Abstract
The main organic input for the elaboration of growing media is peat (Sphagnum spp.), due to its physical and chemical characteristics. However, the mining of this material creates a considerable impact in the local ecosystems from which this is obtained, along with a global impact because of the emission of greenhouse gasses. Thus, sustainable materials that can replace, or reduce the use of peat, while maintaining or improving attributes in the growing media and plant growth, are greatly needed. Therefore, this work aims to evaluate the effects of the use of different proportions of compost and biochar on the biological characteristics of growing media and (Lactuca sativa L.) seedling growth prior to transplanting. Out of the biological variables evaluated, the β-glucosidase activity showed the greatest results in growing media based on 80% peat and based on 70% peat, 5% compost and 5% biochar. Moreover, growing media based on the combination of compost, biochar and peat maintained most of the Lactuca sativa L. (Oak Leaf variety) seedling traits obtained in the growing media based on only peat. These findings emphasize the need to further investigate further biological conditions for alternative materials to peat, and the need to pay attention to feedstock initial characteristics and processing in order to obtain high quality organic inputs for optimum growing media.
1. Introduction
Growing media is a solid, porous, and light material of natural or synthetic origin, mineral or organic, used for sowing seeds or growing plants in pots or containers. Regardless of their origin, a common characteristic of the materials used for the preparation of growing media is that: they need to be chemically stable; free of weed seeds, pathogens, insects, or toxic substances for plants; homogeneous in size; and available in a specific place throughout the year [1,2,3].
Peat (Sphagnum spp.) is the main organic material used as part of growing media for seedling production in the horticultural industry [4]. This material is extracted from peat bogs; a type of wetland in cold to cold-temperate climate zones, that is considered a non-renewable resource, due to its slow formation process. The drainage and overexploitation of peatlands causes a rapid degradation of organic matter (OM) and consequently a high emission of greenhouse gasses (GHG), which compromises the sustainability of these ecosystems and intensifies the effects of climate change [5]. Thus, the search for novel and sustainable materials obtained locally under a circular economy model, that can replace or reduce the use of peat, to maintain or improve attributes in growing media and plant growth, is of vital importance for a modern and low carbon horticultural industry. In this sense, local materials such as bark, coir products, wood fiber, composts derived from various agricultural residues, and vermicompost, used either alone or in combination, have shown potential to replace peat as a bulk material [6,7]. Moreover, there is an increasing interest for bulk materials obtained from biotechnological processes that allow the reuse and recycling of its organic materials and increase their value as a sustainable way in horticultural production.
Among the promising alternatives to replace the use of peat is compost, material obtained by a bio-oxidative process conducted by the succession of different microorganisms. This product is recognized as an adequate material for the elaboration of growing media as it improves chemical, physical, and biological properties (e.g., pH, CE, OM%, CEC, among others), thus, favoring crop growth. Compost is even promoted as a material that provides beneficial microorganisms that favor the availability of nutrients for plants and that suppress the presence of pathogenic organisms [8]. In this context, the addition of compost based on green waste and food waste have been shown to increase enzyme activity when added in a 30% (v/v) to peat [9]. Additionally, other studies have highlighted the synergistic nature of the combination of peat and compost in substrates, where the peat improves aeration and water retention, and the compost improves the nutrition of the substrate [10,11].
Recently, another material that has attracted interest in the production of growing media is biochar [12], which is obtained from thermochemical transformation in the absence of oxygen. The incorporation of biochar has been shown not only to influence physical and chemical properties, but also the biological conditions of growing media [12,13,14]. For example, previous studies testing biochar elaborated based on sludge from sewage treatment plants, have shown that adding 10% of this material to a mixture with peat improved the biomass production of lettuce (Lactuca sativa L.), compared to a conventional growing media based on peat, due to an increase in the concentrations of N, P, K, and microbial activities [15]. Other studies have reported the increase of nutrient uptake by lettuce seedling in growing media based on biochar derived from forest woods [16]. However, despite these promising findings, feedstock and the parameters of operation should be previously analyzed and considered to produce biochar, since these factors influence the physical and chemical properties of the final product [12,17]. In spite of the growing interest in this material and the current synergies between producers and researchers (e.g., https://www.horti-bluec.eu/en/about, accessed on 23 November 2022) there are several issues to be addressed before to promote its wide utilization in growing media.
Novel materials, obtained from biotechnological approaches to replace peat, might have specific and variable physicochemical properties with the concomitant resulting differences in the availability of nutrients in growing media. In addition, microbial characteristics, in terms of the microbial biomass, microbial activity, microbial diversity, and microbial functionality, may also have critical differences between the alternative components of growing media [13,14]. These biological conditions and related characteristics in growing media will depend strongly on the type of feedstock, composition, and treatment of the materials for the design of growing media [18,19]. Microorganisms play important roles in nutrient recycling, the degradation of OM, and the control of plant pathogens in different growing systems [20]. For this, microbes mediate their resource allocation toward targeted substrates through the enzyme production to meet their stoichiometric needs [21,22]. Therefore, microorganisms and their mediated activity will have different effects on the quality of the growing media, which can affect the yield and production of biomass or ecophysiological properties related to the plants [23]. Under these conditions, to analyze the effect of the organic materials in the biological activity and properties of the growing media is priority for the formulation of suitable and optimized substrates.
This work seeks to evaluate alternative organic materials to peat, in the form of compost and biochar, for the elaboration of growing media for horticultural use. It is anticipated that the partial substitution of peat by compost and biochar will improve the biological properties of the substrates, which are expected to contribute to the root and aerial growth of a model horticultural crop (Lactuca sativa L.) during plant nursery prior to transplant. Therefore, the objective was to evaluate the effects of the use of different proportions of compost and biochar on the presence of heterotrophic microorganisms and enzymatic activity of substrates for horticultural use, and subsequently to establish the relationship of these biological properties with the aerial and root growth of Lactuca sativa L. seedlings before transplanting. The biological properties of the elaborated substrates will be evaluated by the quantification of the colony-forming units (CFUs) of heterotrophic microorganisms and the determination of the urease and beta glucosidase enzymatic activities since these allow understanding the nutritional behavior and the levels of microbial status in the substrates.
2. Materials and Methods
2.1. Elaboration of Growing Media
Growing media were prepared based on proportions commonly used by horticultural crops nurseries in central Chile, with a 1:4 (v/v) ratio of inorganic to organic components (personal communication, Alejandro Rojas, Orchard Sabor a Tierra owner, Idahue, Coltauco, Chile). Based on this, there were five treatments tested, using a fixed 20% of perlite (IMERYS, Protekta), and variable proportions of commercial peat (KEKKILÄ, Protekta), commercial compost (ANASAC) and own elaborated biochar materials up to 80% of volume (Table 1). Compost (pH 8.0; EC 1.9 dS/m) was elaborated from raw materials consisting of agro-industrial organic wastes (manufacturer communication) and biochar (pH 9.1; EC 0.5 dS/m) from forest residues pyrolyzed at 450 °C for 4 h under a slow pyrolysis process.

Table 1.
Treatments evaluated based on a fixed 20% of perlite and variable percentages of peat, compost, and biochar up to 80% of volume.
2.2. Initial Physical and Chemical Characterization
Physical and chemical properties typically evaluated for growing media were measured to characterize the quality of materials before experiment establishment. The gravimetric water content (GWC) and dry mass (DM), the particle size distribution (PSD) and the bulk density (BD) were determined through the methodology described in Sadzawka et al. (2005) [24]. Briefly, an aliquot of 10 g of fresh material was over dried in a forced convection oven (FDS 056, Binden, Tuttlingen, Germany) for 72 h at 70 ± 5 °C to determine GWC and DM. The particle size distribution was determined using 250 cm3 of oven dried sample (70 ± 5 °C), which was transferred to a stack of seven diameter stainless-steel sieves, of eight inch ring diameter, with the following mesh opening: 4.75 mm, 3.35 mm, 2 mm, 1.18 mm, 600 µm, 250 µm, and 150 µm and arranged in a sieve shaker (RX-29–10, W.S. TYLER, Mentor, OH, United States). The percentage of each fraction was calculated based on the weight of the total mass sieved, and each of them were identified as A (>4.75 mm), B (3.35–2 mm), C (1.18–0.6 mm), D (0.25–0.15 mm), and E (<0.15 mm). Bulk density (BD) was determined using 750 cm3 of fresh sample (70 ± 5 °C). The Water Holding Capacity (WHC) was determined based on the methodology described in De la Rosa et al. (2014) [25] and Nocentini et al. (2021) [26] by saturating and drying the substrate to determine the mass difference. The pH and electrical conductivity (EC) were determined in a 1:10 (w/v) ratio of dry sample and distilled water Sadzawka et al. (2005) [24].
A phytotoxicity test was carried out under the methodology proposed by Zucconi et al. (1981) [27], testing the incidence on the germination of Lactuca sativa seeds (Oak Leaf variety, Las Encinas brand). Two dilutions 1:10 w/v (10 g substrate: 100 mL distilled water) and 1:15 w/v (10 g substrate: 150 mL distilled water) were used. Of each extract, 10 mL were dispensed in Petri dishes (polystyrene, diameter 90 × 15 mm) with 150 mm filter paper (MN 650 MD, MN Macherey-Nagel, Düren, Germany) at the bottom, on which 10 lettuce seeds were arranged. Then, the Petri dishes were covered with aluminum foil and kept at room temperature (20 ± 5 °C) for 72 h. The germination index (GI) was obtained considering seed germination and radicle length in each extract and in distilled water (control), parameters that were compared as follows:
where RSG is the relative seed germination, RRG is the relative radicle growth and GI is the seed germination index.
2.3. Experimental Design
The duration of the experiment was designed based on the growth period required for Lactuca sativa seedlings (Oak Leaf variety, Las Encina brand) to achieve 4 to 5 true leaves required for transplant. Thus, the assay was run from 4 October to of 30 November 2021, period over which the previously defined growth state was achieved. The experiment was carried out under a completely randomized design, using thermoformed plastic growth trays (54 cm × 28 cm), with 128 cells of 21 cm3 each. In these trays the five treatments were implemented in triplicates using 20 experimental units (i.e., tray cell) per replicate, resulting in 60 experimental units per treatment. Each experimental unit was sown with one Lactuca sativa seed (Oak Leaf variety, Las Encina brand), which germinated to seedlings grown up to 4 to 5 true leaves required for transplant. The experiment was kept at room temperature, with ventilation and indirect natural light and was daily irrigated with 5 mL of distilled water. During this period, room temperature and air humidity were monitored with a thermometer-hygrometer (HTC-2, GVF, London, UK).
2.4. Evaluation of Biological Conditions of Growing Media (Treatments)
From a total of 60 seedlings per treatment (20 seedlings per replica), an equal number of seedlings was selected at the end of the study based on the lowest number of seedling survival obtained in one of the replicates of the study. This corresponded to 15 seedlings (Figure 1); therefore 45 seedlings were selected per treatment (15 plants × 3 replicates). From the set of 15 seedlings, a composite growing medium sample was obtained for each of the replicates per treatment (n = 3), by manually shaking seedlings and collecting the growing medium in a sterile plastic bag.

Figure 1.
Lactuca sativa seedlings (Oak Leaf variety) with 4 to 5 true leaves harvested at the end of the trial (30 November 2021). T1 80P:0C:0B; T2 70P:10C:0B; T3 70P:5C:5B; T4 60P:20C:0B; T5 60P:10C:10B. All treatment with a fixed 20% of perlite.
The quantification of the Colony Forming Units (CFUs) of culturable aerobic heterotrophic microorganisms was determined through the methodology described in Maier et al. (2009) [28]. This was achieved by obtaining serial dilutions arranged in triplicate in Petri dishes (polystyrene, diameter 90 × 15 mm). The first dilution (10−1) was obtained with 3 g of fresh sample and 27 mL of peptone solution (Bacto, BD, France) at 0.1%, second, third and fourth dilution were obtained by transferring 1 mL of previous dilution into 9 mL of 0.1% peptone solution. Dilutions (0.1 mL) were dispensed in Petri dishes (polystyrene, diameter 90 × 15 mm) with R2A agar (Difco, BD, France). Plates were kept in an incubator (KT115, Binder, Tuttlingen, Germany) for 48 h at 25 °C. After this time, the plates that contained between 30 and 300 CFUs, which in this case corresponded to those that came from the 10−3 dilution, were used for quantification. This was achieved with the help of a colony counter (LTECH Chile, Barcelona, Spain), and was expressed in dry mass.
The enzymatic activity of the β-glucosidase was determined using the methodology described by Alef and Nannipieri (1995) [29], quantified by the p-nitrophenol released after soil incubation with p- nitrophenyl-β-D-glucopyranoside. The enzymatic activity of the urease was determined using the methodology described by Kandeler and Geber (1988) [30], measured by the release of ammonium after soil incubation with urea as the substrate. The colorimetric assessment was performed by a microplate spectrophotometer (Epoch2, BioTek Instruments, Winooski, VT, USA).
2.5. Determination of Aerial and Root Growth
The evaluations of the aerial state and roots of lettuce plants were carried out in 10 out of the 15 seedlings selected previously, resulting in a total of 30 plants per treatment. The plants were placed in a plastic container and roots submerged in tap water where they were carefully washed, to obtain root samples and aerial plant portions (Figure 2).
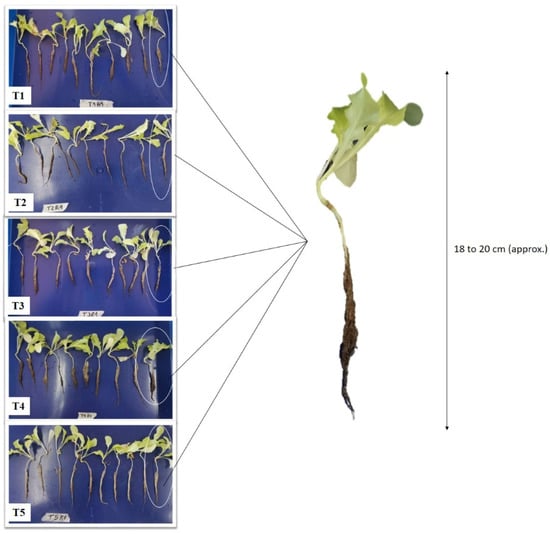
Figure 2.
Lactuca sativa plants selected by treatment for the evaluation of the state of aerial and underground growth. T1 80P:0C:0B; T2 70P:10C:0B; T3 70P:5C:5B; T4 60P:20C:0B; T5 60P:10C:10B. All treatment with a fixed 20% of perlite.
The height of the plants was determined by direct dimensional measurement using ruler graduated in centimeters, from the neck to the apex of the lettuce plants. The dry mass was obtained by placing the aerial part of the plant in an oven (FDS 056, Binder, Tuttlingen, Germany) for 48 h at 70 ± 5 °C and determining the difference between fresh mass and dry mass. The washed roots of each plant were individually placed in Petri dishes (polystyrene, diameter 90 × 15 mm) with 40 mL of tap water for digitalization. For root digitization, the EPSON scanner (driver Perfection V800/V850 3.9.3) was used. The scanner was connected with WinRHIZO 2019a software (Regent Instruments Inc., Quebec, QC, Canada) to obtain the measurement of the root traits. In the software, the image acquisition parameters were selected, which corresponded to the delimitation of the digitization area at 10 cm wide × 10 cm high, since this was adapted to the size of the Petri dishes and choosing the use of the scanner interface. Then, the classification of roots was configured based on the range of root diameter as exposed by Zobel and Waisel (2010) [31], which corresponded to Class 1 (0 to 0.5 mm) and Class 2 (0.5 to 0.75 mm). Root digitization and subsequent analysis with WinRHIZO software provided detailed values for root length L (measured in cm), root surface area SA (measured in cm2), number of tips NT (measured as a simple count) and root volume V (measured in cm3), for each root diameter class (classes measured in mm).
2.6. Statistical Analysis
All the analyses were performed in R statistic version 4.1.1 (R Core Development Team, 2020, Vienna, WU, Austria). Colony forming unit data were log-transformed to analyses. An ANOVA and LSD test were performed with the package “agricolae” [32] after verifying the normality and homoscedasticity assumptions through the Shapiro-Wilks test and Levene test, respectively. Variables associated with roots were analyzed with a generalized linear model (GLM) in which the variance structure of the treatments was modeled using the VarIdent option. Additionally, discrete root variables were analyzed with GLM based on the Poisson distribution and Log link function. Then, for the treatment effect, an LSD multiple comparison test was performed with p ≤ 0.05. To evaluate the relationship between the biological properties of the substrates and the variables of the state of aerial and root growth of the lettuce plants, a Pearson linear correlation analysis was carried out. Additionally, a principal component analysis (PCA) was generated using the “FactoMineR” and “factoextra”.
3. Results
3.1. Initial Physical and Chemical Characterization of the Substrates
Values of gravimetric water content and water holding capacity fluctuated from 47.2 to 55.6% and 233 to 405%, respectively, with the lowest values obtained T4 (with 60 % of peat and 20% of compost) and the highest value in T1 (based on 80 % peat and 20% perlite) (Table 2). In terms of bulk density, the greatest value was observed in T4 and the lowest in T1. Values obtained for pH measurements reflected that substrates fluctuate from slight acidity to moderate alkalinity, where extremes were also observed for T1 (6.2) and T4 (8.0), while all growing media showed low EC values (<0.6 dS/m).

Table 2.
Mean values ± standard deviation (SD) for gravimetric water content (GWC), dry mass (DM), bulk density (BD), water holding capacity (WHC), pH and electrical conductivity (EC).
Particle Size Distribution determination evidenced that fraction C (1.18 to 0.6 mm) was the most abundant for all substate, while the second highest percentage was observed in fraction D (0.25 to 0.15 mm) for all treatments, except for T4 where fraction B (3.55 to 2 mm) was the second most abundant (Figure 3). The lowest percentage was observed for fraction E (<0.15 mm), except for T1 where the lowest percentage was distinguished in fraction A (>4.75 mm) (Figure 3).
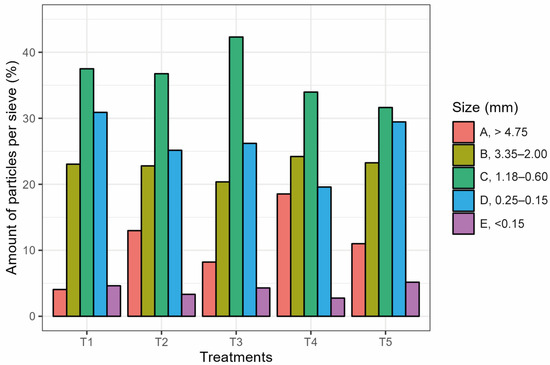
Figure 3.
Bar graph of the percentage of each particle size fraction in mm. T1 80P:0C:0B; T2 70P:10C:0B; T3 70P:5C:5B; T4 60P:20C:0B; T5 60P:10C:10B. All treatment with a fixed 20% of perlite.
3.2. Phytotoxicity Test
The results obtained for phytotoxicity tests reflected a seed germination index in the 1:10 dilution varied between 314% and 555%, and in the 1:15 dilution between 276% and 410% (Table 3). The 1:10 dilution showed a higher germination index in T5 (555%) and a lower value in T3 (314%), while for the 1:15 dilution the highest seed germination index value was observed in T3 (410%) and the lowest value in T4 (276%) (Table 3).

Table 3.
Mean ± standard deviation (SD) for seed germination index (GI) values of 1:10 (10 g: 100 mL) and 1:15 (10 g: 150 mL) dilutions of the phytotoxicity test.
3.3. Biological Conditions of Growing Media (Treatments)
The number of colony forming units (CFUs) did not show significant differences among treatments (Figure 4). The values observed fluctuated between 2.14 × 104 and 4.68 × 104 (CFU g dry substrate−1). The highest number of CFUs was observed in T2 (4.68 × 104 CFUs g dry substrate−1), followed by T4 (3.99 × 104 CFUs g dry substrate−1). The β-glucosidase activity showed significant differences (p ≤ 0.05) between the treatments and fluctuated between 301 to 706 (µg pNF g dry substrate−1h−1) (Figure 4). The highest value was observed in T1 (706 µg p-NF g dry substrate−1h−1) and T3 (591 µg p-NFg dry substrate−1h−1), corresponding to the control treatment consisting of only 80%, and the treatment made of 70% peat, 5% compost and 5% biochar, respectively. The lowest activity was shown in T5 (301 µg p-NF g dry substrate−1h−1) composed of 60% peat, 10% compost and 10% biochar. The urease activity did not reflect significant differences (p > 0.05) between the treatments and fluctuated between 89 and 127 (µg N-NH4 g dry substrate−1h−1) (Figure 4).
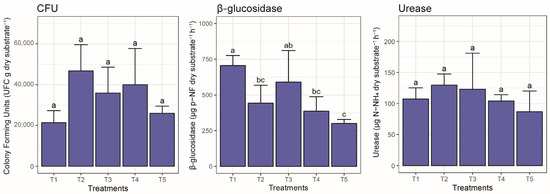
Figure 4.
Bar graph (mean ± standard deviation) of CFUs, β-glucosidase, and urease per treatment. Significant differences (p ≤ 0.05) between treatments are indicated by different letters. T1 80P:0C:0B; T2 70P:10C:0B; T3 70P:5C:5B; T4 60P:20C:0B; T5 60P:10C:10B. All treatment with a fixed 20% of perlite.
3.4. Plant Aerial and Root Growth
Plant Aerial and Root Growth
The plant height of Lactuca sativa seedlings did not show significant differences between the treatments, however, it is observed that the biochar treatments (T3 and T5) had lower height and dry weight compared to the other treatments (Table 4). Likewise, the aerial dry matter did not have significant differences between the use of peat, compost, or biochar, although biochar treatments (T3 and T5) showed the lowest weight of dry matter in the aerial part (Table 4).

Table 4.
Mean ± standard deviation (SD) for shoot length (SL, in cm) and aerial dry matter (ADM, in g) of Lactuca sativa grown on different treatments of growing media.
The mixed model implemented to analyze root length (L), root surface area (SA), and root volume (V) showed significant differences (p ≤ 0.05) between treatments in both root diameter classes (1 and 2) in all traits (Table 5). In class 1, the highest L values were obtained by T3 (220.62 cm) and T2 (216.97 cm), while the lowest value was obtained by T5 (194.25 cm) (Table 5). Similarly, T2 showed the highest values for SA (13.37 cm2) and V (0.083 cm3), while the lowest SA and V traits were obtained by T5 (9.94 cm2 and 0.055 cm3, respectively). In the case of class 2, the highest L, SA and V values were reached by T2 (14.76 cm, 3.43 cm2 and 0.074 cm3, respectively) followed by T1 for the L (14.37 cm) and T4 for the SA and V (3.28 cm2 and 0.070 cm3, respectively), while the shortest L, SA and V were obtained by T5 (8.28 cm, 1.99 cm2 and 0.042 cm3) (Table 5). Notably, the T2 is significantly higher than T3, T4 and T5 in the SA and V traits for the root diameter class 1, while in class 2 T2 is significantly superior to treatments T3 and T5 in the L2, SA2 and V2 traits.

Table 5.
Mean ± standard deviation (SD) for root length (L), root surface area (SA), root volume (V) and root tip numbers (NT) of Lactuca sativa. The root traits were classified in two root diameter classes: 0 to 0.5 mm (1) and 0.5 to 0.75 mm (2).
The generalized linear model implemented to analyze the variable number of tips showed significant differences (p ≤ 0.05) between treatments in class 1 (NT1), unlike class 2 (NT2) where no significant differences were found (ns, p > 0.05) (Table 5). In class 1, the highest NT were observed in T3 (617.43 tips) and T5 (593.6 tips), while the lowest NT was determined in T2 (330.63 tips). On the other hand, for class 2, the highest value was observed in T1 (3.97 tips), followed by T2 (3.13 tips), while the lowest number corresponded to T5 (1.9 tips). This may indicate that the use of biochar favors the number of tips in the fine roots (class 1) of the T3 and T5 treatments, while the use of peat alone helps to increase the number of tips in thicker roots (class 2) in treatment T1.
3.5. Relationship between Biological Properties of Growing Media and Lactuca sativa L. (Oak Leaf variety) Seedling Traits
The relationship between properties of the growing media tested and the variables of seedling traits of lettuce plants (Oak Leaf variety) was determined from a linear correlation analysis (Figure 5). Positive correlations were observed among aerial growth and morphology of the roots of Lactuca sativa L. Particularly, aerial dry matter was significantly correlated with volume (only in class diameter 1), length (in both class diameters) and surface area (in both class diameters) root traits. On the other hand, significant positive correlations between growing media biological properties and plant traits were not observed (Figure 5).
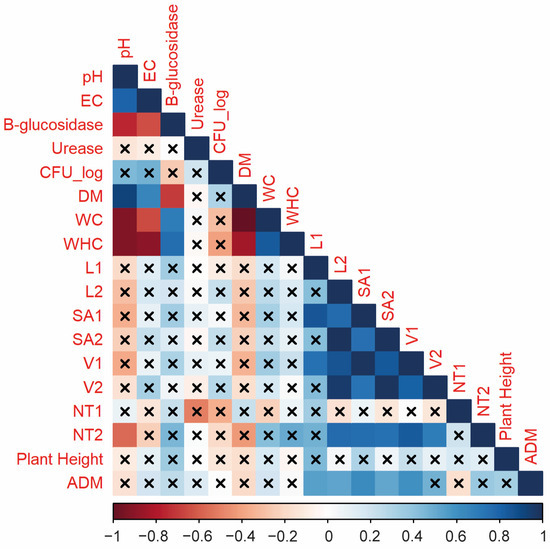
Figure 5.
Pearson correlation coefficient matrix between growing media properties (pH; EC; gravimetric water content, GWC; water holding capacity, WHC; colony forming units, CFUs; β-glucosidase activity; urease activity), aerial growth variables (plant height, aerial dry matter, ADM), and root morphology (L1 = root length for class 1; L2 = root length for class 2; SA1 = surface area for class 1; SA2 = surface area for class 2; V1 = root volume for class 1; V2 = root volume for class 2; NT1 = number of tips for class 1; NT2 = number of tips for class 2; RLD = root length diameter) of lettuce seedlings. Non-crossed boxes show significant correlations (p < 0.05). Red boxes show negative correlation, while blue boxes show positive correlations.
A biplot analysis was carried out to discover the traits more associated with each treatment (Figure 6). The two first principal components (PCs) explained 64.7% of the cumulative variance observed, with 39.5% at the first and 25.2% at the second axes. The parameters that contributed to negative loading to the first principal component (PC1) were EC, pH, DM, and CFUs; while for the second component (PC2) the parameters were NT1, WC, β-glucosidase and WHC. In contrast, the plant aerial and root (except NT) growth traits contributed with positive loading to the first and second components. Notably, treatments based on peat and compost (T2 and T4) were associated with positive loading in PC2, and particularly T2 also contributed with positive loading to PC1, which indicates that this treatment is associated with aerial and root growth traits.
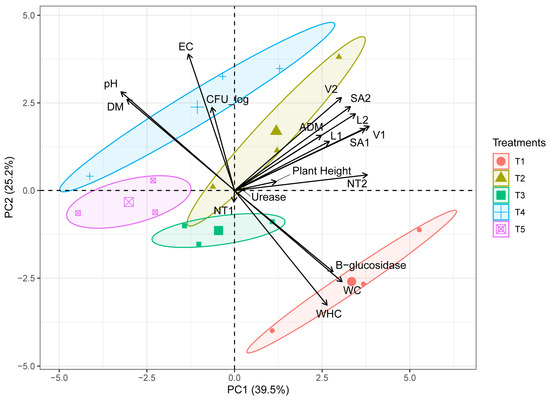
Figure 6.
Principal components analysis (PCA) biplot for compost and biochar treatments based on water holding capacity (WHC), β-glucosidase activity, gravimetric water content (GWC), urease, plant height, dry mass (DM), aerial dry matter (ADM), pH, Bulk density (BD), Electrical Conductivity (EC), colony forming units (CFU_log) and root traits (total root length: L, root surface area: SA, root volume: V and root tip numbers: NT).
4. Discussion
The use of peat as a component of growing media for horticultural crops has generated great environmental concern not only due to the intervention of natural ecosystems from where this material is harvested, but also because of the emission of GHG [5,11,13]. Consequently, there is a latent need to search for new alternatives to partially or totally substitute the use of peat, by high quality and low cost feedstock materials to support plant growth in containers [13,14,33,34]. In this context, the use of organic materials, such as compost and biochar, emerge as sustainable and environmentally friendly alternatives. However, before integrating them as alternatives to peat they must be evaluated physically, chemically, and biologically.
4.1. Physical and Chemical Characterization of Growing Media
The physical and chemical characterization of substrates is a requested step to ensure optimal germination, survival, development, and quality of a crop [3,4,6]. It is considered essential to initially evaluate the physical properties of substrate since these cannot be modified after the establishment of the crop, and have direct effect on water availability and aeration capacity [3]. On the other hand, the chemical properties determine the nutrient bioavailability, resulting in the optimum development of the plants [6].
Among the most important physical properties in substrates are the particle size distribution, the bulk density, the water holding capacity and the porosity, which are determining factors of the success of plant growth [3]. In our study, the particle size distribution of the most abundant fractions were similar to the optimum size suggested as optimum for growing media based on compost (0.25 to 2.50 mm), a fraction related to the optimal range of porosity and water availability, while bulk density were closer to values considered low, particularly for growing media used in pots outside greenhouses due to the risk of falling during windy conditions [6]. On the contrary, the water holding capacity of the substrates evaluated exceeded those recommended (50 to 60%) for such plant growth media [6], regardless of the proportion of peat used, which is known for its high capacity to retain water [7]. The water holding capacity is inversely related to the bulk density of the solid material evaluated, since when this increases, the pore space where water is retained decreases [6,35]. In addition, the water holding capacity is also related to the content of organic matter, as this increases the water retention capacity [36], and also with the type of organic matter, due to compounds more or less water-repellent [37]. Indeed, studies have suggested caution on the handling of growing media based on peats, as this can be extremely hydrophobic when it is dried [38]. In this sense, other studies have highlighted the potential of biochar as a partial or complete substitute of peat in growing media, since biochar has better re-wettability behavior than peat, in spite of the hydrophobicity exhibited by fresh biochar [13,38]. Moreover, it is well known that charring OM enhances the aromaticity commonly associated with higher hydrophobicity. Nevertheless, and despite the pyrolyzed nature of biochar, there was a high water holding capacity observed in the growing media based on 60% peat, 10% compost and 10% biochar, probably due to the fact that the temperatures setup in our study might have increased the porosity of biochar [38].
Some of the most important chemical properties for substrates are pH, electrical conductivity, in addition to others that were not included in this study such as cation exchange capacity (CEC) and atomic ratios (e.g., C:N, H:C, among others). The pH and the EC of the substrate are properties that affect the availability of nutrients for crops and microbes, and an indicator of potential toxicities that prevent the development of the plants, respectively. An optimal pH between 6.0 and 6.8 has been established as a recommendation for lettuce production [39]. In the present study, the increased pH in treatments with biochar and compost are concordant with studies in Syngonium podophyllum [40], Capsicum annuum [41], and Lactuca sativa [16,42,43], where the biochar and/or compost addition leads to an increase in pH (with values ranging from 6.8–9.5 with biochar and 6.1–7.7 with compost) in comparison to peat pH. Studies points out that the use of biochar is beneficial when used with acidic materials also used to prepare growing media, since biochar can replace calcium oxide used to increase the pH [16]. Moreover, it is widely described that compost as a result of OM mineralization, and biochar as a consequence of high temperatures during pyrolysis, reaches higher pH and EC associated with an increased salinity of the growing media [35].
For this reason, the initial characteristics of the feedstock must be considered when preparing substrates. Regarding EC, for growing media used for a variety of plants, including lettuces in greenhouse conditions, ≤0.5 dS m−1 values are suggested [44,45]. In our study, the EC ranged from 0.3 (in the treatment based on only peat) and 0.6 (in the treatment based on 60% peat and 20% compost). These results are in agreement with other studies where EC values have been reported to increase as compost proportions in growing media also increase [16,46]. The low levels of EC, and therefore of salts, are consistent with the results obtained for phytotoxicity in both dilutions (1:10 and 1:15), which fluctuated with seed germination index between 298 and 555%, values considerable above 80% which indicates the absence of toxicity in the medium [47]. These high GI values were particularly due to considerable greater relative radicle growth (RRG) obtained in the test extracts (0.1 to 7.8 cm), than in the control (0.3 to 1.5 cm), which would indicate that more than phytotoxic components in the extract, these would be rather improving vegetative growth due to nutritional conditions. Surprisingly high germination index values obtained in our study after 72 h of incubation are difficult to compare with results reported in the literature due to different procedures utilized (e.g., incubation time, extraction method, seed species) [48]; however, this widely used bioassay accomplished its purpose of indicating potential inhibitory conditions for plant growth.
4.2. Evaluation of the Biological Properties of the Growing Media Tested
Biological properties are essential to consider when determining the quality of solid media used for plant growth, due to the benefits they generate in terms of nutritional conditions and the suppression of pathogens, among others [1,2]. Biological properties are important not only to determine the microbial status of the growing media and its relationship with nutrient availability [10], but also to assess the stability of the organic matter, since it can go through processes of degradation before and after establishment in the containers [6]. The quantification of the colony forming units (CFUs) is a simple way to quantify microbial biomass, although its resolution is low (around 10% of the community can be cultured) it is still informative. As the term indicates, this method quantifies the colonies of aerobic microorganisms, which use available organic carbon sources to supply their carbon requirements. In our study, although known differences in compost, biochar, and peat carbon source stability, CFUs did not show statistical differences among treatments; therefore, this method is better complement with other assays, such as enzyme assays, to evaluate the effect of available carbon sources from compost and the influence of biochar in microbial in growing media [47,49].
Enzyme activity is an indicator of the supply of nutrients for plants and the decomposition of OM [9,50]. Extracellular enzymes (which are outside living cells) released by roots and microorganisms are a good biological indicator of soil quality [51,52]. β-glucosidase is an enzyme involved in the hydrolysis of cellulose to glucose (energy source for microorganisms), therefore, it is related to the mineralization of OM and the biogeochemical carbon [29,51,52]. The highest value of the activity of this enzyme registered for the treatment based on peat and the treatment based on 70% peat, 5% compost, and 5% biochar, might be possibly due to the composition of the sources of OM used in these growing media, compounds with commonly high cellulose concentration, which can lead to a higher β-glucosidase activity since such enzyme hydrolyzes cellobiose and water-soluble cellodextrins [53]. Previous studies have reported higher β-glucosidase activity in soils after organic fertilization [54,55], which is associated with higher availability of the enzyme substrates from organic materials. In this context, studies have reported higher enzyme activity (dehydrogenase and fluorescein diacetate hydrolysis) when adding compost (especially at 30% v/v) to peat [9]. This supports the possible effect of a higher cellulose concentration from organic materials on the β-glucosidase activity from the peat/compost evaluated in this study, especially compared with biochar since the higher temperature can decompose cellulose and hemicellulose [56]. On the other hand, the higher water holding capacity registered in the treatment based on peat and the treatment based on 70% peat, 5% compost and 5% biochar might have improved the solute diffusion and enzyme activities [57], which might be supported by the positive relationship between β-glucosidase and WHC (r = 0.76, Figure 5). The urease, an enzyme involved in the hydrolysis of urea (breaks the C-N bond), is an indicator of inorganic nitrogen available for microorganisms and plants [50]. In our study no significant differences in urease activity was observed, as opposed to other studies in soils where the use of compost and biochar have shown an increase in the activity of this enzyme [50,58]. Contrarily, previous studies have reported a decrease in urease activity under high N availability [59], which can be expected in the evaluated growing media and organic amendments. These findings contribute to the reduced literature on the study of the relationship between the use of biochar and microorganisms in growing media [11], and shed light on the need to further investigate particular biological conditions that make substitutes of peat a feasible alternative for optimal growing media.
4.3. Relationship between the Biological Properties of Growing Media and Lactuca sativa L. (Oak Leaf variety) Seedling Traits
No significant differences were observed in the plant height of Lactuca sativa among treatments. Nonetheless, the biplot analysis performed with all plant traits showed that treatments based on compost as a partial substitute of peat (T2 and T4) are associated with higher values in plant aerial and root growth traits (except NT), urease activity, and CFU, while β-glucosidase activity, WC, and WHC, are related with the control treatment based on peat (T1). This suggests that compost has the potential to increase aerial and root traits in lettuce as opposed to biochar in combination with compost, which under the conditions evaluated did not seem to relate with plant traits. However, a great variation of plant growth responses to the use of biochar in growing media have been reported, due to the high dependence of the characteristic of such material to the feedstock used and the handling of the pyrolysis process [60]. Some studies have stated the benefit of using biochar in lettuce seedlings as it relates to the increase in nutrient uptake [16], with several studies highlighting the benefits of using levels of 10 to 50% compost, biochar, and their combination, in growth, leaf turgor potential, photosynthesis, and nutrient content in crops [58,59,60]. Other studies, on the contrary, have reported no effects of biochar on similar traits in plant crops [36,38]. These contrasting findings could be likely related to the different forms of production previously mentioned, the sources of the origin or the quality of the biochar [61]. Still, materials such as compost and biochar evaluated in this study offer an alternative that allows the partial substitution of peat while maintaining plant traits comparable to those observed for growing media based on peat.
5. Conclusions
Compost and biochar are potential substitutes of peat in the elaboration of growing media upon the achievement of physical, biological, and biological properties that secure the stability of the material and the optimal plant growth. In our study, the use of compost or biochar did not seem to improve biological conditions of growing media, which likely could be due to the quality of the feedstock, or the optimization of biotechnological approaches used to obtain these products. However, these materials maintained most of the Lactuca sativa L. (Oak Leaf variety) seedling traits obtained in the growing media based on peat. These findings shed light on the necessity to further investigate the biotic and abiotic properties of potential substitutes of peat to evaluate the feasible use for optimal growing media, and the need to consider the quality and processing of raw materials to obtain high quality growing media.
Author Contributions
Conceptualization C.R., R.C.-S. and J.M.; methodology C.R., R.C.-S., J.M., C.M. and H.A.; software R.C.-S., C.M. and H.A.; validation C.R., R.C.-S. and J.M.; formal analysis R.C.-S., C.M. and H.A.; investigation A.R., C.R., R.C.-S. and J.M.; resources C.R. and J.M.; data curation H.A., C.M. and R.C.-S.; writing—original draft preparation A.R.; writing—review and editing C.R., R.C.-S., J.M., C.M. and H.A.; visualization, A.R, C.M. and H.A.; supervision C.R. and R.C.-S.; project administration, C.R.; funding acquisition, C.R. and J.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by ANID through the PIA/BASAL FB00002, Centro de Ecología Aplicada y sustentabilidad (CAPES) and the Fondecyt initiation project N° 11201107. H.A. was funded by ANID/Fondecyt 3210752 and C.M. was funded by the University de O’Higgins postdoctoral program.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Borrero, C.; Ordovas, J.; Trillas, M.I.; Aviles, M. Tomato Fusarium wilt suppressiveness. The relationship between the organic plant growth media and their microbial communities as characterized by Biolog (R). Soil. Biol. Biochem. 2006, 38, 1631–1637. [Google Scholar] [CrossRef]
- Ozores-Hampton, M.; Obreza, T.A.; Stoffella, P.J. Weed control in vegetable crops with composted organic mulches. In Compost Utilization in Horticultural Cropping Systems; CRC Press: Boca Raton, FL, USA, 2001; pp. 275–286. [Google Scholar]
- Verdonck, O.; Rd, P.; De Boodt, M. The physical properties of different horticultural substrates. In Proceedings of the International Symposium on Substrates in Horticulture other than Soils In Situ; Acta Horticulturae: Barcelona, Spain, 1983; Volume 150, pp. 155–160. [Google Scholar]
- Amha, Y.; Bohne, H.; Schmilewski, G.; Picken, P.; Reinikainen, O. Physical, chemical and botanical characteristics of peats used in the horticultural industry. Eur. J. Hortic. Sci. 2010, 75, 177–183. [Google Scholar]
- Ceglie, F.; Bustamante, M.; Amara, M.; Tittarelli, F. The challenge of peat substitution in organic seedling production: Optimization of growing media formulation through mixture design and response surface analysis. PLoS ONE 2015, 10, e0128600. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.; Saha, S.; Hariprasad, P. Agro-industrial-residues as potting media: Physicochemical and biological characters and their influence on plant growth. Biomass Convers. Biorefinery 2021, 1–24. [Google Scholar] [CrossRef]
- Barrett, G.E.; Alexander, P.D.; Robinson, J.S.; Bragg, N.C. Achieving environmentally sustainable growing media for soilless plant cultivation systems—A review. Sci. Hortic. 2016, 212, 220–234. [Google Scholar] [CrossRef]
- Pascual, J.; Ceglie, F.; Tuzel, Y.; Koller, M.; Koren, A.; Hitchings, R.; Tittarelli, F. Organic substrate for transplant production in organic nurseries. A review. Agron. Sustain. Dev. 2018, 38, 35. [Google Scholar] [CrossRef]
- Prasad, M.; Ni Chualain, D.; Maher, M.J. The effect of addition of composted greenwaste and biowaste on enzyme activity of peats of two degrees of decomposition. Acta Hortic. 2008, 779, 59–67. [Google Scholar] [CrossRef]
- Jayasinghe, G. Composted sewage sludge as an alternative potting media for lettuce cultivation. Commun. Soil Sci. Plant Anal. 2012, 43, 2878–2887. [Google Scholar] [CrossRef]
- Kern, J.; Tammeorg, P.; Shanskiy, M.; Sakrabani, R.; Knicker, H.; Kammann, C.; Tuhkanen, E.M.; Smidt, G.; Prasad, M.; Tiilikkala, K.; et al. Synergistic use of peat and charred material in growing media–an option to reduce the pressure on peatlands. J. Environ. Eng. Landsc. Manag. 2017, 25, 160–174. [Google Scholar] [CrossRef]
- Huang, L.; Gu, M. Effects of biochar on container substrate properties and growth of plants—A review. Horticulturae 2019, 5, 14. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Allaire, S.E.; Akram, N.A.; Méndez, A.; Younis, A.; Peerzada, A.M.; Shaukat, N.; Wright, S.R. Challenges in organic component selection and biochar as an opportunity in potting substrates: A review. J. Plant Nutr. 2019, 42, 1386–1401. [Google Scholar] [CrossRef]
- Nemati, M.R.; Simard, F.; Fortin, J.-P.; Beaudoin, J. Potential use of biochar in growing media. Vadose Zone J. 2015, 14, vzj2014.06.0074. [Google Scholar] [CrossRef]
- Méndez, A.; Cárdenas-Aguiar, E.; Paz-Ferreiro, J.; Plaza, C.; Gascó, G. The effect of sewage sludge biochar on peat-based growing media. Biol. Agric. Hortic. 2017, 33, 40–51. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Prasad, M.; Kavanagh, A.; Tzortzakis, N. Biochar type, ratio, and nutrient levels in growing media affects seedling production and plant performance. Agronomy 2020, 10, 1421. [Google Scholar] [CrossRef]
- Jindo, K.; Sánchez-Monedero, M.A.; Mastrolonardo, G.; Audette, Y.; Satoshi Higashikawa, F.; Silva, C.A.; Akashi, K.; Mondini, C. Role of biochar in promoting circular economy in the agriculture sector. Part 2: A review of the biochar roles in growing media, composting and as soil amendment. Chem. Biol. Technol. Agric 2020, 7, 16. [Google Scholar] [CrossRef]
- Grunert, O.; Reheul, D.; Van Labeke, M.; Perneel, M.; Hernandez-Sanabria, E.; Vlaeminck, S.E.; Boon, N. Growing media constituents determine the microbial nitrogen conversions in organic growing media for horticulture. Microb. Biotechnol. 2016, 9, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Grunert, O.; Robles-Aguilar, A.A.; Hernandez-Sanabria, E.; Schrey, S.D.; Reheul, D.; Labeke, M.-C.V.; Vlaeminck, S.E.; Vandekerckhove, T.G.L.; Mysara, M.; Monsieurs, P.; et al. Tomato plants rather than fertilizers drive microbial community structure in horticultural growing media. Sci. Rep. 2019, 9, 9561. [Google Scholar] [CrossRef]
- Kasozi, N.; Abraham, B.; Kaiser, H.; Wilhelmi, B. The complex microbiome in aquaponics: Significance of the bacterial ecosystem. Ann. Microbiol. 2021, 71, 1. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Lauber, C.L.; Weintraub, M.N.; Ahmed, B.; Allison, S.D.; Crenshaw, C.; Contosta, A.R.; Cusack, D.; Frey, S.; Gallo, M.E.; et al. Stoichiometry of soil enzyme activity at global scale. Ecol. Lett. 2008, 11, 1252–1264. [Google Scholar] [CrossRef]
- Mooshammer, M.; Wanek, W.; Zechmeister-Boltenstern, S.; Richter, A. Stoichiometric imbalances between terrestrial decomposer communities and their resources: Mechanisms and implications of microbial adaptations to their resources. Front. Microbiol. 2014, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Vandecastelee, B.; Van Loo, K.; Ommeslag, S.; Vierendeels, S.; Rooseleer, M.; Vandaele, E. Sustainable Growing Media Blends with Woody Green Composts: Optimizing the N Release with Organic Fertilizers and Interaction with Microbial Biomass. Agronomy 2022, 12, 422. [Google Scholar] [CrossRef]
- Sadzawka, A.; Carrasco, M.; Grez, R.; De La Luz, M. Métodos de Análisis de Compost; Serie Actas INIA, no. 30; Instituto de Investigaciones Agropecuarias (INIA): Santiago, Chile, 2005; ISSN 0717-4810. [Google Scholar]
- De la Rosa, P.M.; Miller, Z.A.; Knicker, H. Relating physical and chemical properties of four different biochars and their application rate to biomass production of Lolium perenne on a Calcic Cambisol during a pot experiment of 79 days. Sci. Total Environ. 2014, 499, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Nocentini, M.; Panettieri, M.; García, J.; Mastrolonardo, G.; Knicker, H. Recycling pyrolyzed organic waste from plant nurseries, rice production and shrimp industry as peat substitute in potting substrates. J. Environ. Manag. 2021, 277, 111436. [Google Scholar] [CrossRef] [PubMed]
- Zucconi, F. Evaluating toxicity of immature compost. Biocycle 1981, 22, 54–57. [Google Scholar]
- Maier, R.M.; Pepper, I.L.; Gerba, C.P. Environmental Microbiology, 2nd ed.; Academic Press, Elsevier: London, UK, 2009. [Google Scholar]
- Alef, K.; Nannipieri, P. Enzyme activities. In Methods in Applied Soil Microbiology and Biochemistry; Academic Press, Elsevier: Amsterdam, The Netherlands, 1995; pp. 311–373. [Google Scholar] [CrossRef]
- Kandeler, E.; Gerber, H. Short-term assay of soil urease activity using colorimetric determination of ammonium. Biol. Fertil. Soils 1988, 6, 68–72. [Google Scholar] [CrossRef]
- Zobel, R.; Waisel, Y. A plant root system architectural taxonomy: A framework for root nomenclature. Plant Biosyst. 2010, 144, 507–512. [Google Scholar] [CrossRef]
- de Mendiburu, F. Agricolae: Statistical Procedures for Agricultural Research. R package version 1.4.0, 2020. Available online: https://cran.r-project.org/package=agricolae (accessed on 23 November 2022).
- Stewart, S. Efficacy of organic amendments used in containerized plant production: Part 1–Compost-based amendments. Sci. Hortic. 2020, 266, 108856. [Google Scholar] [CrossRef]
- Fryda, L.; Visser, R.; Schmidt, J. Biochar replaces peat in horticulture: Environmental impact assessment of combined biochar & bioenergy production. Detritus 2019, 5, 1. [Google Scholar] [CrossRef]
- Abujabhah, I.; Bound, S.; Doyle, R.; Bowman, J. Effects of biochar and compost amendments on soil physico-chemical properties and the total community within a temperate agricultural soil. Appl. Soil Ecol. 2015, 98, 243–253. [Google Scholar] [CrossRef]
- Banitalebi, G.; Mosaddeghi, M.R.; Shariatmadari, H. Feasibility of agricultural residues and their biochars for plant growing media: Physical and hydraulic properties. Waste Manag. 2019, 87, 577–589. [Google Scholar] [CrossRef]
- Perdana, L.R.; Ratnasari, N.G.; Ramadhan, M.L.; Palamba, P.; Nasruddin; Nugroho, Y.S. Hydrophilic and hydrophobic characteristics of dry peat. In IOP Conference Series: Earth and Environmental Science; Institute of Physics Publishing: Bali, Indonesia, 2018; Volume 105. [Google Scholar]
- Steiner, C.; Harttung, T. Biochar as a growing media additive and peat substitute. Solid Earth 2014, 5, 995–999. [Google Scholar] [CrossRef]
- Flynn, R.P.; Wood, C.W.; Guertal, E.A. Lettuce response to composted broiler litter as a potting substrate component. J. Am. Soc. Hortic. Sci. 1995, 120, 964–970. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Younis, A.; Chen, J. Biochar or biochar-compost amendment to a peat-based substrate improves growth of Syngonium podophyllum. Agronomy 2019, 9, 460. [Google Scholar] [CrossRef]
- Nair, A.; Carpenter, B. Biochar rate and transplant tray cell number have implications on pepper growth during transplant production. HortTechnology 2016, 26, 713–719. [Google Scholar] [CrossRef]
- Christou, A.; Stylianou, M.; Georgiadou, E.C.; Gedeon, S.; Ioannou, A.; Michael, C.; Fatta-Kassinos, D. Effects of biochar derived from the pyrolysis of either biosolids, manure or spent coffee grounds on the growth, physiology and quality attributes of field-grown lettuce plants. Environ. Technol. Innov. 2022, 26, 102263. [Google Scholar]
- Suwor, P.; Jeakkhajorn, S.; Kramchote, S. Effects of different compost manures application on growth of lettuces (Lactuca sativa L.). Int. J. Agric. Technol. 2020, 16, 1257–1266. [Google Scholar]
- Nobile, C.; Denier, J.; Houben, D. Linking biochar properties to biomass of basil, lettuce and pansy cultivated in growing media. Sci. Hortic. 2020, 261, 109001. [Google Scholar]
- Abad, M.; Noguera, P.; Burés, S. National inventory of organic wastes for use as growing media for ornamental potted plant production: Case study in Spain. Bioresour. Technol. 2001, 77, 197–200. [Google Scholar] [CrossRef]
- de Falco, E.; Vitti, A.; Celano, G.; Ronga, D. Suitability of On-Farm Green Compost for the Production of Baby Leaf Species. Horticulturae 2021, 7, 512. [Google Scholar] [CrossRef]
- Emino, E.; Warman, P. Biological Assay for Compost Quality. Compost. Sci. Util. 2004, 12, 342–348. [Google Scholar] [CrossRef]
- Luo, Y.; Liang, J.; Zeng, G.; Chen, M.; Mo, D.; Li, G.; Zhang, D. Seed germination test for toxicity evaluation of compost: Its roles, problems and prospects. Waste Manag. 2018, 71, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Palansooriya, K.N.; Wong, J.T.F.; Hashimoto, Y.; Huang, L.; Rinklebe, J.; Chang, S.X.; Bolan, N.; Wang, H.; Ok, Y.S. Response of microbial communities to biochar-amended soils: A critical review. Biochar 2019, 1, 3–22. [Google Scholar] [CrossRef]
- Nannipieri, P.; Giagnoni, L.; Renella, G.; Puglisi, E.; Ceccanti, B.; Masciandaro, G.; Fornadier, F.; Moscatelli, M.; Marinari, S. Soil enzymology: Classical and molecular approaches. Biol. Fertil. Soils 2012, 48, 743–762. [Google Scholar]
- Pokharel, P.; Ma, Z.; Chang, S. Biochar increases soil microbial biomass with changes in extra-and intracellular enzyme activities: A global meta-analysis. Biochar 2020, 2, 65–79. [Google Scholar]
- Aponte, H.; Medina, J.; Butler, B.; Meier, S.; Cornejo, P.; Kuzyakov, Y. Soil quality indices for metal (loid) contamination: An enzymatic perspective. Land Degrad. Dev. 2020, 31, 2700–2719. [Google Scholar]
- Deng, S.; Popova, I. Carbohydrate Hydrolases. In Methods of Soil Enzymology; Soil Science Society of America: Madison, WI, USA, 2011; Volume 9, pp. 185–209. [Google Scholar] [CrossRef]
- Maarit Niemi, R.; Vepsäläinen, M.; Wallenius, K.; Erkomaa, K.; Kukkonen, S.; Palojärvi, A.; Vestberg, M. Conventional versus organic cropping and peat amendment: Impacts on soil microbiota and their activities. Eur. J. Soil Biol. 2008, 44, 419–428. [Google Scholar] [CrossRef]
- Paillat, L.; Cannavo, P.; Barraud, F.; Huché-Thélier, L.; Guénon, R. Growing Medium Type Affects Organic Fertilizer Mineralization and CNPS Microbial Enzyme Activities. Agronomy 2020, 10, 1955. [Google Scholar] [CrossRef]
- Lin, Y.-L.; Zheng, N.-Y.; Lin, C.-S. Repurposing Washingtonia filifera petiole and Sterculia foetida follicle waste biomass for renewable energy through torrefaction. Energy 2021, 223, 120101. [Google Scholar] [CrossRef]
- Moyano, F.E.; Manzoni, S.; Chenu, C. Responses of soil heterotrophic respiration to moisture availability: An exploration of processes and models. Soil Biol. Biochem. 2013, 59, 72–85. [Google Scholar] [CrossRef]
- Pinto, R.; Brito, L.M.; Gonçalves, F.; Mourão, I.; Torres, L.; Coutinho, J. Lettuce growth and nutrient uptake response to winery waste compost and biochar. In III International Symposium on Growing Media, Composting and Substrate Analysis; ISHS: Milan, Italy, 2019; Volume 1305, pp. 233–240. [Google Scholar]
- Marcote, I.; Hernández, T.; García, C.; Polo, A. Influence of one or two successive annual applications of organic fertilisers on the enzyme activity of a soil under barley cultivation. Bioresour. Technol. 2001, 79, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Rae Kim, K.; Yang, J.-E.; Sik Ok, Y.; Il Kim, W.; Kunhikrishnan, A.; Kim, K.-H. Amelioration of Horticultural Growing Media Properties Through Rice Hull Biochar Incorporation. Waste Biomass Valorization 2017, 8, 483–492. [Google Scholar]
- Zahra, M.B.; Aftab, Z.E.H.; Akhter, A.; Haider, M.S. Cumulative effect of biochar and compost on nutritional profile of soil and maize productivity. J. Plant Nutr. 2021, 44, 1664–1676. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).