The Application of Entomophagous and Acariphagous Species in Biological Protection Systems of an Apple Orchard (Malus domestica Borkh)
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Laboratory Investigation of the Parasitism of H. hebetor Natural Population on C. pomonella
3.2. Molecular Genetic Analysis for H. hebetor Population Quality Assessment
3.3. Sensitivity of Entomophagous Species to Biological and Chemical Insecticides
3.4. Field Evaluations of the Efficacy of the Entomophagous Species in Pests Control
3.4.1. Habrobracon Hebetor to Control C. pomonella
3.4.2. Aphidophagous Predators to Control Aphids
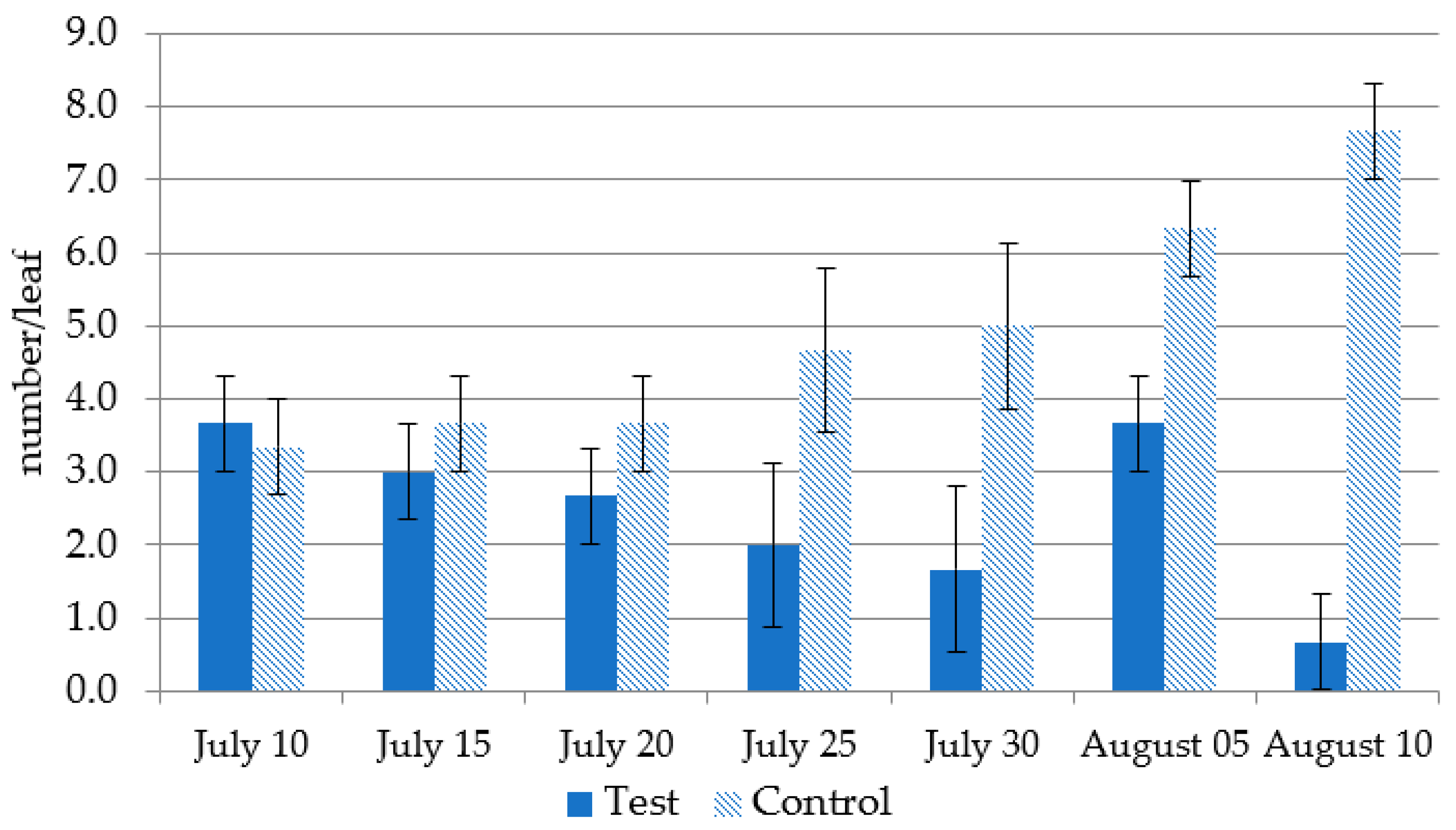
3.4.3. Application of Acariphagous Species to Suppress Tetraniquid Mite Populations during the 2019–2020 Growing Seasons; Two Dominant Species Were Identified on the Apple Tree: The Red Spider Mite T. urticae and the European Red Mite P. ulmi
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- FAOSTAT—Food and Agricultural Organization of the United Nations. Apples—Production Quantity in 2017. 2021. Available online: https://www.fao.org/faostat/en/#data/QCL/visualize (accessed on 30 January 2023).
- Cherniy, A.M.; Balykina, E.B. Ecological niches and their role in the formation of the arthropod fauna of the apple orchard. Plant Prot. Quar. 2014, 5, 15–20. [Google Scholar]
- Mazzi, D.; Dorn, S. Movement of insect pests in agricultural landscapes. Ann. Appl. Biol. 2012, 160, 97–113. [Google Scholar] [CrossRef]
- Ju, D.; Mota-Sanchez, D.; Fuentes-Contreras, E.; Zhang, Y.L.; Wang, X.Q.; Yang, X.Q. Insecticide resistance in the Cydia pomonella (L): Global status, mechanisms, and research directions. Pestic. Biochem. Physiol. 2021, 178, 104925. [Google Scholar] [CrossRef] [PubMed]
- Bass, C.; Denholm, I.; Williamson, M.S.; Nauen, R. The global status of insect resistance to neonicotinoid insecticides. Pestic. Biochem. Physiol. 2015, 121, 78–87. [Google Scholar] [CrossRef]
- Glinushkin, A.P.; Yakovleva, I.N.; Meshkov, Y.I. Monitoring Spider Mites For Resistance To Pesticides In The Russia Protected Ground, All-Russian conference with international participation Economic and Phytosanitary Rationale for the Introduction of Feed Plants. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Bol’shie Vyazemy, Russia, 10–11 June 2020. [Google Scholar] [CrossRef]
- Agasyeva, I.S.; Ismailov, V.Y. The role of biotechnology in biological plant protection. Work. Kuban State Agrar. Univ. 2016, 58, 67–74. [Google Scholar]
- Baudry, J.; Pointereau, P.; Seconda, L.; Vidal, R.; Taupier-Letage, B.; Langevin, B.; Allès, B.; Galan, P.; Hercberg, S.; Amiot, M.J.; et al. Improvement of diet sustainability with increased level of organic food in the diet: Findings from the BioNutriNet cohort. Am. J. Clin. Nutr. 2019, 109, 1173–1188. [Google Scholar] [CrossRef]
- Giampieri, F.; Mazzoni, L.; Cianciosi, D.; Alvarez-Suarez, J.M.; Regolo, L.; Sánchez-González, C.; Capocasa, F.; Xiao, J.; Mezzetti, B.; Battino, M. Organic vs conventional plant-based foods: A review. Food Chem. 2022, 383, 132352. [Google Scholar] [CrossRef]
- Samnegård, U.; Alins, G.; Boreux, V.; Bosch, J.; García, D.; Happe, A.-K.; Klein, A.-M.; Miñarro, M.; Karsten, M.; Porcel, M.; et al. Management trade-offs on ecosystem services in apple orchards across Europe: Direct and indirect effects of organic production. J. Appl. Ecol. 2019, 56, 802–811. [Google Scholar] [CrossRef]
- Herz, A.; Cahenzli, F.; Penvern, S.; Pfiffner, L.; Tasin, M.; Sigsgaard, L. Managing Floral Resources in Apple Orchards for Pest Control: Ideas, Experiences and Future Directions. Insects 2019, 10, 247. [Google Scholar] [CrossRef]
- Piekarska-Boniecka, H.; Rzanska-Wieczorek, M.; Siatkowski, I.; Zyprych-Walczak, J. Controlling the Abundance of the Rose Tortrix Moth [Archips Rosana (L.)] by Parasitoids in Apple Orchards in Wielkopolska, Poland. Plant Prot. Sci. 2019, 55, 265–272. [Google Scholar] [CrossRef]
- Gontijo, L.M.; Cockfield, S.D.; Beers, E.H. Natural enemies of woolly apple aphid (Hemiptera: Aphididae) in Washington State. Environ. Entomol. 2012, 41, 1364–1371. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, R.Z.; Zhang, F. Research progress on biology and ecology of Harmonia axyridis Pallas (Coleoptera: Coccinellidae). Ying Yong Sheng Tai Xue Bao 2007, 18, 26. [Google Scholar]
- Balykina, E.B.; Yagodinskaya, L.P.; Rybareva, T.S.; Korzh, D.A.; Ivanova, O.V. Regulation of spider mites number in apple orchards of the Crimea by the method of "Flooding" of phytoseid mites. Zemledelie 2020, 7, 30–34. [Google Scholar] [CrossRef]
- Kaçar, G. Bioecologies of Pests, Natural Enemies in Apple Orchards of Seben (Bolu). Uluslararası Tarım Ve Yaban Hayatı Bilim. Derg. 2019, 5, 286–291. [Google Scholar] [CrossRef]
- Schmidt-Jeffris, R.A.; Beers, E.H. Potential impacts of orchard pesticides on Tetranychus urticae: A predator-prey perspective. Crop Prot. 2018, 103, 56–64. [Google Scholar] [CrossRef]
- Ismailov, V.Y.; Agasyeva, I.S.; Nastasy, A.S. Habrobracon hebetor Say-an effective parasite against codling moth. Hort. Vitic. 2020, 2, 52–57. [Google Scholar] [CrossRef]
- Agas’eva, I.S.; Ismailov, V.Y.; Fedorenko, E.V.; Nevedova, M.V. Results of approbation of entomoacariphagous in biological protection systems of organic orchards. Inf. Byulleten VPRS MOBB 2017, 52, 24–27. [Google Scholar]
- Shirinyan, Z.A.; Ismailov, V.Y.; Kvasenkov, O.I. Method for the Production of a Nutrient Medium for Breeding Galleria mellonella L. RU Patent 2215428 C2; 2001131299/13, 21 November 2001. [Google Scholar]
- Kovalenkov, V.G.; Tyurina, N.M.; Shirinyan, Z.A.; Ismailov, V.Y. Breeding technology of entomophagous. Production of environmentally safe crop production: Regional recommendations. Pushchino 1996, 2, 22–34. [Google Scholar]
- Konovalova, T.V. Laboratory maintenance and breeding of the large wax moth Galleria mellonella L. Rus. Vet. J. Farm Anim. 2009, 4, 46–48. [Google Scholar]
- Agasieva, I.S.; Ismailov, V.Y.; Fedorenco, E.V.; Sagitov, A.O.; Mukhtarkhanova, A.A.; Chadinova, A.M. Improving the methods of breeding, storing, and using beaked mites for biological pest control. Ecol. Environ. Conserv. 2020, 26, S243–S246. [Google Scholar]
- Yeh, F.C.; Yang, R.C.; Boyle, T.B.J.; Ye, Z.H.; Mao, J.X. POPGENE, the User-Friendly Shareware for Population Genetic Analysis, Version 1.31; University of Alberta and Centre for International Forestry Research: Edmonton, AB, Canada, 1999; pp. 102–120. [Google Scholar]
- Agasyeva, I.S.; Fedorenko, E.F.; Nefedova, M.V.; Ismailov, V.Y. Effectiveness assessment of biological plant protection products against the main corn pests. Oil Cult. 2019, 3, 124–129. [Google Scholar] [CrossRef]
- Agasieva, I.S.; Ismailov, V.Y.; Nefedova, M.V.; Fedorenko, E.F.; Nastasy, A.S. Development of methods for combating cotton bollworm on corn for eco-friendly farming. Tauride Bull. Agrar. Sci. 2020, 3, 8–17. [Google Scholar] [CrossRef]
- Frolov, A.N. Biotic factors of depression in the European corn borer. Her. Plant Prot. 2014, 2, 37–47. [Google Scholar]
- Kovalenkov, V.G.; Tyurina, N.M.; Ismailov, V.Y. Technology of Cultivation and Application of the Ectoparasitoid H. Hebetor. Methodical Instructions; Rossel’hozakademiya: Moscow, Russia, 1995; p. 48. [Google Scholar]
- Kostyukov, V.V.; Kosheleva, O.V.; Balakhnina, I.V. Determinant of Parasites of Fruit Orchard Pests; DSM: Rostov-on-Don, Russia, 2007; p. 254. [Google Scholar]
- Kil, V.I. Using RAPD PCR Method for Studying DNA Polymorphism of Agricultural Importance Arthropods. Agric. Res. Technol. 2017, 9, 555769. [Google Scholar] [CrossRef]
- Suchail, S.; Le Navenant, A.; Capowiez, Y.; Thiéry, A.; Rault, M. An exploratory study of energy reserves and biometry as potential tools for assessing the effects of pest management strategies on the earwig, Forficula auricularia L. Environ. Sci. Pollut. Res. Int. 2018, 25, 22774. [Google Scholar] [CrossRef]
- Braak, N.; Neve, R.; Jones, A.K.; Gibbs, M.; Breuker, C.J. The effects of insecticides on butterflies—A review. Environ. Pollut. 2018, 242, 507–518. [Google Scholar] [CrossRef]
- Ricupero, M.; Desneux, N.; Zappalà, L.; Biondi, A. Target and non-target impact of systemic insecticides on a polyphagous aphid pest and its parasitoid. Chemosphere 2020, 247, 125728. [Google Scholar] [CrossRef]
- Umina, P.A.; Bass, C.; van Rooyen, A.; Chirgwin, E.; Arthur, A.L.; Pym, A.; Mackisack, J.; Mathews, A.; Kirkland, L. Spirotetramat resistance in Myzus persicae (Sulzer) (Hemiptera: Aphididae) and its association with the presence of the A2666V mutation. Pest. Manag. Sci. 2022, 78, 4822–4831. [Google Scholar] [CrossRef]
- Wang, W.; Huang, Q.; Liu, X.; Liang, G. Differences in the Sublethal Effects of Sulfoxaflor and Acetamiprid on the Aphis gossypii Glover (Homoptera: Aphididae) Are Related to Its Basic Sensitivity Level. Insects 2022, 13, 498. [Google Scholar] [CrossRef]
- Margaritopoulos, J.T.; Kati, A.N.; Voudouris, C.C.; Skouras, P.J.; Tsitsipis, J.A. Long-term studies on the evolution of resistance of Myzus persicae (Hemiptera: Aphididae) to insecticides in Greece. Bull. Entomol. Res. 2021, 111, 1–16. [Google Scholar] [CrossRef]
- Van Leeuwen, T.; Vontas, J.; Tsagkarakou, A.; Dermauw, W.; Tirry, L. Acaricide resistance mechanisms in the two-spotted spider mite Tetranychus urticae and other important Acari: A review. Insect Biochem. Mol. Biol. 2010, 40, 563–572. [Google Scholar] [CrossRef]
- Parmagnani, A.S.; Mannino, G.; Brillada, C.; Novero, M.; Dall’Osto, L.; Maffei, M.E. Biology of Two-Spotted Spider Mite (Tetranychus urticae): Ultrastructure, Photosynthesis, Guanine Transcriptomics, Carotenoids and Chlorophylls Metabolism, and Decoyinine as a Potential Acaricide. Int. J. Mol. Sci. 2023, 24, 1715. [Google Scholar] [CrossRef]
- Rameshgar, F.; Khajehali, J.; Nauen, R.; Bajda, S.; Jonckheere, W.; Dermauw, W.; Van Leeuwen, T. Point mutations in the voltage-gated sodium channel gene associated with pyrethroid resistance in Iranian populations of the European red mite Panon. Pestic. Biochem. Physiol. 2019, 157, 80–87. [Google Scholar] [CrossRef]



| Option | Release Rate, Individuals/ha |
|---|---|
| aphidophages | |
| Coccinellidae (Harmonia axyridis Pallas, Leis dimidiata Fabr., Cycloneda sangvinea L.)—larvae | 2000–3000 |
| Aphidius colemani Vier.—imago | 10,000 |
| Control | without release |
| acariphagous | |
| Metaseiulus occidentalis Nesb. + Amblyseius andersoni Athias-Henriot | 10,000–12,000 |
| Control | without release |
| Codling Moth, Caterpillars | Number of Caterpillars, Ind. | Including: | The Number of Cocoons Formed, Ind. | Number of Emerged Parasitoids, % | |
|---|---|---|---|---|---|
| Paralyzed, % | Parasitized, % | ||||
| old age | 42 e * | 100 | 75.6 | 33 a | 78.6 |
| middle age | 34 a | 100 | 68.7 | 20 c | 54.8 |
| young age | 37 d | 0 | 0 | 0 b | 0 |
| Sample from a Population | P (%) | Na ± SD * | Ne ± SD * | H ± SD * | I ± SD * |
|---|---|---|---|---|---|
| Belgorod | 35.9 | 1.36 ± 0.48 | 1.22 ± 0.35 | 0.13 ± 0.19 | 0.19 ± 0.27 |
| Chimkent | 26.4 | 1.26 ± 0.45 | 1.16 ± 0.31 | 0.09 ± 0.17 | 0.14 ± 0.25 |
| Stavropol | 45.3 | 1.45 ± 0.50 | 1.26 ± 0.34 | 0.15 ± 0.19 | 0.23 ± 0.28 |
| Krasnodar | 64.2 | 1.64 ± 0.48 | 1.37 ± 0.35 | 0.22 ± 0.19 | 0.33 ± 0.28 |
| Sample from a Population | Belgorod | Chimkent | Krasnodar | Stavropol |
|---|---|---|---|---|
| Belgorod | – | 0.979 | 0.770 | 0.789 |
| Chimkent | 0.022 | – | 0.743 | 0.736 |
| Krasnodar | 0.261 | 0.297 | – | 0.849 |
| Stavropol | 0.237 | 0.307 | 0.164 | – |
| Preparation, Active Substance | Consumption, L/ha, kg/ha, g/ha | Cocoons before Treatment, Ind. | Imago Emergence | ||||
|---|---|---|---|---|---|---|---|
| Upon the Time of Accounting, Ind. | Total, Ind. | From the Initial Number, % | |||||
| 3rd Day | 5th Day | 7th Day | |||||
| Biological insecticides | |||||||
| Lepidocid® (Bacillus thuringiensis var. kurtsaki) | 2.0 | 69.2 | 10.2 ± 2.1 | 37.6 ± 1.6 | 7.4 ± 1.8 | 55.2 | 79.8 c * |
| FermoVirin/YP® (codling moth granulosis virus) | 1.0 | 83.4 | 22.5 ± 3.7 | 39.6 ± 2.1 | 21.3 ± 3.4 | 83.4 | 100 b |
| Biorational insecticides | |||||||
| Insegar® (phenoxycarb) | 0.6 | 80.3 | 14.7 ± 1.5 | 51.9 ± 3.3 | 12.4 ± 1.1 | 79.0 | 98.4 bc |
| Atabron® (chlorfluazuron) | 0.75 | 76.2 | 24.8 ± 3.2 | 44.3 ± 1.3 | 9 ±1.6 | 76.2 | 100 b |
| Chemical insecticides | |||||||
| Decis Expert® (deltamethrin) | 0.1 | 87.5 | 0 | 0 | 0 | 0 | 0 a |
| Control | 93.0 | 20.9 ± 1.6 | 56.2 ± 4.2 | 13.9 ± 2.3 | 93.0 | 100 b | |
| Variant | Consumption, L/ha, kg/ha | Number of Beetles before Treatment, Ind. | After Treatment by Counting Days, Ind. | Surviving Individuals on Day 5, % | ||
|---|---|---|---|---|---|---|
| 1st Day | 3rd Day | 5th Day | ||||
| Spintor® (Spinosad) | 0.5 | 26 ± 0.0 | 26 ± 0.0 | 25.7 ± 0.3 | 16.1 ± 1.9 | 61.9 |
| Madex twin® (Codling moth granulosis virus) | 0.1 | 26 ± 0.0 | 26 ± 0.0 | 25.7 ± 0.3 | 25.3 ± 0.7 | 97.3 |
| Admiral® (Piriproxifen) | 0.7 | 26 ± 0.0 | 26 ± 0.0 | 21.3 ± 1.7 | 13.0 ± 1 | 50.0 |
| Koragen® (Chloranthraniliprol) | 0.2 | 26 ± 0.0 | 26 ± 0.0 | 23 ± 1.0 | 21.3 ± 0.7 | 81.9 |
| Atabron® (Chlorofluazuron) | 0.7 | 26 ± 0.0 | 26 ± 0.0 | 25.7 ± 0.3 | 23.3 ± 0.7 | 89.6 |
| Akkar®, (Verticillum lecanii, Hirsutella thompsonii, Beauveria bassiana, Bacillus thuringiensis) | 5.0 | 26 ± 0.0 | 26 ± 0.0 | 24.7 ± 0.3 | 18.7 ± 0.3 | 71.9 |
| Control | - | 26 ± 0.0 | 26 ± 0.0 | 26 ± 0.5 | 26 ± 0.9 | - |
| Variant | Number of Caterpillars, Ind. | Number of the Infected Caterpillars, Ind. | Number of Cocoons, Pcs. | Parasitized, % | The Number of the Emerged Adult Parasitoids, % |
|---|---|---|---|---|---|
| Parasite release | 6.6 | 5.4 | 6.2 | 79.2 | 100 |
| Control (without parasite release) | 6.2 | 0.4 | 0.4 | 6.5 | 100 |
| Year | Average Number of Aphids Per Shoot | |
|---|---|---|
| Before Release of Aphidophagous Species, Ind. | 3 Weeks after the Release, Ind. | |
| 2019 | 18.4 ± 1.5 * | 2.1 ± 1.2 |
| 2020 | 27.3 ± 2.5 | 4.7 ± 1.9 |
| Initial Number of Phytophagous Mites (Ind./Leaf) | The Number of Phytophagous Mites on a Certain Day (Ind./Leaf) | ||||||
|---|---|---|---|---|---|---|---|
| 7 Day | 14 Day | 21 Day | |||||
| Mobile Stages | Eggs | Mobile Stages | Eggs | Mobile Stages | Eggs | Mobile Stages | Eggs |
| Number of spider mites, ind./leaf | |||||||
| 9.4 d * | 5.7 d | 7.9 c | 4.3 c | 3.7 b | 2.1 b | 1.6 a | 0.4 a |
| Number of European red mites, ind./leaf | |||||||
| 6.2 d | 3.8 a | 5.0 c | 3.3 a | 3.3 b | 1.8 c | 1.3 a | 0.5 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ismailov, V.; Agasyeva, I.; Nastasy, A.; Nefedova, M.; Besedina, E.; Komantsev, A. The Application of Entomophagous and Acariphagous Species in Biological Protection Systems of an Apple Orchard (Malus domestica Borkh). Horticulturae 2023, 9, 379. https://doi.org/10.3390/horticulturae9030379
Ismailov V, Agasyeva I, Nastasy A, Nefedova M, Besedina E, Komantsev A. The Application of Entomophagous and Acariphagous Species in Biological Protection Systems of an Apple Orchard (Malus domestica Borkh). Horticulturae. 2023; 9(3):379. https://doi.org/10.3390/horticulturae9030379
Chicago/Turabian StyleIsmailov, Vladimir, Irina Agasyeva, Anton Nastasy, Maria Nefedova, Ekaterina Besedina, and Alexandr Komantsev. 2023. "The Application of Entomophagous and Acariphagous Species in Biological Protection Systems of an Apple Orchard (Malus domestica Borkh)" Horticulturae 9, no. 3: 379. https://doi.org/10.3390/horticulturae9030379
APA StyleIsmailov, V., Agasyeva, I., Nastasy, A., Nefedova, M., Besedina, E., & Komantsev, A. (2023). The Application of Entomophagous and Acariphagous Species in Biological Protection Systems of an Apple Orchard (Malus domestica Borkh). Horticulturae, 9(3), 379. https://doi.org/10.3390/horticulturae9030379







