Effect of Drying Post-Harvest on the Nutritional Compounds of Edible Flowers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Post-Harvest Treatments
2.2. Water Loss Percentage, Crude Protein Percentage and Mineral/Trace Content
2.3. Biochemical Analyses
2.4. Statistical Analysis
3. Results and Discussion
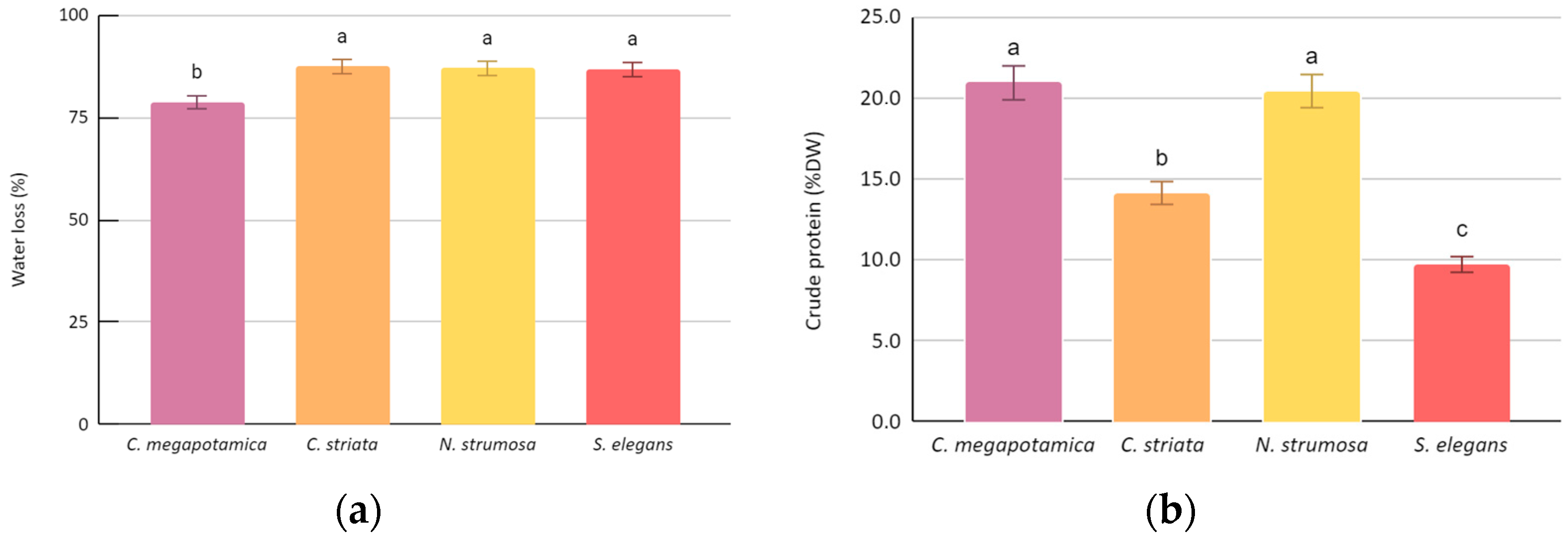
3.1. Water Loss Percentage, Crude Protein Percentage and Mineral/Trace Content
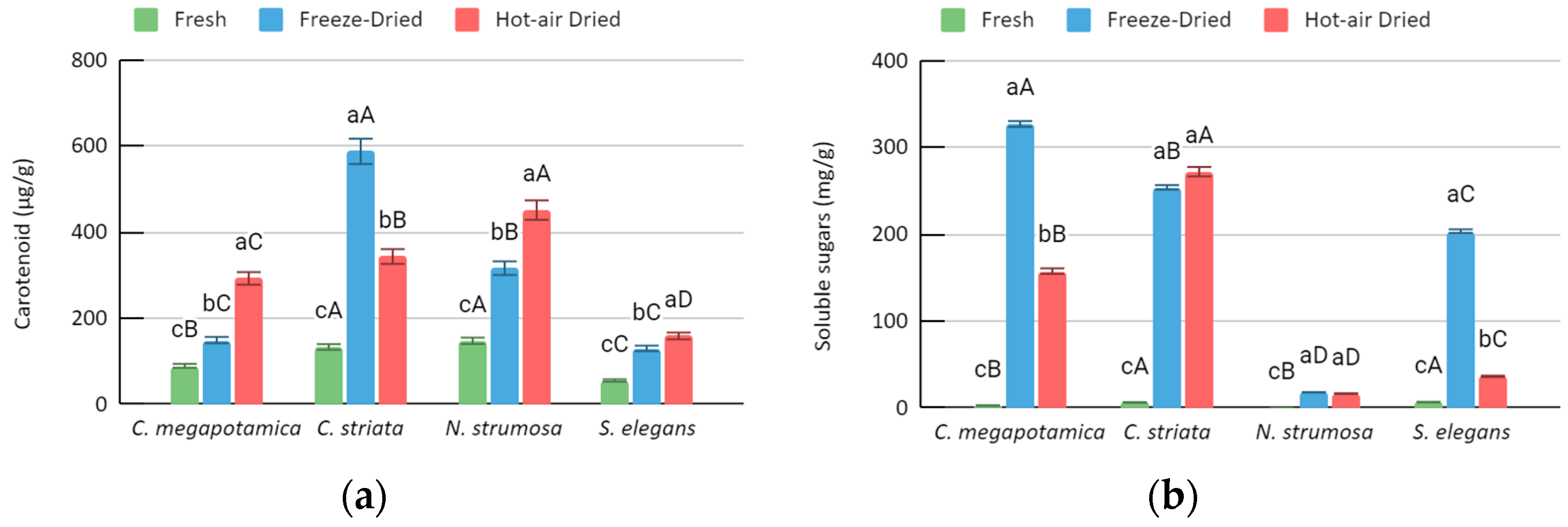
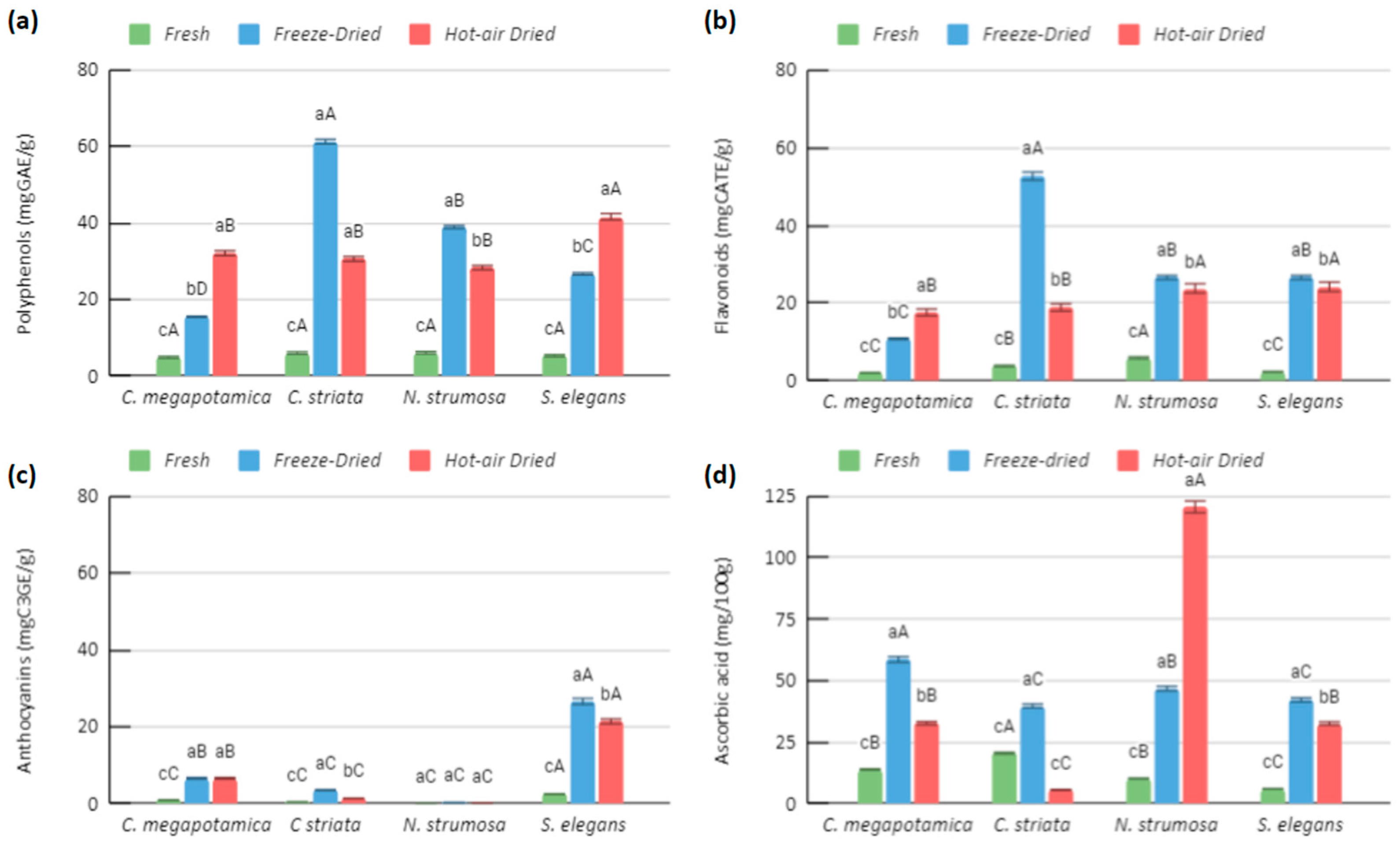
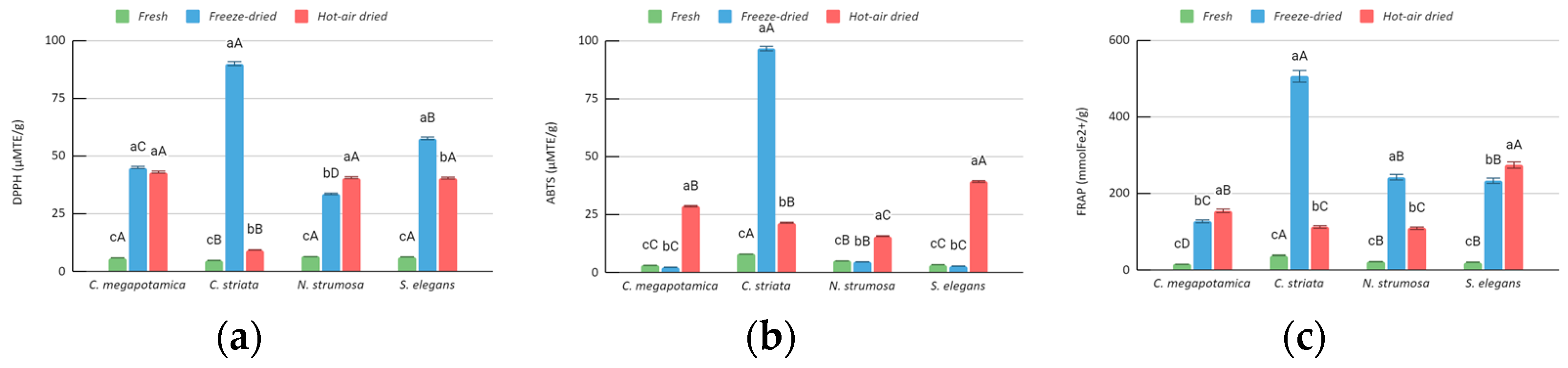
3.2. Biochemical Analysis
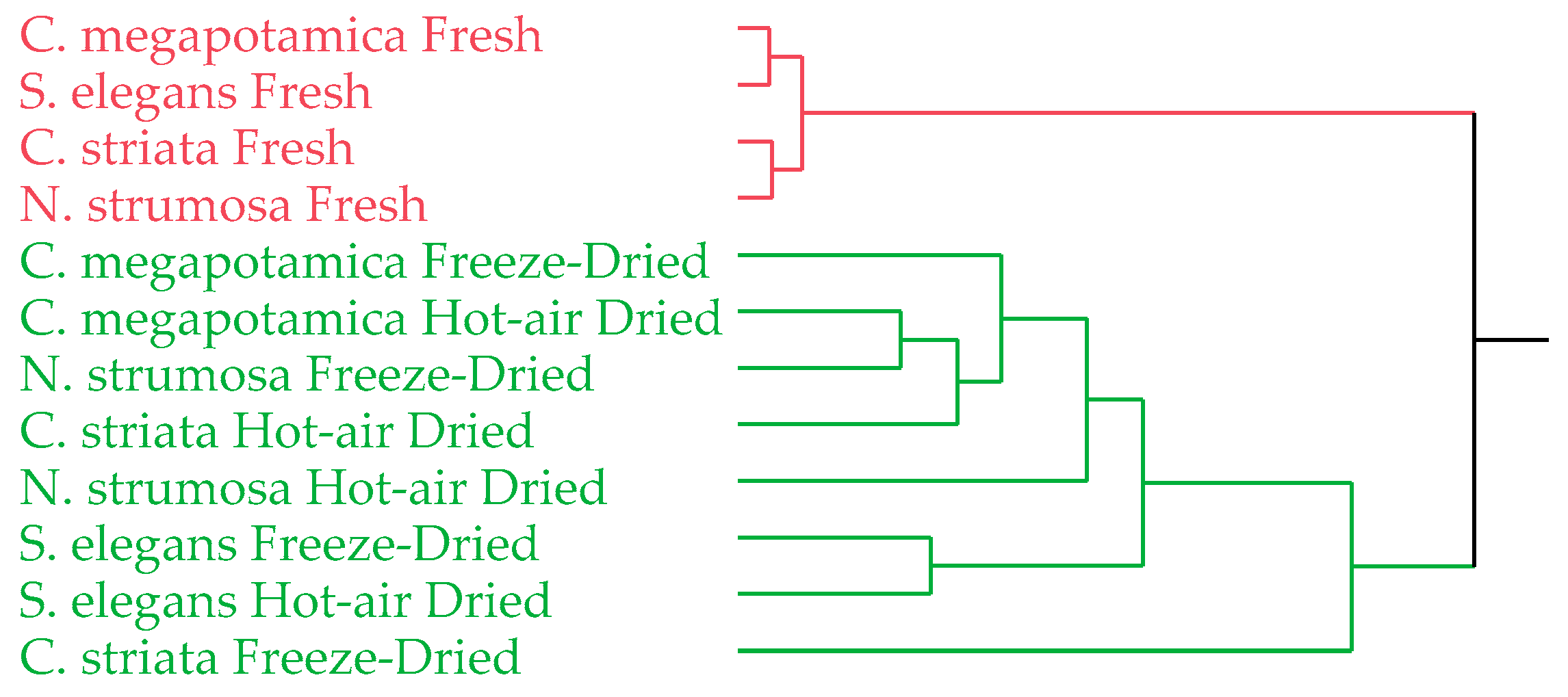
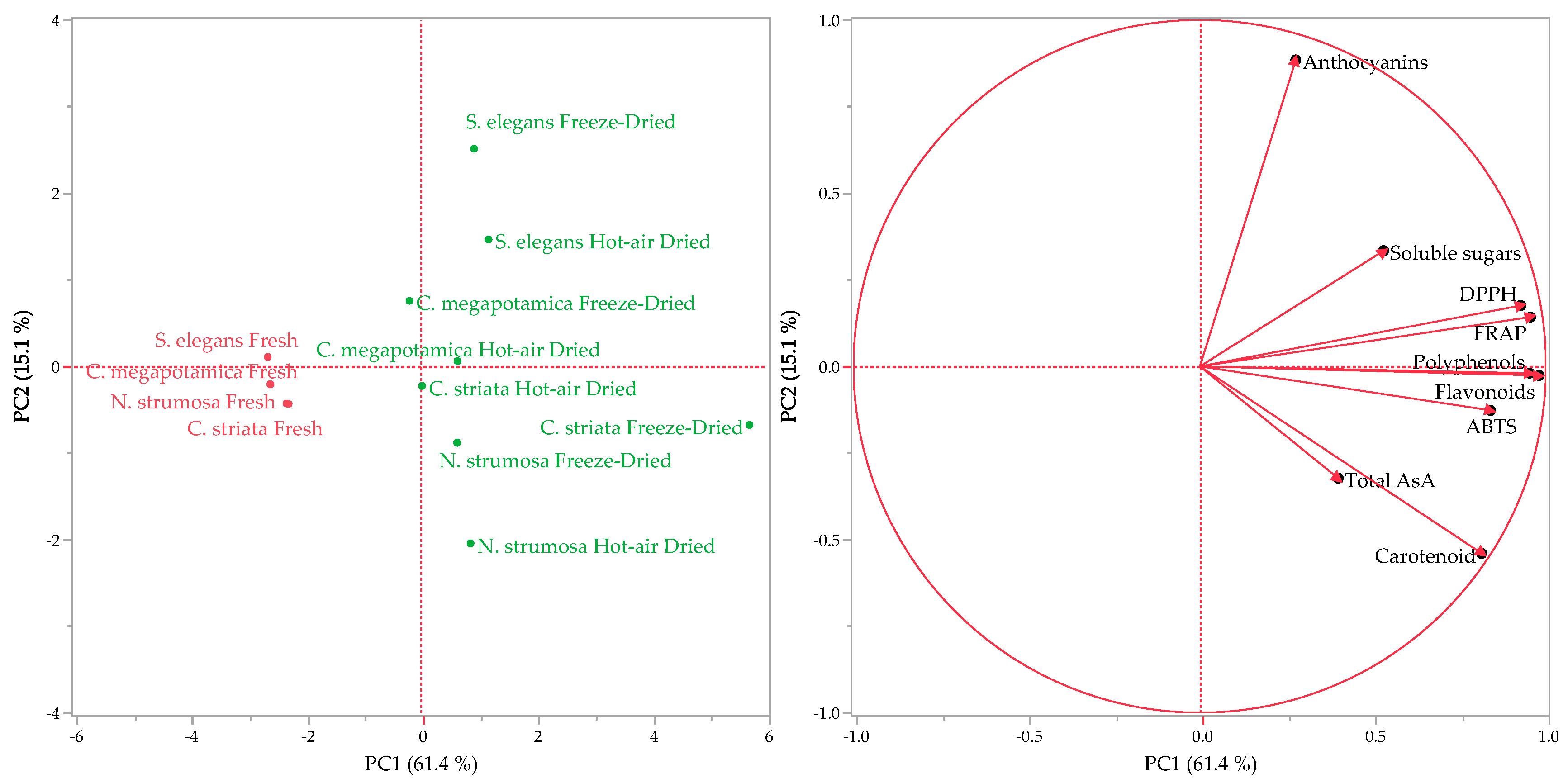
3.3. Statistical Analyses
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Takahashi, J.A.; Rezende, F.A.G.G.; Moura, M.A.F.; Dominguete, L.C.B.; Sande, D. Edible flowers: Bioactive profile and its potential to be used in food development. Food Res. Int. 2020, 129, 108868. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, L.; Casal, S.; Pereira, J.A.; Saraiva, J.A.; Ramalhosa, E. Edible flowers: A review of the nutritional, antioxidant, antimicrobial properties and effects on human health. J. Food Compos. Anal. 2017, 60, 38–50. [Google Scholar] [CrossRef]
- Pires, T.C.; Barros, L.; Santos-Buelga, C.; Ferreira, I.C. Edible flowers: Emerging components in the diet. Trends Food Sci. Technol. 2019, 93, 244–258. [Google Scholar] [CrossRef]
- González-Barrio, R.; Periago, M.J.; Luna-Recio, C.; Garcia-Alonso, F.J.; Navarro-González, I. Chemical composition of the edible flowers, pansy (Viola wittrockiana) and snapdragon (Antirrhinum majus) as new sources of bioactive compounds. Food Chem. 2018, 252, 373–380. [Google Scholar] [CrossRef]
- Copetta, A.; Marchioni, I.; Ruffoni, B. The edible flowers from woody ornamental plants. In Proceedings of the IV International Symposium on Woody Ornamentals of the Temperate Zone 1331, Torino, Italy, 16 December 2021; pp. 195–204. [Google Scholar]
- Pires, E.D.O., Jr.; Di Gioia, F.; Rouphael, Y.; García-Caparrós, P.; Tzortzakis, N.; Ferreira, I.C.; Barros, L.; Petropoulos, S.A.; Caleja, C. Edible flowers as an emerging horticultural product: A review on sensorial properties, mineral and aroma profile. Trends Food Sci. Technol. 2023, 137, 31–54. [Google Scholar] [CrossRef]
- Brazil 2023 Abutilon in Flora e Funga do Brasil. Jardim Botânico do Rio de Janeiro. Available online: https://floradobrasil.jbrj.gov.br/FB106884 (accessed on 6 July 2023).
- Tropicos.org 2023 Callianthe megapotamica, Missouri Botanical Garden. Available online: https://tropicos.org/name/100397683 (accessed on 19 October 2023).
- Kew, 2023, Callianthe megapotamica, Royal Botanic Gardens. Available online: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:77146519-1 (accessed on 19 October 2023).
- Lim, T.K.; Lim, T.K. Abutilon megapotamicum. In Edible Medicinal and Non Medicinal Plants: Volume 8, Flowers; Springer: Berlin/Heidelberg, Germany, 2014; pp. 290–291. [Google Scholar]
- Donnell, A.A.; Ballard, H.E.; Cantino, P.D. Callianthe (Malvaceae): A new genus of neotropical Malveae. Syst. Bot. 2012, 37, 712–722. [Google Scholar] [CrossRef]
- Takeuchi, C.; Esteves, G.L. Synopsis of Abutilon (Malvoideae, Malvaceae) in the state of São Paulo, Brazil. Phytotaxa 2012, 44, 39–57. [Google Scholar] [CrossRef]
- Takeuchi 2023, Callianthe striata, in Flora e Funga do Brasil. Jardim Botânico do Rio de Janeiro. Available online: https://floradobrasil.jbrj.gov.br/xxxx (accessed on 6 July 2023).
- Corrêa, M.P. Dicionário das Plantas Úteis do Brasil e das Exóticas Cultivadas; Ministério da Agricultura IBDF: Rio de Janeiro, Brazil, 1984; Volume 1, p. 297. [Google Scholar]
- De Oliveira, M.F.; Vaz, L.M.D.C.; Rocha, M.D.M. Plantas Alimentícias Não Convencionais (PANC) no Parque Municipal Shangrilá (São Paulo). Rev. Bras. Meio Ambiente 2022, 10, 204–217. [Google Scholar]
- SANBI, South African National Biodiversity Institute, Oliver, R. Nemesia strumosa (Herb. Banks ex Benth.) Benth. (Scrophulariaceae). October 2012. Available online: http://pza.sanbi.org/nemesia-strumosa (accessed on 20 October 2023).
- Martino, L.D.; Roscigno, G.; Mancini, E.; Falco, E.D.; Feo, V.D. Chemical composition and antigerminative activity of the essential oils from five Salvia species. Molecules 2010, 15, 735–746. [Google Scholar] [CrossRef]
- Herrera-Ruiz, M.; García-Beltrán, Y.; Mora, S.; Díaz-Véliz, G.; Viana, G.S.; Tortoriello, J.; Ramírez, G. Antidepressant and anxiolytic effects of hydroalcoholic extract from Salvia elegans. J. Ethnopharmacol. 2006, 107, 53–58. [Google Scholar] [CrossRef]
- Mora, S.; Millán, R.; Lungenstrass, H.; Díaz-Véliz, G.; Morán, J.A.; Herrera-Ruiz, M.; Tortoriello, J. The hydroalcoholic extract of Salvia elegans induces anxiolytic-and antidepressant-like effects in rats. J. Ethnopharmacol. 2006, 106, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Ombra, M.N.; d’Acierno, A.; Nazzaro, F.; Fratianni, F. Health attributes of ten Mediterranean edible flowers: Anti-proliferative and enzyme-inhibitory properties. Trends Phytochem. Res. 2019, 3, 251–260. [Google Scholar]
- Araújo, S.; Matos, C.; Correia, E.; Antunes, M.C. Evaluation of phytochemicals content, antioxidant activity and mineral composition of selected edible flowers. Qual. Assur. Saf. Crops Foods. 2019, 11, 471–478. [Google Scholar] [CrossRef]
- Makino, T.; Ohno, T.; Iwbuchi, H. Aroma components of pineapple sage (Salvia elegans Vahl). Foods Food Ingred. J. 1996, 169, 121–124. [Google Scholar]
- Fernandes, L.; Saraiva, J.A.; Pereira, J.A.; Casal, S.; Ramalhosa, E. Post-harvest technologies applied to edible flowers: A review: Edible flowers preservation. Food Rev. Int. 2019, 35, 132–154. [Google Scholar] [CrossRef]
- Najar, B.; Marchioni, I.; Ruffoni, B.; Copetta, A.; Pistelli, L.; Pistelli, L. Volatilomic analysis of four edible flowers from Agastache genus. Molecules 2019, 24, 4480. [Google Scholar] [CrossRef]
- Jones, J.B.J.; Wolf, B.; Mills, H.A. Plant Analysis Handbook: A Practical Sampling, Preparation, Analysis, and Interpretation Guide; Micro-Macro Publishing, Inc.: Athens, GA, USA, 2021. [Google Scholar]
- Regulation (EU) No 1168/2011 of the European Parliament and of the Council of 25 October 2011 amending Council Regulation (EC) No 2007/2004 establishing a European Agency for the Management of Operational Cooperation at the External Borders of the Member States of the European Union. Off. J. Eur. Union 2011, 54, L 304.
- Rop, O.; Mlcek, J.; Jurikova, T.; Neugebauerova, J.; Vabkova, J. Edible flowers—A new promising source of mineral elements in human nutrition. Molecules 2012, 17, 6672–6683. [Google Scholar] [CrossRef]
- Ceccanti, C.; Brizzi, A.; Landi, M.; Incrocci, L.; Pardossi, A.; Guidi, L. Evaluation of major minerals and trace elements in wild and domesticated edible herbs traditionally used in the Mediterranean area. Biol. Trace Elem. Res. 2021, 199, 3553–3561. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Najar, B.; Nardi, V.; Cervelli, C.; Pistelli, L. Volatilome analyses of four South African Helichrysum spp. grown in Italy. Nat. Prod. Res. 2020, 36, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Viticult. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Kim, D.; Jeong, S.; Lee, C. Antioxidant capacity of phenolic phytochemicals from various cultivars of plums. Food Chem. 2003, 81, 321–326. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Giusti, M.; Wrolstad, R.E. Characterization and measurement of anthocyanins by UV-Visible Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, F1.2.1–F1.2.13. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Szôllôsi, R.; Szôllôsi Varga, I. Total antioxidant power in some species of Labiatae (adaptation of FRAP method). Acta Biol. Szeged. 2002, 46, 125–127. [Google Scholar]
- Degl’innocenti, E.; Guidi, L.; Pardossi, A.; Tognoni, F. Biochemical study of leaf browning in minimally processed leaves of lettuce (Lactuca sativa L. var. Acephala). J. Agric. Food Chem. 2005, 53, 9980–9984. [Google Scholar] [CrossRef]
- Kampfenkel, K.; Vanmontagu, M.; Inzé, D. Extraction and determination of ascorbate and dehydroascorbate from plant tissue. Anal. Biochem. 1995, 225, 165–167. [Google Scholar] [CrossRef]
- Li, P.; Gao, J.; Hu, H.; Luo, S.; Zhang, L. Postharvest senescence of fresh lotus pods and seeds is delayed by treatment with 1-methylcyclopropene. Ann. Appl. Biol. 2016, 169, 440–452. [Google Scholar] [CrossRef]
- Teixeira, R.S.S.; Da Silva, A.S.; Ferreira-Leitão, V.S.; Bon, E.P.D.S. Amino acids interference on the quantification of reducing sugars by the 3,5-dinitrosalicylic acid assay mislead carbohydrase activity measurements. Carbohydr. Res. 2012, 363, 33–37. [Google Scholar] [CrossRef]
- Marchioni, I.; Taglieri, I.; Dimita, R.; Ruffoni, B.; Zinnai, A.; Venturi, F.; Sanmartin, C.; Pistelli, L. Postharvest treatments on sensorial and biochemical characteristics of Begonia cucullata Willd edible flowers. Foods 2022, 11, 1481. [Google Scholar] [CrossRef] [PubMed]
- Guimaraes, R.; Barros, L.; Carvalho, A.M.; Ferreira, I.C. Studies on chemical constituents and bioactivity of Rosa micrantha: An alternative antioxidants source for food, pharmaceutical, or cosmetic applications. J. Agric. Food Chem. 2010, 58, 6277–6284. [Google Scholar] [CrossRef] [PubMed]
- Marchioni, I.; Najar, B.; Ruffoni, B.; Copetta, A.; Pistelli, L.; Pistelli, L. Bioactive compounds and aroma profile of some Lamiaceae edible flowers. Plants 2020, 9, 691. [Google Scholar] [CrossRef] [PubMed]
- Grzeszczuk, M.; Stefaniak, A.; Meller, E.; Wysocka, G. Mineral composition of some edible flowers. J. Elem. 2018, 23, 151–162. [Google Scholar] [CrossRef]
- Drava, G.; Iobbi, V.; Govaerts, R.; Minganti, V.; Copetta, A.; Ruffoni, B.; Bisio, A. Trace elements in edible flowers from Italy: Further insights into health benefits and risks to consumers. Molecules 2020, 25, 2891. [Google Scholar] [CrossRef]
- Mlcek, J.; Plaskova, A.; Jurikova, T.; Sochor, J.; Baron, M.; Ercisli, S. Chemical, nutritional and sensory characteristics of six ornamental edible flowers species. Foods 2021, 10, 2053. [Google Scholar] [CrossRef]
- Udensi, K.; Tchounwou, P.B. Potassium homeostasis, oxidative stress, and human disease. Int. J. Clin. Exp. Physiol. 2017, 4, 111. [Google Scholar]
- Fernandes, L.; Ramalhosa, E.; Pereira, J.A.; Saraiva, J.A.; Casal, S. The unexplored potential of edible flowers lipids. Agriculture 2018, 8, 146. [Google Scholar]
- Marchioni, I.; Dimita, R.; Gioe, G.; Pistelli, L.; Ruffoni, B.; Pistelli, L.; Najar, B. The effects of post-harvest treatments on the quality of Agastache aurantiaca edible flowers. Horticulturae 2021, 7, 83. [Google Scholar] [CrossRef]
- Tai, C.Y.; Chen, B.H. Analysis and stability of carotenoids in the flowers of daylily (Hemerocallis disticha) as affected by various treatments. J. Agric. Food Chem. 2000, 48, 5962–5968. [Google Scholar] [CrossRef] [PubMed]
- Siriamornpun, S.; Kaisoon, O.; Meeso, N. Changes in colour, antioxidant activities and carotenoids (lycopene, β-carotene, lutein) of marigold flower (Tagetes erecta L.) resulting from different drying processes. J. Funct. Foods 2012, 4, 757–766. [Google Scholar] [CrossRef]
- Shi, L.; Gu, Y.; Wu, D.; Wu, X.; Grierson, D.; Tu, Y.; Wu, Y. Hot air drying of tea flowers: Effect of experimental temperatures on drying kinetics, bioactive compounds and quality attributes. Int. J. Food Sci. Technol. 2019, 54, 526–535. [Google Scholar] [CrossRef]
- Mlcek, J.; Rop, O. Fresh edible flowers of ornamental plants—A new source of nutraceutical foods. Trends Food Sci. Technol. 2011, 22, 561–569. [Google Scholar] [CrossRef]
- Zhao, L.; Fan, H.; Zhang, M.; Chitrakar, B.; Bhandari, B.; Wang, B. Edible flowers: Review of flower processing and extraction of bioactive compounds by novel technologies. Food Res. Int. 2019, 126, 108660. [Google Scholar] [CrossRef]
- Marchioni, I.; Rodolfi, M.; Najar, B.; Ruffoni, B.; Machado, J.; Pistelli, L. Phytonutritional compounds and antioxidant activity of eight new edible flowers. Nat. Prod. Res. 2023, 1, 7. [Google Scholar] [CrossRef]
- Hazar, D.; Boyar, I.; Dincer, C.; Ertekin, C. Investigation of color and bioactive compounds of different color pansy (Viola × wittrockiana Gams.) dried in hot air dryer. Horticulturae 2023, 9, 186. [Google Scholar] [CrossRef]
- Aquino-Bolaños, E.N.; Urrutia-Hernández, T.A.; López Del Castillo-Lozano, M.; Chavéz-Servia, J.L.; Verdalet-Guzmán, I. Physicochemical parameters and antioxidant compounds in edible squash (Cucurbita pepo) flower stored under controlled atmospheres. J. Food Qual. 2013, 36, 302–308. [Google Scholar] [CrossRef]
- Revansiddaya, P.; Kalyani, B.; Veerangouda, A.; Shivkumar, H.; Santosh, P. Hepatoprotective and antioxidant role of flower extract of Abutilon indicum. Int. J. Pharm. Biol. Arch. 2011, 2, 541–545. [Google Scholar]
- Gomaa, A.A.R.; Samy, M.N.; Desoukey, S.Y.; Kamel, M.S. Phytochemistry and pharmacological activities of genus Abutilon: A review (1972–2015). J. Adv. Med. Pharm. Sci. 2018, 1, 56–74. [Google Scholar] [CrossRef]

| Mineral/Trace | C. megapotamica | C. striata | N. strumosa | S. elegans |
|---|---|---|---|---|
| Ca (g/Kg DW) | 42.0 ± 28.0 a | 61.0 ± 17.0 a | 40.0 ± 28.0 a | 35.0 ± 13.0 a |
| K (g/Kg DW) | 20.0 ± 1.0 a | 19.0 ± 1.0 a | 36.0 ± 5.0 a | 21.0 ± 1.0 a |
| Na (g/Kg DW) | 0.7 ± 0.7 a | 1.0 ± 0.2 a | 1.8 ± 1.4 a | 0.8 ± 0.4 a |
| Mg (g/Kg DW) | 3.3 ± 0.3 a | 2.4 ± 0.8 a | 2.9 ± 0.2 a | 1.4 ± 0.0 b |
| Cu (mg/Kg DW) | 4.0 ± 4.0 a | 5.2 ± 0.8 a | 2.4 ± 2.4 a | 1.5 ± 0.8 a |
| Fe (mg/Kg DW) | nd * | 5.0 ± 5.0 a | 13.0 ± 13.0 a | nd * |
| Mn (mg/Kg DW) | 0.1 ± 0.1 a | 13.0 ± 5.0 a | 9.4 ± 9.0 a | 6.2 ± 3.0 a |
| Zn (mg/Kg DW) | 0.1 ± 0.1 a | 6.0 ± 6.0 a | 14.0 ± 14.0 a | 4.0 ± 2.0 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machado, J.S.; Pieracci, Y.; Carmassi, G.; Ruffoni, B.; Copetta, A.; Pistelli, L. Effect of Drying Post-Harvest on the Nutritional Compounds of Edible Flowers. Horticulturae 2023, 9, 1248. https://doi.org/10.3390/horticulturae9111248
Machado JS, Pieracci Y, Carmassi G, Ruffoni B, Copetta A, Pistelli L. Effect of Drying Post-Harvest on the Nutritional Compounds of Edible Flowers. Horticulturae. 2023; 9(11):1248. https://doi.org/10.3390/horticulturae9111248
Chicago/Turabian StyleMachado, Jean Santos, Ylenia Pieracci, Giulia Carmassi, Barbara Ruffoni, Andrea Copetta, and Laura Pistelli. 2023. "Effect of Drying Post-Harvest on the Nutritional Compounds of Edible Flowers" Horticulturae 9, no. 11: 1248. https://doi.org/10.3390/horticulturae9111248
APA StyleMachado, J. S., Pieracci, Y., Carmassi, G., Ruffoni, B., Copetta, A., & Pistelli, L. (2023). Effect of Drying Post-Harvest on the Nutritional Compounds of Edible Flowers. Horticulturae, 9(11), 1248. https://doi.org/10.3390/horticulturae9111248









