Impact of Dry Processing on Secondary Metabolites in the Petals of Marigold (Tagetes spp.) Cultivar
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Plant Materials
2.2. Dry Processing
2.3. Determination of Individual Metabolites Using HPLC
2.3.1. Determination of Carotenoid by Reverse-Phase HPLC
2.3.2. Determination of Flavonoid by Reverse-Phase HPLC
2.4. Measurements of TFC and TPC
2.5. Measurements of Antioxidant Activities
2.6. Statistical Analysis
3. Results and Discussion
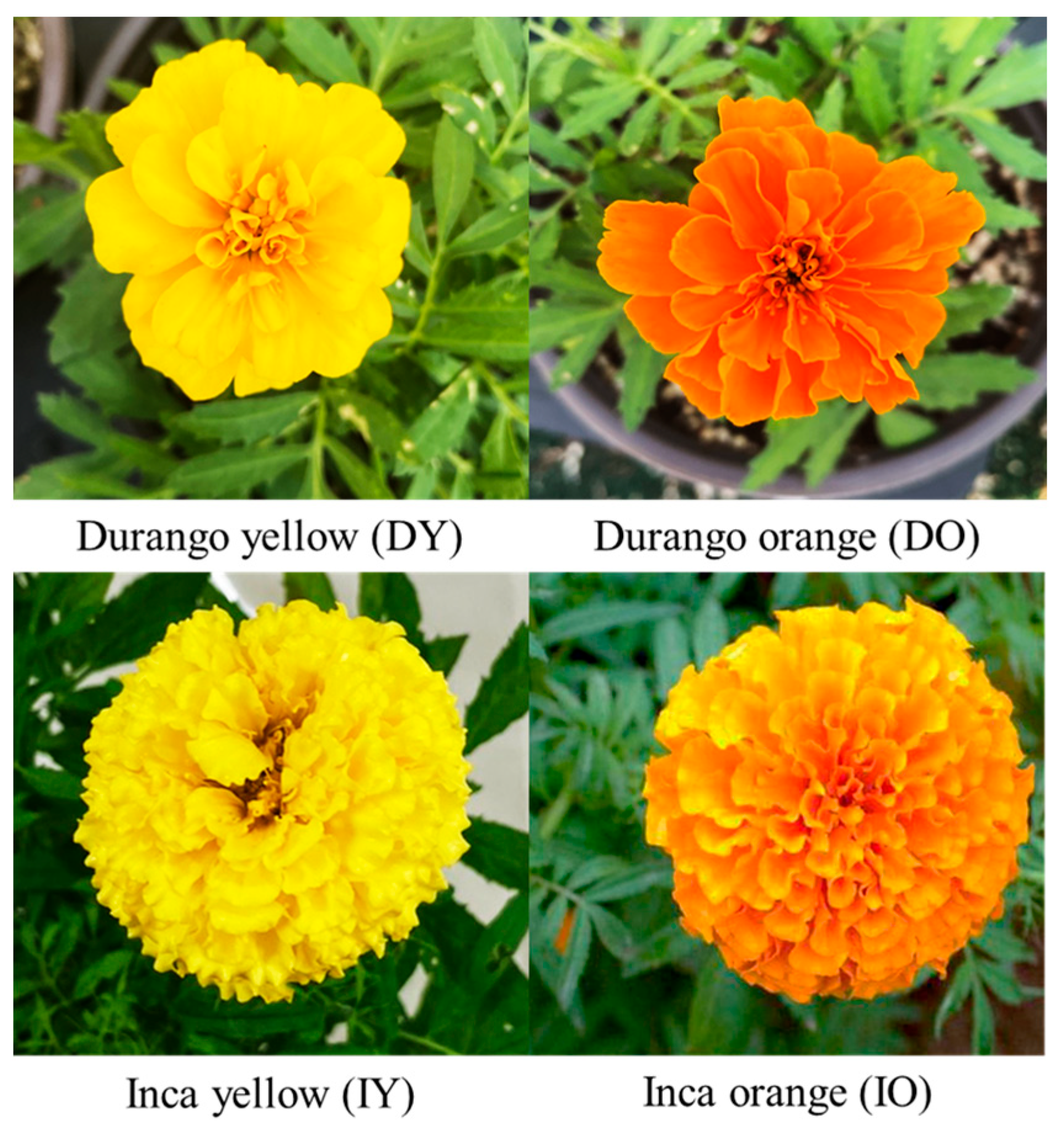
3.1. Morphological Appearances and Characteristics of Four Marigold Cultivars
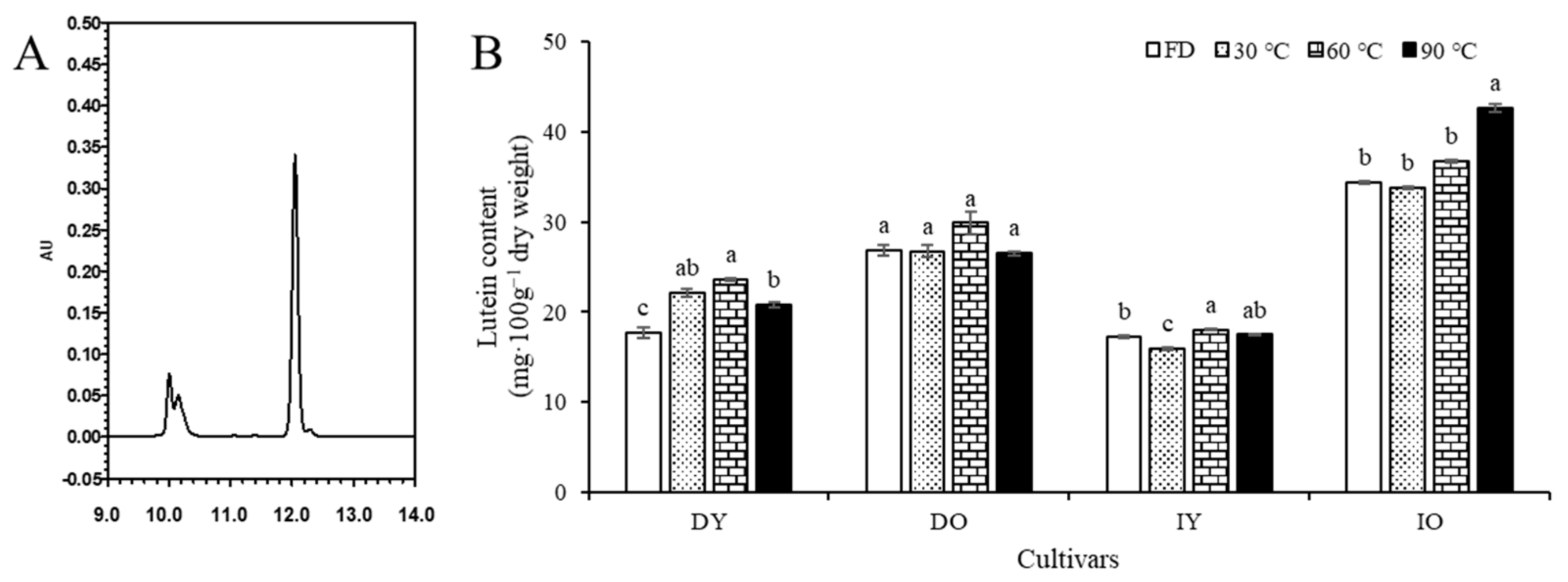
3.2. Individual Carotenoid Content
3.3. Individual Flavonoid Content
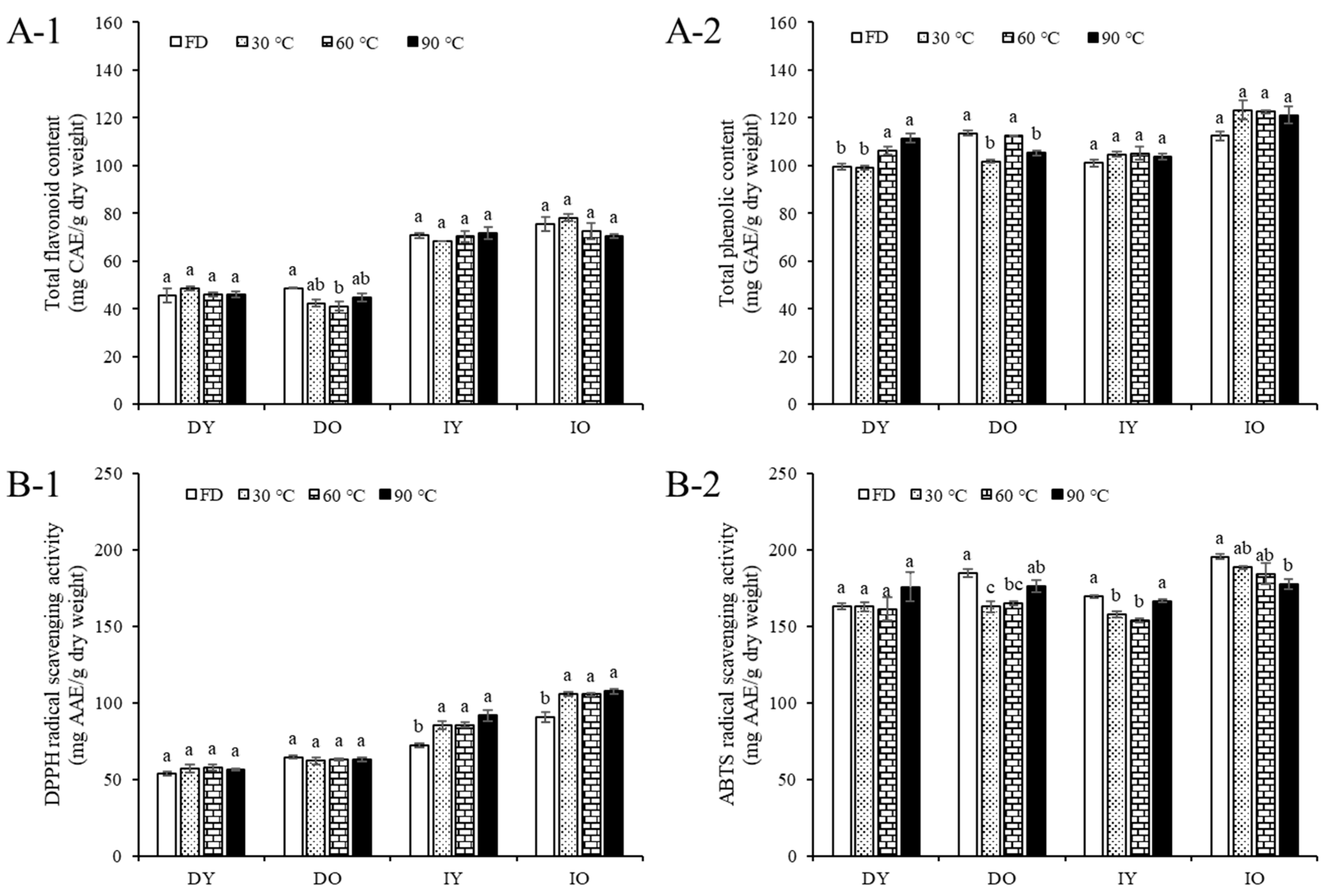
3.4. Total Flavonoids and Phenolics
3.5. Antioxidant Activities
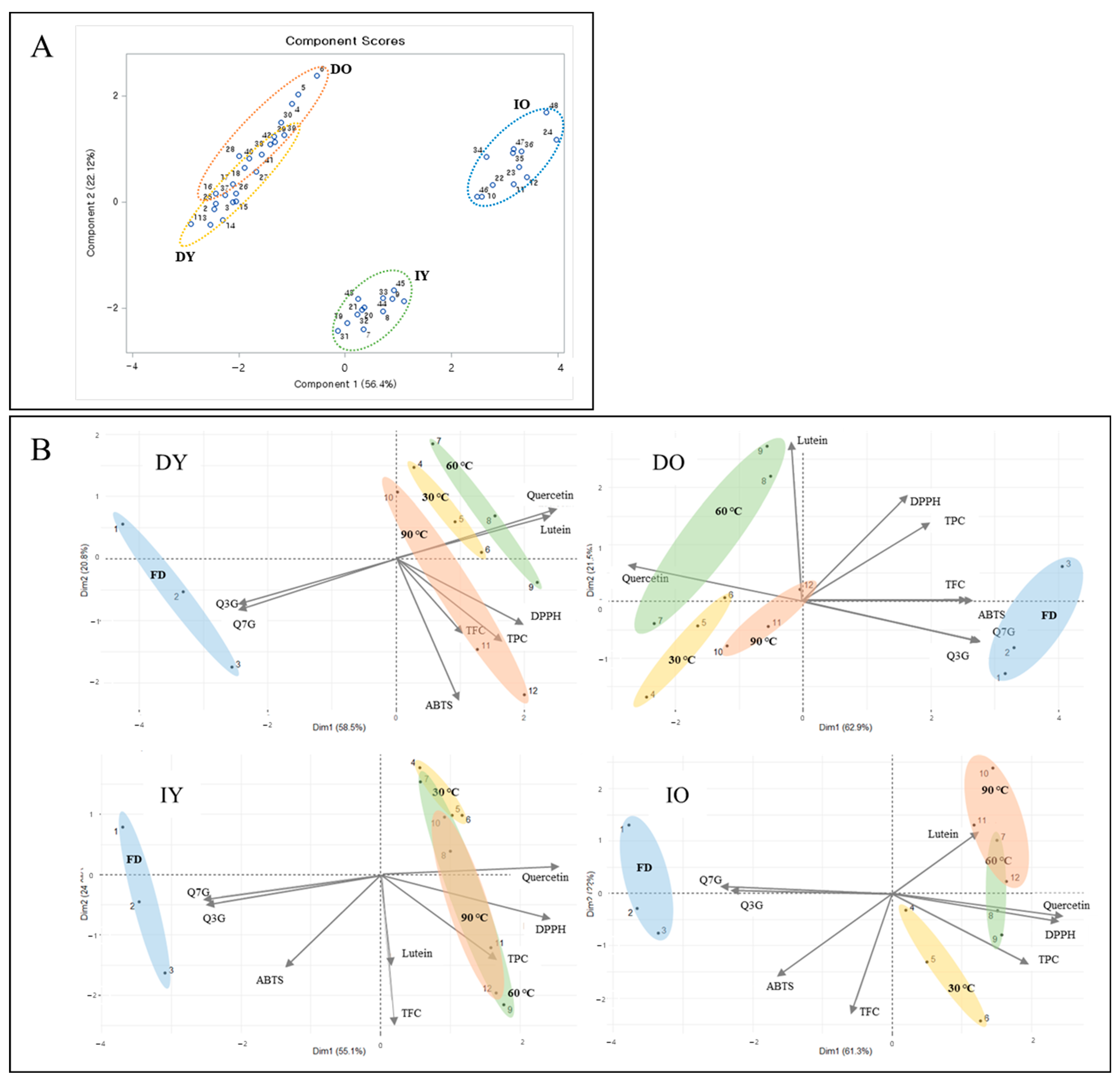
3.6. Correlation Analysis and PCA
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sowbhagya, H.B.; Sampathu, S.R.; Krishnamurthy, N. Natural colorant from marigold-chemistry and technology. Food Rev. Int. 2004, 20, 33–50. [Google Scholar] [CrossRef]
- Chitrakar, B.; Zhang, M.; Bhandari, B. Edible flowers with the common name “marigold”: Their therapeutic values and processing. Trends Food Sci. 2019, 89, 76–87. [Google Scholar] [CrossRef]
- Mlcek, J.; Plaskova, A.; Jurikova, T.; Sochor, J.; Baron, M.; Ercisli, S. Chemical, nutritional and sensory characteristics of six ornamental edible flowers species. Foods 2021, 10, 2053. [Google Scholar] [CrossRef] [PubMed]
- Dorozko, J.; Kunkulberga, D.; Sivicka, I.; Kruma, Z. The influence of various drying methods on the quality of edible flower petals. CABI 2019, 32, 182–187. [Google Scholar]
- Sowbhagya, H.B.; Sushma, S.B.; Rastogi, N.K.; Naidu, M.M. Effect of pretreatments on extraction of pigment from marigold flower. J. Food Sci. Technol. 2013, 50, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Sommano, S.; Suksathan, R.; Colelli, G.; Kumpuan, W. Potentials of Thai tropical edible flowers as fresh cut salad mix. ISHS 2016, 1194, 1187–1194. [Google Scholar] [CrossRef]
- Park, Y.J.; Park, S.Y.; Valan Arasu, M.; Al-Dhabi, N.A.; Ahn, H.G.; Kim, J.K.; Park, S.U. Accumulation of carotenoids and metabolic profiling in different cultivars of Tagetes flowers. Molecules 2017, 22, 313. [Google Scholar] [CrossRef] [PubMed]
- Sing, Y.; Gupta, A.; Kannojia, P. Tagetes erecta (Marigold)—A review on its phytochemical and medicinal properties. Curr. Med. Drugs Res. 2020, 4, 201. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Datta, S.; Mallick, B.; Dhar, P.; Ghosh, S. Lutein content and in vitro antioxidant activity of different cultivars of Indian marigold flower (Tagetes patula L.) extracts. J. Agric. Food Chem. 2010, 58, 8259–8264. [Google Scholar] [CrossRef]
- Calvo, M.M. Lutein: A valuable ingredient of fruit and vegetables. Crit. Rev. Food Sci. Nutr. 2005, 45, 671–696. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Roychowdhury, A.; Ghosh, S. Lutein content, fatty acid composition and enzymatic modification of lutein from marigold (Tagetes patula L.) flower petals. J. Indian Chem. Soc. 2008, 85, 942–944. [Google Scholar]
- Piccaglia, R.; Marotti, M.; Grandi, S. Lutein and lutein ester content in different types of Tagetes patula and T. erecta. Ind. Crops Prod. 1998, 8, 45–51. [Google Scholar] [CrossRef]
- Kim, J.H.; Duan, S.; Lim, Y.J.; Eom, S.H. Changes in quercetin derivatives and antioxidant activity in marigold petals (Tagetes patula L.) Induced by Ultraviolet-B irradiation and methyl jasmonate. Plants 2022, 11, 2947. [Google Scholar] [CrossRef] [PubMed]
- Akshaya, H.R.; Namita, N.; Singh, K.P.; Saha, S.; Panwar, S.; Bharadwaj, C. Determination and correlation of carotenoid pigments and their antioxidant activities in marigold (Tagetes sp.) flowers. Indian J. Agric. Sci. 2017, 87, 390–396. [Google Scholar] [CrossRef]
- Mir, R.A.; Ahanger, M.A.; Agarwal, R.M. Marigold: From mandap to medicine and from ornamentation to remediation. Am. J. Plant Sci. 2019, 10, 309. [Google Scholar] [CrossRef]
- Ratti, C. Hot air and freeze-drying of high-value foods: A review. J. Food Eng. 2001, 49, 311–319. [Google Scholar] [CrossRef]
- Kurniawan, J.M.; Yusuf, M.M.; Azmi, S.S.; Salim, K.P.; Utami Prihastyanti, M.N.; Indrawati, R.; Heriyanto; Shioi, Y.; Limantara, L.; Panintingjati Brotosudarmo, T.H. Effect of drying treatments on the contents of lutein and zeaxanthin in orange-and yellow-cultivars of marigold flower and its application for lutein ester encapsulation. IOP Conf. Ser. Mater. Sci. Eng. 2019, 509, 012060. [Google Scholar] [CrossRef]
- Zhao, L.; Fan, H.; Zhang, M.; Chitrakar, B.; Bhandari, B.; Wang, B. Edible flowers: Review of flower processing and extraction of bioactive compounds by novel technologies. Food Res. Int. 2019, 126, 108660. [Google Scholar] [CrossRef] [PubMed]
- Rathod, P.; Vala, M.; Maitreya, B. Drying techniques of some selected ornamental flowers—A reviews. EPRA Int. J. Res. Dev. (IJRD) 2021, 6, 180–186. [Google Scholar] [CrossRef]
- Ahluwalia, P.; Kaur, A.; Dhillon, G.K. Effect of different drying methods on chemical and functional properties of marigold petals. Int. J. Food Nutr. Sci. 2012, 3, 53–59. [Google Scholar]
- Buser, M.D.; Stone, M.L.; Brusewitz, G.H.; Maness, N.O.; Whitelock, D.P. Thin-layer drying of marigold flowers and flower components for petal removal. Trans. ASAE 1999, 42, 1367–1374. [Google Scholar] [CrossRef]
- Siriamornpun, S.; Kaisoon, O.; Meeso, N. Changes in colour, antioxidant activities and carotenoids (lycopene, β-carotene, lutein) of marigold flower (Tagetes erecta L.) resulting from different drying processes. J. Funct. Foods 2012, 4, 757–766. [Google Scholar] [CrossRef]
- Bhave, A.; Schulzová, V.; Mrnka, L.; Hajšlová, J. Influence of harvest date and postharvest treatment on carotenoid and flavonoid composition in French marigold flowers. J. Agric. Food Chem. 2020, 68, 7880–7889. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.J.; Eom, S.H. The different contributors to antioxidant activity in thermally dried flesh and peel of astringent persimmon fruit. Antioxidants 2022, 11, 597. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.J.; Kwon, S.J.; Qu, S.; Kim, D.G.; Eom, S.H. Antioxidant contributors in seed, seed coat, and cotyledon of γ-ray-induced soybean mutant lines with different seed coat colors. Antioxidants 2021, 10, 353. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, S.; Zhang, H.; Chen, X.; Liang, F.; Qin, H.; Zhang, Y.; Cong, R.; Xin, H.; Zhang, Z. Carotenoid metabolite and transcriptome dynamics underlying flower color in marigold (Tagetes erecta L.). Sci. Rep. 2020, 10, 16835. [Google Scholar] [CrossRef]
- Rubio-Diaz, D.E.; Santos, A.; Francis, D.M.; Rodriguez-Saona, L.E. Carotenoid stability during production and storage of tomato juice made from tomatoes with diverse pigment profiles measured by infrared spectroscopy. J. Agric. Food Chem. 2010, 58, 8692–8698. [Google Scholar] [CrossRef]
- Muliterno, M.M.; Rodrigues, D.; de Lima, F.S.; Ida, E.I.; Kurozawa, L.E. Conversion/degradation of isoflavones and color alterations during the drying of okara. LWT 2017, 75, 512–519. [Google Scholar] [CrossRef]
- Chaaban, H.; Ioannou, I.; Chebil, L.; Slimane, M.; Gérardin, C.; Paris, C.; Charbonnel, C.; Chekir, L.; Ghoul, M. Effect of heat processing on thermal stability and antioxidant activity of six flavonoids. J. Food Process. Preserv. 2017, 41, e13203. [Google Scholar] [CrossRef]
- Ozdemir, M.; Gungor, V.; Melikoglu, M.; Aydiner, C. Solvent selection and effect of extraction conditions on ultrasound-assisted extraction of phenolic compounds from galangal (Alpinia officinarum). J. Appl. Res. Med. 2024, 38, 100525. [Google Scholar] [CrossRef]
- Kim, J.H.; Duan, S.; Park, Y.R.; Eom, S.H. Tissue-specific antioxidant activities of germinated seeds in lentil cultivars during thermal processing. Antioxidants 2023, 12, 670. [Google Scholar] [CrossRef]
- Elfalleh, W.; Nasri, N.; Marzougui, N.; Thabti, I.; M’rabet, A.; Yahya, Y.; Lachiheb, B.; Guasmi, F.; Ferchichi, A. Physico-chemical properties and DPPH-ABTS scavenging activity of some local pomegranate (Punica granatum) ecotypes. Int. J. Food Sci. Nutr. 2009, 60, 197–210. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, Y.F.; Zhou, Z.Q. Phenolic and flavonoid contents of mandarin (Citrus reticulata Blanco) fruit tissues and their antioxidant capacity as evaluated by DPPH and ABTS methods. J. Integr. Agric. 2018, 17, 256–263. [Google Scholar] [CrossRef]

| Antioxidants | Cultivar | ||||
|---|---|---|---|---|---|
| DY | DO | IY | IO | ||
| Carotenoid | Lutein | 17.70 ± 0.63 c | 26.84 ± 0.63 b | 17.20 ± 0.17 c | 31.45 ± 0.15 a |
| Flavonoid | Q7G | 7.65 ± 0.04 d | 14.44 ± 0.03 c | 140.93 ± 0.69 b | 145.80 ± 0.49 a |
| Q3G | 69.40 ± 0.52 b | 102.44 ± 0.05 a | 20.82 ± 0.19 c | 14.57 ± 0.11 d | |
| Quercetin | 71.19 ± 0.07 a | 56.07 ± 0.22 b | 0.59 ± 0.03 d | 1.19 ± 0.25 c | |
| Total flavonoids | 45.73 ± 3.01 b | 48.63 ± 0.19 b | 70.81 ± 0.98 a | 75.60 ± 3.00 a | |
| Total phenolics | 99.48 ± 1.19 b | 113.66 ± 0.92 a | 101.16 ± 1.51 b | 112.44 ± 1.87 a | |
| Antioxidant activity | DPPH | 53.40 ± 1.30 d | 64.28 ± 1.01 c | 72.13 ± 1.28 b | 90.60 ± 3.51 a |
| ABTS | 163.08 ± 2.10 c | 184.57 ± 2.47 b | 169.33 ± 0.87 c | 195.50 ± 1.67 a | |
| Cultivars | Variables | DPPH | ABTS | TPC | TFC | Lutein | Q7G | Q3G | Quercetin |
|---|---|---|---|---|---|---|---|---|---|
| DY | DPPH | 1 | |||||||
| ABTS | 0.48 ns | 1 | |||||||
| TPC | 0.48 ns | 0.73 * | 1 | ||||||
| TFC | 0.63 * | 0.31 ns | 0.04 ns | 1 | |||||
| Lutein | 0.68 * | 0.00 ns | 0.36 ns | 0.37 ns | 1 | ||||
| Q7G | −0.50 ns | −0.13 ns | −0.44 ns | −0.16 ns | −0.86 ** | 1 | |||
| Q3G | −0.52 ns | −0.16 ns | −0.48 ns | −0.15 ns | −0.86 ** | 1.00 *** | 1 | ||
| Quercetin | 0.55 ns | 0.12 ns | 0.45 ns | 0.18 ns | 0.90 *** | −0.99 *** | −0.99 *** | 1 | |
| DO | DPPH | 1 | |||||||
| ABTS | 0.60 * | 1 | |||||||
| TPC | 0.43 ns | 0.51 ns | 1 | ||||||
| TFC | 0.67 * | 0.91 *** | 0.38 ns | 1 | |||||
| Lutein | 0.48 ns | −0.11 ns | 0.49 ns | −0.12 ns | 1 | ||||
| Q7G | 0.31 ns | 0.78 ** | 0.60 * | 0.74 * | −0.23 ns | 1 | |||
| Q3G | 0.31 ns | 0.76 ** | 0.59 * | 0.73 * | −0.23 ns | 1.00 *** | 1 | ||
| Quercetin | −0.30 ns | −0.77 ** | −0.64 * | −0.71 * | 0.21 ns | −1.00 *** | −0.99 *** | 1 | |
| IY | DPPH | 1 | |||||||
| ABTS | −0.2 ns | 1 | |||||||
| TPC | 0.69 * | −0.13 ns | 1 | ||||||
| TFC | 0.32 ns | 0.48 ns | 0.55 ns | 1 | |||||
| Lutein | 0.14 ns | 0.07 ns | 0.13 ns | 0.42 ns | 1 | ||||
| Q7G | −0.89 *** | 0.53 ns | −0.46 ns | 0.07 ns | 0.04 ns | 1 | |||
| Q3G | −0.88 ** | 0.55 ns | −0.46 ns | 0.40 ns | 0.11 ns | 1.00 *** | 1 | ||
| Quercetin | 0.91 *** | −0.53 ns | 0.50 ns | 0.00 ns | 0.13 ns | −0.98 *** | −0.97 *** | 1 | |
| IO | DPPH | 1 | |||||||
| ABTS | −0.51 ns | 1 | |||||||
| TPC | 0.89 ** | −0.10 ns | 1 | ||||||
| TFC | 0.03 ns | 0.61 * | 0.25 ns | 1 | |||||
| Lutein | 0.51 ns | −0.51 ns | 0.30 ns | −0.42 ns | 1 | ||||
| Q7G | −0.85 ** | 0.59 * | −0.70 * | 0.20 ns | −0.30 ns | 1 | |||
| Q3G | −0.76 ** | 0.57 ns | −0.61 * | 0.25 ns | −0.20 ns | 0.99 *** | 1 | ||
| Quercetin | 0.92 *** | −0.54 ns | 0.78 ** | −0.07 ns | 0.31 ns | −0.98 *** | −0.95 *** | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.H.; Lim, Y.J.; Kim, J.-H.; Eom, S.H. Impact of Dry Processing on Secondary Metabolites in the Petals of Marigold (Tagetes spp.) Cultivar. Horticulturae 2024, 10, 382. https://doi.org/10.3390/horticulturae10040382
Kim JH, Lim YJ, Kim J-H, Eom SH. Impact of Dry Processing on Secondary Metabolites in the Petals of Marigold (Tagetes spp.) Cultivar. Horticulturae. 2024; 10(4):382. https://doi.org/10.3390/horticulturae10040382
Chicago/Turabian StyleKim, Ji Hye, You Jin Lim, Jae-Hee Kim, and Seok Hyun Eom. 2024. "Impact of Dry Processing on Secondary Metabolites in the Petals of Marigold (Tagetes spp.) Cultivar" Horticulturae 10, no. 4: 382. https://doi.org/10.3390/horticulturae10040382
APA StyleKim, J. H., Lim, Y. J., Kim, J.-H., & Eom, S. H. (2024). Impact of Dry Processing on Secondary Metabolites in the Petals of Marigold (Tagetes spp.) Cultivar. Horticulturae, 10(4), 382. https://doi.org/10.3390/horticulturae10040382






