collaborators []) can have a profound effect while too much will have the opposite effect []. Gibberellins have a number of effects on plant development. They can stimulate rapid stem and root growth, induce mitotic division in the leaves of some plants, and increase seed germination rates []. It has been shown that the application of GA3 stimulated bud growth, increased flowering, and promoted the number and weight of flowers and yield of saffron [].
The effect of light quality on the optimization of phytochemical concentration in plant species has been indicated in many studies [,,,,,]. The lighting spectra has been shown to affect the flower production of saffron through altering biomass partitioning toward flowers []; however, there is limited knowledge about the optimal lighting environment for saffron growth [], particularly on the effect of light quality on saffron quality. On the other hand, the effects of indoor farming have not been widely studied on the flower yield and quality in bulbous plants such as saffron. Little is known about the role of exogenously applied PGRs in the yield and stigma quality of the saffron plant. Meanwhile, the interaction of PGRs and different light spectra on the amount of essence and the level of its composition in saffron has not been addressed so far. Given the information provided above, the present experiment was conducted to evaluate flowering and phytochemical composition in fogponically-grown saffron exposed to different PGRs and various light qualities. This research mainly aimed to address whether or how an optimal light quality and an ideal PGR can increase the yield and quality of saffron in the controlled environment. Specifically, we envisaged that, during the flowering period, an optimal light spectrum with an ideal PGR would have a synergistic effect, and subsequently, saffron yield and quality would increase.
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
To evaluate the impacts of two PGRs and four different light qualities on saffron corms, this research was carried out as a two-factor factorial experiment in a randomized complete block design with three replications. The experimental factors included PGR at three levels (GA3, GABA, control (just tap water) and light quality at four levels (W = white, B = blue, R = red, BR = combined blue and red) (Table S1). Saffron corms (Crocus sativus L.) were chosen from an Iranian ecotype called Qayen, which is native to the South Khorasan province (the main saffron production region of the world) in Iran. After digging up in early July (the true dormant period), healthy, wound-free and equal-size saffron corms were sorted (10–12 g). They were subsequently soaked into an acaricide (0.1% Propargite) and fungicide (0.15% Thiophanate-methyl) solution and dried for 1–2 h. The corms were incubated at 25 °C under dark conditions at a relative humidity of 85 ± 2% for 3 months, since previous studies showed that flower formation starts in this condition [,].
In early October, corms were stacked on metal mesh shelves and separately primed by GA3 (Merck KGaA Co., Darmstadt, Germany) and GABA (Sigma-Aldrich Co., Darmstadt, Germany) standard solutions with 1 mM and 25 μM concentration, respectively, since previous literature showed that the highest growth was observed in plants receiving these concentrations [,]. Tween-20 (0.1%, w/v) was added as a surfactant to ensure the adhesion of PGRs to the treated corms. Spraying PGRs was repeated after 3 days. Then, corms were kept in a controlled phytotron (85% relative humidity, day/night temperature of 20 °C/15 °C with a photoperiod of 8 h day/16 h night, according to Fallahi and collaborators []). The irradiance was provided by LED modules with customized built spectra (12W, Parto Roshde Novin, Iran Grow Light, Tehran, Iran) at a photosynthetic photon flux density (PPFD) of 80 ± 10 μmol m−2 s−1. Four light qualities including white (wavelength 400–700 nm), monochromatic blue (peak wavelength at 465 nm), monochromatic red (peak wavelength at 660 nm), and a combination of blue and red lights (50% blue: 50% red) were set as light treatments (Figure 1). The light regimes were segregated using panels made of aluminum foil to avoid light transmission, and they were applied until the end of the flowering period. Light intensity and spectrum were checked by PAR-FluorPen FP 100-MAX (Photon Systems Instruments, Drasov, Czech Republic) and Sekonic C-7000 spectrometer (Sekonic Corp, Tokyo, Japan), respectively. For each treatment, 100 corms were used.
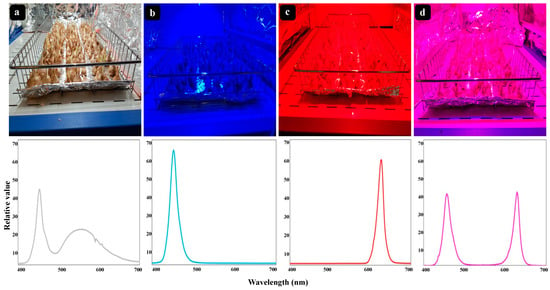
Figure 1.
The lighting conditions together with the relative spectral distribution of each lighting treatment. Four lighting treatments are illustrated: White (a), monochromatic blue (b), monochromatic red (c), and a combination of blue and red lights (d). The light treatments were separated with a panel wall to segregate light regimes.
After 25 days from light treatments, corms were cold-shocked at 13 °C to induce flowering, something that is difficult to achieve in outdoor conditions where the temperature cannot be controlled.
2.2. Determining the Yield of Saffron Flower
Flowering was initiated 32–38 days after light treatments. During the flowering period, three plants were sampled for each replication. The flowers were harvested by hand and their fresh weights were recorded instantly after harvesting. Afterward, stigmas were pulled out from the flowers and their fresh weight was recorded immediately. The flowers and stigmas were weighed again after oven-drying (Binder, Tuttlingen, Germany) at 80 °C for 72 h to determine their dry weights []. Fresh and dry weights of flower and stigma were measured using a digital scale with a precision of 0.001 g (Mettler Toledo, Greifensee, Switzerland).
2.3. Determination of Phytochemical Compounds
To assay qualitative compounds in stigma, secondary metabolites of saffron stigmas were measured regarding ISO 3632-2 []. Accordingly, the dried stigmas were ground into a fine powder using a pestle and mortar, 0.5 g of which was transferred into a 1 L volumetric flask, and then brought to 1 L volume with distilled water. The mixture was stirred in the dark for 20 min using a magnetic stirrer. After filtration through quantitative filter paper (No131; Advantec Toyo Kaisha Co., Tokyo, Japan), the aqueous saffron extract was analyzed for spectral features using an UV-Vis spectrophotometer (Lambda 365, PerkinElmer, Waltham, MA, USA). Consistent with ISO, the absorbance of each aqueous solution was measured at a wavelength of 257, 330, and 440 nm to determine active substances of picrocrocin, safranal, and crocin, respectively. The percentage of three types of qualitative compounds was calculated by the following equation:
where E is the amount of specific qualitative compound in %; A is the obtained absorbance number at the relevant wavelength, and M is the stigma dry weight in milligrams.
2.4. Statistical Analysis
To test the normality of data distribution, Anderson-Darling criterion was used at a significance level of 0.05 using the program MINITAB, version 14.1 (Minitab LLC, State College, PA, USA). The measured data were statistically assessed by analysis of variance (ANOVA) according to the ‘factorial experiment in a randomized complete block design’ method using two PGRs and four different light treatments with three replications. The data mean with standard errors were compared using Duncan’s multiple range test at the p ≤ 0.05 probability level using the SAS software, version 9.4 (SAS Institute, Cary, NC, USA). Principal component analysis (PCA) was carried out using the prcomp command of the R statistical software []. Since variables had different units of measurement, PCA was performed based on the correlation matrix [].
3. Results
3.1. The Floral Yield of Saffron Plants
While light quality and PGRs are effective factors influencing plant’s growth and development, studies on their interactions on the flower yield and stigma quality of saffron plants are scarce. In the present research, to evaluate the interactions of light quality and PGRs on the growth of saffron flowers, three months after incubation in the dark at 25 °C, corms were incubated at 13 °C under the PGRs and light treatments to induce flowering. Flowers emerged 32–38 days after light treatments. The flower number per corm, flower, and stigma fresh weight and dry weight were evaluated. The impacts of treatments on the number of flowers per corm, flower, and stigma fresh weight and dry weight were significantly different (Table 1). Not only were the levels of both factors significantly different, but their interaction was also significant in all of the investigated traits, which implies the different effects of treatments on flower characteristics of saffron. The results showed that the applications of PGRs and different light qualities were effective treatments for enhancing the floral yield of saffron plants. The maximum number of flowers per corm, flower, and stigma fresh and dry weights were obtained in the saffron plants grown under GABA+BR treatment (Figure 2).

Table 1.
Analysis of variance for the effect of treatments on flower characteristics of saffron.

Figure 2.
The number of flowers (a), flower fresh weight (b), flower dry weight (c), stigma fresh weight (d), and stigma dry weight (e) of saffron plants grown under two PGRs including GA3 (gibberellic acid) and GABA (γ-aminobutyric acid), and four light qualities including white (W), blue (B), red (R) and combined B&R (50% blue: 50% red) (BR). Different letters indicate that values are significantly different at p ≤ 0.05 according to Duncan’s multiple range test. Error bars represent the mean value of three replications ± standard deviation.
The number of flowers in GABA+BR plants was almost three-fold higher than their numbers in plants grown under control treatments (i.e., control+W, control+B, control+R, and control+BR) (Figure 2a). No significant difference was noted regarding flower fresh and dry weights between GABA+BR and GABA+B. An intermediate flower fresh weight and dry weight was also detected under GABA+W and GABA+R. Fresh and dry weights of stigmas followed the same trend as of flowers. Stigma dry weight in GABA+BR saffron plants was significantly higher than the others. In this way, it was more than four times higher than its value in all control treatments regardless of the light spectrum.
3.2. The Phytochemicals Content
Phytochemical concentration in the flowers of saffron plants was analyzed to evaluate the influence of light quality and PGRs on its accumulation. According to the results, all characteristics were significantly affected by the different treatments (Table 2). The application of PGRs was the most effective treatment for inducing the production of secondary metabolites in the stigmas of saffron. The maximum amount of safranal (perfume) and picrocrocin (flavor) was observed in GABA+BR treatment, with values of 5.03% and 15.8%, respectively (Figure 3a,b). The highest enhancement in crocin content (color) was recorded in stigmas of corms treated with GA3+BR (Figure 3c). The concentration of safranal in flowers of GABA+BR was 2.1 times higher than its concentration in flowers of control+R. The results demonstrated that there is a positive interaction between PGRs and combined blue and red lights.

Table 2.
Analysis of variance for the effect of treatments on phytochemical traits of saffron.
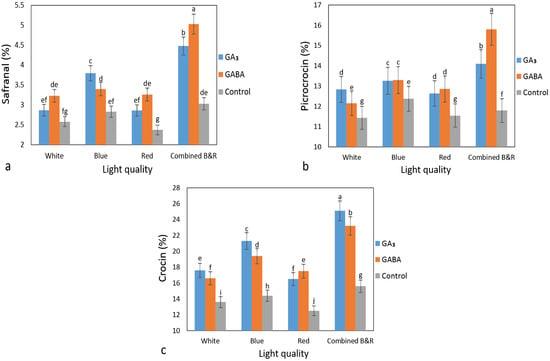
Figure 3.
Safranal (a), picrocrocin (b), and crocin (c) accumulation of saffron flowers grown under two PGRs including GA3 (gibberellic acid) and GABA (γ-aminobutyric acid), and four light quality including white, blue, red and combined B&R (50% blue: 50% red). Different letters indicate that values are significantly different at p ≤ 0.05 according to Duncan’s multiple range test. Error bars represent the mean value of three replications ± standard deviation.
In saffron, the concentration of the three major secondary metabolites is of great importance since it determines its quality []. Accordingly, PGRs+BR treatments had the highest efficiency among the treatments by improving the concentration of the main chemical ingredients.
3.3. Principal Component Analysis (PCA) of Variables
For an overall interpretation of the results obtained, PCA was used. The matrix of the analysis consisted of 12 cases corresponding to the treatments and eight variables (measured characteristics). The first two PCs account for 92.5% and 4.3%, respectively, of the total variation in the dataset. Because of using the correlation matrix, two PCs are desirable, according to Jolliffe []. The results were presented as a two-dimensional scatter-plot of the data (Figure 4), which is, by definition, the best variance-preserving a two-dimensional plot of the data, representing over 96% of the total variation. The first PC separated the treatments control and red light from the others. The second PC separated the BR treatment from the other treatments.
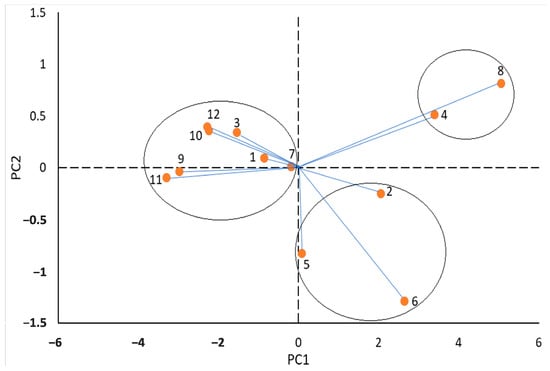
Figure 4.
PCA biplot graphics of measured variables in saffron flowers grown under different conditions: 1 = GA3+white light, 2 = GA3+blue light, 3 = GA3+red light, 4 = GA3+combined B&R light, 5 = GABA+white light, 6 = GABA+blue light, 7 = GABA+red light, 8 = GABA+combined B&R light, 9 = control+white light, 10 = control+blue light, 11 = control+red light, and 12 = control+combined B&R light.
PCA biplot divided the treatments into three separate groups. The GA3+BR treatment was associated with the GABA+BR treatment. These two treatments have the most positive effect on the yield and quality of saffron flowers, as previously shown (Figure 2 and Figure 3), and the PC analysis could put them into one group. Finally, it can be largely claimed that the biplot graphic with the first two PCs was able to group the treatments well.
4. Discussion
The importance of light for plant growth and development is already well-known. Light quality is one of the important features of the lighting environment detected by very sophisticated sensory networks. Photomorphogenesis is a light-mediated development, where plant growth patterns respond to the light spectrum, direction and quantity [,,]. Phytochromes, cryptochromes, and phototropins are photochromic sensory receptors that restrict the photomorphogenic effect of light to the UV-A, UV-B, blue, and red portions of the electromagnetic spectrum []. Light quality can also affect the synthesis of bioactive compounds and the secondary metabolism of plants []. Therefore, regulating light quality can be considered as a strategic tool to improve the yield of secondary metabolites [].
PGRs are organic compounds that affect the physiological processes of plants. More specific responses include alteration of C partitioning, greater root-to-shoot ratios, enhanced photosynthesis, altered nutrient uptake, improved water status, and altered crop canopy []. PGRs play a significant role in the growth, development, and distribution of essential nutrients in plant systems []. PGRs can potentially play a fundamental role in regulating plant responses to various abiotic stresses and hence, contribute to plant adaptation under adverse environments [].
In the present study, the floral yield and phytochemicals content of saffron plants were influenced by light quality and PGR application. PGRs are positive regulators of secondary metabolic pathways, and the highest improvement in phytochemicals content was observed in corms treated with PGRs. The results showed that the combination of 50% blue: 50% red light caused the generation of more flowers with the highest content of phytochemicals.
Certain quantitative and qualitative responses are expected when plants are grown under different light conditions. Literature data shows that a combination of blue and red light is of great importance for the normal growth and physiological performance of plants. For instance, the maximum photosynthetic capacity is reported in the leaves of cucumber plants grown under 50% red: 50% blue lights []. Klein and collaborators, and Naznin and collaborators found that mixed red and blue light led to higher Chlorophyll a, b and total Chlorophyll levels, increased electron transport rate (ETR) and an early onset of non-photochemical quenching (NPQ), all of which led to improvements in photosynthetic efficiency [,]. Recent experimental evidence indicates that the highest flower number and stigma fresh and dry weight of saffron plants were detected under monochromatic blue light, whereas no significant change was noted among monochromatic blue and 50% red: 50% blue lights []. Lettuce, spinach, kale, basil, and pepper plants grown under 100% red light showed lower fresh and dry mass compared to those cultured under a combination of red and blue lights []. The previous report has shown that mixed blue and red lights alter plant photomorphogenesis and photosynthesis, mainly through their effects on leaf anatomy, photosynthetic electron transport and the expression and activities of Calvin cycle-related enzymes, including Rubisco, FBPase and GAPDH []. Similar to our findings, Moradi and collaborators observed the lowest number of flowers as well as fresh and dry weight of flowers and stigmas in the saffron plants grown under monochromatic red light [].
It is well known that light is a physical factor that can affect the composition of secondary metabolites. Kajikawa and collaborators found that a light environment with a low red to far-red (R/FR) photon flux density ratio increased the crocin concentration in the stigmas of hydroponically-cultivated saffron corms []. They assumed that the decrease in the R/FR ratio from 15.8 to 1.8 enhanced the accumulation of photosynthetic products that are sources of crocin biosynthesis. Sng and collaborators found that a combination of red and blue light induced the expression of genes involved in secondary metabolite biosynthesis [].
GABA is a very important candidate for controlling a wide variety of physio-biochemical processes in plants. Regulations in cytosolic pH, anti-oxidative enzymatic systems, buffering agent in C and N metabolism, osmoregulation, armoring against oxidative stress, and signal transduction are the key roles of GABA that might improve overall plant performance []. Due to the different roles of GABA in a diverse range of processes, research on GABA can provide fundamental insight into various aspects of a plant’s developmental system []. Fait and collaborators suggested that GABA is one of the main components providing C resources for the TCA (Tricarboxylic acid) cycle in tobacco plants []. In melon seedlings, a GABA-induced increase in nitrate absorption was associated with enhancing nitrate reductase activity []. There is evidence that N2 fixation and nodule formation in legumes are regulated by GABA []. The indirect effect of GABA on the photochemical efficiency of lettuce plants by regulating the C:N ratio has been argued by Kalhor and collaborators []. Based on the results obtained by Vijayakumari and Puthur in black pepper plants, photosynthetic performance was improved by GABA exposure []. A growing body of evidence demonstrated the role of GABA as an endogenous plant signaling molecule and metabolite. According to the results of Hijaz and collaborators in Citrus sinensis, levels of plant hormones such as salicylic acid, benzoic acid, jasmonic acid, abscisic acid, and indole-3-acetic acid are significantly enhanced by GABA exposure []. In another study, the exogenous application of GABA led to the improvement in seedling growth and net photosynthesis of maize []. Being an essential intermediate of nitrogen metabolism and amino acid biosynthesis, GABA plays a key role in primary and secondary metabolite synthesis []. The overall study by Kaur and Zhawar indicated GABA as an important regulator of secondary and carbohydrate metabolisms [].
It has been shown that fluctuations in gibberellin concentration influence light-regulated seed germination, photomorphogenesis during de-etiolation, and photoperiod regulation of stem elongation and flowering []. It was reported that saffron corms treated with GA3 have a stimulated decomposition of starch and an increased accumulation of soluble sugars, particularly sucrose []. They found that the accumulation of total pentoses and total ketoses was increased by GA3. As starch breakdown is influenced by the sink demand, sucrose has been suggested to play a signal role in the systemic control of starch synthesis and breakdown []. GA3 has been reported to increase the availability of carbohydrates in flowers, and since carbohydrates are the main source of nutrition for flowers [], hormonal priming with GA3 improved the yield of flowers and the quality of saffron stigma []. GA3 affects the gene expression of invertase, and invertase activity in target tissues causes the production of hexoses (fructose and glucose), which are sources of carbon and energy for plant growth [].
Mollafilabi reported that saffron produced under controlled conditions was graded as excellent according to Iranian national standards for stigma quality []. The author also stated that 1.38 flowers per corm were produced when saffron corms were kept under a controlled environment for 90 days during the flower initiation stage and then transferred to the flowering room during the flower emergence stage.
5. Conclusions
As the main chemical compounds, secondary metabolites play an important role in the saffron quality. The percentage of secondary metabolites determines the quality of saffron flowers. This study, for the first time, evaluated the interaction of PGRs and different light spectra on the flowering yield and phytochemical performance of saffron plants. Improvement of yield and quality of saffron in indoor farming was targeted in this study. According to the findings of the present study, the application of GABA with a combination of red and blue lights led to better flower production with a higher stigma yield. Additionally, this treatment also positively affected the contents of safranal and picrocrocin. Meanwhile, the application of GA3 with a combination of red and blue lights led to the highest content of crocin. Ultimately, the utilization of PGRs and artificial light plays a crucial role in the enhancement of secondary metabolites and the improvement of physiological attributes of saffron. PCA was used for interpretation of the results and it confirmed our findings. The positive effect of PGRs on flowers number per corm, flower and stigma fresh weight and dry weight was determined for saffron plants. This experiment revealed that quality of stigma is influenced by the exogenous application of PGRs, especially GABA. Red and blue light spectra are necessary for plant photomorphogenesis, and their combination led to better yield and quality of saffron flowers.
Overall, the results of this research set up the best production protocol for saffron, and developing this protocol guarantees optimized conditions to produce top-quality saffron indoors.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/horticulturae9020169/s1, Table S1. Experimental design for arranging the treatments in saffron.
Author Contributions
Conceptualization, M.E. and M.G.J.; methodology, M.G.J. and S.A.; formal analysis, M.E.; investigation, M.E.; data curation, M.E.; writing—original draft preparation, M.E.; writing—review and editing, M.G.J., S.A. and S.N.; supervision, M.G.J. and S.A.; project administration, M.G.J.; funding acquisition, S.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the University of Tehran and partly by the Italian Ministry of Education and Research, Project MUR - PRIN 2020, Ref. 2020ELWM82.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding authors. The data are not public.
Acknowledgments
The authors would like to thank Hamid Ahadi for his technical support in the phytochemical analyses.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gernot Katzer’s Spice Pages. Available online: http://gernot-katzers-spice-pages.com/engl/Croc_sat.html (accessed on 23 October 2022).
- McGee, H. On Food and Cooking: The Science and Lore of the Kitchen, 2nd ed.; Scribner: New York, NY, USA, 2004; p. 423. [Google Scholar]
- Cardone, L.; Castronuovo, D.; Perniola, M.; Cicco, N.; Candido, V. Saffron (Crocus sativus L.), the king of spices: An overview. Sci. Hortic. 2020, 272, 109560. [Google Scholar] [CrossRef]
- Gresta, F.; Lombardo, G.M.; Siracusa, L.; Ruberto, G. Saffron, an alternative crop for sustainable agricultural systems. A review. Agron. Sustain. Dev. 2008, 28, 95–112. [Google Scholar] [CrossRef]
- Ghorbani, R.; Koocheki, A. Sustainable cultivation of saffron in Iran. In Sustainable Agriculture Reviews; Lichtfouse, E., Ed.; Springer International Publishing: Cham, Switzerland, 2017; Volume 25, pp. 170–171. [Google Scholar]
- Business Insider. Available online: https://www.businessinsider.com/why-saffron-world-most-expensive-spice-2018-4 (accessed on 29 October 2022).
- BBC News. Available online: https://www.bbc.com/news/business-41110151 (accessed on 23 October 2022).
- Kothari, D.; Thakur, R.; Kumar, R. Saffron (crocus sativus L.): Gold of the spices-a comprehensive review. Hortic. Environ. Biotechnol. 2021, 62, 661–677. [Google Scholar] [CrossRef]
- Good Practices for Saffron Production in Kashmir. Available online: https://diragrijmu.nic.in/saffron/saffron-manual.pdf (accessed on 9 January 2023).
- Ting, K.C.; Lin, T.; Davidson, P.C. Integrated urban controlled environment agriculture systems. In LED Lighting for Urban Agriculture; Kozai, T., Fujiwara, K., Runkle, E.S., Eds.; Springer: Singapore, 2016; pp. 19–36. [Google Scholar]
- Moradi, S.; Kafi, M.; Aliniaeifard, S.; Salami, S.A.; Shokrpour, M.; Pedersen, C.; Moosavi-Nezhad, M.; Wróbel, J.; Kalaji, H.M. Blue light improves photosynthetic performance and biomass partitioning toward harvestable organs in saffron (Crocus sativus L.). Cells 2021, 10, 1994. [Google Scholar] [CrossRef] [PubMed]
- Controlled Environment Agriculture Center. The University of Arizona. Available online: https://ceac.arizona.edu/about/about-us (accessed on 29 October 2022).
- Birkby, J. Vertical Farming. ATTRA Sustain. Agric. 2016. Available online: https://attra.ncat.org/publication/Vertical-Farming/ (accessed on 16 January 2022).
- Tunio, M.H.; Gao, J.M.; Qureshi, W.A.; Sheikh, S.A.; Chen, J.D.; Chandio, F.A.; Lakhiar, I.A.; Solangi, K.A. Effects of droplet size and spray interval on root-to-shoot ratio, photosynthesis efficiency, and nutritional quality of aeroponically grown butterhead lettuce. Int. J. Agric. Biol. Eng. 2022, 15, 79–88. [Google Scholar] [CrossRef]
- Uddin, M.R.; Suliaman, M.F. Energy Efficient Smart Indoor Fogponics Farming System. In Proceedings of the 3rd International Conference on Smart City Innovation, Bali, Indonesia, 5–6 August 2020; IOP Conference Series: Earth and Environmental Science. Volume 673, p. 012012. [Google Scholar]
- Aghhavani-Shajari, M.; Fallahi, H.R.; Sahabi, H.; Kaveh, H.; Branca, F. Production systems and methods affect the quality and the quantity of saffron (Crocus sativus L.). Span. J. Agric. Res. 2021, 19, 14. [Google Scholar] [CrossRef]
- Zhou, T.; Qiu, X.; Zhao, L.; Yang, W.; Wen, F.; Wu, Q.; Yan, J.; Xu, B.; Chen, J.; Ma, Y.; et al. Optimal light intensity and quality increased the saffron daughter corm yield by inhibiting the degradation of reserves in mother corms during the reproductive stage. Ind. Crops Prod. 2022, 176, 114396. [Google Scholar] [CrossRef]
- Jung, W.S.; Chung, I.M.; Hwang, M.H.; Kim, S.H.; Yu, C.Y.; Ghimire, B.K. Application of light-emitting diodes for improving the nutritional quality and bioactive compound levels of some crops and medicinal plants. Molecules 2021, 26, 1477. [Google Scholar] [CrossRef]
- Kozai, T.; Niu, G.; Takagaki, M. Plant Factory: An Indoor Vertical Farming System for Efficient Quality Food Production, 2nd ed.; Academic Press: London, UK, 2020; pp. 7–33. [Google Scholar]
- Benke, K.; Tomkins, B. Future food-production systems: Vertical farming and controlled-environment agriculture. Sustain. Sci. Pract. Policy 2017, 13, 13–26. [Google Scholar] [CrossRef]
- Aalifar, M.; Aliniaeifard, S.; Arab, M.; Mehrjerdi, M.Z.; Daylami, S.D.; Serek, M.; Woltering, E.; Li, T. Blue light improves vase life of carnation cut flowers through its effect on the antioxidant defense system. Front. Plant Sci. 2020, 11, 511. [Google Scholar] [CrossRef] [PubMed]
- Amiri, A.; Kafi, M.; Kalate-Jari, S.; Matinizadeh, M. Tulip response to different light sources. Anim. Plant Sci. 2018, 28, 539–545. [Google Scholar]
- Ghorbanzadeh, P.; Aliniaeifard, S.; Esmaeili, M.; Mashal, M.; Azadegan, B.; Seif, M. Dependency of growth, water use efficiency, chlorophyll fluorescence, and stomatal characteristics of lettuce plants to light intensity. Plant Growth Regul. 2020, 40, 2191–2207. [Google Scholar] [CrossRef]
- Seif, M.; Aliniaeifard, S.; Arab, M.; Mehrjerdi, M.Z.; Shomali, A.; Fanourakis, D.; Li, T.; Woltering, E. Monochromatic red light during plant growth decreases the size and improves the functionality of stomata in chrysanthemum. Funct. Plant Biol. 2021, 48, 515–528. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Rasul, F.; Li, W.; Tian, H.; Mo, Z.; Duan, M.; Tang, X. Roles of plant growth regulators on yield, grain qualities and antioxidant enzyme activities in super hybrid rice (Oryza sativa L.). Rice 2013, 6, 9. [Google Scholar] [CrossRef]
- Martinez, M.E.; Jorquera, L.; Poirrier, P.; Díaz, K.; Chamy, R. Effect of the carbon source and plant growth regulators (PGRs) in the induction and maintenance of an in vitro callus culture of Taraxacum officinale (L.) weber Ex F.H. Wigg. Agronomy 2021, 11, 1181. [Google Scholar] [CrossRef]
- Prins, C.L.; Vieira, I.J.C.; Freitas, S.P. Growth regulators and essential oil production. Braz. J. Plant Physiol. 2010, 22, 91–102. [Google Scholar] [CrossRef]
- Shelp, B.J.; Bown, A.W.; McLean, M.D. Metabolism and functions of gamma-aminobutyric acid. Trends Plant Sci. 1999, 4, 446–452. [Google Scholar] [CrossRef]
- Ramesh, S.A.; Tyerman, S.D.; Xu, B.; Bose, J.; Kaur, S.; Conn, V.; Domingos, P.; Ullah, S.; Wege, S.; Shabala, S.; et al. GABA signalling modulates plant growth by directly regulating the activity of plant-specific anion transporters. Nat. Commun. 2015, 6, 7879. [Google Scholar] [CrossRef]
- Ramesh, S.A.; Tyerman, S.D.; Gilliham, M.; Xu, B. γ-Aminobutyric acid (GABA) signalling in plants. Cell. Mol. Life Sci. 2017, 74, 1577–1603. [Google Scholar] [CrossRef]
- Seifikalhor, M.; Aliniaeifard, S.; Hassani, B.; Niknam, V.; Lastochkina, O. Diverse role of γ-aminobutyric acid in dynamic plant cell responses. Plant Cell Rep. 2019, 38, 847–867. [Google Scholar] [CrossRef]
- Bouché, N.; Fromm, H. GABA in plants: Just a metabolite? Trends Plant Sci. 2004, 9, 110–115. [Google Scholar] [CrossRef]
- Roberts, M.R. Does GABA act as a signal in plants? Hints from molecular studies. Plant Signal Behav. 2007, 2, 408–409. [Google Scholar] [CrossRef]
- Silva, A.L.L.; Rodrigues, C.; Costa, J.D.L.; Machado, M.P.; Penha, R.D.O.; Biasi, L.A.; Vandenberghe, L.P.D.S.; Soccol, C.R. Gibberellic acid fermented extract obtained by solid-state fermentation using citric pulp by Fusarium moniliforme: Influence on Lavandula angustifolia Mill. cultivated in vitro. Pak. J. Bot. 2013, 45, 2057–2064. [Google Scholar]
- Camara, M.C.; Rodrigues, C.; da Silva, A.L.L.; Vandenberghe, L.; Soccol, C. General aspects and applications of gibberelins and gibberellic acid in plants. In Gibberellins and Gibberellic Acid: Biosynthesis, Regulation and Physiological Effects, 1st ed.; Hardy, J., Ed.; Nova Science Publishers: New York, NY, USA, 2015; pp. 1–21. [Google Scholar]
- Keshtkar, H.R.; Azarnivand, H.; Etemad, V.; Moosavi, S.S. Seed dormancy-breaking and germination requirements of Ferula ovina and Ferula gummosa. Desert 2008, 13, 45–51. [Google Scholar]
- Riley, J.M. Gibberellic acid for fruit set and seed germination. CRFG 1987, 19, 10–12. [Google Scholar]
- Edwards, M. Dormancy in seeds of charlock (Sinapis arvensis L.): Early effects of gibberellic acid on the synthesis of amino acids and proteins. Plant Physiol. 1976, 58, 626–630. [Google Scholar] [CrossRef] [PubMed]
- Chrungoo, N.K.; Farooq, S. Influence of GA3 and NAA on certain carbohydrate fractions in corms of saffron crocus (Crocus sativus L.) during development. Acta Soc. Bot. Pol. 1989, 58, 237–246. [Google Scholar] [CrossRef]
- Braidot, E.; Petrussa, E.; Peresson, C.; Patui, S.; Bertolini, A.; Tubaro, F.; Wählby, U.; Coan, M.; Vianello, A.; Zancani, M. Low-intensity light cycles improve the quality of lamb’s lettuce (Valerianella olitoria L. Pollich) during storage at low temperature. Postharvest Biol. Technol. 2014, 90, 15–23. [Google Scholar] [CrossRef]
- Chen, X.; Guo, W.; Xue, X.; Wang, L.; Qiao, X. Growth and quality responses of ‘Green Oak Leaf’ lettuce as affected by monochromic or mixed radiation provided by fluorescent lamp (FL) and light-emitting diode (LED). Sci. Hortic. 2014, 172, 168–175. [Google Scholar] [CrossRef]
- Chung, I.M.; Paudel, N.; Kim, S.H.; Yu, C.Y.; Ghimire, B.K. The influence of light wavelength on growth and antioxidant capacity in Pachyrhizus erosus (L.) Urban. Plant Growth Regul. 2020, 39, 296–312. [Google Scholar] [CrossRef]
- Kopsell, D.A.; Sams, C.E.; Barickman, T.C.; Morrow, R.C. Sprouting broccoli accumulate higher concentrations of nutritionally important metabolites under narrow-band light-emitting diode lighting. Amer. Soc. Hort. Sci. 2014, 139, 469–477. [Google Scholar] [CrossRef]
- Samuolienė, G.; Brazaitytė, A.; Jankauskienė, J.; Viršilė, A.; Sirtautas, R.; Novičkovas, A.; Sakalauskienė, S.; Sakalauskaitė, J.; Duchovskis, P. LED irradiance level affects growth and nutritional quality of Brassica microgreens. Cent. Eur. J. Biol. 2013, 8, 1241–1249. [Google Scholar] [CrossRef]
- Samuolienė, G.; Brazaitytė, A.; Sirtautas, R.; Viršilė, A.; Sakalauskaitė, J.; Sakalauskienė, S.; Duchovskis, P. LED illumination affects bioactive compounds in romaine baby leaf lettuce. Sci. Food Agric. 2013, 93, 3286–3291. [Google Scholar] [CrossRef]
- Renau-Morata, B.; Nebauer, S.G.; Sánchez, M.; Molina, R.V. Effect of corm size, water stress and cultivation conditions on photosynthesis and biomass partitioning during the vegetative growth of saffron (Crocus sativus L.). Ind. Crops Prod. 2012, 39, 40–46. [Google Scholar] [CrossRef]
- Molina, R.V.; Valero, M.; Navarro, Y.; Guardiola, J.L.; García-Luis, A. Temperature effects on flower formation in saffron (Crocus sativus L.). Sci. Hortic. 2005, 103, 361–379. [Google Scholar] [CrossRef]
- Molina, R.V.; Valero, M.; Navarro, Y.; García-Luis, A.; Guardiola, J.L. Low temperature storage of corms extends the flowering season of saffron (Crocus sativus L.). Hortic. Sci. Biotechnol. 2005, 80, 319–326. [Google Scholar] [CrossRef]
- Kalhor, M.S.; Aliniaeifrad, S.; Seif, M.; Asayesh, E.J.; Bernard, F.; Hassani, B.; Li, T. Enhanced salt tolerance and photosynthetic performance: Implication of γ-amino butyric acid application in salt-exposed lettuce (Lactuca sativa L.) plants. Plant Physiol. Biochem. 2018, 130, 157–172. [Google Scholar] [CrossRef]
- Mzabri, I.; Rimani, M.; Kouddane, N.; Berrichi, A. Study of the effect of pretreatment of corms by different concentrations of gibberellic acid and at different periods on the growth, flowering, and quality of saffron in eastern Morocco. Adv. Agric. 2021, 2021, 9979827. [Google Scholar] [CrossRef]
- Fallahi, H.R.; Abbasi Aval Bohlooli, S.; Pahlavan, Z.; Hosseini, S.M.; Hosseini, S.A.H.; Ghohestani-Bojd, P. Saffron vegetative growth as affected by transplanting and direct corm planting under field conditions. J. Hortic. Postharvest Res. 2021, 4, 1–10. [Google Scholar] [CrossRef]
- ISO 3632-2:2010(en); Spices—Saffron (Crocus sativus L.)—Part 2: Test methods. International Organization for Standardization: Geneva, Switzerland, 2010. Available online: https://www.iso.org/obp/ui/#iso:std:iso:3632:-2:ed-2:v1:en (accessed on 29 October 2022).
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria; Available online: https://www.r-project.org (accessed on 29 October 2022).
- Jolliffe, I.T. Principal Component Analysis, 2nd ed.; Springer-Verlag Press: New York, NY, USA, 1986. [Google Scholar]
- Lage, M.; Cantrell, C.L. Quantification of saffron (Crocus sativus L.) metabolites crocins, picrocrocin and safranal for quality determination of the spice grown under different environmental Moroccan conditions. Sci. Hortic. 2009, 121, 366–373. [Google Scholar] [CrossRef]
- Esmaeili, S.; Aliniaeifard, S.; Dianati Daylami, S.; Karimi, S.; Shomali, A.; Didaran, F.; Telesiński, A.; Sierka, E.; Kalaji, H.M. Elevated light intensity compensates for nitrogen deficiency during chrysanthemum growth by improving water and nitrogen use efficiency. Sci. Rep. 2022, 12, 10002. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, R.T. Distinct responses to light in plants. Plants 2020, 9, 894. [Google Scholar] [CrossRef]
- Parks, B.M. The red side of photomorphogenesis. Plant Physiol. 2003, 133, 1437–1444. [Google Scholar] [CrossRef] [PubMed]
- Khalighi, S.; Zare Mehrjerdi, M.; Aliniaefard, S. Light quality affects phytochemicals content and antioxidant capacity of Satureja hortensis. South-West. J. Hortic. Biol. Environ. 2021, 12, 99–117. [Google Scholar]
- Yang, L.; Wen, K.S.; Ruan, X.; Zhao, Y.X.; Wei, F.; Wang, Q. Response of plant secondary metabolites to environmental factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef]
- Meshram, J.H.; Singh, S.B.; Raghavendra, K.P.; Waghmare, V.N. Drought stress tolerance in cotton: Progress and perspectives. In Climate Change and Crop Stress: Molecules to Ecosystems, 1st ed.; Shanker, A.K., Shanker, C., Anand, A., Maheswari, M., Eds.; Academic Press: London, UK, 2022; pp. 135–169. [Google Scholar]
- Patel, A.; Tiwari, S.; Parihar, P.; Singh, R.; Prasad, S.M. Carbon nanotubes as plant growth regulators: Impacts on growth, reproductive system, and soil microbial community. In Nanomaterials in Plants, Algae and Microorganisms: Concepts and Controversies, 1st ed.; Tripathi, D.K., Ahmad, P., Sharma, S., Chauhan, D.K., Dubey, N.K., Eds.; Academic Press: London, UK, 2019; Volume 2, pp. 23–42. [Google Scholar]
- Sabagh, A.E.; Mbarki, S.; Hossain, A.; Iqbal, M.A.; Islam, M.S.; Raza, A.; Llanes, A.; Reginato, M.; Rahman, M.A.; Mahboob, W.; et al. Potential role of plant growth regulators in administering crucial processes against abiotic stresses. Front. Agron. 2021, 3, 648694. [Google Scholar] [CrossRef]
- Hogewoning, S.W.; Trouwborst, G.; Maljaars, H.; Poorter, H.; van Ieperen, W.; Harbinson, J. Blue light dose-responses of leaf photosynthesis, morphology, and chemical composition of Cucumis sativus grown under different combinations of red and blue light. Exp. Bot. 2010, 61, 3107–3117. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.; Fiebig, A.; Noga, G.; Hunsche, M. Influence of light quality on leaf physiology of sweet pepper plants grown under drought. Theor. Exp. Plant Physiol. 2018, 30, 287–296. [Google Scholar] [CrossRef]
- Naznin, M.T.; Lefsrud, M.; Gravel, V.; Azad, M.O.K. Blue light added with red LEDs enhance growth characteristics, pigments content, and antioxidant capacity in lettuce, spinach, kale, basil, and sweet pepper in a controlled environment. Plants 2019, 8, 93. [Google Scholar] [CrossRef]
- Li, Y.; Xin, G.; Liu, C.; Shi, Q.; Yang, F.; Wei, M. Effects of red and blue light on leaf anatomy, CO2 assimilation and the photosynthetic electron transport capacity of sweet pepper (Capsicum annuum L.) seedlings. BMC Plant Biol. 2020, 20, 318. [Google Scholar] [CrossRef] [PubMed]
- Kajikawa, N.; Uno, Y.; Kuroki, S.; Miyagawa, S.; Yamashita, Y.; Hamaguchi, Y.; Ueda, Y.; Kobayashi, M.; Kaji, K.; Itoh, H. Effect of far-red light on saffron (crocus sativus L.) growth and crocin yields. Environ. Control Biol. 2018, 56, 51–57. [Google Scholar] [CrossRef]
- Sng, B.J.R.; Mun, B.; Mohanty, B.; Kim, M.; Phua, Z.W.; Yang, H.; Lee, D.Y.; Jang, I.C. Combination of red and blue light induces anthocyanin and other secondary metabolite biosynthesis pathways in an age-dependent manner in Batavia lettuce. Plant Sci. 2021, 310, 110977. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, J.; Ashraf, U.; Li, G.; Li, Y.; Lu, W.; Gao, L.; Han, F.; Hu, J. Exogenous γ-aminobutyric acid (GABA) application improved early growth, net photosynthesis, and associated physio-biochemical events in maize. Front. Plant Sci. 2016, 7, 919. [Google Scholar] [CrossRef]
- Fait, A.; Nesi, A.N.; Angelovici, R.; Lehmann, M.; Pham, P.A.; Song, L.; Haslam, R.P.; Napier, J.A.; Galili, G.; Fernie, A.R. Targeted enhancement of glutamate-to-γ-aminobutyrate conversion in Arabidopsis seeds affects carbon-nitrogen balance and storage reserves in a development-dependent manner. Plant Physiol. 2011, 157, 1026–1042. [Google Scholar] [CrossRef] [PubMed]
- Suo-ling, S.; Jing-rui, L.; Hong-bo, G.; Qing-yun, L.; Li-wen, Y.; Rui-juan, G. Effects of exogenous γ-aminobutyric acid on inorganic nitrogen metabolism and mineral elements contents of melon seedling under hypoxia stress. Acta Hortic. 2012, 4, 012. [Google Scholar]
- Jin, H.; Dilworth, M.; Glenn, A. 4-Aminobutyrate is not available to bacteroids of cowpea rhizobium MNF2030 in snake bean nodules. Arch. Microbiol. 1990, 153, 455–462. [Google Scholar] [CrossRef]
- Vijayakumari, K.; Puthur, J.T. γ-Aminobutyric acid (GABA) priming enhances the osmotic stress tolerance in Piper nigrum Linn. plants subjected to PEG-induced stress. Plant Growth Regul. 2016, 78, 57–67. [Google Scholar] [CrossRef]
- Hijaz, F.; Nehela, Y.; Killiny, N. Application of gamma-aminobutyric acid increased the level of phytohormones in Citrus sinensis. Planta 2018, 248, 909–918. [Google Scholar] [CrossRef]
- Ramos-Ruiz, R.; Martinez, F.; Knauf-Beiter, G. The effects of GABA in plants. Cogent Food Agric. 2019, 5, 1670553. [Google Scholar] [CrossRef]
- Kaur, R.; Zhawar, V.K. Regulation of secondary antioxidants and carbohydrates by gamma-aminobutyric acid under salinity-alkalinity stress in rice (Oryza sativa L.). Biologia Futura 2021, 72, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, S. Gibberellin metabolism and its regulation. Annu. Rev. Plant Biol. 2008, 59, 225–251. [Google Scholar] [CrossRef] [PubMed]
- Stitt, M.; Zeeman, S.C. Starch turnover: Pathways, regulation and role in growth. Curr. Opin. Plant Biol. 2012, 15, 282–292. [Google Scholar] [CrossRef]
- Silva, J.A.T. The cut flower: Postharvest considerations. J. Biol. Sci. 2003, 3, 406–442. [Google Scholar] [CrossRef]
- Javid, M.G.; Hoseinifard, M.S.; Allahdadi, I.; Soltani, E. Hormonal priming with BAP and GA3 induces improving yield and quality of saffron flower through promotion of carbohydrate accumulation in corm. Plant Growth Regul. 2022, 41, 205–215. [Google Scholar] [CrossRef]
- Mortazavi, H.; Asil, M.H. Effects of temperature and gibberellic acid on forcing and quality improvement of Iris (Iris hollandica cv. ‘Blue Magic’) cut flowers. Agric. Sci. Sustain. Prod. 2010, 20, 1–14. [Google Scholar]
- Mollafilabi, A. Effect of New Cropping Technologies on Growth Characteristics, Yield, Yield Components of Flower and Corm Criteria of Saffron (Crocus sativus L.). Ph. D. Thesis, Ferdowsi University of Mashhad, Mashhad, Iran, 2014. (In Persian with English summary). [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).