Abstract
Orchid seeds are predominantly wind-dispersed, often developed within dry, dehiscent fruits that typically release millions of dust-like seeds into the air. Animal-mediated seed dispersal is a lesser-known phenomenon in the family and predominantly occurs in groups belonging to early-diverging lineages bearing indehiscent, fleshy fruits with hard, rounded, dark seeds. In this review, we explore the evolutionary trends of seed dispersal mechanisms in Orchidaceae, focusing on the pantropical genus Vanilla. Notably, certain Neotropical species of Vanilla produce vanillin-aromatic compounds synthesized naturally in their fruits, which plays a pivotal role in seed dispersal. Ectozoochory occurs in dry, dehiscent fruits, whose seeds are dispersed by (i) male euglossine bees collecting the fruit’s vanillin aromatic compounds and (ii) female stingless bees collecting the fruit’s mesocarp. Endozoochory occurs in (iii) highly nutritious, indehiscent fruits consumed by terrestrial mammals or (iv) fleshy, dehiscent fruits whose mesocarp is consumed by arboreal mammals. Wind dispersal appears to be a derived state in Orchidaceae and, given its predominance, a trait likely associated with enhanced speciation rates. Zoochory primarily occurs in groups derived from early-diverging lineages; occasional reversions suggest a link between dispersal mode and fruit and seed traits. Interestingly, fruit dehiscence and fleshiness in Vanilla lack phylogenetic signal despite their role in determining dispersal modes, suggesting potential environmental adaptability.
1. Background
Vanilla is an economically significant spice of global importance, widely used in a broad spectrum of products offered by the food, cosmetics, and pharmaceutical industries. Vanilla flavoring is derived from vanillin and related aromatic compounds, which are naturally extracted from the fruits, better known as beans or pods, of orchids belonging to the genus Vanilla Mill. [1,2]. Vanilla orchids grow as climbing vines in the tropical regions of Africa, America, and Asia, where over 120 species are known to occur in the wild [1,3]. However, only one specific clade of Vanilla species native to tropical America (Vanilla sect. Xanata) produces fruits that contain highly esteemed vanillin and related compounds. This includes Vanilla planifolia Andrews, the commercially most important species. Although Mexico is generally recognized as the birthplace of vanilla cultivation [4], the leading vanilla-producing countries today are Madagascar (3070 tons/yr) and Indonesia (1456 tons/yr) [5]. Both countries lack the natural pollinators and seed dispersers of vanilla, as they are located outside the native growing range of all members of Vanilla sect. Xanata, including V. planifolia. Consequently, vanilla plants introduced into these areas do not naturally develop fruits or reproduce sexually by cross-pollination. Therefore, flowers are hand-pollinated, and plants are reproduced through vegetative cloning. As a result, these practices are now widespread among all vanilla-producing countries, even in the Neotropics, because they result in high reproduction rates and satisfying yields [4]. Unfortunately, these practices have also led to a decrease in genetic diversity in the cultivated species of the vanilla crop, which in turn has made vanilla plants highly vulnerable to abiotic and biotic stressors [6,7,8,9]. Given that climate change is expected to negatively impact the agricultural sector [10,11], vanilla cultivation practices need to be improved to safeguard the future of this beloved spice and the associated economic activities of this industry.
A possible solution lies In the use of the gene pool available among the wild relatives of the vanilla crop, which includes wild populations of V. planifolia but also closely related species with potential traits of interest for crop improvement and breeding [9,12]. Advancements in molecular biology tools have substantially improved our knowledge of phylogenetic relationships, inter- and intra-specific diversity, reproductive systems, and mutualistic interactions within Vanilla. Studies range from assessing the genetic variation within V. planifolia and closely related species (e.g., [6,13,14,15,16,17,18,19,20,21,22]) to enhancing Vanilla taxonomy [3,23,24], confirming hybrid progeny and establishing marker-trait associations for crop improvement and breeding (e.g., [22,25,26,27]). Furthermore, evidence for the presence of diverse mating systems (allogamy vs. autogamy) in V. planifolia and related species was provided via molecular studies using isozyme and microsatellite markers [28,29].
Anthropogenic pressures, however, are putting a severe strain on the survival of wild Vanilla populations, with most species already having a (critically) endangered status on the IUCN red list [30]. Despite their critical role in species survival, ecological interactions between vanilla plants and their hosts, patrolling insects, pollinators, seed-dispersers, as well as wild seed germination and mycorrhizal associations remain largely undocumented and poorly understood, with numerous knowledge gaps persisting [31,32,33,34,35,36,37,38,39,40,41,42,43]. However, unlike most other orchid genera, using character and observational data from extant species suggests a diversity of dispersal modes co-existing within Vanilla. Therefore, phylogenetic inferences using current data can reveal novel insights into the evolutionary history of seed dispersal in Vanilla and Orchidaceae in general. Seed dispersal is an important stage in the life cycle of a plant, as it influences its reproductive success and, ultimately, survival, thus directly affecting gene frequencies and geographical range of populations [44]; the evolutionary dynamics favoring specific dispersal modes are mostly expected to respond to increased fitness. For centuries, scientists have wondered how vanilla seeds are dispersed and what ecological role vanillin and related aromatic compounds have. The answer—at least part of it—has finally been revealed, and these insights could serve as the foundation for an integral conservation plan across Vanilla’s natural distribution range. A recent study by Karremans et al. [42] evidenced a multi-modal seed dispersal mechanism based on the presence of vanillin in species belonging to Vanilla sect. Xanata, including both ectozoochory and endozoochory. Furthermore, the study showed the occurrence of both dehiscent and indehiscent fruits, even within the same species, and suggests that this fruit trait may be one of the main drivers determining the dispersal mode.
The aim of this review is to summarize the available information on seed dispersal modes within the Orchidaceae, thereby highlighting evolutionary trends and current knowledge gaps. Given the diversity and multimodality of seed dispersal mechanisms in Vanilla, combining molecular biology tools with the latest ecological evidence allows us to elucidate patterns in fruit and seed character evolution. Lastly, we identify potential research frontiers, applying molecular biology tools to facilitate evaluation and prediction of seed dispersal modes within Vanilla and the Orchidaceae.
2. Animal-Mediated Seed Dispersal in Orchidaceae
Orchid seeds are predominantly adapted to wind dispersal since orchid fruits or pods are typically dry and dehisce longitudinally while still attached to the plant [31]. As such, the millions of dust-like seeds can be easily uplifted and carried away by the wind. Nevertheless, there are a few notable exceptions [31,42,45,46,47,48,49,50,51,52,53,54,55,56,57,58] (Table 1; Figure 1). Hydrochory, or the dispersal of seeds by water, is an extremely rare phenomenon that has been documented in only two species within the Epidendroideae subfamily, Disa uniflora P.J.Bergius [52,59] and Epipactis gigantea Dougl. ex Hook. [52]. These species are typically found near waterbodies, and their fruit and seed traits differ from those of animal-dispersed orchids [52]. Certain orchids—primarily found in early diverging clades—show fleshy fruits that bear hard, rounded, dark seeds. These orchids are known to occur in Apostasia Blume and Neuwiedia Blume, the two genera of the Apostasioideae subfamily, the genus Selenipedium Rchb.f. of the Cypripedioideae subfamily, and in the genera Cyrtosia Blume and Vanilla of the Vanilloideae subfamily [31,42,46,49,50,51,52,53,54,55,56,57,58]. Fleshy fruits and sclerified seeds seem to be very rare in the Orchidoideae and Epidendroideae subfamilies. Among the former, it has only been reported in the genus Rhizanthella R.S.Rogers, while in the second, it is known to occur in the genera Palmorchis Barb. Rodr. [31,46,49,51,52] and Yoania Maxim. [55,56]. In the subfamily Vanilloideae, sclerified seeds are also found in some genera that bear non-fleshy, non-aromatic fruits, such as Epistephium Kunth, Erythrorchis Blume, Galeola Lour., and Pseudovanilla Garay. However, the seeds of those orchids are winged and believed to be dispersed by anemochory [48,51]. This may indicate a transitional phase, potentially leading to animal dispersal or a regression to wind dispersal.

Table 1.
Exceptions to wind dispersal in Orchidaceae.
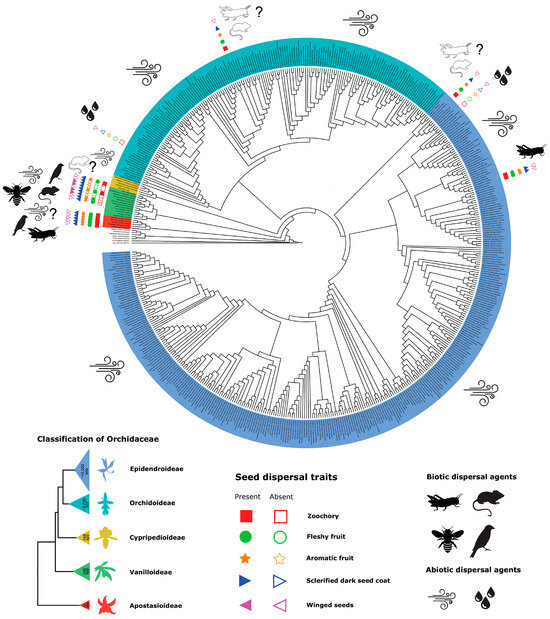
Figure 1.
Evolution of seed dispersal mechanisms and fruit traits in the Orchidaceae.
The fleshy, often aromatic fruits containing sclerified seeds are suggested to employ animal vectors rather than being adapted to wind dispersal, and this has indeed been proven in certain cases. For example, in tropical Asia, avian dispersal has been observed in Cyrtosia [54] and Neuwiedia [58], while seed dispersal by crickets was shown to occur in Apostasia [57] and Yoania (Y. amagiensis Nakai & Maek. and Y. japonica Maxim.) [55,56]. Both Cyrtosia septentrionalis (Rchb.f.) Garay and Neuwiedia singapureana (Wall. ex Baker) Rolfe have fleshy fruits that turn from green to bright red as they mature, a color that has been shown to attract birds [60,61]. Fruits of Apostasia and Yoania are eaten by crickets and camel crickets and display green and pinkish-white colors, respectively [55,56,57]. In these examples, the dark, rounded seeds were recovered from the feces of animals that fed on the fleshy fruits, and germination trials showed that the seeds are viable after passing the digestive system, confirming the presence of zoochory among these orchid genera.
Zoochory is suggested to be the main dispersal mechanism in Vanilla, and no other orchid genus seems to have such diverse dispersal modes and agents as this large pantropical group. Indeed, unlike the tiny, dust-like, transparent seeds of most other wind-dispersed orchids, like other zoochorous orchids, Vanilla seeds are relatively heavy, rounded, and covered by a hard, black seed coat. Moreover, seeds of certain Vanilla species are packed into a notoriously vanillin-fragrant fruit. This fragrance has been suspected to play a role in the dispersal of Vanilla seeds, but evidence for this important ecological function has been absent until very recently. Animals ranging from ants and bats, bees, crickets and reptiles, birds, rodents, marsupials, and even monkeys have been reported in the literature as possible Vanilla seed dispersers. Fruit and seed traits, therefore, are suggested to result from the interplay of abiotic factors, such as habitat, and biotic factors, in the sense of dispersing agents.
3. Animal-Mediated Seed Dispersal in Vanilla
3.1. Vanilla Fruit Trait Diversity
Bouetard et al. [62] noted that vanillin-bearing species, so-called ‘fragrant’ Vanilla, come exclusively from the Neotropics and belong to a single clade: Vanilla sect. Xanata. Except for a few early diverging species (e.g., V. bicolor Lindl., V. palmarum Lindl.), most other members of this section seem to bear fruits that contain vanillin and related compounds. Hereafter, we will refer to these as the fragrant Vanilla. This clade is sister to Vanilla sect. Tethya Soto Arenas & P.J.Cribb, which includes the Old World Vanilla species found in tropical Asia and Africa and the leafless species from the Caribbean. Together, these two sections comprise Vanilla subgen. Xanata Soto Arenas & P.J.Cribb, which in turn is sister to the exclusively Neotropical Vanilla subgen. Vanilla, known as the ‘membranaceous’ group. As far as is known, both Vanilla subgen. Vanilla and Vanilla sect. Tethya consistently lack vanillin relatives in their fruits, and we will hereafter refer to these as the non-aromatic Vanilla. However, we emphasize that the lack of vanillin and related compounds should not be confused with a complete lack of odor. Species belonging to Vanilla sect. Tethya produce a sweet aroma and members of Vanilla subgen. Vanilla have fruits with a rather grassy, slightly sweet, fermented aroma. The non-aromatic members of Vanilla sect. Tethya and Vanilla subgen. Vanilla are, therefore, not completely odorless; their fruits emit a slightly sweet, fermented smell that is likely appealing to certain animals.
Another important and often neglected fruit trait that shows variation among Vanilla species is fruit dehiscence. Dehiscent fruits split open successively while they mature on the vine, with two valves recoiling from the apex to the base. Indehiscent fruits do not split at all. Instead, they turn yellow and drop to the ground, where they finish maturing. Unfortunately, this fruit trait is rarely noted in the literature and needs to be better understood. Among the fragrant species, Vanilla hartii Rolfe, V. insignis Ames, V. odorata C.Presl., and V. trigonocarpa Hoehne, for example, have dehiscent fruits [1]. Interestingly, the dehiscent fruits of V. hartii and V. odorata are rather non-fleshy and expose their seeds when still attached to the vine. In contrast, the dehiscent fruits of V. trigonocarpa are fleshy, exposing its thick pulp as the dorsal valve of the mature fruit recoils. Dehiscence has also been described in Vanilla bahiana Hoehne (a taxonomical synonym of V. phaeantha Rchb.f.) and V. labellopapillata A.K.Koch, Fraga, J.U. Santos & Ilk.-Borg. [63,64], as well as in V. cristato-callosa Hoehne, V. karen-christianae Karremans & P.Lehm. (as V. riberoi Hoehne), V. palmarum Lindl. and V. pompona Schiede [33]. Interestingly, unlike the dehiscent Peruvian V. pompona populations, Costa Rican populations produce indehiscent fruits that drop to the ground before turning brown, soft, and fragrant [42]. Even though these two populations are sometimes treated as different at the species or subspecies level, they are nevertheless close sisters [3,19]. The same occurs within V. planifolia, where the Mexican and commercial cultivars are dehiscent, while plants native to Costa Rica, forming a sister clade [19,21], have indehiscent fruits. Indehiscence has also been observed in V. dressleri and the commercial hybrid Vanilla × tahitensis. The latter is particularly curious, given that both putative parents (V. odorata and V. planifolia) have dehiscent fruits.
Based on our phylogenetic reconstruction combined with dispersal features of fragrant Vanilla species (Figure 2), dehiscence appears to be more common and is likely to be the ancestral condition [65]. Recent evolutionary studies in other plant families suggest that indehiscent fruits evolved from dehiscent ancestors (e.g., Brassicaceae) [66,67,68]. Curiously, fruit dehiscence (and dispersal modes) appear not to be phylogenetically conserved, and both dehiscence and indehiscence may occur in different populations of a single species. However, within the same population or individual, this fruit trait seems to be invariable. So, the question now arises: has indehiscence evolved several times independently among the fragrant Vanilla species, or is gene expression involved in fruit dehiscence plastic and, therefore, expressed in some populations of a single species and not in others?
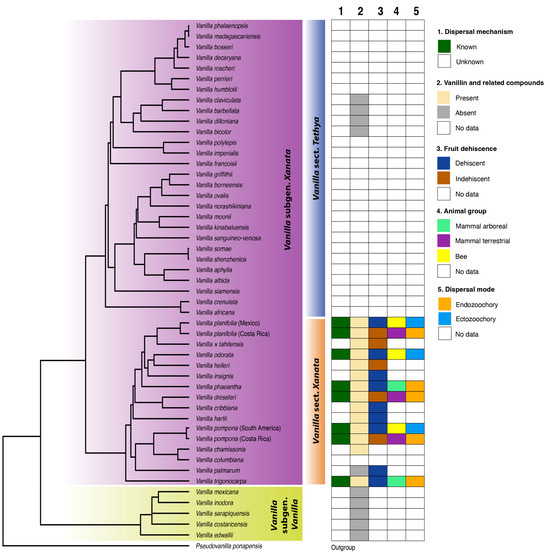
Figure 2.
Maximum likelihood phylogenetic Vanilla tree based on nrITS dataset linked with compiled data on known dispersal modes and fruit traits within Vanilla.
3.2. Linking Vanilla Fruit Traits with Dispersal Modes
3.2.1. Evidence for Ectozoochory
Vanillin, which is found on the inner side of the Vanilla fruit, attracts euglossine bees (Apidae: Euglossini)—known as orchid bees for their importance in the pollination of many large-flowered Neotropical orchids. Male euglossine bees search and collect fragrances from diverse sources to concoct a perfume they use for courtship. Among other plant resources, they are known to actively collect the aromatic compounds produced by Vanilla fruits. In Mexico, Lozano Rodríguez et al. [69] found that the euglossine bees Euglossa hemichlora, Eug. variabilis, Eulaemaexicanta, Eul. Polychroma, and Exaerete frontalis visited the mature, dehiscent fruits of Vanilla planifolia. Eulaemaexicanta was also recorded visiting the dehiscent fruits of Vanilla odorata in Mexico [70] and V. pompona in Peru [32]. Furthermore, euglossine males demonstrated typical fragrance collection behavior when visiting the dehiscent fruits of Vanilla cristato-callosa and V. pompona in Peru (Householder et al. [33]). A broad study by Karremans et al. [42] showed that numerous Euglossini species visit the dehiscent fruits of Vanilla planifolia and V. odorata in Costa Rica. Males of fifteen different species were identified: Eufriesea surinamensis, Euglossa allostricta, Eug. asarophora, Eug. bursigera, Eug. cybelia, Eug. dodsoni, Eug. hansonii, Eug. heterostricta, Eug. ignita, Eug.exicantea, Eug. tridentata, Eug. villosiventris, Eulaemaexicanta, Eul. meriana and Eul.exicantea. Of these, Eug. bursigera, Eug. ignita, Eug. tridentata, and Eul.exicanta were confirmed to remove seeds of both V. planifolia and V. odorata, while Eul.exicantea removed seeds of V. planifolia. In total, about 10% of all Euglossini species, belonging to four out of the five genera, have now been observed visiting Vanilla fruits, with several bee species visiting more than one Vanilla species. One common and widespread species, Eul.exicanta, has been observed visiting V. odorata, V. planifolia, and V. pompona in Mexico, Costa Rica, and Peru. This suggests that there is broad interest from Euglossini in fragrant Vanilla fruits of different species across a wide geographical range. Given that fragrance collection is a common behavioral trait among these bees and that the dispersal mechanism is not size-dependent, many more Euglossini species are expected to be able to disperse Vanilla seeds.
Euglossine males are not the only bees that show interest in Vanilla fruits. Householder et al. [33], for example, suggested that a species of stingless bee of the genus Trigona could be responsible for collecting seeds from the dehiscent fruits of Vanilla pompona in Peru. In Costa Rica, Karremans et al. [42] observed female stingless bees (Apidae: Meliponini) collecting the fruit’s seed-rich pulp of V. odorata and, especially, cultivated V. planifolia. More specifically, female bees belonging to the genera Trigona and Scaptotrigona were seen to actively collect the seeds and store them in specialized pouches in their hind legs, together with other nest-building materials. Recent studies in the Neotropics have shown that stingless bees are indeed attracted to, and may actively collect, vanillin [71,72]. The fact that seeds of V. odorata and V. planifolia were found on the hind legs of generalist foragers Trigona fulviventris and Scaptotrigona subobscuripennis, respectively, suggests that other stingless bee species may also visit the fruits and potentially remove and disperse seeds while collecting the fruit’s pulp. Such is likely to be the case for dehiscent V. pompona fruits as well, confirming Householder et al.’s [33] observations in Peru. Seed dispersal by bees, or melittochory, is an extremely rare mutualistic relationship. It is known to occur in only three rainforest tree species: Corymbia torelliana (F. Muell.) K.D.Hill & L.A.S.Johnson (Myrtaceae), Coussapoa asperifolia subsp. magnifolia (Trécul) Akkermans & C.C.Berg (Urticaeae), and Zygiaexicana (Ducke) Barneby & J.W.Grimes (Fabaceae). The seeds of these tree species are dispersed by Meliponini bees that collect the resin inside the fruits [73,74,75,76]. The genus Vanilla is the first among the monocots with confirmed seed dispersal by bees, making Orchidaceae only the fourth melittochorous family among plants.
3.2.2. Evidence for Endozoochory
Vanilla seed dispersal has long been suggested to be mediated by large animals through endozoochory. Unfortunately, most reports have been anecdotal, and none have shown any proof of animals manipulating Vanilla fruits until recently, let alone ingesting and defecating the seeds. The diversity of animals that have been suspected to be involved in the dispersal of Vanilla seeds includes birds [31,49], reptiles [77], and diverse mammals, such as monkeys [78], marsupials [33], and bats [1,79]. Yet, the subject has remained rather cryptic, lacking resolution as to which and how animals truly interact with fragrant Vanilla fruits and the consequences of these interactions.
This changed when the first conclusive evidence of rodents consuming the mature, indehiscent fruits of Vanilla pompona was presented by Karremans et al. [35,36,37]. Videos obtained from motion-activated camera traps placed in several V. pompona populations naturally occurring in the Southern Pacific of Costa Rica displayed the Central American spiny rat, Proechimys semispinosus (Rodentia: Echimyidae), and Central American agouti Dasyprocta punctata (Rodentia: Dasyproctidae) actively handling the indehiscent fruits before consuming them entirely. Karremans et al. [42] further expanded these observations with additional evidence of the rodents Proechimys semispinosus and Sigmodontomys alfari (Rodentia: Cricetidae), and the marsupial Didelphis marsupialis (Didelphimorphia: Didelphidae) consuming the indehiscent fruits of the native Vanilla planifolia growing on the Caribbean side of Costa Rica. The study not only provided clear video and photographic proof of the mammals consuming fruits of these two Vanilla species in their natural habitat, but intact V. planifolia seeds were recovered from the feces of both the spiny rat (P. semispinosus) and common opossum (D. marsupialis), showing that seeds passed through their digestive systems. Furthermore, in situ and ex situ experiments demonstrate that passing through the mammal’s digestive system is not harmful to the seeds but is also not a strict requirement for germination. Protocorms of V. planifolia were shown to develop 3.5 months after packets with fecal material containing seeds were placed in the soil at independent locations within the natural populations in Costa Rica. Our preliminary observations on the fleshy, fragrant, indehiscent fruits of V. dressleri suggest that this species also attracts rodents that consume the fruits.
Virtually simultaneous observations of animals interacting with Vanilla fruits were made in Brazil. A study by Pansarin [38] found that birds interacted with the fruits of multiple Vanilla species at the campus of the University of São Paulo in southeastern Brazil. Unfortunately, the fruits of the different Vanilla species were displayed simultaneously, in unequal proportions, in an unnatural disposition, and in an intervened environment outside their natural habitat. The mix of mature and immature fruits, belonging to both fragrant and non-fragrant species, simultaneously offered on an artificial platform, discounts any individual attractiveness. Moreover, it disregards the possibility of a magnet effect of one or more particular Vanilla fruits, as well as prior-learned food cues and the exploratory behavior of birds given the presentation. It is, therefore, impossible to conclusively say which, if any, of the fruits of the Vanilla species tested actually attract birds and if the same occurs under natural conditions in a wild setting. Consumption and passing of the seeds were not shown for any of the Vanilla and bird species tested. In contrast, motion-activated camera traps placed at several locations in Costa Rica showed several bird species, belonging to multiple families, passing close to or over the highly fragrant, mature fruits of V. odorata, V. planifolia, and V. pompona without ever attempting to consume or even reacting to the presence of the fruits [42]. The video footage shows that the fruits of these three species do not elicit any response from birds. It is noteworthy, however, that Vanilla palmarum, which differs by having non-fragrant, compact fruits, showed the highest visitation by birds in southeastern Brazil [38]. Therefore, it would certainly be worthwhile to separately re-evaluate if the fruits of that species, and any other non-aromatic Vanilla, do, in fact, attract birds or other animals in their natural habitat and if seeds are truly consumed and dispersed through these means.
A follow-up paper by Pansarin and Suetsugu [40] provided evidence that the mature, aromatic, dehiscent fruits of Vanilla bahiana (=V. phaeantha) are visited by birds, marsupials, and rodents in a semi-natural setting. The bird species were said to be mainly Mimus saturninus (Passeriformes: Mimidae) without any further specification. Unfortunately, no evidence was provided regarding the birds’ interaction with the fruits. In contrast, the arboreal marsupial Gracilinanus agilis (Didelphimorphia: Didelphidae) and the rodent Oligoryzomys nigripes (Rodentia: Cricetidae) were documented, using camera traps, actively engaging with, and nibbling on the fruits. The interest from these mammals in the mature Vanilla fruits is consistent with aforementioned studies of mammals consuming fragrant fruits and dispersing the seeds. Contrary to the indehiscent V. planifolia and V. pompona, the dehiscent fruits of V. phaeantha do not fall to the ground as they mature, and it was suggested that the observed animals solely consume the exposed pulp without chewing the mesocarp. In southeastern Costa Rica, the arboreal rodent Nyctomys sumichrasti (Rodentia: Cricetidae) was recorded consuming the pulp of Vanilla trigonocarpa (Karremans et al., pers. obs.), a species that, like V. phaeantha, has fleshy, fragrant, dehiscent fruits that are persistent on the vine. Bite and puncture marks on another mature fruit of V. trigonocarpa, which had the pulp removed, were identified as being made by bats. Moreover, another arboreal mammal, Potos flavus (Carnivora: Procynoidae), was recently recorded consuming mature fruits of V. planifolia at Cahuita National Park in Costa Rica (Karremans et al., pers. obs.). Even though ingestion and passing of the seeds remains to be shown in these cases, G. agilis, N. sumichrasti, O. nigripes, and P. flavus are all fruit-eating arboreal animals that have been shown to disperse seeds. These observations indicate that seed dispersal through direct pulp consumption by mammals is likely another endozoochory mechanism in Vanilla. Claims by Pansarin and Suetsugu [40] that the mesocarp of fragrant Vanilla species is generally toxic to mammals are probably premature given that, at least for Vanilla planifolia and V. pompona, several mammal species have shown to be rather keen on consuming the entire fruit. The authors’ own video published with the supporting information shows a Gracilinanus agilis individual ripping a piece of the mesocarp of Vanilla phaeantha and evidently chewing it. Whether palatability changes with the fruit’s maturity is worth investigating further.
Current data show a possible correlation between certain fruit traits and the dispersal agent or mode in Vanilla (Figure 2). For example, indehiscent fruits (e.g., Vanilla dressleri, V. pompona) are dispersed by terrestrial mammals, which consume the mature, nutritious fruits as they fall onto the ground (endozoochory). Dehiscent fruits (e.g., Vanilla odorata, V. phaeantha) are dispersed by bees, which displace seeds while collecting fragrances or nest-building materials from the fruits (ectozoochory) or arboreal mammals that consume the fruit’s pulp (endozoochory). However, the question remains, which fruit traits trigger the attraction of different animal groups (bees vs. arboreal mammals) to dehiscent fruits. Fruit fleshiness and richness of the pulp possibly play an important role therein. The rather dry fruits of V. odorata attract bees, while the succulent fruits of V. phaeantha and V. trigonocarpa attract arboreal mammals that consume the rich pulp. However, only bees have been recorded visiting the dehiscent, fleshy fruits of V. pompona (Peruvian populations). The attraction of different animal groups may also depend on the concentration and composition of aromatic compounds in each fruit, which differs significantly among Vanilla species and can vary even within a single species [80]. Our understanding of these interactions is still too partial to provide a clear overview of the mysterious ecology of Vanilla seed dispersal. Future in-depth studies with robust field experimentation are needed to explore (i) the relationships between various Vanilla fruit traits and corresponding dispersal modes and (ii) the genetic and ecological triggers that promote certain traits and dispersal modes above others.
3.3. Flexible Dispersal Modes in Vanilla: A Gene–Environment-Induced Regulation?
An intriguing question that arises is why certain fruit traits and seed dispersal modes vary among populations within a single species. For example, V. planifolia exhibits indehiscent fruits in Costa Rica but dehiscent fruits in Mexico, thereby attracting distinct dispersal agents. This variation leads to a shift in dispersal modes, namely a transition from endozoochory to ectozoochory or vice versa. The same holds for V. pompona, which produces indehiscent fruits in Costa Rica but dehiscent fruits in Peru. The coexistence of both dehiscent and indehiscent fruits, and, thus, varying dispersal modes and agents within the same species, suggests the possibility of a shift between dispersal strategies in response to, for example, spatiotemporal environmental variability. As previously argued, fruit dehiscence is a critical characteristic that influences seed maturation and dispersal in Brassicaceae [81,82], and specific environmental factors may influence genes regulating fruit (in)dehiscence. For instance, fruit dehiscence has been shown to be regulated by temperature via specific thermosensory activation pathways [83].
Based on our preliminary observations of varying dispersal modes, we see great potential in future studies that provide insights into (i) the molecular and genetic mechanisms underlying the evolutionary transition between specific fruit traits (e.g., dehiscent to indehiscent fruits or vice versa) and (ii) the responses of genes regulating these particular fruit characteristics to (local) environmental conditions, such as climate, light intensity, or habitat type, amongst others. Considering that genes regulating fruit dehiscence and other fruit traits have been successfully studied in several plant species [81,82,84,85] and epidendrioid orchids [86,87], we believe that applying similar molecular approaches will greatly advance our understanding of seed dispersal mechanisms within Vanilla. For this, whole-genome and transcriptome sequencing combined with resequencing technologies could be applied to identify and isolate candidate genes responsible for the expression of fruit traits affecting fruit ripening and, thus, seed dispersal. Later studies could then track down these genes using molecular markers to reveal their presence in Vanilla species, specifically at the population level. Furthermore, experimental evaluations within controlled settings combined with transcriptome analyses could reveal certain interactions between these candidate genes and specific environmental conditions. This could be especially of interest given climate change causing potential shifts in dispersal modes.
4. Conclusions
Orchid fruits and seeds are mainly adapted to anemochory, and this derived state has likely been an important driver of speciation within the family. However, zoochory occurs primarily in groups belonging to the early diverging clades of the family. This ancestral state is often linked to morphological features, including fruit indehiscence and fleshiness and darkening and sclerification of the seed coat. Fruit and seed features also appear to determine dispersal modes within Vanilla, the largest and most diverse zoochorous genus in the family. Vanilla joins Apostasia, Cyrtosia, Neuwiedia, and Yoania as the only orchid genera in which animal dispersal has been proven beyond doubt. Despite lacking a phylogenetic signal for fruit traits within Vanilla, fruit fragrance, dehiscence, and fleshiness play a role in determining the attraction to particular animals and, therefore, the dispersal modes. Fragrant Vanilla species present multi-modal seed dispersal mechanisms that exploit different behaviors of several groups of animals (Figure 3) and are likely to depend on vanillin and related aromatic compounds to attract seed-dispersing animals. The mature, slender, dehiscent fruits of Vanilla odorata, V. planifolia (Mexico), and V. pompona (Peru) attract male euglossine bees and female stingless bees that displace or collect seeds as they collect fragrances and nest-building materials from the fruits, respectively. As such, Vanilla represents the first case of plants having their seeds dispersed through fragrance collection, with the above-mentioned species being the only monocot species known to date to have their seeds dispersed through melittochory. Together with Corymbia torelliana (Myrtaceae), Coussapoa asperifolia subsp. magnifolia (Urticaeae), and Zygiaexicana (Fabaceae), they are the only known plant species sharing this exceedingly rare mutualistic relationship.
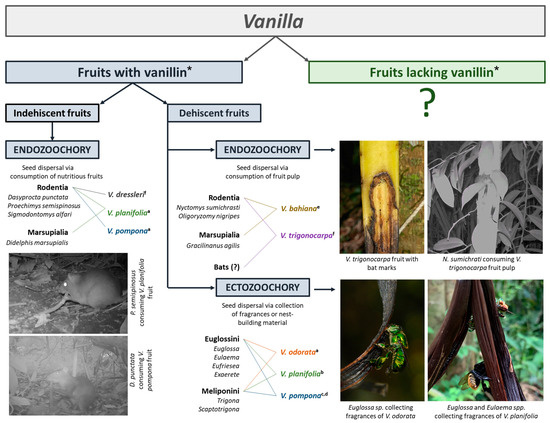
Figure 3.
Dispersal mechanisms in the orchid genus Vanilla. © Adam Karremans. a Karremans et al. [42]. b Lozano Rodríguez et al. [69]. c Lubinsky et al. [32]. d Householder et al. [33]. e Pansarin and Suetsugu [40]. f Karremans et al., pers. obs. * We refer to both vanillin and its related compounds.
The indehiscent fruits of V. dressleri, V. planifolia (Costa Rica), and V. pompona (Costa Rica) are unique among the Orchidaceae in being highly nutritious and attracting terrestrial mammals, especially rodents, that consume the whole fruit and can pass the entire seeds through their digestive system. Mammals also visit and consume the dehiscent fruits of V. phaeantha and V. trigonocarpa. In this case, the fleshy, persistent fruit is visited by arboreal or flying mammals that mainly consume the pulp and presumably disperse the seeds after passing them through their digestive system. As such, there may be multiple other mammals involved in seed dispersal across the Vanilla genus. Secondary dispersal by other animals is surely a possibility in certain cases, too. Crickets, which have been shown to disperse the seeds of other orchids, were found by Karremans et al. [42] feeding upon the indehiscent fruits of V. planifolia that had been left out in the field. The crickets, which belong to a species in genus Idiarthron (Tettigoniidae), passed the Vanilla seeds intact and could also act as effective dispersers.
The identity of seed dispersers for Vanilla species that lack vanillin remains enigmatic. Given the diversity of seed dispersers and mechanisms among the fragrant, New World members of Vanilla, one may speculate that numerous interactions involving different animal groups may exist across the broad geographical range of this genus. Birds, which have been observed visiting Vanilla fruits, may potentially consume and disperse the seeds of certain Vanilla species, as they do for other orchids. However, this remains somewhat speculative. Due to their underdeveloped sense of smell, birds primarily rely on visual cues to locate the fleshy fruits they consume, showing a particular attraction to reddish colors while often ignoring greens. This raises controversy regarding their role in seed dispersal of Vanilla species with fragrant, but visually unappealing, fruits. However, it does not exclude them from being potential Vanilla seed dispersers. The fleshy, compact, non-fragrant fruits of the greenish V. palmarum and the reddish V.exicantea and their relatives, for example, are good candidates for dispersal by birds or other groups of animals.
Significant progress has been made recently toward understanding the ecological interactions involving the dispersal mechanisms of Vanilla orchids. However, much remains to be studied, particularly under natural conditions within their native habitats. Robust field experimentation is crucial in understanding the intricate details of these complex relationships and how they may have evolved. Furthermore, detailed anatomical and morphological observations of fruits and seeds from groups potentially dispersed by animals are essential to explore the evolution of these traits and their role in seed dispersal. These traits are often underrepresented in the literature and require more comprehensive documentation. Additionally, insights into the genetic basis of these multidimensional dispersal modes could reveal potential adaptations of Vanilla species and their populations to specific environmental conditions. Although describing this genetic basis may be highly challenging, we believe that a combination of molecular tools could provide great potential to study these underlying mechanisms. We eagerly expect upcoming and exciting advances in Vanilla ecology to further expand our knowledge on fundamental biological aspects and to allow the development of a proper conservation plan for this commercially very important orchid genus.
Author Contributions
Conceptualization, A.P.K. and C.W.; methodology, D.B. and O.A.P.-E.; software, D.B. and O.A.P.-E.; formal analysis, D.B. and O.A.P.-E.; investigation, A.P.K., C.W., D.S., D.B. and O.A.P.-E.; data curation, D.B. and O.A.P.-E.; writing—original draft preparation, A.P.K., C.W. and D.S.; writing—review and editing, A.P.K., C.W. and D.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Vicerrectory of Research of the University of Costa Rica under project numbers 814-C0-049 and 814-C3-464.
Data Availability Statement
No new data has been created for the current review.
Acknowledgments
We are very thankful to the staff at JBL and CIBET, Cahuita National Park, Tirimbina Biological Reserve, Las Brisas Nature Reserve, Piro Biological Station, and Finca Christina. The Costa Rican Ministry of Environment and Energy (MINAE) and its National System of Conservation Areas (SINAC) kindly provided the permits and access to protected areas.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Soto Arenas, M.A.; Dressler, R.L. A revision of the Mexican and Central American species of Vanilla Plumier ex Miller with a characterization of their ITS region of the nuclear ribosomal DNA. Lankesteriana 2010, 9, 285–354. [Google Scholar] [CrossRef]
- Cameron, K.M. Vanilla Orchids: Natural History and Cultivation, 1st ed.; Timber Press Inc.: Portland, OR, USA, 2011. [Google Scholar]
- Karremans, A.P.; Chinchilla, I.F.; Rojas-Alvarado, G.; Cedeño-Fonseca, M.; Damián, A.; Léotard, G. A reappraisal of Neotropical Vanilla. With a note on taxonomic inflation and the importance of alpha taxonomy in biological studies. Lankesteriana 2020, 20, 395–497. [Google Scholar] [CrossRef]
- Havkin-Frenkel, D.; Belanger, F.C. Handbook of Vanilla Science and Technology, 2nd ed.; Blackwell Publishing Ltd.: Ames, IA, USA, 2018. [Google Scholar]
- Faostat. Food and Agriculture Organization of the United Nations. 2021. Available online: https://www.fao.org/faostat/ (accessed on 15 June 2023).
- Besse, P.; Da Silva, D.; Bory, S.; Grisoni, M.; Le Bellec, F.; Duval, M.F. RAPD genetic diversity in cultivated vanilla: Vanilla planifolia, and relationships with V. tahitensis and V. pompona. Plant Sci. J. 2004, 167, 379–385. [Google Scholar] [CrossRef]
- Bory, S.; Grisoni, M.; Duval, M.F.; Besse, P. Biodiversity and preservation of vanilla: Present state of knowledge. Genet. Resour. Crop Evol. 2008, 55, 551–571. [Google Scholar] [CrossRef]
- Hernández, H.J.; Lubinsky, P. Cultivation systems. In Vanilla, 1st ed.; Odoux, E., Grisoni, M., Eds.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2010; pp. 75–95. [Google Scholar]
- Flanagan, N.S.; Mosquera-Espinosa, A.T. An integrated strategy for the conservation and sustainable use of native Vanilla species in Colombia. Lankesteriana 2016, 16, 201–218. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Prueger, J.H. Temperature extremes: Effect on plant growth and development. Weather. Clim. Extremes 2015, 10, 4–10. [Google Scholar] [CrossRef]
- Lesk, C.; Rowhani, P.; Ramankutty, N. Influence of extreme weather disasters on global crop production. Nature 2016, 529, 84–87. [Google Scholar] [CrossRef]
- Bramel, P.; Frey, F. Global Strategy for the Conservation and Use of Vanilla Genetic Resources; Global Crop Diversity Trust: Bonn, Germany, 2021. [Google Scholar]
- Duval, M.F.; Bory, S.; Andrzejewski, S.; Grisoni, M.; Besse, P.; Causse, S.; Charon, C.; Dron, M.; Odoux, E.; Wong, M. Diversité Génétique des Vanilliers Dans Leurs Zones de Dispersion Secondaire. Les Actes BRG 2006, 6, 181–196. [Google Scholar]
- Schlüter, P.M.; Arenas, M.A.S.; Harris, S.A. Genetic variation in Vanilla planifolia (Orchidaceae). Econ. Bot. 2007, 61, 328–336. [Google Scholar] [CrossRef]
- Sreedhar, R.V.; Venkatachalam, L.; Roohie, K.; Bhagyalakshmi, N. Molecular analyses of Vanilla planifolia cultivated in India using RAPD and ISSR markers. OSB 2007, 1, 29–33. [Google Scholar]
- Minoo, D.; Jayakumar, V.N.; Veena, S.S.; Vimala, J.; Basha, A.; Saji, K.V.; Nirmal Babu, K.; Peter, K.V. Genetic variations and interrelationships in Vanilla planifolia and few related species as expressed by RAPD polymorphism. Genet. Resour. Crop. Evol. 2008, 55, 459–470. [Google Scholar] [CrossRef]
- Verma, P.C.; Chakrabarty, D.; Jena, S.N.; Mishra, D.K.; Singh, P.K.; Sawant, S.V.; Tuli, R. The extent of genetic diversity among Vanilla species: Comparative results for RAPD and ISSR. Ind. Crops. Prod. 2009, 29, 581–589. [Google Scholar] [CrossRef]
- Hu, Y.; Resende, M.F., Jr.; Bombarely, A.; Brym, M.; Bassil, E.; Chambers, A.H. Genomics-based diversity analysis of Vanilla species using a Vanilla planifolia draft genome and Genotyping-By-Sequencing. Sci. Rep. 2019, 9, 3416. [Google Scholar] [CrossRef]
- Chambers, A.; Cibrián-Jaramillo, A.; Karremans, A.P.; Martinez, D.M.; Hernandez-Hernandez, J.; Brym, M.; Resende, M.F.R.; Moloney, R.; Sierra, S.N.; Hasing, T.; et al. Genotyping-By-Sequencing diversity analysis of international Vanilla collections uncovers hidden diversity and enables plant improvement. Plant Sci. 2021, 311, 111019. [Google Scholar] [CrossRef] [PubMed]
- Ellestad, P.; Pérez-Farrera, M.A.; Buerki, S. Genomic insights into cultivated Mexican Vanilla planifolia reveal high levels of heterozygosity stemming from hybridization. Plants 2022, 11, 2090. [Google Scholar] [CrossRef]
- Favre, F.; Jourda, C.; Grisoni, M.; Piet, Q.; Rivallan, R.; Dijoux, J.-B.; Hascoat, J.; Lepers-Andrzejewski, S.; Besse, P.; Charron, C. A genome-wide assessment of the genetic diversity, evolution and relationships with allied species of the clonally propagated crop Vanilla planifolia Jacks. ex Andrews. Genet. Resour. Crop. Evol. 2022, 69, 2125–2139. [Google Scholar] [CrossRef]
- Anderson, J.D.; Gastelbondo, M.; Chambers, A.H. Diagnostic KASP markers differentiate Vanilla planifolia, V. odorata, V. pompona, and their hybrids using leaf or cured pod tissues. Mol. Biol. Rep. 2023, 50, 707–717. [Google Scholar] [CrossRef]
- Azofeifa-Bolaños, J.B.; Gigant, L.R.; Nicolás-García, M.; Pignal, M.; Tavares-González, F.B.; Hágsater, E.; Salazar-Chávez, G.A.; Reyes-Lopez, D.; Archila-Morales, F.L.; García-García, J.A.; et al. A new Vanilla species from Costa Rica closely related to V. planifolia (Orchidaceae). Eur. J. Taxon. 2017, 284, 1–26. [Google Scholar] [CrossRef]
- Grisoni, M.; Nany, F. The beautiful hills: Half a century of vanilla (Vanilla planifolia Jacks. ex Andrews) breeding in Madagascar. Genet. Resour. Crop Evol. 2021, 68, 1691–1708. [Google Scholar] [CrossRef]
- Divakaran, M.; Babu, K.N.; Peter, K.V. Conservation of Vanilla species, in vitro. Sci. Hortic. 2006, 110, 175–180. [Google Scholar] [CrossRef]
- Lubinsky, P.; Bory, S.; Hernández Hernández, J.; Kim, S.C.; Gómez-Pompa, A. Origins and dispersal of cultivated vanilla (Vanilla planifolia Jacks [Orchidaceae]). Econ. Bot. 2008, 62, 127–138. [Google Scholar] [CrossRef]
- Li, J.; Demesyeux, L.; Brym, M.; Chambers, A.H. Development of species-specific molecular markers in Vanilla for seedling selection of hybrids. Mol. Biol. Rep. 2020, 47, 1905–1920. [Google Scholar] [CrossRef] [PubMed]
- Soto Arenas, M.A. Filogeografia y Recursos Genéticos de las Vainillas de México. 1999. Available online: http://www.conabio.gob.mx/institucion/proyectos/resultados/InfJ101.pdf (accessed on 2 November 2023).
- Gigant, R.; Bory, S.; Grisoni, M.; Besse, P. Biodiversity and evolution in the Vanilla. In The Dynamical Processes of Biodiversity; Case Studies of Evolution and Spatial, Distribution, Grillo, O., Venora, G., Eds.; IntechOpen: Rijeka, Croatia, 2011; pp. 1–26. [Google Scholar]
- Goettsch, B.; Urquiza-Haas, T.; Koleff, P.; Gasman, F.A.; Aguilar-Meléndez, A.; Alavez, V.; Alejandre-Iturbide, G.; Cuevas, F.A.; Pérez, C.A.; Carr, J.A.; et al. Extinction risk of Mesoamerican crop wild relatives. Plants People Planet 2021, 3, 775–795. [Google Scholar] [CrossRef]
- Arditti, J.; Ghani, A.K.A. Numerical and physical properties of orchid seeds and their biological implications. New Phytol. 2000, 145, 367–421. [Google Scholar] [CrossRef] [PubMed]
- Lubinsky, P.; Van Dam, M.; Van Dam, A. Pollination of Vanilla and evolution in Orchidaceae. Lindleyana 2006, 75, 926–929. [Google Scholar]
- Householder, E.; Janovec, J.; Balarezo, M.A.; Huinga, M.J.; Wells, J.; Valega, R. Diversity, natural history, and conservation of Vanilla (Orchidaceae) in Amazonian wetlands of Madre de Dios, Peru. J. Bot. Res. Inst. Tex. 2010, 4, 227–243. [Google Scholar]
- Karremans, A.P.; Lehmann, C.P. A highly threatened new species of Vanilla from Costa Rica. Lindleyana 2018, 87, 304–307. [Google Scholar]
- Karremans, A.P.; Pillco Huarcaya, R.; Watteyn, C.; Cedeño-Fonseca, M.; Chinchilla, I.F.; Rojas-Alvarado, G.; López-Morales, M.; Whitworth, A.; Rodríguez-Herrera, B.; Ramírez, S.; et al. Irresistibly fragrant: A key innovation for the long-distance dispersal of wild Vanilla seeds. In Proceedings of the 6th Scientific Conference on Andean Orchids (IISCAO), Medellin, Colombia, 6–8 August 2019. [Google Scholar]
- Karremans, A.P.; Pillco Huarcaya, R.; Watteyn, C.; Cedeño-Fonseca, M.; Chinchilla, I.F.; Rojas-Alvarado, G.; López-Morales, M.; Whitworth, A.; Rodríguez-Herrera, B.; Ramírez, S.; et al. Irresistibly fragrant: The mysterious ecology of Vanilla. In Proceedings of the 7th International Orchid Conservation Congress (IOCC), London, UK, 29 May 2019. [Google Scholar]
- Karremans, A.P.; Pillco Huarcaya, R.; Watteyn, C.; Cedeño-Fonseca, M.; Chinchilla, I.F.; Rojas-Alvarado, G.; López-Morales, M.; Whitworth, A.; Rodríguez-Herrera, B.; Ramírez, S.; et al. Irresistiblemente fragante: La misteriosa ecología de Vanilla. In Proceedings of the 5th Meeting of Mexican Orchidology, Merida, Mexico, 21 November 2019. [Google Scholar]
- Pansarin, E.R. Unravelling the enigma of seed dispersal in Vanilla. Plant Biol. 2021, 23, 974–980. [Google Scholar] [CrossRef]
- Watteyn, C.; Scaccabarozzi, D.; Muys, B.; van der Schueren, N.; van Meerbeek, K.; Guizar Amador, M.F.; Ackerman, J.D.; Cedeño Fonseca, M.; Chinchilla, I.F.; Reubens, B.; et al. Trick or Treat? Pollinator attraction in Vanilla pompona (Orchidaceae). Biotropica 2021, 54, 268–274. [Google Scholar] [CrossRef]
- Pansarin, E.R.; Suetsugu, K. Mammal-mediated seed dispersal in Vanilla: Its rewards and clues to the evolution of fleshy fruits in orchids. Ecology 2022, 103, e3701. [Google Scholar] [CrossRef]
- Karremans, A.P. Demystifying Orchid Pollination: Stories of Sex, Lies and Obsession; Kew Publishing: Richmond, UK, 2023; p. 440. [Google Scholar]
- Karremans, A.P.; Bogarín, D.; Otárola, M.F.; Sharma, J.; Watteyn, C.; Warner, J.; Herrera, B.R.; Chinchilla, I.F.; Carman, E.; Valerio, E.R.; et al. First evidence for multimodal animal seed dispersal in orchids. Curr. Biol. 2023, 33, 364–371. [Google Scholar] [CrossRef]
- Watteyn, C.; Scaccabarozzi, D.; Muys, B.; Reubens, B.; Ackerman, J.D.; Fernández Otárola, M.; Guizar Amador, M.F.; Karremans, A.P. Sweet as Vanilla hartii: Evidence for nectar rewards in Vanilla (Orchidaceae) flowers. Flora 2023, 303, 152294. [Google Scholar] [CrossRef]
- Webb, C.J. The selection of pollen and seed dispersal in plants. Plant Species Biol. 1998, 13, 57–67. [Google Scholar] [CrossRef]
- Dressler, R.L. The Orchids: Natural History and Classification; Harvard University Press: Cambridge, MA, USA, 1981. [Google Scholar]
- Dressler, R.L. Phylogeny and Classification of the Orchid Family, 1st ed.; Dioscorides Press: Portland, OR, USA, 1993. [Google Scholar]
- Dixon, K.W.; Pate, J.S.; Kuo, J. The Western Australian subterranean orchid Rhizanthella gardneri Rogers, the underground orchid of Western Australia. In Orchid Biology, Reviews and Perspectives 5; Timber Press: Portland, OR, USA, 1990; pp. 37–62. [Google Scholar]
- Cameron, K.M.; Chase, M.W. Seed morphology of the vanilloid orchids. Lindleyana 1998, 13, 148–169. [Google Scholar]
- Molvray, M.; Chase, M.W. Seed Morphology. In Genera Orchidacearum; Pridgeon, A.M., Criib, P.J., Chase, M.W., Ramusen, F.N., Eds.; General Introduction, Apastosiodea, Cypripedioideae; Oxford University Press: Oxford, UK, 1999; pp. 59–66. [Google Scholar]
- Kocyan, A.; Endress, P.K. Floral structure and development of Apostasia and Neuwiedia (Apostasioideae) and their relationships to other Orchidaceae. Int. J. Plant Sci. 2001, 162, 847–867. [Google Scholar] [CrossRef]
- Cameron, K.M. Vanilloideae. In Genera Orchidacearum; Pridgeon, A.M., Cribb, P.J., Chase, M.W., Rasmussen, F.N., Eds.; Oxford University Press: Oxford, UK, 2003; Volume 3, pp. 281–334. [Google Scholar]
- Barthlott, W.; Große-Veldmann, B.; Korotkova, N. Orchid seed diversity. Englera 2014, 32, 1–245. [Google Scholar]
- Yang, C.-K.; Lee, Y.-I. The seed development of a mycoheterotrophic orchid, Cyrtosia javanica Blume. Bot. Stud. 2014, 55, 44. [Google Scholar] [CrossRef]
- Suetsugu, K.; Kawakita, A.; Kato, M. Avian seed dispersal in a mycoheterotrophic orchid Cyrtosia septentrionalis. Nat. Plants 2015, 1, 15052. [Google Scholar] [CrossRef]
- Suetsugu, K. Seed dispersal in the mycoheterotrophic orchid Yoania japonica: Further evidence for endozoochory by camel crickets. Plant Biol. 2018, 20, 707–712. [Google Scholar] [CrossRef]
- Suetsugu, K. Independent recruitment of a novel seed dispersal system by camel crickets in achlorophyllous plants. N. Phytol. 2018, 217, 828–835. [Google Scholar] [CrossRef] [PubMed]
- Suetsugu, K.A. Novel seed dispersal mode of Apostasia nipponica could provide some clues to the early evolution of the seed dispersal system in Orchidaceae. Evol. Lett. 2020, 4, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Y.Y.; Liu, J.; Luo, F.; Lee, Y.I. Seed dispersal in Neuwiedia singapureana: Novel evidence for avian endozoochory in the earliest diverging clade in Orchidaceae. Bot. Stud. 2021, 62, 3. [Google Scholar] [CrossRef] [PubMed]
- Kurzweil, H. Seed morphology in Southern African Orchidoideae (Orchidaceae). Plant Syst. Evol. 1993, 185, 229–247. [Google Scholar] [CrossRef]
- Gosper, C.R.; Stansbury, C.D.; Vivian-Smith, G. Seed dispersal of fleshy-fruited invasive plants by birds: Contributing factors and management options. Divers. Distrib. 2005, 11, 549–558. [Google Scholar] [CrossRef]
- Duan, Q.; Goodale, E.; Quan, R.-C. Bird fruit preferences match the frequency of fruit colours in tropical Asia. Sci. Rep. 2014, 4, 5627. [Google Scholar] [CrossRef] [PubMed]
- Bouetard, A.; Lefuvre, P.; Gigant, R.; Bory, S.; Pignal, M.; Besse, P.; Grisoni, M. Evidence of transoceanic dispersion of the genus Vanilla based on plastid DNA phylogenetic analysis. Mol. Phylogenet. Evol. 2010, 55, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, T.A.; Furtado, M.S.C.; Pereira, W.C.; Barbarena, F.F.V.A. Vanilla bahiana Hoehne (Orchidaceae): Studies on fruit development and new perspectives into crop improvement for the Vanilla planifolia group. Biota Neotrop. 2019, 19, e20180696. [Google Scholar] [CrossRef]
- Koch, A.K.; de Fraga, C.N.; dos Santos, J.U.M.; Ilkiu-Borges, A.L. Taxonomic notes on Vanilla (Orchidaceae) in the Brazilian Amazon, and the description of a new species. Syst. Bot. 2013, 38, 975–981. [Google Scholar] [CrossRef]
- Pramanik, D.; Becker, A.; Roessner, C.; Rupp, O.; Bogarín, D.; Pérez-Escobar, O.A.; Dirks-Mulder, A.; Droppert, K.; Kocyan, A.; Smets, E.; et al. Evolution and development of fruits of Erycina pusilla and other orchid species. PLoS ONE 2023, 18, e0286846. [Google Scholar] [CrossRef]
- Mühlhausen, A.; Polster, A.; Theißen, G.; Mummenhoff, K. Evolution of fruit dehiscence in Brassicaceae–Examples from Aethionema and Lepidium. Acta Hortic. 2010, 867, 207–220. [Google Scholar] [CrossRef]
- Mühlhausen, A.; Lenser, T.; Mummenhoff, K.; Theißen, G. Evidence that an evolutionary transition from dehiscent to indehiscent fruits in Lepidium (Brassicaceae) was caused by a change in the control of valve margin identity genes. Plant J. 2013, 73, 824–835. [Google Scholar] [CrossRef] [PubMed]
- Gramzow, L.; Klupsch, K.; Fernández-Pozo, N.; Hölzer, M.; Marz, M.; Rensing, S.A.; Theißen, G. Comparative transcriptomics identifies candidate genes involved in the evolutionary transition from dehiscent to indehiscent fruits in Lepidium (Brassicaceae). BMC Plant Biol. 2022, 22, 340. [Google Scholar] [CrossRef]
- Lozano Rodríguez, M.A.; Rodríguez, M.L.; Canche, J.M.P.; García, R.A.M.; Cabrera, C.R.C. Visit frequency of Euglossine bees (Hymenoptera: Apidae) to mature fruits of Vanilla planifolia (Orchidaceae). Acta Bot. Mex. 2022, 129, e2001. [Google Scholar] [CrossRef]
- Madison, M. Vanilla beans and bees. Mo. Bot. Gard. bull. 1981, 8, 8. [Google Scholar]
- Nemésio, A.; Seixas, D.P.; Rasmussen, C. Trigona pallens (Fabricius, 1798) (Hymenoptera: Apidae) strongly attracted to vanillin in northeastern Peru. Braz. J. Biol. 2013, 73, 677–678. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Roubik, D.W.; de Camargo, J.M.F. The Panama microplate, island studies and relictual species of Melipona (Melikerria) (Hymenoptera: Apidae: Meliponini). Syst. Entomol. 2013, 37, 189–199. [Google Scholar] [CrossRef]
- Wallace, H.M.; Trueman, S.J. Dispersal of Eucalyptus torelliana seeds by the resin-collecting stingless bee, Trigona carbonaria. Oecologia 1995, 104, 12–16. [Google Scholar] [CrossRef]
- Bacelar-Lima, C.G.; Freire, D.C.B.; Coletto-Silva, A.; Costa, K.B.D.; Laray, J.P.B.; Vilas-Boas, H.C.; Carvalho-Zilse, G.A. Melitocory of Zygia racemosa (Ducke) Barneby & Grimes by Melipona seminigra merrillae Cockerell 1919 and Melipona compressipes manaosensis Schwarz 1932 (Hymenoptera, Meliponina) in Central Amazon, Brazil. Acta Amazon. 2006, 36, 343–348. [Google Scholar]
- Nunez, C.V.; de Oliveira, M.L.; Lima, R.D.; Diaz, I.E.C.; Sargentini, E., Jr.; Pereira, O.L., Jr.; Araújo, L.M. Chemical analyses confirm a rare case of seed dispersal by bees. Apidologie 2008, 39, 618–626. [Google Scholar] [CrossRef]
- Leonhardt, S.D.; Baumann, A.M.; Wallace, H.M.; Brooks, P.; Schmitt, T. The chemistry of an unusual seed dispersal mutualism: Bees use a complex set of olfactory cues to find their partner. Anim. Behav. 2014, 98, 41–51. [Google Scholar] [CrossRef]
- Flanagan, N.S.; Otero, J.T.; Molineros, F.H.; Mosquera-Espinosa, A.T.; Vásquez, E.; Garnica, S.; Rosero, D.; Parra, S.; Martínez, C.; Palma, R. Aprovechamiento Sostenible de Recursos Biológicos Nativos del Distrito de Manejo Integrado de Atuncela; Pontificia Universidad Javeriana Cali: Cali, Colombia, 2012; p. 11. [Google Scholar]
- Hartwig, G. The Tropical World: A Popular Scientificaccount of the Natural History of the Animal and Vegetablekingdoms in the Equatorial Regions; Spottiswoode and Co.: London, UK, 1863; p. 201. [Google Scholar]
- Pohl, J.B.E. Reise Im Innern Von Brasilien; Nabu Press: Vienna, Austria, 1832; pp. 416–417. [Google Scholar]
- Pérez-Silva, A.; Nicolás-García, M.; Petit, T.; Dijoux, J.B.; de los Ángeles Vivar-Vera, M.; Besse, P.; Grisoni, M. Quantification of the aromatic potential of ripe fruit of Vanilla planifolia (Orchidaceae) and several of its closely and distantly related species and hybrids. Eur. Food Res. Technol. 2021, 247, 1489–1499. [Google Scholar] [CrossRef]
- Liljegren, S.J.; Ditta, G.S.; Eshed, Y.; Savidge, B.; Bowman, J.L.; Yanofsky, M.F. SHATTERPROOF MADS-box genes control seed dispersal in Arabidopsis. Nature 2000, 404, 766–770. [Google Scholar] [CrossRef]
- Tamayo-García, R.; Narváez-Zapata, J.A.; Ku-González, A.; Aguilar-Espinosa, M.; Gutiérrez-Pacheco, L.C.; Rivera-Madrid, R. Gene expression analysis during the fruit development in dehiscent and indehiscent Bixa orellana L. accessions. Physiol. Mol. Biol. Plants 2022, 28, 709–718. [Google Scholar] [CrossRef]
- Li, X.R.; Deb, J.; Kumar, S.V.; Østergaard, L. Temperature modulates tissue-specification program to control fruit dehiscence in Brassicaceae. Mol. Plant. 2018, 11, 598–606. [Google Scholar] [CrossRef]
- Giovannoni, J.J. Genetic regulation of fruit development and ripening. Plant Cell 2004, 16, S170–S180. [Google Scholar] [CrossRef]
- Kumar, R.; Khurana, A.; Sharma, A.K. Role of plant hormones and their interplay in development and ripening of fleshy fruits. J. Exp. Bot. 2013, 65, 4561–4575. [Google Scholar] [CrossRef]
- Dirks-Mulder, A.; Ahmed, I.; uit het Broek, M.; Krol, L.; Menger, N.; Snier, J.; van Winzum, A.; de Wolf, A.; van’t Wout, M.; Zeegers, J.J.; et al. Morphological and molecular characterization of orchid fruit development. Front. Plant Sci. 2019, 10, 137. [Google Scholar] [CrossRef]
- Pramanik, D.; Spaans, M.; Kranenburg, T.; Bogarin, B.; Heijungs, R.; Lens, F.; Smets, E.; Gravendeel, B. Inflorescence lignification of natural species and horticultural hybrids of Phalaenopsis orchids. Sci. Hortic. 2022, 295, 110845. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).