Abstract
Viticulture is a perennial cropping system that provides large inter-row space as a non-crop habitat for a range of different taxa. Extensive vegetation management has been shown to increase biodiversity and ecosystem service provision in vineyards. Important soil ecosystem services are decomposition, nutrient cycling, and pest regulation provided by the mesofauna (e.g., Acari and Collembola). However, studies investigating the effects of inter-row management on soil mesofauna are scarce. We studied the effect of inter-row management intensity (complete vegetation cover, alternating vegetation cover, and bare ground) and local pedoclimatic conditions on Acari and Collembola in nine Austrian vineyards. Our results showed that the clay content of the soil was the most important factor and increased the abundances of both analyzed taxa. Complete and alternating vegetation cover increased their abundance in comparison to bare ground management. Higher soil respiration slightly contributed to higher abundances of those two taxa in both years. In conclusion, besides the positive effects of the clay content in the soil, complete and alternating vegetation cover are feasible management practices for increasing soil mesofauna in vineyards.
1. Introduction
Grapevines (Vitis vinifera L. ssp. vinifera) are perennial crops covering 7.3 million hectares worldwide, shaping viticultural landscapes and contributing significantly to regional and national economies [1]. Various viticultural practices (e.g., pesticide use, vegetation management, and the absence or presence of semi-natural habitats) influence biodiversity and ecosystem services in vineyards and their surrounding landscape [2,3,4,5,6,7,8,9]. Vegetation cover in vineyards at the local scale and habitat heterogeneity at the landscape scale have been identified as the key factors increasing biodiversity in vineyards in a global-scale review [10].
Vegetation cover in the form of cover crops or spontaneous vegetation is known to improve soils (e.g., reducing soil erosion, increasing soil organic matter content, and regulating soil temperature) and is favorable for soil biodiversity in vineyards [4,5,11,12,13]. The preservation of soil quality itself is the most important environmental attitude and belief for winegrowers driving decision-making and, consequently, vineyard management practices across Europe [14].
Soils in vineyards have, furthermore, a special relevance in viticulture [4], especially in the context of the term terroir, which is influenced by factors such as climate, cultivar, and soil type [15]. The type of soil affects the development and ripening of grapes as well as several grape quality parameters, such as sugar content, total acidity, and pH [15]. The inter-row management is also an important factor that may affect grape quality parameters and vine vigor through inter-row vegetation cover [16].
Soils harbor a great diversity of different organisms categorized into four groups according to their body size: microbiota, microfauna, mesofauna, and macrofauna [17,18]. The majority of the mesofauna in the soil is represented by Acari, Collembola, and Enchytraeids [18]. Soil mesofauna are an important part of the soil food web, contributing to many soil-related ecosystem services such as pest regulation, nutrient cycling, soil structure formation, and organic matter decomposition, which build the basis for sustainable agriculture [19,20,21,22,23].
Disturbance frequency and magnitude are two well-described main factors influencing soil biota across different ecosystems [19,24,25,26]. In general, high mechanical disturbance is associated with negative effects on biodiversity and an abundance of macro- and mesofauna [27], with higher sensitivity associated with increasing body size of soil biota [26]. Management practices also influence plant communities, particularly plant diversity, which is one of the main drivers for preserving diverse soil organisms (e.g., Acari and Collembola) and their role in soil functioning [28]. Furthermore, plant community composition and diversity might be able to buffer the negative effects of disturbance, with potential positive feedback for plant productivity [29].
In vineyards, little research has been conducted on mesofauna, and consequently, the knowledge about their functional diversity and main drivers is not well understood [4]. In general, most studies showed that undisturbed vegetation cover increased soil mesofauna abundance [11] and diversity [12,30,31] in comparison to disturbed inter-row vegetation by tillage (bare ground) or herbicides. Pesticide use is, in general, known as a detrimental factor for the soil biota [4]. The abundance of Collembola and Acari are, furthermore, sensitive indicators of soil degradation in vineyards [32]. However, recent studies [6,33,34] indicate that a certain level of tillage increased the Collembola population, e.g., alternating vegetation with tillage in every second inter-row increased the abundance of Collembola compared to tillage in both inter-rows in Romanian vineyards [34], whereas Collembola abundance was lower in Austrian vineyards with permanent vegetation cover [33]. A recent study in French vineyards showed, furthermore, that organic management in combination with high tillage intensity favored Collembola abundance in comparison to conventional management [6]. In contrast, Acari abundance in vineyards in Switzerland was not significantly influenced by tillage [35]. Nevertheless, a recent study showed that higher tillage intensities had negative effects on oribatid mites (Acari) in vineyard soils [31].
In summary, these inconclusive results call for an integrative analysis of the effects of inter-row management strategies under consideration of local pedoclimatic conditions on Acari and Collembola abundances. In the presented study, we applied an experimental setting within nine commercial vineyards in Austria for three consecutive years to identify beneficial management practices for soil mesofauna across study sites. We hypothesized that (1) complete (permanent) vegetation cover in vineyard inter-rows would gradually increase the abundance of Acari and Collembola in comparison to alternating cover or bare ground. Furthermore, (2) higher plant diversity was expected to increase their abundance by buffering the negative effects of intensive soil tillage.
2. Materials and Methods
2.1. Study Sites
This study was conducted in nine commercially used vineyards in Austria with a humid continental climate and warm summers [36]. Four vineyards were located in the federal state of Burgenland (Code: AT03, AT04, AT05, and AT06), and five vineyards in Lower Austria (Code: AT01, AT02, AT07, AT08, and AT09) (Figure 1a, Table S1). The annual mean temperature ranged from 11.2 °C in Lower Austria to 12 °C in Burgenland, with a mean precipitation from 487 mm to 567 mm per year, respectively, during the study period from 2015–2017 (Table S1, Figure S1). All vineyards were trained with a Vertical Shoot Positioned Trellis (VSP) system and rainfed without further irrigation. Management practices among the vineyards for vine nutrition and plant protection were applied by the respective winegrowers according to common local practices. Six vineyards (AT01–AT06) were managed according to integrated regulations (conventional), and three vineyards (AT07–AT09) were certified organic.
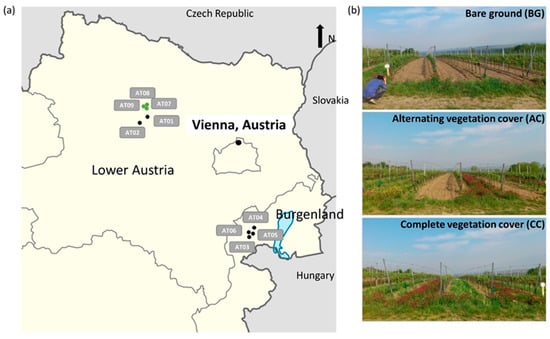
Figure 1.
(a) Location of the nine study vineyards in Lower Austria vineyards AT01, AT02, AT07–AT09 and in Burgenland vineyards AT03–AT06 (green dots: organic management; black dots: conventional management), and (b) representative pictures of the ground cover treatments established in each of the vineyard sites.
2.2. Experimental Setup
The experimental setup consisted of three different inter-row ground cover treatments, which were each established in four neighboring inter-rows in all vineyards in 2015: (i) alternating vegetation cover (AC) with continuous vegetation cover in every second inter-row and soil tillage in the other inter-row, (ii) complete vegetation cover (CC) in every inter-row, and (iii) bare ground (BG) through soil tillage in every inter-row (Figure 1b). Soil tillage was conducted three times per season in April, end of May, and mid-July in the tilled inter-rows. The vegetation cover in inter-rows was established in 2015 by sowing seed mixtures in one inter-row of the CC and AC treatment (the used seed mixtures are listed in Table S1). Inter-rows with vegetation cover were frequently mulched throughout the vegetation period (May, June, July, and August), and no herbicides were applied. Before the start of the experiment, all vineyards were managed with alternating vegetation cover; organic inter-rows were tilled slightly less frequently, and cover crop mixtures were used more often in the organic vineyards. The two central inter-rows out of the four inter-rows were used for measurements and sampling for each treatment. The vascular plant species richness was determined within two 1 m2 squares per treatment in two neighboring inter-rows in spring 2016 and 2017. The cover of all vascular plant species was estimated in percent, and all plants were determined at species level with the exception of two specimens identified only at genus level.
2.3. Soil Analyses and Microbial Soil Respiration
Physico-chemical soil parameters were determined in 2016 and 2017 through a 250 g soil sample from two neighboring inter-rows for each treatment and vineyard at the Geisenheim University using standard methods after Schaller (2000) [37] and as previously described [16,38]. Accordingly, soil pH, total elemental carbon and nitrogen, plant-available phosphorus, potassium and magnesium content, and calcium-carbonate fractions were measured in both years [37]. In addition, the inorganic carbon fraction was determined through the calcium-carbon fraction, and soil organic matter and organic carbon were calculated from the amount of total and inorganic carbon. Bioavailable copper content, as well as the clay, silt, and sand content of the soil, were measured only in 2016 because these parameters stay relatively stable across time [37]. A detailed summary of the obtained results is given in Table S2.
Microbial soil respiration rates were measured in 2016 with a micro-respirometer [39] from two neighboring inter-rows per treatment and vineyard, with 4.5 g of fresh soil moisturized to water saturation and measured for about 22 h. The microbial soil respiration was calculated as the mean consumption rate of oxygen (µg O2 × h−1 × 1 g dry soil). Results obtained from the samples collected in 2016 were used for model calculation (for detailed results see Table S4) for both years as relatively stable values were determined between years in study vineyards in Switzerland within the European project PromESSinG (correlation coefficient 0.718 **; see also comment for soil respiration stability across years in Table S4, further general details in Steiner et al. (2023) [40]).
2.4. Soil Mesofauna Sampling
The soil mesofauna was sampled three times throughout the vegetation period in 2016 and 2017: in spring (2 May 2016; 7 May 2017), at grapevine flowering (6 June 2016, 2 June 2017), and at harvest (5 September 2016; 13 September 2017). A core borer (2.5 cm diameter) was used for collecting soil samples from the upper 10 cm for mesofauna extraction. Two pooled samples from both neighboring inter-rows within all treatments of each vineyard were taken, resulting in 54 samples per sampling date. The pooled samples were obtained by collecting and mixing eight subsamples extracted with the soil core borer per inter-row. Mesofauna extraction was performed with a Berlese funnel with a 2 mm mesh size and 5 days with continuous light exposure. Acari and Collembola were separated and counted. Other soil fauna in the sample was also counted, but because of possible bias due to the sampling method, it was not used for further statistical analysis: Centipedes, Coleoptera, Diplura, Diptera, Enchytraeidae, Hemiptera, Hymenoptera, Isopoda, Milipedes, Nematoda, Pauropoda, Protura, Pseudoscorpiones, Phylloxera, Symphyla, and Thysanoptera [41]. The abundance of Acari and Collembola was summarized across the sampling date for each year and used for further analyses.
2.5. Statistical Analyses
The statistical analyses and visualization of the data were done with R Version 4.1.2 [42] and RStudio [43] using the R packages “tidyr” [44], “lme4” [45], “MASS“ [46], “DHARMa” [47], “car“ [48], “effects“ [49], “lattice“ [50], “MuMIn“ [51], “AICcmodavg“ [52], “corrplot” [53], and “ggplot2” [54].
Acari and Collembola abundance, which were aggregated across all sampling dates for each year for statistical modeling, were selected as response variables. Data exploration was performed as recommended by Zuur et al. (2010) [55]. The results from the inter-row treatment AC and BG in vineyard AT06 from 2017 were removed before statistical analysis because of missing values for the variable plant species richness. We initially used generalized linear mixed models (GLMM) with a Poisson distribution to analyze the effects of explanatory variables on both response variables. Potential explanatory variables were selected based on biological relevance and a Spearman correlation with the response variable of rs > ±0.15. Consequently, explanatory variables were as follows: inter-row treatment, management type (organic vs. conventional), inter-row plant species richness, total vegetation cover, microbial soil respiration, bioavailable copper content, and pH and clay content, as well as bioavailable copper content for Acari abundance and plant available phosphorus for Collembola abundance. “Vineyard site” and “year” were used as independent random effects for both response variables. Explanatory variables were rescaled (z-score) due to different scaling before modeling [56]. Accordingly, global models for Acari and Collembola abundances were computed with all possible combinations of the explanatory variables. The threshold of a maximum variance inflation factor (VIF) of 3 was used for the exclusion of explanatory variables [55]. Accordingly, the variables’ management type and total vegetation cover were excluded for both response variables. Overdispersion was detected during model validation, and therefore, we refit the global models with a negative binomial distribution [47,57]. As the integration of the random effect “vineyard site” for the Collembola abundance models led to boundary issues caused by a low variance of the random effect estimated at zero [56], we refitted the global models with generalized linear mixed models (GLMM) and a negative binomial distribution only with the random effect “year”. Explanatory variables with a collinearity of r ≥ ±0.5 [58] were excluded in the same models. The second-order Akaike’s Information Criterion corrected for small sample size (AICc) was used to select the most parsimonious models with a minimum difference of ∆i of 2 between them [58]. These resulted in a set of most parsimonious models for bot explanatory variables. These sets were then used for model averaging (zero method) [58] to show the relative importance and strongest effect of the response variables on the Acari and Collembola abundance [59,60]. In addition, for the most parsimonious models, the explained deviance was calculated [57], and effect plots were visualized.
3. Results
During the study period, a total number of 15,367 individuals (microfauna, mesofauna, and macrofauna) representing the 18 different taxa (see Section 2) were determined in the soil samples. By far, the most abundant group in total was Acari, represented by 6381 individuals in 2016 and 3267 in 2017, followed by Collembola, represented by 2295 individuals in 2016 and 478 in 2017 (see Table S5).
3.1. Factors Influencing Acari Abundance
The five most parsimonious models showed that the Acari abundance was influenced by the inter-row treatment as well as soil-related parameters (Table 1). The Acari abundance increased with higher clay content in the soil (Figure 2a), which was also the most important variable with the strongest positive effect size (Figure 3a). Surprisingly, their abundance increased with a higher bioavailable copper content in the soil (Figure 2e), which was the second most important variable according to the model averaging (Figure 3a). The inter-row treatment was the third most important variable (Figure 3a). Furthermore, the complete vegetation cover and the alternating vegetation cover showed the highest Acari abundance compared to the bare ground cover treatment (Figure 2b). The most parsimonious models indicated that the Acari abundance increased with higher microbial soil respiration (Figure 2c) but slightly decreased with higher plant species richness in the inter-row (Figure 2d and Figure 3a). Plant species richness had the lowest importance for the Acari abundance according to model averaging (Figure 3a). Therefore, the minor effect of this variable should be interpreted with care to avoid possible misinterpretations.

Table 1.
Summary of the most parsimonious models (GLMM) for the Acari and Collembola abundance, which were selected through the second-order Akaike’s Information Criterion corrected for small sample size (AICc), with ∆i as the difference in AICc to the best model, R2 marginal (R2 m), and R2 conditional (R2 c). Most parsimonious models are highlighted in bold. Vineyard site and year were used as random effects for Acari abundance, and year was used as random effect for Collembola abundance.
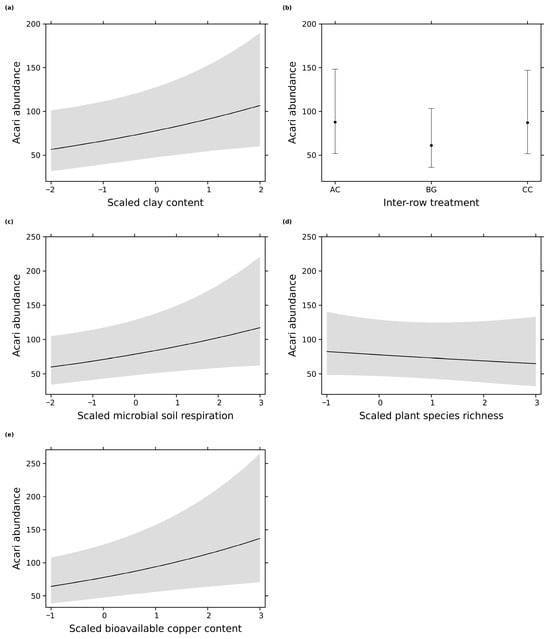
Figure 2.
Effect plots from the most parsimonious models of Acari abundance with scaled explanatory variables: (a) clay content in the soil; (b) inter-row treatment: AC = alternating vegetation cover, CC = complete vegetation cover, and BG = bare ground; (c) microbial soil respiration; (d) plant species richness in the inter-row; (e) bioavailable copper content in the soil. Error bars/gray shading indicate 0.95 confidence intervals.
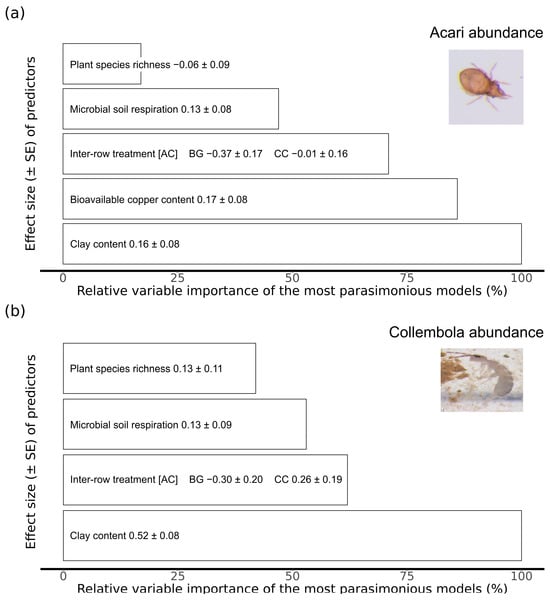
Figure 3.
Model averaging output with the relative importance of the explanatory variables from the set of most parsimonious models for the (a) Acari and (b) Collembola abundance expressed in percentage. Effect size (Estimate ± SE (Standard Error), positive and/or negative) for each variable is indicated within the bars. Squared brackets indicate the base category for the parameter estimation. Inter-row treatment: AC = alternating vegetation cover, CC = complete vegetation cover, and BG = bare ground.
3.2. Factors Influencing Collembola Abundance
The six most parsimonious models showed that Collembola abundance was influenced by similar parameters as the Acari abundance (Table 1). An increasing clay content increased the Collembola abundance (Figure 4a), which also resulted in the strongest positive effect size (Figure 3b). The inter-row treatment was the second most important variable (Figure 3b). The complete vegetation cover treatment showed the highest Collembola abundance compared to alternating vegetation cover and bare ground (Figure 4b). Bare ground resulted in a negative effect size for Collembola abundance (Figure 3b), which was similar to the results of the Acari (Figure 3a). An increasing microbial soil respiration (Figure 4d) and plant species richness (Figure 4c) slightly increased Collembola abundance. These two parameters showed the lowest importance and positive effect size for Acari abundance (Figure 3b).
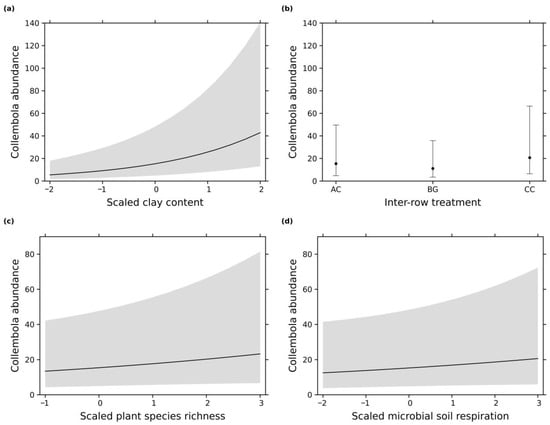
Figure 4.
Effect plots from the most parsimonious models of the Collembola abundance with scaled explanatory variables: (a) clay content in the soil; (b) inter-row treatment: AC = alternating vegetation cover, CC = complete vegetation cover, and BG = bare ground; (c)plant species richness in the inter-row; (d) microbial soil respiration. Error bars/gray shading indicate 0.95 confidence intervals.
4. Discussion
In accordance with our hypothesis, we found a gradual and slight difference between the different inter-row management regarding Acari and Collembola abundance. These effects were modulated by soil parameters like clay content, which significantly increased Acari and Collembola abundance. Furthermore, higher plant species richness resulted in small negative effects for Acari and positive for Collembola abundance.
Our study showed that the mesofauna in the investigated vineyards mainly consisted of the taxa Acari and Collembola, which was expected and corresponds to existing knowledge of different soil ecosystems in forests and annual and perennial crops [18,25,61]. A large-scale French study showed that total mean microarthropod abundance was highest in vineyard soils dominated by Acari, whereas Collembola abundances were lowest compared to other land use types like forests, grasslands, arable land, or gardens [62]. The dominance of Acari in vineyard soils is in line with two studies from Italy [11,63]. The recognizable difference in the Acari and Collembola abundance between the two sampling years may be related to the different rainfall patterns as an influencing factor [63]. Higher soil moisture is, thereby, especially beneficial for Collembola and oribatid mites [61].
Higher clay content in the soils was the most important positive factor for Acari and Collembola abundance in the investigated vineyards. The physical properties of the soil texture or other soil properties such as pH or organic matter are factors that influence Collembola and Acari abundances [64,65]. A recent meta-analysis also showed that Collembola densities were highest in clayey and loamy soils and that Acari densities were higher in clayey soils in combination with reduced tillage [65]. These findings support the observed influence of the clay content in our study.
In accordance with our hypothesis, complete vegetation cover in vineyard inter-rows resulted in the highest Collembola abundance and gradual decrease compared to alternating vegetation cover and bare soil due to intensive tillage. The Acari abundance showed a similar trend, with only a slight gradual difference between alternating and complete vegetation cover. In general, a reduced tillage frequency benefits Collembola and Acari densities [65]. Gagnarli et al. (2015) [11] connected higher mite (mainly oribatid mites) and springtail populations with permanent vegetation cover in organically managed vineyards. Collembola abundance also profited more from alternating inter-row cover compared to tilled inter-rows in conventional Romanian vineyards [34]. Seniczak et al. (2018) [31] also found negative effects of tillage on oribatid mites in the Spanish vineyard inter-rows. Accordingly, their sensitivity to soil disturbance like tillage can influence their populations negatively [66].
According to our second hypothesis, higher plant species richness in the inter-rows slightly increased Collembola abundance but slightly decreased Acari abundance. In our study, this factor was of minor importance and should be interpreted with great care. Studies in vineyards in France and South Africa found a general positive effect of diverse herbaceous cover crops on the abundance of natural enemies of vineyard pest species [2] and arthropod diversity [67] in comparison to low-diversity grassy vegetation. In a long-term grassland experiment, plant diversity was the strongest driver for increasing abundance and diversity of soil organisms, which stresses the overall importance of the conservation of plant diversity for biodiversity and soil functioning [28]. Overall, there is still a significant lack of studies investigating the interactions of plant communities and below-ground microarthropods for most ecosystems.
Microbial soil respiration was another driver of Acari and Collembola abundances in the investigated vineyard soils. Microbial soil respiration in this context is mostly used as an indicator of the metabolic activity of soil organisms [25] and can be positively influenced through vegetation cover [40]. Several authors showed a synergistic effect of microbial soil respiration and microarthropod abundance [68,69,70], e.g., grazing Collembola can increase fungal respiration [68]. Accordingly, Collembola plays an important role in decomposition [21] and microbial soil respiration by feeding on food sources like fungal hyphae and rotted plant material [71].
The positive effect of an increased bioavailable copper content on Acari abundance was an unexpected and surprising result in our study. Inorganic fungicides such as copper are known for their negative effects on soil biota [72,73,74]. However, several factors, like the soil type or copper concentration, influence copper toxicity in soils [73]. A recent study showed that an increasing copper concentration also increased the uptake by the oribatid mite Oppia nitens [72]. Similar results of an increase in Acari abundance were also found in France [6]. Skubala and Zaleski (2012) [75] were able to show that small concentrations of heavy metals (Cd, Ni, Pb, and Zn) increased oribatid abundance in grassland ecosystems. Similar results were found for copper in pine forests [76]. Oribatid mites are, furthermore, categorized into species that are tolerant to heavy metals, sensitive to heavy metals, or tolerant to low but sensitive to high heavy metal concentrations [76]. Nevertheless, this effect may not only be species dependent since copper toxicity in soils affects the whole soil biota [73] and can be, therefore, an indirect effect through affecting other competitors or food sources (e.g., soil fungi), see also [75]. Our results suggest, therefore, a higher complexity between the soil bioavailable copper content, soil type, and abundance of mesofauna, such as Acari, which calls for further studies analyzing this relationship.
5. Conclusions
Several studies investigated the effects of agricultural management practices on soil fauna in different agricultural systems, whereas vineyards are still rather poorly explored. Our study showed that mesofauna abundance was influenced by the soil texture and benefited from complete or alternating vegetation cover compared to bare ground management. Furthermore, the mesofauna increased parallel to higher microbial soil respiration, which is an indicator of the metabolic activity of soil fungi and bacteria. Plant species richness showed a weak effect; however, vegetation cover in the inter-row clearly resulted in higher Collembola and Acari abundances in the current study. Consequently, plant vegetation cover in vineyard inter-rows should be supported by agri-environmental programs to preserve biodiversity and related ecosystem services.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/horticulturae9121249/s1. Table S1: Description of site locations of the experimental vineyards. Table S2: Results of soil chemical and physical analysis. Table S3: Vegetation cover composition of the treatments complete vegetation cover (CC) and alternating vegetation cover (AC) as a percentage of grasses, percentage of herbs, and percentage of legumes coverage. Table S4: Soil respiration determined in June 2016 in all study sites and ground cover treatments. Table S5: Summary table of arthropod fauna for summarizing the mesofauna. Figure S1: Summary of the climatic conditions in 2016 and 2017 in the different wine-growing regions.
Author Contributions
A.F. and M.G. conceived the study design in cooperation with the PromESSinG project group; M.G., S.K. and R.R. collected and determined the mesofauna samples from all vineyards; M.S. determined the soil respiration; M.G. and S.K. validated the explanatory and response data; S.M. performed the statistical analyses and prepared the figures under supervision of S.W.; S.M., M.G. and S.W. drafted the manuscript with inputs from A.F., S.B. and P.Q. All authors have read and agreed to the published version of the manuscript.
Funding
Results presented here are part of the PromESSinG project, funded by the 2013–2014 BiodivERsA/FACCE JPI joint call for research proposals (assessed on 17 June 2014). With the national funders, I.L. received funding from BMBF, DE, grant number 01LC1405A; B.G. received funding ANR, FR, ANR-14-EBID-0004; C.P. received funding from ANCS, RO, CCCDI–UEFISCDI, RO, project number PN3-P3-61 contract 21/2015, within PNCDI III; A.F. received funding from FWF, AT, project number I 2053-B25; and S.B. received funding from SNSF Swiss National Science Foundation, CH, project number 40FA40_158390. S.W. and S.M. were funded by the research project SECBIVIT, which was funded through the 2017–2018 Belmont Forum and BiodivERsA joint call for research proposals under the BiodivScen ERA-Net COFUND programme, with the following funding organizations: AEI/SP, BMBF and Projektträger VDI/VDE Innovation + Technik GmbH/DE, ANR/FR, NWO/NL, UEFISCDI/RO, FWF/AT (Grant number I 4025-B32), and the NSF/USA (Grant #1850943). P.Q. is funded by the Austria Academy of Science grant: Heritage_2020-043_Modeling-Museum.
Data Availability Statement
The data presented in this study are openly available in Zenodo at https://doi.org/10.5281/zenodo.7405287 (accessed on 21 October 2023).
Acknowledgments
We would like to thank all farmers supporting the project by providing study vineyards and establishing the ground cover treatments within each vineyard. We would like to thank Open Access Funding by the Austrian Science Fund (FWF).
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Organisation Internationale de la Vigne et du Vin State of the World Vitivinicultural Sector in 2020. Available online: https://www.oiv.int/de/normen-und-technische-dokumente/statistischen-analysen/konjunkturanalyse (accessed on 24 August 2022).
- Beaumelle, L.; Auriol, A.; Grasset, M.; Pavy, A.; Thiéry, D.; Rusch, A. Benefits of increased cover crop diversity for predators and biological pest control depend on the landscape context. Ecol. Solut. Evid. 2021, 2, e12086. [Google Scholar] [CrossRef]
- Etienne, L.; Franck, P.; Lavigne, C.; Papaïx, J.; Tolle, P.; Ostandie, N.; Rusch, A. Pesticide use in vineyards is affected by semi-natural habitats and organic farming share in the landscape. Agric. Ecosyst. Environ. 2022, 333, 107967. [Google Scholar] [CrossRef]
- Giffard, B.; Winter, S.; Guidoni, S.; Nicolai, A.; Castaldini, M.; Cluzeau, D.; Coll, P.; Cortet, J.; Le Cadre, E.; D’Errico, G.; et al. Vineyard management and its impacts on soil biodiversity, functions, and ecosystem services. Front. Ecol. Evol. 2022, 10, 1–21. [Google Scholar] [CrossRef]
- Karimi, B.; Cahurel, J.Y.; Gontier, L.; Charlier, L.; Chovelon, M.; Mahé, H.; Ranjard, L. A Meta-analysis of the ecotoxicological impact of viticultural practices on soil biodiversity. Environ. Chem. Lett. 2020, 18, 1947–1966. [Google Scholar] [CrossRef]
- Ostandie, N.; Giffard, B.; Bonnard, O.; Joubard, B.; Richart-Cervera, S.; Thiéry, D.; Rusch, A. Multi-community effects of organic and conventional farming practices in vineyards. Sci. Rep. 2021, 11, 11979. [Google Scholar] [CrossRef]
- Paredes, D.; Rosenheim, J.A.; Chaplin-Kramer, R.; Winter, S.; Karp, D.S. Landscape simplification increases vineyard pest outbreaks and insecticide use. Ecol. Lett. 2021, 24, 73–83. [Google Scholar] [CrossRef]
- Sáenz-Romo, M.G.; Veas-Bernal, A.; Martínez-García, H.; Campos-Herrera, R.; Ibáñez-Pascual, S.; Martínez-Villar, E.; Pérez-Moreno, I.; Marco-Mancebón, V.S. Ground cover management in a mediterranean vineyard: Impact on insect abundance and diversity. Agric. Ecosyst. Environ. 2019, 283, 106571. [Google Scholar] [CrossRef]
- Winter, S.; Bauer, T.; Strauss, P.; Kratschmer, S.; Paredes, D.; Popescu, D.; Landa, B.; Guzmán, G.; Gómez, J.A.; Guernion, M.; et al. Effects of vegetation management intensity on biodiversity and ecosystem services in vineyards: A meta-analysis. J. Appl. Ecol. 2018, 55, 2484–2495. [Google Scholar] [CrossRef]
- Paiola, A.; Assandri, G.; Brambilla, M.; Zottini, M.; Pedrini, P.; Nascimbene, J. Exploring the potential of vineyards for biodiversity conservation and delivery of biodiversity-mediated ecosystem services: A Global-Scale Systematic Review. Sci. Total Environ. 2020, 706, 135839. [Google Scholar] [CrossRef]
- Gagnarli, E.; Goggioli, D.; Tarchi, F.; Guidi, S.; Nannelli, R.; Vignozzi, N.; Valboa, G.; Lottero, M.R.; Corino, L.; Simoni, S. Case study of microarthropod communities to assess soil quality in different managed vineyards. Soil 2015, 1, 527–536. [Google Scholar] [CrossRef]
- Gonçalves, F.; Nunes, C.; Carlos, C.; López, Á.; Oliveira, I.; Crespí, A.; Teixeira, B.; Pinto, R.; Costa, C.A.; Torres, L. Do soil management practices affect the activity density, diversity, and stability of soil arthropods in vineyards? Agric. Ecosyst. Environ. 2020, 294, 106863. [Google Scholar] [CrossRef]
- Renaud, A.; Poinsot-Balaguer, N.; Cortet, J.; Le Petit, J. Influence of four soil maintenance practices on collembola communities in a mediterranean vineyard. Pedobiologia 2004, 48, 623–630. [Google Scholar] [CrossRef]
- Chen, Y.; Herrera, R.A.; Benitez, E.; Hoffmann, C.; Möth, S.; Paredes, D.; Plaas, E.; Popescu, D.; Rascher, S.; Rusch, A.; et al. Winegrowers’ decision-making: A Pan-European perspective on pesticide use and inter-row management. J. Rural Stud. 2022, 94, 37–53. [Google Scholar] [CrossRef]
- Van Leeuwen, C.; Friant, P.; Choné, X.; Tregoat, O.; Koundouras, S.; Dubourdieu, D. Influence of climate, soil, and cultivar on terroir. Am. J. Enol. Vitic. 2004, 55, 207–217. [Google Scholar] [CrossRef]
- Griesser, M.; Steiner, M.; Pingel, M.; Uzman, D.; Preda, C.; Giffard, B.; Tolle, P.; Memedemin, D.; Forneck, A.; Reineke, A.; et al. General trends of different inter-row vegetation management affecting vine vigor and grape quality across European vineyards. Agric. Ecosyst. Environ. 2022, 338, 108073. [Google Scholar] [CrossRef]
- Briones, M.J.I. Soil fauna and soil functions: A jigsaw puzzle. Front. Environ. Sci. 2014, 2, 1–22. [Google Scholar] [CrossRef]
- Lavelle, P.; Spain, A.V. Soil Ecology; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2001; ISBN 978-0-7923-7123-6. [Google Scholar]
- Barrios, E. Soil biota, ecosystem services and land productivity. Ecol. Econ. 2007, 64, 269–285. [Google Scholar] [CrossRef]
- Lavelle, P.; Decaëns, T.; Aubert, M.; Barot, S.; Blouin, M.; Bureau, F.; Margerie, P.; Mora, P.; Rossi, J.P. Soil invertebrates and ecosystem services. Eur. J. Soil Biol. 2006, 42, S3–S15. [Google Scholar] [CrossRef]
- Potapov, A.; Bellini, B.; Chown, S.; Deharveng, L.; Janssens, F.; Kováč, Ľ.; Kuznetsova, N.; Ponge, J.-F.; Potapov, M.; Querner, P.; et al. Towards a global synthesis of collembola knowledge: Challenges and potential solutions. Soil Org. 2020, 92, 161–188. [Google Scholar] [CrossRef]
- Potapov, A.M.; Guerra, C.A.; van den Hoogen, J.; Babenko, A.; Bellini, B.C.; Berg, M.P.; Chown, S.L.; Deharveng, L.; Kováč, Ľ.; Kuznetsova, N.A.; et al. Globally invariant metabolism but density-diversity mismatch in springtails. Nat. Commun. 2023, 14, 674. [Google Scholar] [CrossRef] [PubMed]
- Pulleman, M.; Creamer, R.; Hamer, U.; Helder, J.; Pelosi, C.; Pérès, G.; Rutgers, M. Soil Biodiversity, biological indicators and soil ecosystem services—An overview of European approaches. Curr. Opin. Environ. Sustain. 2012, 4, 529–538. [Google Scholar] [CrossRef]
- Brussaard, L.; de Ruiter, P.C.; Brown, G.G. Soil biodiversity for agricultural sustainability. Agric. Ecosyst. Environ. 2007, 121, 233–244. [Google Scholar] [CrossRef]
- Coleman, D.C.; Crossley, D.A.; Hendrix, P.F. Fundamentals of Soil Ecology, 2nd ed.; Elsevier: London, UK, 2004; ISBN 0-12-179726-0. [Google Scholar]
- Kladivko, E.J. Tillage systems and soil ecology. Soil Tillage Res. 2001, 61, 61–76. [Google Scholar] [CrossRef]
- Cortet, J.; Ronce, D.; Poinsot-Balaguer, N.; Beaufreton, C.; Chabert, A.; Viaux, P.; Cancela De Fonseca, J.P. Impacts of different agricultural practices on the biodiversity of microarthropod communities in arable crop systems. Eur. J. Soil Biol. 2002, 38, 239–244. [Google Scholar] [CrossRef]
- Eisenhauer, N.; Dobies, T.; Cesarz, S.; Hobbie, S.E.; Meyer, R.J.; Worm, K.; Reich, P.B. Plant diversity effects on soil food webs are stronger than those of elevated CO2 and N deposition in a long-term grassland experiment. Proc. Natl. Acad. Sci. USA 2013, 110, 6889–6894. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.A.; Koch, A.M.; Forsythe, J.; Johnson, N.C.; Tilman, D.; Klironomos, J. Resistance of soil biota and plant growth to disturbance increases with plant diversity. Ecol. Lett. 2020, 23, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Bordoni, M.; Vercesi, A.; Maerker, M.; Ganimede, C.; Reguzzi, M.C.; Capelli, E.; Wei, X.; Mazzoni, E.; Simoni, S.; Gagnarli, E.; et al. Effects of vineyard soil management on the characteristics of soils and roots in the lower Oltrepò Apennines (Lombardy, Italy). Sci. Total Environ. 2019, 693, 133390. [Google Scholar] [CrossRef]
- Seniczak, A.; Seniczak, S.; García-Parra, I.; Ferragut, F.; Xamaní, P.; Graczyk, R.; Messeguer, E.; Laborda, R.; Rodrigo, E. Oribatid mites of conventional and organic vineyards in the valencian community, Spain. Acarologia 2018, 58, 119–133. [Google Scholar] [CrossRef]
- Costantini, E.A.C.; Castaldini, M.; Diago, M.P.; Giffard, B.; Lagomarsino, A.; Schroers, H.J.; Priori, S.; Valboa, G.; Agnelli, A.E.; Akça, E.; et al. Effects of soil erosion on agro-ecosystem services and soil functions: A multidisciplinary study in nineteen organically farmed European and Turkish vineyards. J. Environ. Manag. 2018, 223, 614–624. [Google Scholar] [CrossRef]
- Buchholz, J.; Querner, P.; Paredes, D.; Bauer, T.; Strauss, P.; Guernion, M.; Scimia, J.; Cluzeau, D.; Burel, F.; Kratschmer, S.; et al. Soil biota in vineyards are more influenced by plants and soil quality than by tillage intensity or the surrounding landscape. Sci. Rep. 2017, 7, 17445. [Google Scholar] [CrossRef] [PubMed]
- Fiera, C.; Ulrich, W.; Popescu, D.; Bunea, C.I.; Manu, M.; Nae, I.; Stan, M.; Markó, B.; Urák, I.; Giurginca, A.; et al. Effects of vineyard inter-row management on the diversity and abundance of plants and surface-dwelling invertebrates in central Romania. J. Insect Conserv. 2020, 24, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Linder, C.; Juvara-Bals, I. Soil litter-inhabiting Gamasina species (Acari, Mesostigmata) from a vineyard in western Switzerland. Acarologia 2006, 46, 143–156. [Google Scholar]
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World map of the Köppen-Geiger climate classification updated. Meteorol. Z. 2006, 15, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Schaller, K. Praktikum zur Bodenkunde und Pflanzenernährung, 8th ed.; Gesellschaft zur Förderung der Forschungsanstalt: Geisenheim, Germany, 2000; ISBN 978-3-934742-01-7. [Google Scholar]
- Porter, L.; Kahlil, S.; Forneck, A.; Winter, S.; Griesser, M. Effects of ground cover management, landscape elements and local conditions on carabid (Coleoptera: Carabidae) diversity and vine vitality in temperate vineyards. Agronomy 2022, 12, 1328. [Google Scholar] [CrossRef]
- Scheu, S. Automated measurement of the respiratory response of soil microcompartments: Active microbial biomass in earthworm faeces. Soil Biol. Biochem. 1992, 24, 1113–1118. [Google Scholar] [CrossRef]
- Steiner, M.; Pingel, M.; Falquet, L.; Giffard, B.; Griesser, M.; Leyer, I.; Preda, C.; Uzman, D.; Bacher, S.; Reineke, A. Local conditions matter: Minimal and variable effects of soil disturbance on microbial communities and functions in European vineyards. PLoS ONE 2023, 18, e0280516. [Google Scholar] [CrossRef]
- Eisenbeis, G.; Wichard, W. Atlas Zur Biologie Der Bodenarthropoden; Springer: Berlin/Heidelberg, Germany, 1985; ISBN 978-3-642-39391-4. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.r-project.org/ (accessed on 4 December 2021).
- RStudio Team. RStudio: Integrated Development Environment for R. RStudio; PBC: Boston, MA, USA, 2021; Available online: http://www.rstudio.com/ (accessed on 4 December 2021).
- Wickham, H. Tidyr: Tidy Messy Data. R Package Version 1.1.4. Available online: https://cran.r-project.org/package=tidyr (accessed on 7 December 2021).
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using Lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S, 4th ed.; Springer: New York, NY, USA, 2002; ISBN 0-387-95457-0. [Google Scholar]
- Hartig, F. DHARMa: Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models. R Package Version 0.4.5. Available online: https://cran.r-project.org/package=DHARMa (accessed on 4 April 2022).
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 3rd ed.; Sage: Thousand Oaks, CA, USA, 2019; Available online: https://socialsciences.mcmaster.ca/jfox/Books/Companion/ (accessed on 20 April 2020).
- Fox, J. Effect displays in R for generalised linear models. J. Stat. Softw. 2003, 8, 1–27. [Google Scholar] [CrossRef]
- Sarkar, D. Lattice: Multivariate Data Visualization with R; Springer: New York, NY, USA, 2008; ISBN 978-0-387-75968-5. [Google Scholar]
- Bartoń, K. MuMIn: Multi-Model Inference. R Package Version 1.43.17. Available online: https://cran.r-project.org/package=MuMIn (accessed on 20 April 2020).
- Mazerolle, M.J. AICcmodavg: Model Selection and Multimodel Inference Based on (Q)AIC(c). R P Package Version 2.3-0. Available online: https://cran.r-project.org/web/packages/AICcmodavg/index.html (accessed on 20 April 2020).
- Wei, T.; Simko, V. R Package “Corrplot”: Visualization of a Correlation Matrix (Version 0.84). Available online: https://github.com/taiyun/corrplot (accessed on 20 April 2020).
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- Zuur, A.F.; Ieno, E.N.; Elphick, C.S. A protocol for data exploration to avoid common statistical problems. Methods Ecol. Evol. 2010, 1, 3–14. [Google Scholar] [CrossRef]
- Bolker, B.M.; Gardner, B.; Maunder, M.; Berg, C.W.; Brooks, M.; Comita, L.; Crone, E.; Cubaynes, S.; Davies, T.; de Valpine, P.; et al. Strategies for fitting nonlinear ecological models in R, AD model builder, and bugs. Methods Ecol. Evol. 2013, 4, 501–512. [Google Scholar] [CrossRef]
- Zuur, A.F.; Ieno, E.N.; Walker, N.; Saveliev, A.A.; Smith, G.M. Mixed Effects Models and Extensions in Ecology with R, 1st ed.; Springer: New York, NY, USA, 2009; ISBN 978-0-387-87457-9. [Google Scholar]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference A Practical Information-Theoretic Approach, 2nd ed.; Springer: New York, NY, USA, 2002; ISBN 0-384-95364-7. [Google Scholar]
- Grueber, C.E.; Nakagawa, S.; Laws, R.J.; Jamieson, I.G. Multimodel inference in ecology and evolution: Challenges and solutions. J. Evol. Biol. 2011, 24, 699–711. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, S.; Freckleton, R.P. Model averaging, missing data and multiple imputation: A case study for behavioural ecology. Behav. Ecol. Sociobiol. 2011, 65, 103–116. [Google Scholar] [CrossRef]
- Culliney, T.W. Role of arthropods in maintaining soil fertility. Agriculture 2013, 3, 629–659. [Google Scholar] [CrossRef]
- Joimel, S.; Schwartz, C.; Hedde, M.; Kiyota, S.; Krogh, P.H.; Nahmani, J.; Pérès, G.; Vergnes, A.; Cortet, J. Urban and industrial land uses have a higher soil biological quality than expected from physicochemical quality. Sci. Total Environ. 2017, 584–585, 614–621. [Google Scholar] [CrossRef]
- Costantini, E.A.C.; Agnelli, A.E.; Fabiani, A.; Gagnarli, E.; Mocali, S.; Priori, S.; Simoni, S.; Valboa, G. Short-term recovery of soil physical, chemical, micro- and mesobiological functions in a new vineyard under organic farming. Soil 2015, 1, 443–457. [Google Scholar] [CrossRef]
- Bedano, J.C.; Domínguez, A.; Arolfo, R.; Wall, L.G. Effect of good agricultural practices under no-till on litter and soil invertebrates in areas with different soil types. Soil Tillage Res. 2016, 158, 100–109. [Google Scholar] [CrossRef]
- Betancur-Corredor, B.; Lang, B.; Russell, D.J. Reducing tillage intensity benefits the soil micro- and mesofauna in a global meta-analysis. Eur. J. Soil Sci. 2022, 73, e13321. [Google Scholar] [CrossRef]
- Behan-Pelletier, V.M. Oribatid mite biodiversity in agroecosystems. Agric. Ecosyst. Environ. 1999, 74, 411–423. [Google Scholar] [CrossRef]
- Geldenhuys, M.; Gaigher, R.; Pryke, J.S.; Samways, M.J. Diverse herbaceous cover crops promote vineyard arthropod diversity across different management regimes. Agric. Ecosyst. Environ. 2021, 307, 107222. [Google Scholar] [CrossRef]
- Bengtsson, G.; Rundgren, S. Respiration and growth of a fungus, Mortierella Isabellina, in response to grazing by Onychiurus Armatus (Collembola). Soil Biol. Biochem. 1983, 15, 469–473. [Google Scholar] [CrossRef]
- Kaneko, N.; McLean, M.A.; Parkinson, D. Do mites and collembola affect pine litter fungal biomass and microbial respiration? Appl. Soil Ecol. 1998, 9, 209–213. [Google Scholar] [CrossRef]
- Setälä, H.; Haimi, J.; Huhta, V. A Microcosm study on the respiration and weight loss in birch litter and raw humus as influenced by soil fauna. Biol. Fertil. Soils 1988, 5, 282–287. [Google Scholar] [CrossRef]
- Hopkin, S.P. Biology of the Springtails Insecta: Collembola; Oxford University Press: Oxford, UK, 1997; ISBN 0-19-854084-1. [Google Scholar]
- Akrami, M.A.; Ardestani, M.M.; Verweij, R.A.; van Gestel, C.A.M. Toxicity and bioaccumulation of copper in the oribatid mite Oppia Nitens (Acari: Oribatida). Appl. Soil Ecol. 2022, 179, 104601. [Google Scholar] [CrossRef]
- Komárek, M.; Čadková, E.; Chrastný, V.; Bordas, F.; Bollinger, J.C. Contamination of vineyard soils with fungicides: A review of environmental and toxicological aspects. Environ. Int. 2010, 36, 138–151. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, W.F.; Wong, F.P. Grapevine fungicides. In Compendium of Grape Diseases, Disorders, and Pests; Wilcox, W.F., Gubler, W.D., Uyemoto, J.K., Eds.; The American Phytopathological Society: St. Paul, MN, USA, 2015; pp. 177–184. ISBN 978-0-89054-479-2. [Google Scholar]
- Skubała, P.; Zaleski, T. Heavy metal sensitivity and bioconcentration in oribatid mites (Acari, Oribatida). Gradient study in meadow ecosystems. Sci. Total Environ. 2012, 414, 364–372. [Google Scholar] [CrossRef]
- Seniczak, S.; Dabrowski, J.; Dlugosz, J. Effect of copper smelting air pollution on the mites (Acari) associated with young scots pine forests polluted by a copper smelting works at Giogow, Poland. I. Arboreal mites. Water Air Soil Pollut. 1997, 97, 287–302. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).