Warm Bulb Storage Optimises Flowering Attributes and Foliage Characteristics in Amaryllis belladonna L.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Location
2.2. Plant Material and Preparation
2.3. Experimental Design and Treatment Set-Up
2.4. Data Collection
2.4.1. Determination of Inflorescence Morphological Development
2.4.2. Determination of Inflorescence Flowering Time Course
2.4.3. Determination of Leaf Morphological Growth
2.5. Statistical Analysis
3. Results
3.1. Effect of Warm Bulb Storage Period on Inflorescence Morphological Development
3.1.1. Percentage Flowering Yield
3.1.2. Inflorescence Stem Length and Stem Diameter
3.1.3. Number of Florets
3.1.4. Floret Length and Diameter
3.1.5. Inflorescence Crown Diameter
3.1.6. Inflorescence Fullness Ratio
3.1.7. Inflorescence Longevity
3.2. Effect of Warm Bulb Storage Period on Flowering Time Course of Visible Flower Buds, Anthesis, Opening of 50% Florets, and Complete Floral Senescence
3.3. Effect of Warm Bulb Storage Period on Leaf Morphological Growth
3.3.1. Number of Leaves
3.3.2. Leaf Length, Leaf Width, and Leaf Area
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, P. Floriculture-World Wide Production, Trade, Consumption Pattern, Market Opportunities and Challenges. Available online: https://medium.com/@preetisharma_51610/floriculture-world-wide-production-trade-consumption-pattern-market-opportunities-and-challenges-e797d87c87a7 (accessed on 6 August 2023).
- Anumala, N.V.; Kumar, R. Floriculture Sector in India: Current Status and Export Potential. J. Hortic. Sci. Biotechnol. 2021, 96, 673–680. [Google Scholar] [CrossRef]
- Barnhoorn, C. The Bulb Book: A South African Gardener’s Guide; Sunbird Publishers: Cape Town, South Africa, 2013; ISBN 1920289763. [Google Scholar]
- Darras, A.I. Overview of the Dynamic Role of Specialty Cut Flowers in the International Cut Flower Market. Horticulturae 2021, 7, 51. [Google Scholar] [CrossRef]
- Darras, A.I. Implementation of Sustainable Practices to Ornamental Plant Cultivation Worldwide: A Critical Review. Agronomy 2020, 10, 1570. [Google Scholar] [CrossRef]
- Thörning, R.; Ahlklo, Å.K.; Spendrup, S. The Slow Flower Movement—Exploring Alternative Sustainable Cut-Flower Production in a Swedish Context. Heliyon 2022, 8, e11086. [Google Scholar] [CrossRef] [PubMed]
- Ingram, D.L.; Hall, C.R.; Knight, J. Understanding Carbon Footprint in Production and Use of Landscape Plants. Horttechnology 2019, 29, 6–10. [Google Scholar] [CrossRef]
- Lee, J.; Miller, W.B. Preplant Storage and Greenhouse Temperature Influence Flowering of Ornithogalum. Hortscience 2015, 50, 1784–1790. [Google Scholar] [CrossRef]
- Miller, W.B. Commercial Flower Production Methodology. Encycl. Appl. Plant Sci. 2017, 3, 203–208. [Google Scholar] [CrossRef]
- De Pascale, S.; Romano, D. Potential Use of Wild Plants in Floriculture. Acta Hortic. 2019, 1240, 87–98. [Google Scholar] [CrossRef]
- Adams, T.D. Amaryllis Belladonna|PlantZAfrica. Available online: https://pza.sanbi.org/amaryllis-belladonna (accessed on 3 March 2023).
- Theron, K.I.; De Hertogh, A.A. Amaryllidaceae: Geophytic Growth, Development, and Flowering. Hortic. Rev. Am. Soc. Hortic. Sci. 2001, 25, 1–70. [Google Scholar]
- Manning, J.; Goldblatt, P.; Snijman, D. The Color Encyclopedia of Cape Bulbs; Timber Press: Cambridge, UK, 2002; ISBN 0881925. [Google Scholar]
- Duncan, G.D.; Jeppe, B.; Voigt, L. Field Guide to the Amaryllis Family of Southern Africa and Surrounding Territories; Galley Press: Nelspruit, South Africa, 2020; ISBN 9780620885911. [Google Scholar]
- Campos-Rocha, A.; Meerow, A.W.; Lopes, E.F.M.; Semir, J.; Mayer, J.L.S.; Dutilh, J.H.A. Eithea Lagopaivae, a New Critically Endangered Species in the Previously Monotypic Genus Eithea Ravenna (Amaryllidaceae). PhytoKeys 2017, 85, 45–58. [Google Scholar] [CrossRef]
- Reinten, E.Y.; Coetzee, J.H.; Van Wyk, B.E. The Potential of South African Indigenous Plants for the International Cut Flower Trade. South Afr. J. Bot. 2011, 77, 934–946. [Google Scholar] [CrossRef]
- Wilmot, C.M.; Laubscher, C.P. Amaryllis Belladonna: A Potential Urban Landscape Wonder. Acta Hortic. 2019, 1237, 287–293. [Google Scholar] [CrossRef]
- Gul, F.; Tahir, I.; Shahri, W. Flower Senescence and Some Postharvest Considerations of Amaryllis Belladonna Cut Scapes. Plant Physiol. Rep. 2020, 25, 315–324. [Google Scholar] [CrossRef]
- Duncan, G.D. Amaryllis Magic. Veld. Flora 2004, 90, 142–147. [Google Scholar]
- Corbesier, L.; Coupland, G. The Quest for Florigen: A Review of Recent Progress. J. Exp. Bot. 2006, 57, 3395–3403. [Google Scholar] [CrossRef]
- Horvath, D. Common Mechanisms Regulate Flowering and Dormancy. Plant Sci. 2009, 177, 523–531. [Google Scholar] [CrossRef]
- Denay, G.; Chahtane, H.; Tichtinsky, G.; Parcy, F. A Flower Is Born: An Update on Arabidopsis Floral Meristem Formation. Curr. Opin. Plant Biol. 2017, 35, 15–22. [Google Scholar] [CrossRef] [PubMed]
- De Hertogh, A.; Le Nard, M. The Physiology of Flower Bulbs; Elsevier: Amsterdam, The Netherlands, 1993; ISBN 9780444874986. [Google Scholar]
- Roh, M.S.; Hong, D.K. Inflorescence Development and Flowering of Ornithogalum Thyrsoides Hybrid as Affected by Temperature Manipulation during Bulb Storage. Sci. Hortic. 2007, 113, 60–69. [Google Scholar] [CrossRef]
- Khodorova, N.V.; Boitel-Conti, M. The Role of Temperature in the Growth and Flowering of Geophytes. Plants 2013, 2, 699. [Google Scholar] [CrossRef]
- Capovilla, G.; Schmid, M.; Posé, D. Control of Flowering by Ambient Temperature. J. Exp. Bot. 2015, 66, 59–69. [Google Scholar] [CrossRef]
- Howard, C.C.; Folk, R.A.; Beaulieu, J.M.; Cellinese, N. The Monocotyledonous Underground: Global Climatic and Phylogenetic Patterns of Geophyte Diversity. Am. J. Bot. 2019, 106, 850–863. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, X.; Xu, J.; Yang, Z.; Zhang, Y. Effects of Ambient Temperature on Flower Initiation and Flowering in Saffron (Crocus sativus, L.). Sci. Hortic. 2021, 279, 109859. [Google Scholar] [CrossRef]
- Le Nard, M.; De Hertogh, A.A. Bulb Growth and Development and Flowering. In The Physiology of Flower Bulbs; Le Nard, M., De Hertogh, A.A., Eds.; Elsevier: Amsterdam, The Netherlands, 1993; pp. 29–44. [Google Scholar]
- Miller, W.B. Flower Bulbs Worldwide: Perspectives on the Production Chain and Research. Acta Hortic. 2017, 1171, 1–8. [Google Scholar] [CrossRef]
- Rees, A.R. The Physiology of Ornamental Bulbous Plants. Bot. Rev. 1966, 32, 1–23. [Google Scholar] [CrossRef]
- Kamenetsky-Goldstein, R. Geophyte Cultivation in Changing Climate: Environmental Effects on Development and Production. Acta Hortic. 2019, 1237, 277–285. [Google Scholar] [CrossRef]
- Wilmot, C.M.; Laubscher, C.P. Flowering Initiation in Amaryllis Belladonna. Acta Hortic. 2019, 1237, 137–144. [Google Scholar] [CrossRef]
- Molina, R.V.; Valero, M.; Navarro, Y.; Guardiola, J.L.; García-Luis, A. Temperature Effects on Flower Formation in Saffron (Crocus sativus L.). Sci. Hortic. 2005, 103, 361–379. [Google Scholar] [CrossRef]
- Thompson, D.I.; Mtshali, N.P.; Ascough, G.D.; Erwin, J.E.; Van Staden, J. Flowering Control in Watsonia: Effects of Corm Size, Temperature, Photoperiod and Irradiance. Sci. Hortic. 2011, 129, 493–502. [Google Scholar] [CrossRef]
- Inkham, C.; Piriyapongpitak, P.; Ruamrungsri, S. Storage and Growth Temperatures Affect Growth, Flower Quality, and Bulb Quality of Hippeastrum. Hortic. Env. Biotechnol. 2019, 60, 357–362. [Google Scholar] [CrossRef]
- Carlson, A.S.; Dole, J.M. Determining Optimal Bulb Storage and Production Methods for Successful Forcing of Cut Pineapple Lily. Horttechnology 2015, 25, 608–616. [Google Scholar] [CrossRef]
- Warrington, I.J.; Brooking, I.R.; Fulton, T.A. Lifting Time and Bulb Storage Temperature Influence Nerine sarniensis Flowering Time and Flower Quality. N. Z. J. Crop. Hortic. Sci. 2011, 39, 107–117. [Google Scholar] [CrossRef]
- Viljoen, C.C.; Jimoh, M.O.; Laubscher, C.P. Studies of Vegetative Growth, Inflorescence Development and Eco-Dormancy Formation of Abscission Layers in Streptocarpus Formosus (Gesneriaceae). Horticulturae 2021, 7, 120. [Google Scholar] [CrossRef]
- Kamenetsky, R.; Miller, W.B. The Global Trade in Ornamental Geophytes. Chron. Hortic. 2010, 50, 27–30. [Google Scholar]
- Marasek-Ciolakowska, A.; Sochacki, D.; Marciniak, P. Breeding Aspects of Selected Ornamental Bulbous Crops. Agronomy 2021, 11, 1709. [Google Scholar] [CrossRef]
- Loyola, C.E.; Dole, J.M.; Dunning, R. North American Specialty Cut Flower Production and Postharvest Survey. Horttechnology 2019, 29, 338–359. [Google Scholar] [CrossRef]
- Salachna, P.; Mikiciuk, M.; Zawadzińska, A.; Piechocki, R.; Ptak, P.; Mikiciuk, G.; Pietrak, A.; Łopusiewicz, Ł. Changes in Growth and Physiological Parameters of ×Amarine Following an Exogenous Application of Gibberellic Acid and Methyl Jasmonate. Agronomy 2020, 10, 980. [Google Scholar] [CrossRef]
- Kapczyńska, A. Effect of Bulb Size on Growth, Flowering and Bulb Formation in Lachenalia Cultivars. Hortic. Sci. 2014, 41, 89–94. [Google Scholar] [CrossRef]
- Slezák, K.A.; Mazur, J.; Jezdinský, A.; Kapczyńska, A. Bulb Size Interacts with Lifting Term in Determining the Quality of Narcissus Poeticus L. Propagation Material. Agronomy 2020, 10, 975. [Google Scholar] [CrossRef]
- Duncan, G.D. Grow Bulbs; Kirstenbosch Gardening Series; South African National Biodiversity Institute: Cape Town, South Africa, 2010; ISBN 9781919684567. [Google Scholar]
- Yu, X.; Shi, P.; Schrader, J.; Niklas, K.J. Nondestructive Estimation of Leaf Area for 15 Species of Vines with Different Leaf Shapes. Am. J. Bot. 2020, 107, 1481–1490. [Google Scholar] [CrossRef]
- Shi, P.J.; Li, Y.R.; Niinemets, Ü.; Olson, E.; Schrader, J. Influence of Leaf Shape on the Scaling of Leaf Surface Area and Length in Bamboo Plants. Trees Struct. Funct. 2021, 35, 709–715. [Google Scholar] [CrossRef]
- Hartsema, A.M.; Leupen, F.F. Orgaanvorming En periodiciteit van Amaryllis Belladonna. Meded. Landbouwhoogesch. Wagening. 1942, 46, 1–30. [Google Scholar]
- Van Kilsdonk, M.G.; Nicolay, K.; Franssen, J.M.; Kollöffel, C. Bud Abortion in Tulip Bulbs Studied by Magnetic Resonance Imaging. J. Exp. Bot. 2002, 53, 1603–1611. [Google Scholar] [CrossRef] [PubMed]
- Kapczyńska, A. Effect of Planting Time on Flowering of Four Lachenalia Cultivars. Acta Hortic. 2012, 937, 575–579. [Google Scholar] [CrossRef]
- Du Toit, E.S.; Robbertse, P.J.; Niederwieser, J.G. Effects of Growth and Storage Temperature on Lachenalia Cv. Ronina Bulb Morphology. Sci. Hortic. 2002, 94, 117–123. [Google Scholar] [CrossRef]
- Roh, M.S. Flowering and Inflorescence Development of Lachenalia Aloides “Pearsonii” as Influenced by Bulb Storage and Forcing Temperature. Sci. Hortic. 2005, 104, 305–323. [Google Scholar] [CrossRef]
- Rees, A.R. Ornamental Bulbs, Corms and Tubers; CAB International: Wallingford, UK, 1992; Volume 1. [Google Scholar]
- Dole, J.M.; Wilkins, H.F. Floriculture: Principles and Species, 2nd ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2005; ISBN 0130462500. [Google Scholar]
- Kim, J.; Oh, W. Cold Storage Period of Bulbs Influences Their Sugar Contents and Post-Storage Growth and Flowering in Lilium Oriental Hybrids. Agriculture 2021, 11, 1080. [Google Scholar] [CrossRef]
- Wendell, M.; Ripel, L.; Lee, Y.K.; Rognli, O.A.; Torre, S.; Olsen, J.E. Thermoperiodic Control of Floral Induction Involves Modulation of the Diurnal Flowering Locus T Expression Pattern. Plant Cell Physiol. 2017, 58, 466–477. [Google Scholar] [CrossRef]
- Marković, M.; Momčilov, M.T.; Uzelac, B.; Jevremović, S.; Subotić, A. Bulb Dormancy in Vitro—Fritillaria meleagris: Initiation, Release and Physiological Parameters. Plants 2021, 10, 902. [Google Scholar] [CrossRef]
- Dafni, A.; Cohen, D.; Noy-Mier, I. Life-Cycle Variation in Geophytes. Ann. Mo. Bot. Gard. 1981, 68, 652. [Google Scholar] [CrossRef]
- Howard, C.C.; Cellinese, N. Tunicate Bulb Size Variation in Monocots Explained by Temperature and Phenology. Ecol. Evol. 2020, 10, 2299–2309. [Google Scholar] [CrossRef]
- Miller, W.B. A Review of Carbohydrate Metabolism in Geophytes. Acta Hortic. 1992, 325, 239–246. [Google Scholar] [CrossRef]
- Kapczyńska, A.; Kidawska, A. “Namakwa” Lachenalia’s Response to Flurprimidol and Different Planting Dates. Folia Hortic. 2016, 28, 173–179. [Google Scholar] [CrossRef]
- Chen, Q.X.C.; Guo, Y.; Warner, R.M. Identification of Quantitative Trait Loci for Component Traits of Flowering Capacity across Temperature in Petunia. G3 Genes Genomes Genet. 2019, 9, 3601–3610. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Huo, B.; Lin, S.; Zhang, S.; Mao, C.; Jiang, J.; Chen, S.; Fang, W.; Guan, Z.; Liao, Y.; et al. Germplasm Innovation and Establishment of Comprehensive Evaluation System for Hedgerow Garden Chrysanthemum. Agronomy 2022, 12, 1736. [Google Scholar] [CrossRef]
- Kapczyńska, A.; Stodolak, B. The Morphological and Physiological Response of Lachenalia to Supplemental Irradiation. Hortic. Env. Biotechnol. 2019, 60, 455–465. [Google Scholar] [CrossRef]
- Chandel, A.; Thakur, M.; Singh, G.; Dogra, R.; Bajad, A.; Soni, V.; Bhargava, B. Flower Regulation in Floriculture: An Agronomic Concept and Commercial Use. J. Plant Growth Regul. 2023, 42, 2136–2161. [Google Scholar] [CrossRef]
- Luria, G.; Watad, A.A.; Cohen-Zhedek, Y.; Borochov, A. Growth and Flowering of Ornithogalum Dubium. Acta Hortic. 2002, 570, 113–119. [Google Scholar] [CrossRef]
- Malik, K.M.; Shiekh, M.Q.; Nazki, I.T.; Mir, S.A. Influence of Storage Temperature, Storage Duration and Disbudding on Bulb Production in Asiatic lilium Cv. ‘Royal Trinity’. Biosci. Biotechnol. Res. Asia 2017, 14, 577–585. [Google Scholar] [CrossRef]
- Van Doorn, W.G.; Woltering, E.J. Physiology and Molecular Biology of Petal Senescence. J. Exp. Bot. 2008, 59, 453–480. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhong, D.; Liu, Z.; Gao, J. Study on the Physiological, Cellular, and Morphological Aspects of the Postharvest Development of Cut Lily Flowers. Hortic. Plant J. 2021, 7, 149–158. [Google Scholar] [CrossRef]
- Reinten, E.Y.; Van Wyk, B.E. Floriculture Industry Benefits from Southern African Floral Biodiversity. Acta Hortic. 2018, 1201, 659–663. [Google Scholar] [CrossRef]
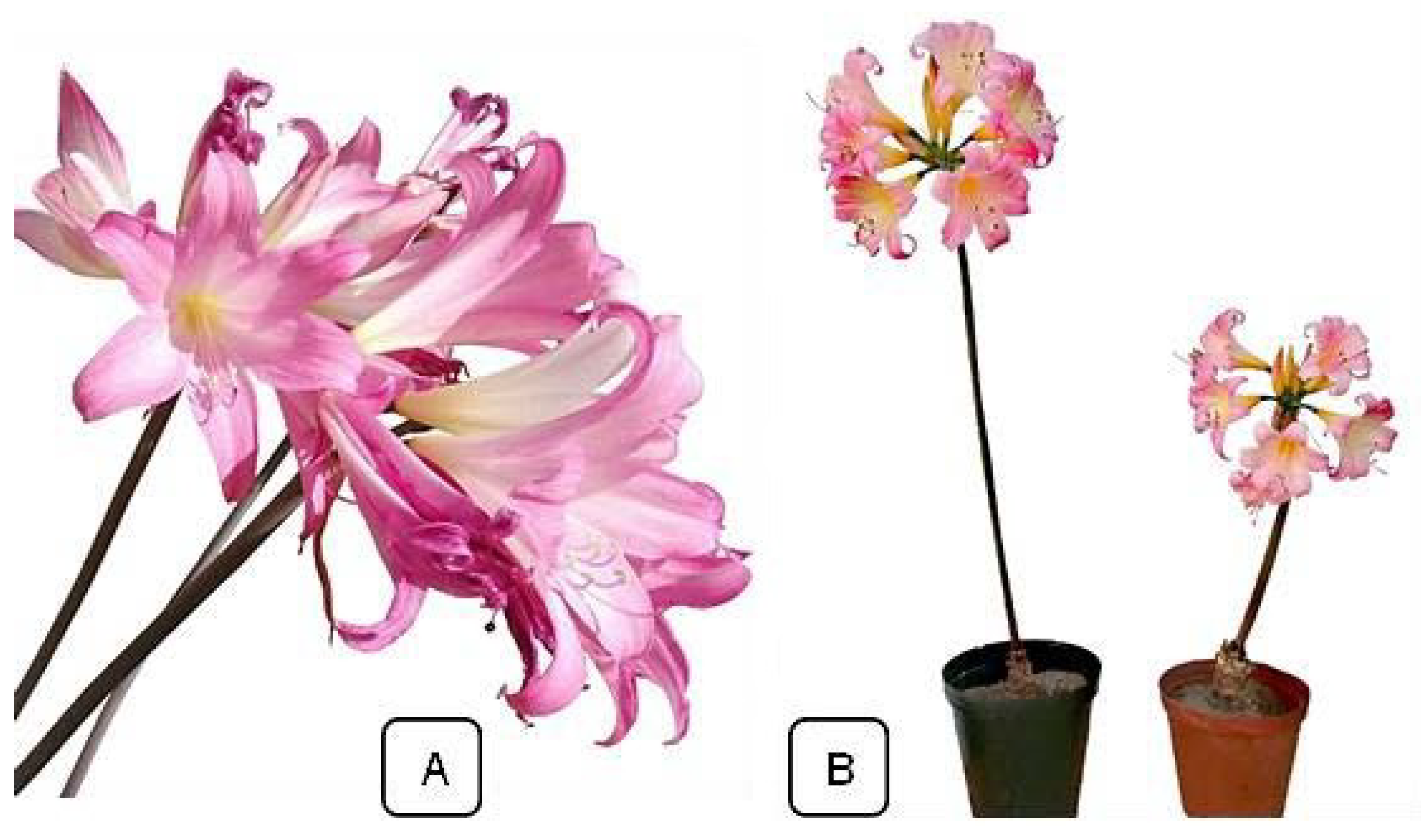

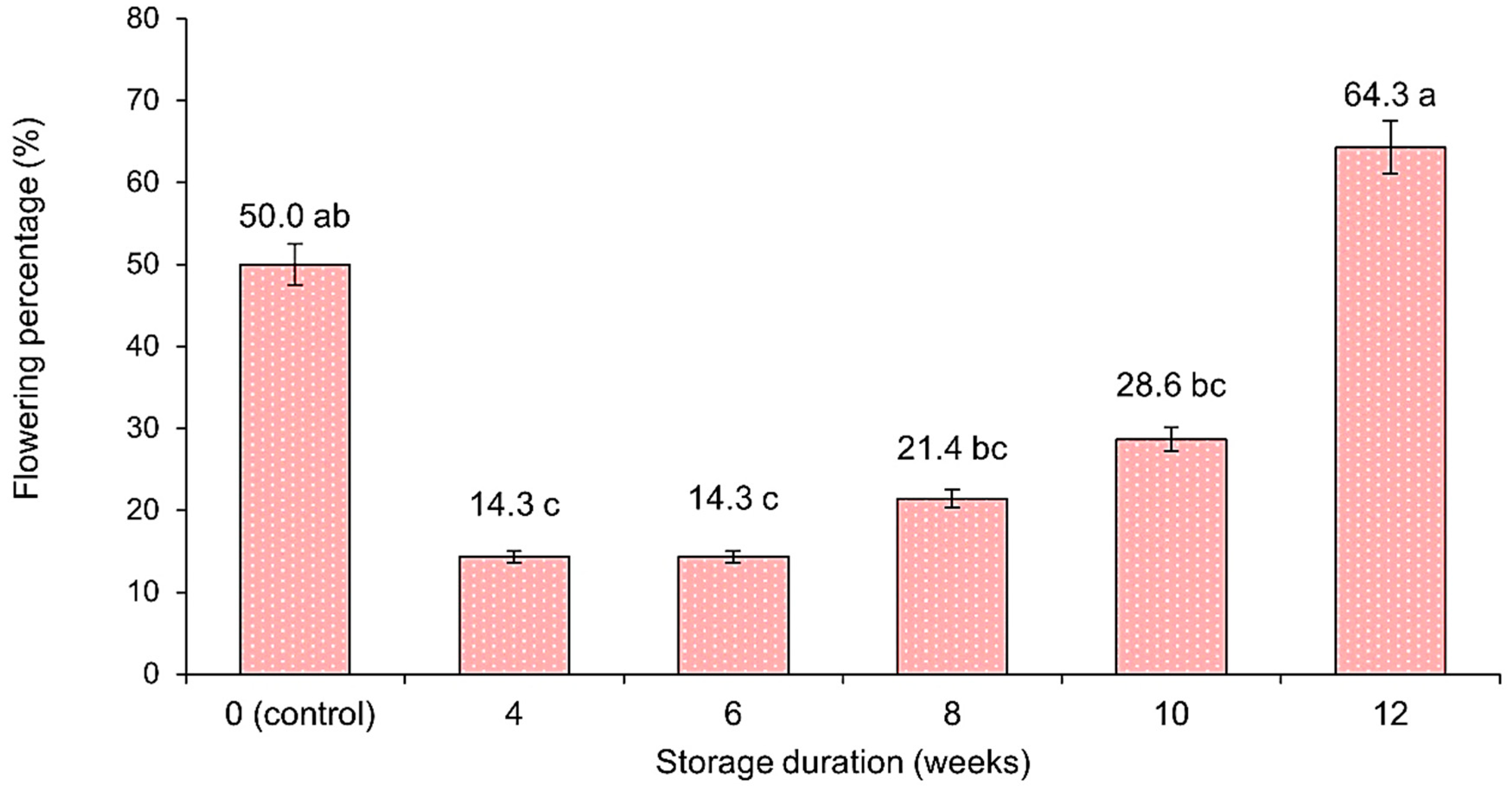
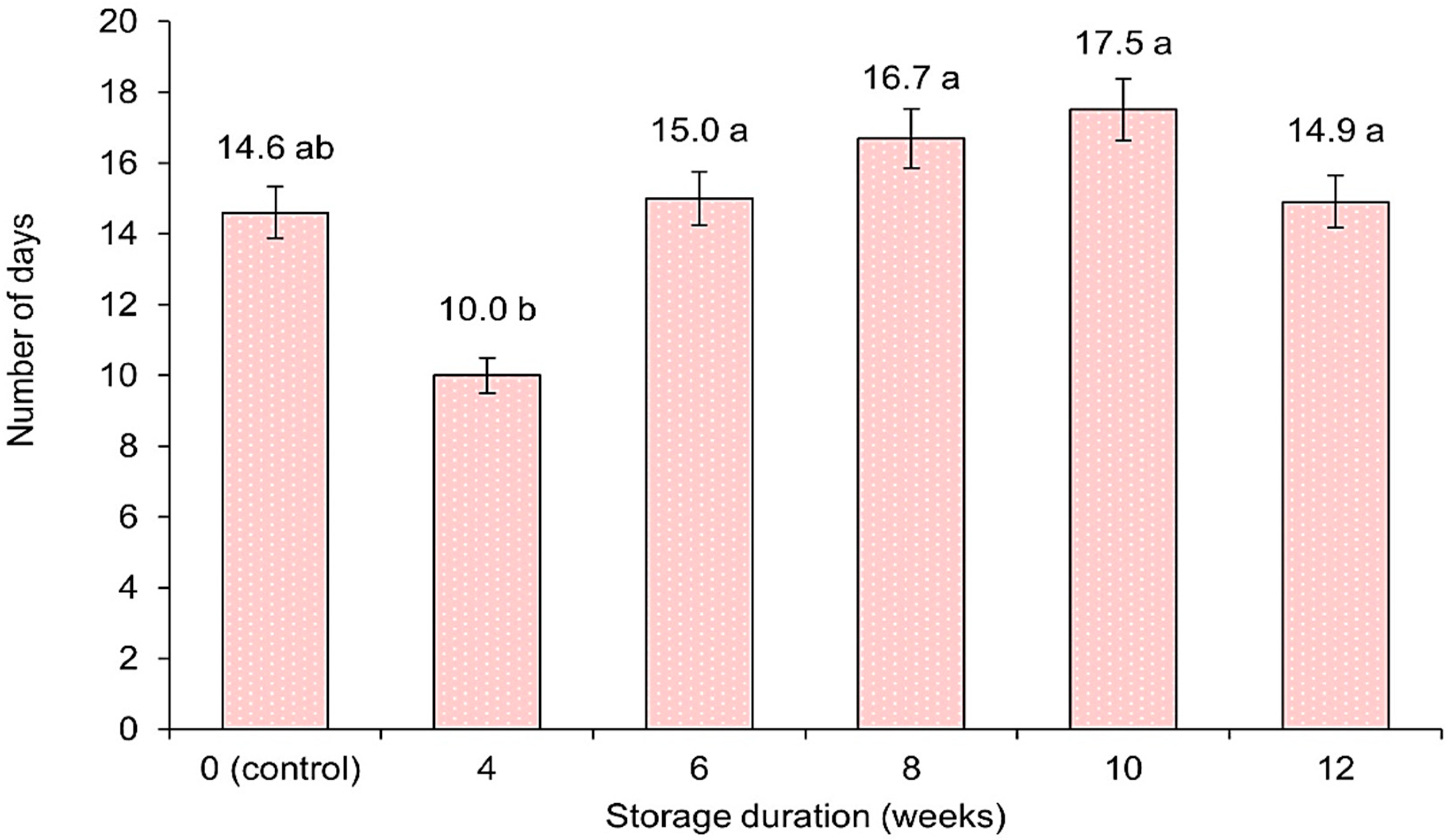
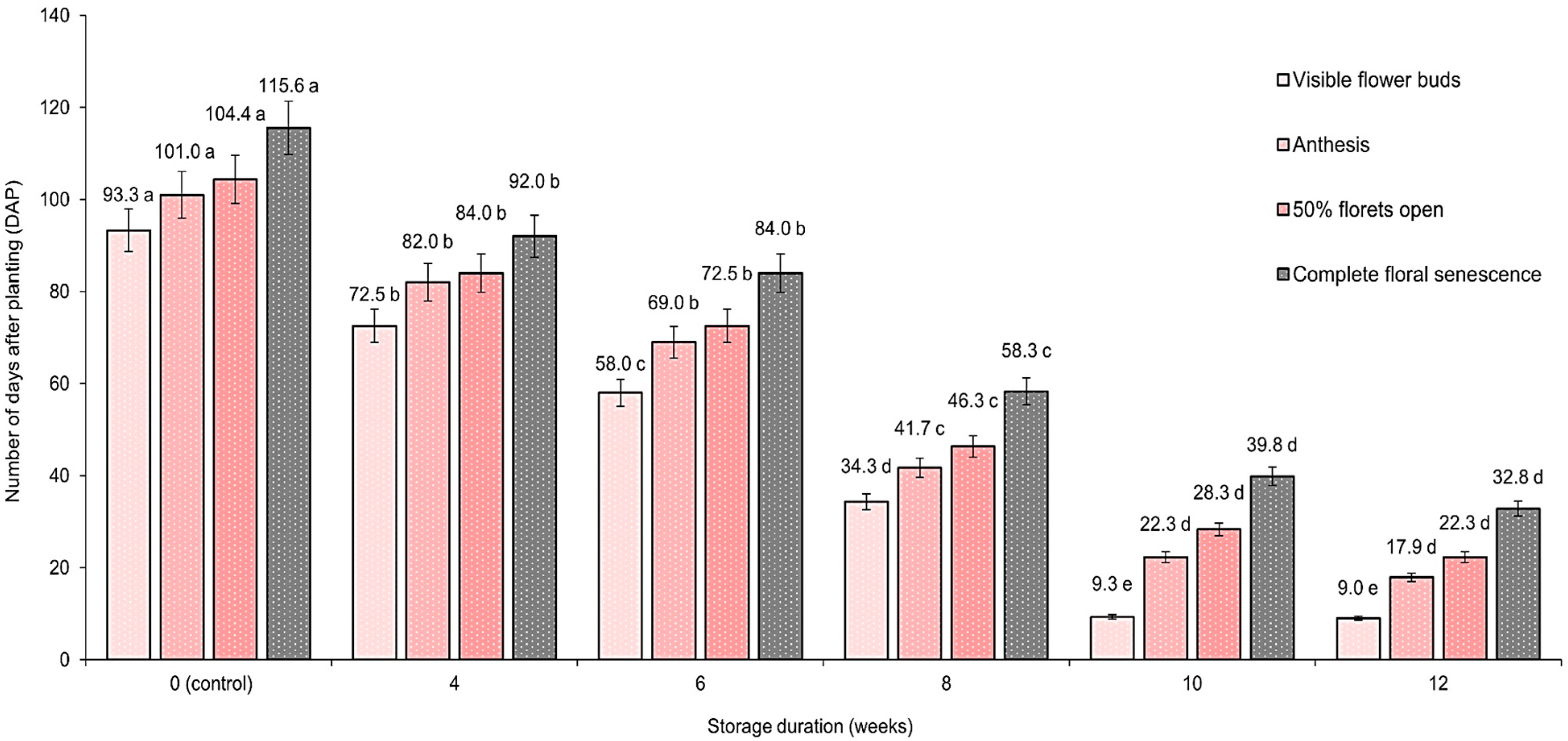
| S/N | Code | Storage Duration Description | Subsequent Planting Time after Storage |
|---|---|---|---|
| 1 | D1 | 0-week bulb storage (c) (0 days) | (mid-November 2021) |
| 2 | D2 | 4-week bulb storage (28 days) | (mid-December 2021) |
| 3 | D3 | 6-week bulb storage (42 days) | (end-December 2021) |
| 4 | D4 | 8-week bulb storage (56 days) | (mid-January 2022) |
| 5 | D5 | 10-week bulb storage (70 days) | (end-January 2022) |
| 6 | D6 | 12-week bulb storage (94 days) | (mid-February 2022) |
| Bulb Storage (Weeks) | Inflorescence Characteristics | ||||||
|---|---|---|---|---|---|---|---|
| Inflorescence Stem Length (cm) | Inflorescence Stem Diameter (mm) | Number of Florets (n) | Floret Length (cm) | Floret Diameter (cm) | Inflorescence Crown Diameter (cm) | Inflorescence Fullness Ratio | |
| 0 (control) | 45.1 ± 2.89 b | 8.9 ± 0.39 d | 9.6 ± 0.90 b | 12.0 ± 0.36 a | 9.8 ± 0.21 a | 22.7 ± 0.64 a | 0.4 ± 0.04 b |
| 4 | 39.2 ± 6.00 b | 9.6 ± 0.66 cd | 8.0 ± 4.00 b | 11.4 ± 0.75 a | 9.2 ± 0.55 a | 21.6 ± 1.35 a | 0.4 ± 0.21 b |
| 6 | 37.0 ± 6.55 b | 10.5 ± 0.09 bcd | 12.5 ± 4.50 ab | 11.8 ± 0.85 a | 9.5 ± 0.70 a | 22.4 ± 1.46 a | 0.6 ± 0.24 ab |
| 8 | 56.7 ± 6.08 a | 12.7 ± 0.02 a | 17.0 ± 5.78 a | 11.5 ± 0.10 a | 9.6 ± 0.07 a | 21.9 ± 0.17 a | 0.8 ± 0.03 a |
| 10 | 43.2 ± 5.72 b | 11.7 ± 0.75 ab | 16.5 ± 1.50 a | 10.8 ± 0.53 a | 8.9 ± 0.42 a | 21.0 ± 0.87 a | 0.8 ± 0.09 a |
| 12 | 44.0 ± 1.23 b | 10.2 ± 0.36 c | 10.2 ± 0.97 b | 11.6 ± 0.39 a | 9.5 ± 0.36 a | 22.0 ± 0.72 a | 0.5 ± 0.05 b |
| One-way ANOVA F-statistic | |||||||
| Bulb storage | 2.15 ns | 7.16 * | 5.31 * | 0.74 ns | 0.67 ns | 0.50 ns | 4.51 * |
| Bulb Storage (Weeks) | Leaf Characteristics | |||
|---|---|---|---|---|
| Number of Leaves (n) | Leaf Length (cm) | Leaf Width (cm) | Leaf Area (cm2) | |
| 0 (control) | 11.9 ± 0.43 ab | 47.1 ± 2.21 a | 3.1 ± 0.12 b | 148.6 ± 10.40 b |
| 4 | 11.9 ± 0.28 ab | 49.4 ± 3.02 a | 3.4 ± 0.13 ab | 170.0 ± 13.30 ab |
| 6 | 11.7 ± 0.34 b | 47.1 ± 2.11 a | 3.4 ± 0.12 ab | 160.90 ± 12.0 ab |
| 8 | 11.7 ± 0.66 b | 50.6 ± 2.28 a | 3.5 ± 0.11 a | 178.6 ± 11.20 a |
| 10 | 12.2 ± 0.43 ab | 45.9 ± 2.08 a | 3.4 ± 0.06 ab | 154.22 ± 7.69 ab |
| 12 | 13.0 ± 0.42 a | 47.1 ± 1.41 a | 3.5 ± 0.09 a | 165.14 ± 8.17 ab |
| One-way ANOVA F-statistic | ||||
| Bulb storage | 1.26 ns | 0.62 ns | 1.59 ns | 1.03 ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilmot, C.M.; Jimoh, M.O.; Laubscher, C.P. Warm Bulb Storage Optimises Flowering Attributes and Foliage Characteristics in Amaryllis belladonna L. Horticulturae 2023, 9, 1271. https://doi.org/10.3390/horticulturae9121271
Wilmot CM, Jimoh MO, Laubscher CP. Warm Bulb Storage Optimises Flowering Attributes and Foliage Characteristics in Amaryllis belladonna L. Horticulturae. 2023; 9(12):1271. https://doi.org/10.3390/horticulturae9121271
Chicago/Turabian StyleWilmot, Carolyn Margaret, Muhali Olaide Jimoh, and Charles Petrus Laubscher. 2023. "Warm Bulb Storage Optimises Flowering Attributes and Foliage Characteristics in Amaryllis belladonna L." Horticulturae 9, no. 12: 1271. https://doi.org/10.3390/horticulturae9121271
APA StyleWilmot, C. M., Jimoh, M. O., & Laubscher, C. P. (2023). Warm Bulb Storage Optimises Flowering Attributes and Foliage Characteristics in Amaryllis belladonna L. Horticulturae, 9(12), 1271. https://doi.org/10.3390/horticulturae9121271





