Greenhouse Screening for pH Stress in Rhododendron Genotypes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Substrate and Experimental Setup
2.3. Measurements
2.4. Statistical Analysis
3. Results
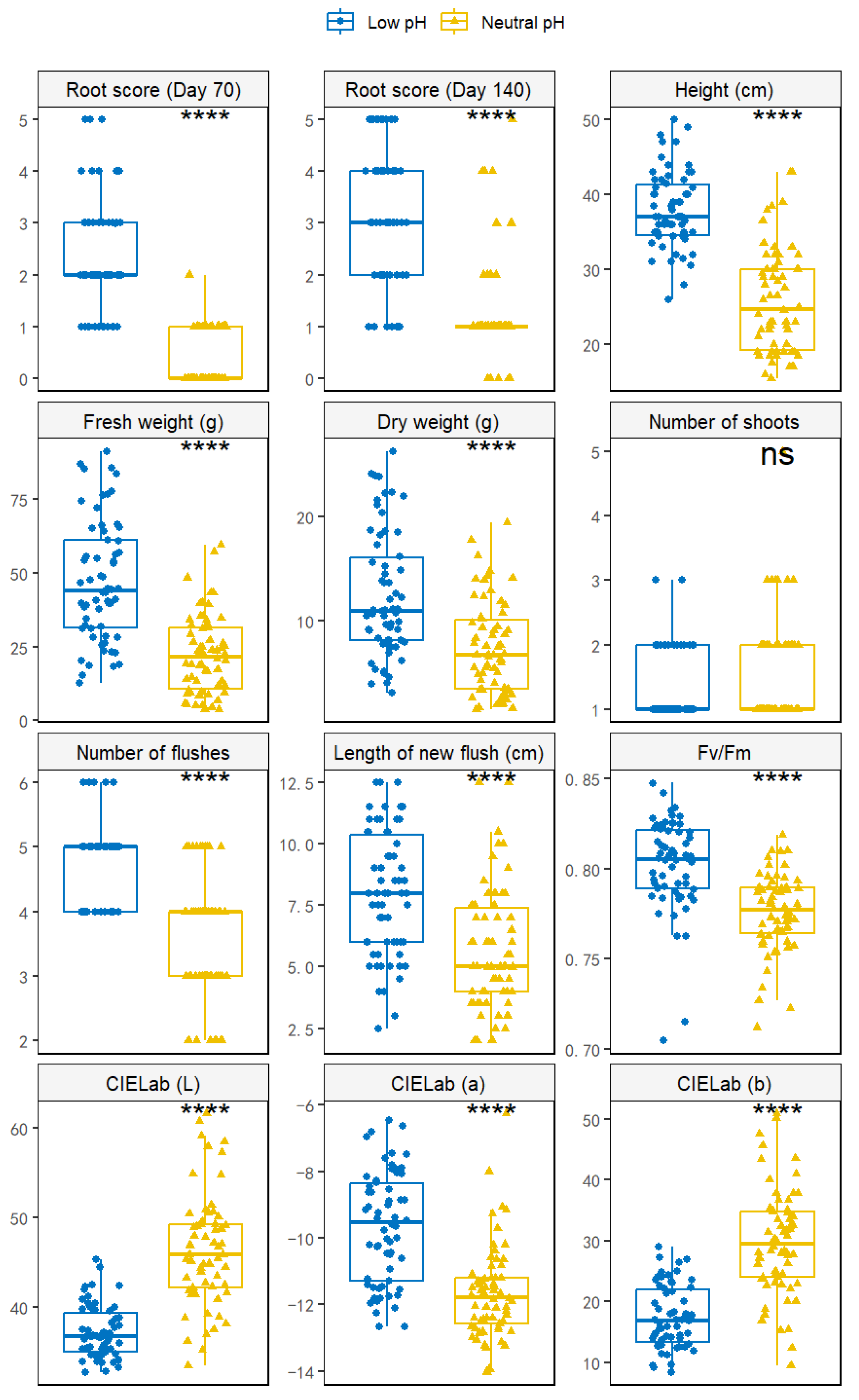
3.1. Comparison of pH Stress between Acidic and Neutral Substrate pH
3.2. Comparison of pH Stress between Genotypes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Demasi, S.; Handa, T.; Scariot, V. Ferric chelate reductase activity under iron deficiency stress in Azalea. Int. J. Hortic. Florcult. 2015, 3, 157–160. [Google Scholar]
- Wang, S.; Leus, L.; Van Labeke, M.-C.; Van Huylenbroeck, J. Prediction of lime tolerance in Rhododendron based on herbarium specimen and geochemical data. Front. Plant Sci. 2018, 9, 1538. [Google Scholar] [CrossRef] [PubMed]
- Boxma, R. Bicarbonate as the most important soil factor in lime-induced chlorosis in The Netherlands. Plant Soil 1972, 37, 233–243. [Google Scholar] [CrossRef]
- Chaanin, A.; Preil, W. Influence of bicarbonate on iron deficiency chlorosis in Rhododendron. Acta Hortic. 1994, 364, 71–77. [Google Scholar] [CrossRef]
- Chaanin, A. Lime tolerance in rhododendron. Comb. Proc. IPPS 1998, 48, 180–182. [Google Scholar]
- Giel, P.; Bojarczuk, K. Effects of high concentrations of calcium salts in the substrate and its pH on the growth of selected. Rhododendron cultivars. Acta Soc. Bot. Pol. 2011, 80, 105–114. [Google Scholar] [CrossRef]
- Ström, L.; Owen, A.G.; Godbold, D.L.; Jones, D.L. Organic acid behaviour in a calcareous soil—Implications for rhizosphere nutrient cycling. Soil Biol. Biochem. 2005, 37, 2046–2054. [Google Scholar] [CrossRef]
- Lee, J.A.; Woolhouse, H.W. A comparative study of bicarbonate inhibition of root growth in calcicole and calcifuge grasses. New Phytol. 1969, 68, 1–11. [Google Scholar] [CrossRef]
- Wang, S.; Leus, L.; Lootens, P.; Van Huylenbroeck, J.; Van Labeke, M.-C. Germination kinetics and chlorophyll fluorescence imaging allow for early detection of alkalinity stress in Rhododendron species. Horticulturae 2022, 8, 823. [Google Scholar] [CrossRef]
- Wang, S.; Zheng, T.; Leus, L.; Van Labeke, M.-C.; Van Huylenbroeck, J. Screening and evaluation of Rhododendron progenies for alkaline pH tolerance. Acta Hort. 2021, 1331, 95–100. [Google Scholar] [CrossRef]
- ACTTR. Available online: https://www.acttr.com/en/en-report/en-report-technology/383-en-tech-color-space-conversion.html (accessed on 4 October 2023).
- The R Project for Statistical Computing. Available online: https://www.R-project.org (accessed on 4 October 2023).
- Li, C.; Fang, B.; Yang, C.; Shi, D.; Wang, D. Effects of various salt–alkaline mixed stresses on the state of mineral elements in nutrient solutions and the growth of alkali resistant halophytechloris virgata. J. Plant Nutr. 2009, 32, 1137–1147. [Google Scholar] [CrossRef]
- Wei, L.; Zhang, R.; Zhang, M.; Xia, G.; Liu, S. Functional analysis of long non-coding RNAs involved in alkaline stress responses in wheat. J. Exp. Bot. 2022, 73, 5698–5714. [Google Scholar] [CrossRef] [PubMed]
- Preil, W.; Ebbinghaus, R. Breeding of lime tolerant Rhododendron rootstocks. Acta Hort. 1994, 364, 61–70. [Google Scholar] [CrossRef]
- Susko, A.Q. Phenotypic and Genetic Variation for Rhizosphere Acidification, a Candidate Trait for pH Adaptability, in Deciduous Azalea (Rhododendron sect. Pentanthera). Master’s Thesis, University of Minnesota Twin Cities, Minneapolis, MN, USA, 2016. [Google Scholar]
- Susko, A.; Rinehart, T.A.; Bradeen, J.; Hokanson, S.C. An evaluation of two seedling phenotyping protocols to assess pH adaptability in deciduous azalea (Rhododendron sect. Pentanthera, G. Don). HortScience 2018, 53, 268–274. [Google Scholar] [CrossRef]
- Rosen, C.J.; Allan, D.L.; Luby, J.J. Nitrogen form and solution pH influence growth and nutrition of two vaccinium clones. J. Am. Soc. Hortic. Sci. 1990, 115, 83–89. [Google Scholar] [CrossRef]
- Marrs, R.H.; Bannister, P. Response of several members of the Ericaceae to soils of contrasting pH and base-status. J. Ecol. 1978, 66, 829–834. [Google Scholar] [CrossRef]
- Finn, C.E.; Rosen, C.J.; Luby, J.J. Nitrogen form and solution pH effects on root anatomy of cranberry. HortScience 1990, 25, 1419–1421. [Google Scholar] [CrossRef]
- Turner, A.J.; Arzola, C.I.; Nunez, G.H. High pH stress affects root morphology and nutritional status of hydroponically grown rhododendron (Rhododendron spp.). Plants 2020, 9, 1019. [Google Scholar] [CrossRef]
- Demasi, S.; Caser, M.; Kobayashi, N.; Kurashige, Y.; Scariot, V. Hydroponic screening for iron deficiency tolerance in evergreen azaleas. Not. Bot. Horti Agrobot. Cluj-Napoca 2015, 43, 210–213. [Google Scholar] [CrossRef]
- Demasi, S.; Caser, M.; Handa, T.; Koboyashi, N. Adaptation to iron deficiency and high pH in evergreen azaleas (Rhododendron spp.): Potential resources for breeding. Euphytica 2017, 213, 148. [Google Scholar] [CrossRef]
- Li, X.; Dong, J.L.; Chu, W.Y.; Chen, Y.; Duan, Z. The relationship between root exudation properties and root morphological traits of cucumber grown under different nitrogen supplies and atmospheric CO2 concentrations. Plant Soil 2018, 425, 415–432. [Google Scholar] [CrossRef]
- Ji, Y.; Zhang, X.Q.; Peng, Y.; Liang, X.Y.; Huang, L.K.; Ma, X.; Ma, Y.M. Effects of drought stress on lipid peroxidation, osmotic adjustment and activities of protective enzymes in the roots and leaves of orchardgrass. Acta Pratacult. Sin. 2014, 23, 144–151. [Google Scholar] [CrossRef]
- Robson, J.K.; Ferguson, J.N.; McAusland, L.; Atkinson, J.A.; Tranchant-Dubreuil, C.; Cubry, P.; Sabot, F.; Wells, D.M.; Price, A.H.; Wilson, Z.A.; et al. Chlorophyll fluorescence-based high-throughput phenotyping facilitates the genetic dissection of photosynthetic heat tolerance in African (Oryza glaberrima) and Asian (Oryza sativa) rice. J. Exp. Bot. 2023, 74, 5181–5197. [Google Scholar] [CrossRef] [PubMed]
- Lootens, P.; Devacht, S.; Baert, J.; Van Waes, J.; Van Bockstaele, E.; Roldan-Ruiz, I. Evaluation of cold stress of young industrial chicory (Cichorium intybus L.) by chlorophyll a fluorescence imaging. II. Dark relaxation kinetics. Photosynthetica 2011, 49, 185–194. [Google Scholar] [CrossRef]
- Mishra, A.; Heyer, A.G.; Mishra, K.B. Chlorophyll fluorescence emission can screen cold tolerance of cold acclimated arabidopsis thaliana accessions. Plant Meth. 2014, 10, 38. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, K.; Jiang, Y.L.; Wang, G.L. Flash drought early warning based on the trajectory of solar-induced chlorophyll fluorescence. Proc. Natl. Acad. Sci. USA 2022, 119, 32. [Google Scholar] [CrossRef]
- Arief, M.A.A.; Kim, H.; Kurniawan, H.; Nugroho, A.P.; Kim, T.; Cho, B.K. Chlorophyll fluorescence imaging for early detection of drought and heat stress in strawberry plants. Plants 2023, 12, 1387. [Google Scholar] [CrossRef]
- Shomali, A.; Aliniaeifard, S.; Bakhtiarizadeh, M.R.; Lotfi, M.; Mohammadian, M.; Vafaei Sadi, M.S.; Rastogi, A. Artificial neural network (ANN)-based algorithms for high light stress phenotyping of tomato genotypes using chlorophyll fluorescence features. Plant Phys. Biochem. 2023, 201, 107893. [Google Scholar] [CrossRef]
- Latifinia, E.; Eisvand, H.R. Soybean physiological properties and grain quality responses to nutrients, and predicting nutrient deficiency using chlorophyll fluorescence. J. Soil Sci. Plant Nutr. 2022, 22, 2346. [Google Scholar] [CrossRef]
- Zheng, L.; Steppe, K.; Van Labeke, M.C. Spectral quality of monochromatic LED affects photosynthetic acclimation to high-intensity sunlight of Chrysanthemum and Spathiphyllum. Physiol. Plant. 2020, 169, 10–26. [Google Scholar] [CrossRef]
- Senesi, G.S.; De Pascale, O.; Marangoni, B.S.; Caires, A.R.L.; Nicolodelli, G.; Pantaleo, V.; Leonetti, P. Chlorophyll fluorescence imaging (CFI) and laser-induced breakdown spectroscopy (LIBS) applied to investigate tomato plants infected by the root knot nematode (RKN) Meloidogyne incognita and tobacco plants infected by cymbidium ringspot virus. Photonics 2022, 9, 627. [Google Scholar] [CrossRef]
- Meng, L.J.; Mestdagh, H.; Ameye, M.; Audenaert, K.; Hofte, M.; Van Labeke, M.C. Phenotypic variation of Botrytis cinerea isolates is influenced by spectral light quality. Front. Plant Sci. 2020, 11, 1233. [Google Scholar] [CrossRef] [PubMed]
- Valadares, N.R.; Soares, M.A.; Ferreira, E.A.; de Sa, V.G.M.; Azevedo, A.M.; Leite, G.L.D.; Zanuncio, J.C. Physiological responses in genetically modified cotton and its isohybrid attacked by Aphis gossypii Glover (Hemiptera: Aphididae). Arthropod-Plant Interact. 2023, 17, 167–172. [Google Scholar] [CrossRef]
- Guerinot, M.L.; Yi, Y. Iron: Nutritious, noxious, and not readily available. Plant Physiol. 1994, 104, 815–820. [Google Scholar] [CrossRef]
- Kaisheva, M.E. The Effect of Metals and Soil pH on the Growth of Rhododendron and Other Alpine Plants in Limestone Soil. Ph.D. Dissertation, University of Edinburgh, Edinburgh, UK, 2006. Available online: https://www.era.lib.ed.ac.uk/handle/1842/2606 (accessed on 1 October 2023).
- Krebs, S.L. Rhododendron. In Ornamental Crops; Van Huylenbroeck, J., Ed.; Springer: Cham, Switzerland, 2018; pp. 673–718. [Google Scholar] [CrossRef]
- Dunemann, F.; Kahnau, R.; Stange, I. Analysis of complex leaf and flower characters in Rhododendron using a molecular linkage map. Theor. Appl. Genet. 1999, 98, 1146–1155. [Google Scholar] [CrossRef]





| Genotypes | Not Selected (NS)/Selected (S) in Previous Seedling Selection in Tissue Culture | pH Used for Selection | Number of Plants per Treatment |
|---|---|---|---|
| PB-T3-1 | S | 7.7 | 4 |
| PB-T3-4 | S | 7.7 | 4 |
| PB-T4-2 | S | 7.5 | 4 |
| PB | NS | 5.8 | 12 |
| RF-T-2 | S | 8.9 | 3 |
| RF-T-3 | S | 8.9 | 3 |
| RF-T-5 | S | 8.9 | 3 |
| RF-T-6 | S | 8.9 | 3 |
| RF-C-1 | NS | 6.2 | 3 |
| GW | NA | NA | 12 |
| CW | NA | NA | 12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Van Labeke, M.-C.; Dhooghe, E.; Van Huylenbroeck, J.; Leus, L. Greenhouse Screening for pH Stress in Rhododendron Genotypes. Horticulturae 2023, 9, 1302. https://doi.org/10.3390/horticulturae9121302
Wang S, Van Labeke M-C, Dhooghe E, Van Huylenbroeck J, Leus L. Greenhouse Screening for pH Stress in Rhododendron Genotypes. Horticulturae. 2023; 9(12):1302. https://doi.org/10.3390/horticulturae9121302
Chicago/Turabian StyleWang, Shusheng, Marie-Christine Van Labeke, Emmy Dhooghe, Johan Van Huylenbroeck, and Leen Leus. 2023. "Greenhouse Screening for pH Stress in Rhododendron Genotypes" Horticulturae 9, no. 12: 1302. https://doi.org/10.3390/horticulturae9121302
APA StyleWang, S., Van Labeke, M.-C., Dhooghe, E., Van Huylenbroeck, J., & Leus, L. (2023). Greenhouse Screening for pH Stress in Rhododendron Genotypes. Horticulturae, 9(12), 1302. https://doi.org/10.3390/horticulturae9121302







