Abstract
Cannabis sativa L., subsp. ruderalis Janish., ‘Finola’ is a dioecious cultivar of Finnish origin. This cultivar is very interesting because its cultivation cycle lasts less than 3 months. The aim of this study was to define an efficient micropropagation protocol to ensure in vitro multiplication and rooting and in vivo acclimatization. Two different explant sources were tested: seed-derived in vitro explants and nodal segments containing axillary buds from selected mother plants. Shoot proliferation was tested on different growth media enriched with cytokinin alone or cytokinin in combination with auxins. Among all combinations, the best results were obtained by combining the Basal Medium (BM—a Murashige and Skoog modified medium) with sucrose (20 g L−1), thidiazuron (TDZ 0.4 mg L−1), and 1-naphthalenacetic acid (NAA 0.2 mg L−1). Regarding rooting induction, the plants developed an extensive root system under red/blue lights on BM enriched with sucrose (30 g L−1) and indol-3 butyric acid (0.1 mg L−1), which allowed the survival of more than 90 percent of the plantlets once transplanted into the climate-controlled greenhouse.
1. Introduction
In recent years, there has been renewed interest in hemp (Cannabis sativa L., genus Cannabis family Cannabaceae), a multipurpose crop cultivated in many parts of the world for its nutritional, medicinal, and industrial uses [1,2,3,4].
The taxonomic determinations of Cannabis genus have been deeply studied and discussed. Based on genetic evaluations, Cannabis sativa can be subdivided into three subspecies: sativa, indica, and ruderalis [5]. C. sativa subsp. ruderalis is a lesser-known hemp that originated in regions with harsh climates, such as Russia and Eastern Europe. This subspecies is typically smaller in size and contains very low Δ9-tetrahydrocannabinol (THC), the psychoactive compound in the plant, but is often rich in CBD (cannabidiol, a non-intoxicating compound), known for its potential therapeutic benefits [6]. Furthermore, Ruderalis strains are auto-flowering, which means that they are photoperiod-insensitive. For this reason, the transition from the vegetative stage to the flowering stage is based on the plant’s age, rather than relying on light cycles [7]. Conversely, the cultivation cycle of the other subspecies of Cannabis sativa is typically photo-dependent, strongly linked to seasonal trends and, consequently, influenced by the number of hours of light and darkness per day [5].
The above-mentioned characteristics of ruderalis make vegetative reproduction difficult, while seed propagation can lead to uneven growth and maturity because the flowering process can vary among individual plants. Indeed, because auto-flowering strains containing ruderalis genetics are renowned for their ability to flower without strict light schedules [8], some plants may initiate flowering earlier or later than desired. This factor can be challenging for growers seeking a consistent and synchronized harvest [9]. Belonging to ruderalis, ‘Finola’ is a hemp cultivar of Finnish origin, characterized by high cannabidiol (CBD) and low Δ9-tetrahydrocannabinol (THC) contents. This cultivar is dioecious, independent of the photoperiod, and interesting due to its very short cycle of fewer than 3 months, with early sowing and flowering needed. This cultivar is characterized by its low branching, small size, small seeds, and low biomass recovery [10]. Indeed, ‘Finola’ has high potential at a commercial level and could be used in super-intensive production models of protected environments, allowing significant water savings [11].
To obtain uniform plant production, the micropropagation technique could be a useful tool. Indeed, in vitro propagation has great potential in rapidly producing many true to type clonal and high-quality plants using synthetic growth media in a controlled environment [12,13,14,15]. Micropropagation requires few selected mother plants as explant sources to initiate an in vitro culture [15]. Furthermore, the in vitro plants are grown in reduced spaces under sterile conditions to avoid the transmission of pests, diseases, and pathogens and the influence of weather or soil [16,17]. Due to all these advantages, in recent years, several researchers have developed different protocols to define the in vitro cultures of C. sativa subspecies sativa and indica derived from the leaves, axillary nodes, cotyledons, shoot tips, and epicotyls of explants with the aim to obtain a high multiplication rate, produce disease-free plants, select elite clones, and overcome the limits of heterozygosity from cross-pollination [15,18,19,20,21]. To the best of our knowledge, only two studies focused on the in vitro culture of the subspecies ruderalis. These studies underline the low branching tendency, strong apical dominance [22,23], and influence of the genotype on the explant’s behavior and multiplication rate. The difficulties of in vitro growth and the maintenance of a vegetative state may be due to auto-flowering varieties and photoperiod independence [11,24]. These factors could also be other limits for the micropropagation of this subspecies. Therefore, the main challenge in developing an effective micropropagation protocol is the specificity of species, varieties, and genotypes, resulting in different responses in vitro depending on the explant, nutrient media, and growth regulators, such as the culture conditions [21].
Considering the above-mentioned information, the focus of this experimental research was to develop the most suitable protocol for the in vitro direct propagation of C. sativa L., subsp. ruderalis Janish., cultivar ‘Finola’.
2. Materials and Methods
2.1. Plant Materials and Explant Source of C. sativa L., subsp. ruderalis Janish., Cultivar ‘Finola’
This research began with certified ‘Finola’ seeds. The seeds were sown directly in vitro or under controlled conditions to obtain mother plants. The nodes of the seedlings were then excised to initiate micropropagation via axillary shoots.
2.1.1. In Vitro Germinated Seeds
After initial rinsing under running water to remove impurities, the effects of the sterilant and immersion times on the surface sterilization of ‘Finola’ seeds were evaluated based on the following two treatments:
- (a)
- Sodium hypochlorite (NaClO with 14% active chlorine) at 1.4% for 20 min;
- (b)
- Mercuric chloride (HgCl) at 0.1% for 15 min.
One-hundred seeds in each treatment were then rinsed five times in sterile distilled water. For the in vitro sowing, 50 mL glass tubes containing 10 mL of the nutrient medium (BM [25]: Murashige and Skoog (MS) macro nutrients [26], Nitsch and Nitsch (NN) micronutrients [27], FeNaEDTA (40 mg L−1), and thiamine HCl (0.4 mg L−1)), adding agar (7 g L−1) as a gelling agent, were used. The pH of the medium was adjusted to 5.6–5.8 before autoclaving. The tubes were maintained in a growth chamber equipped with broad-spectrum LED lights between 400 and 700 nm and a light intensity of 50 µm s−1 m−2. The photoperiod was set to 16 h/8 h (day/night–d/n) and the temperature to 22 ± 1 °C.
2.1.2. Mother Plant Production
Thirty seeds were first soaked in distilled water for 48 h at a temperature of 22 ± 1 °C in total darkness. Then, seeds showing open seed coats and the emergence of coleorhiza (root sheath) were sown in polystyrene trays filled with a substrate of pH 5.5–6.5 consisting of a mix of blond peat (fraction 20–40 mm), German black peat (fraction 0–15 mm), and perlite (3:1 v/v). Once the seedlings had emerged, and the first pair of true leaves were grown, the plants were transplanted into pots 9 cm in diameter and 11 cm deep, which were filled with peat and expanded clay and perlite (3:1:1).
Ten selected mother plants were grown in a climate-controlled greenhouse, and the same number of plants was grown in a growth cabinet to compare their behaviors and growth responses under two different conditions. Starting from these plants, five mother plants were selected to collect the nodal buds. The controlled greenhouse, located at the Department of Soil, Plant, and Food Sciences (DiSSPA) (University of Bari Aldo Moro, Bari, Italy), had a temperature control system maintained between 18 °C and 25 °C, with a 15 h/9 h (d/n) photoperiod of natural light per day (in Summer) and relative humidity of 85%. On the other hand, the thermostat-controlled cabinet (F-Cell 707 comfort Blue-Line—MMM Group) featured illumination using white LED lights (VIS LED light Cool White), with a spectrum between 400 nm and 700 nm at an intensity of 80 µm s−1 m−2; the photoperiod set to 24 h/0 h (d/n), and a temperature of 22 ± 1 °C was used.
The growth of the seedlings was monitored to record the response to the growth conditions up to the time of the appearance of flower buds in order to define the time of the end of growth and determine the best method of cultivation to obtain a high number of axillary buds per female plant.
2.2. In Vitro Multiplication
One month after the seeds were planted in test tubes, flower buds appeared at the apex of the seedlings. The nodal buds below the apex of female plants were taken and immediately transferred to jars containing 30 mL of BM culture medium, solidified with agar (7 g L−1) ((Agar No. 1) Oxoid Agar Bacteriological), with the addition of sucrose (20 g L−1) and growth phytoregulators of the cytokinin group to stimulate multiplication. The cytokinins used were Metatopolin (MT) [6-(3-hydroxybenzylaminopurine) at a concentration of 0.5 mg L−1 and 6-Benzylaminopurine (BAP) at a concentration of 0.05 mg L−1. The test was carried out on 80 jars for each cytokinin, with a total of 160 jars containing a single bud. After 21 days, the first subculture was performed, maintaining the same starting cytokinins with equal concentrations.
For the mother plants, when the first female flower buds appeared, the nodal buds below (1 cm in length), that were not yet induced to flower, were excised and sterilized with sodium hypochlorite (NaClO, active chlorine 14%) at 1.4% for 20 min following extensive washing with sterile-distilled water to remove residues of the sterilizing agent. The nodal buds were then placed in 220 mL glass jars containing 30 mL of nutrient media and the growth regulators reported below to enable the in vitro introduction and subsequent induction of multiplication.
Two different growth media were tested:
- (1)
- BM [25], regularly used at the Laboratory of Micropropagation and Microscopy, DiSSPA, University of Bari Aldo Moro (Bari, Italy), for routine culture produced by stocks.
- (2)
- Driver and Kuniyuki (DKW) (1984) [28].
Both media, agarized with 7 g L−1 Agar (No. 1 Oxoid Agar Bacteriological), were enriched with sucrose (20 g L−1).
The tested growth regulators were selected based on several studies exploring in vitro cultures of Cannabis [29,30,31,32] or based on the best results of previous research on other subspecies carried out at the Laboratory of Micropropagation and Microscopy, DiSSPA, University of Bari Aldo Moro (unpublished data):
- -
- Benzylaminopurine (BAP) (0.05 mg L−1);
- -
- Metatopoline (MT) (0.5 mg L−1);
- -
- Thidiazuron (TDZ) (0.4 mg L−1) + 1 naphthalenacetic acid (NAA) (0.2 mg L−1);
- -
- Thidiazuron (TDZ) (0.4 mg L−1) + 2,3,5- Triiodobenzoic acid (TIBA 1 g L−1).
For each growth medium, 80 buds were considered, with 20 buds for each of the four growth regulators tested. When miniaturization of the shoots was recorded (for BAP, MT, and TDZ in combination with TIBA), with lengths less than 2 cm and shortened internodes, the shoots were subjected to an elongation phase by adding gibberellic acid (GA3 0.25 mg L−1) to achieve greater extension between nodes and increased leaf growth. The pH level was adjusted to 5.7 prior to autoclaving at 121 °C for 20 min.
Three subsequent subcultures (lasting three weeks) were performed on the same growth media, and several parameters (number of axillary buds that formed shoots, shoot length, and number of nodes per each shoot) were recorded for each subculture. In each subculture, the apical bud was always removed to reduce apical dominance, promote lateral shoots as much as possible, and delay flowering. During the trial, the explants were maintained in a growth chamber at 22 ± 1 °C, with a photoperiod of 16 h/8 h (d/n).
2.3. In Vitro Rooting
Around 200 shoots that had reached a minimum length of 3 cm were transplanted to the agarized BM supplemented with sucrose (30 g L−1) and indol-3-butyric acid (IBA) at two different concentrations (0.5 mg L−1 and 0.1 mg L−1) to induce rooting. The pH level was adjusted to 5.7 prior to autoclaving at 121 °C for 20 min.
To evaluate the influence of different kinds and combinations of light on the photomorphogenic and physiological responses in the last in vitro step, 100 plantlets for each concentration of IBA were divided under two different light conditions. The first light condition used white (Osram Lumilux De Luxe T8 36 W/954)/pink (Osram Fluora T8 36 W/77) light (LEDs with a broad spectrum between 400 and 700 nm, a white/pink ratio of 2:1, and a light intensity of 50 µm s−1 m−2), as white light is traditionally used as the light source in micropropagation, while pink light enhances plant growth, increasing fresh and dry weights compared to monochromatic light [33]. The second light condition used in the growth cabinet (F-Cell 707 comfort Blue-MMM-Group) was red/blue light (VQ-GLSF0014 W 1:3) LEDs with a wavelength of 700 nm and 400 nm. LEDs with a red/blue ratio of 3:1 at an intensity of 50 µm s−1 m−2 were used for the possible positive induction of root formation [34]. For both groups, the photoperiod was a 16 h/8 h d/n cycle.
After 20 days, the effects of the different light types and phytoregulators on the rooting rate, in terms of root percentage, and the average length and number of roots per shoot were evaluated.
2.4. Ex Vitro Acclimatization
Approximately 50 rooted microplants were removed from the in vitro containers. Then, the roots were gently rinsed in distilled water and the microplants were transplanted for acclimatization in organic Jiffypots® square planting pots (Ø 8 cm2) filled with sterile peat (46% organic carbon, 1–2% organic nitrogen, and 80% organic matter, pH 6.5) and perlite (2:1, v/v ratio) and covered with transparent plastic. Acclimatization conditions in the greenhouse were 18–25 °C and humidity that was reduced from 85–90% to 50–60% over 20 days. At this time, the survival of the plants was also evaluated.
2.5. Statistical Analysis
Data were subjected to an analysis of variance (ANOVA) test using the CoStat software, Version 6.40. The means of the different treatments were compared to each other in terms of significance using the Student Newman Keuls (SNK) Test (p ≤ 0.001). Before the ANOVA analysis, the percentage data were subjected to angular transformation. All the experiments were repeated three times.
3. Results
3.1. Germination of Seeds In Vitro
The sterilization test, carried out on a sample of 100 seeds for each sterilizing agent, demonstrated that exposure to sodium hypochlorite (with 14% active chlorine) at a concentration of 1.4% for 20 min was able to obtain highly contamination-free vital seeds. The percentage of in vitro germinated seeds, not contaminated seeds, was 80%. Among those seeds, only 42% were female, as identified visually by the inflorescence sex as soon as flower buds appeared. Thirty days after in vitro sowing, the morphological parameters measured on ‘Finola’ female seedlings indicated good development of the shoot length (5.3 cm on average), with the shoots containing about 3 nodes (3.1 on average). The shoots were then excised and transferred to a culture medium (BM) enriched with BAP and MT to induce multiplication.
The growth and multiplication responses of the shoots of ‘Finola’ in the presence of the two different phytoregulators tested were recorded after 21 days (Table 1) (Figure 1). The results showed no significant differences in the parameters evaluated, although the data were higher after adding MT to the growth medium for both shoot length (5.8 cm vs. 2.8 cm) and the number of nodes (5 vs. 4) compared to the average multiplication index (5 vs. 3) (Table 1). When the development parameters were recorded, the sufficiently grown shoots were subcultured for the first time to increase the number of shoots obtained from each explant. After a further 21 days from the start of the first subculture, although the growth conditions remained identical, all explants responded to the second cut with completely arrested development (Figure 1b), showing necrosis of the apices, diffuse hyperhidricity, or early flowering.

Table 1.
Influence of different phytoregulators on morphological parameters and mean multiplication index (MMI) measured on ‘Finola’ shoots grown from nodal buds of seedlings 21 days after the multiplication induction.
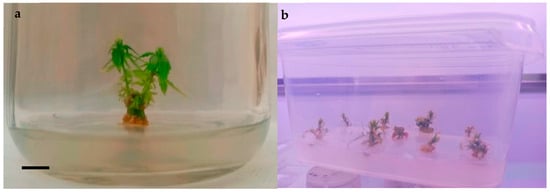
Figure 1.
‘Finola’ shoots multiplied on BM enriched with BAP, scale bar 1 cm (a); decline in the subcultures of ‘Finola’ shoots developed from nodal buds of seedlings sowed directly in vitro (b).
3.2. Mother Plant Production and Micropropagation by Axillary Buds
Table 2 presents the values of the different growth parameters measured under the two different conditions of mother plant development at the time of the first flower’s appearance.

Table 2.
Influence of different growing conditions on the growth of the stem and appearance of female and male flowers in mother plants of ‘Finola’ cultivar.
Plants obtained in the growth cabinet showed significant differences (Table 2, Figure 2) in vigor compared to the vigor of plants obtained in the climate-controlled greenhouse. The length was more than two times greater, and the stem diameter was almost two times greater, for the mother plants obtained in the growth cabinet. To complete the cycle, the time required for the appearance of female flowers differed by only three days between the two growth conditions (32.2 days in the climate-controlled greenhouse vs. 35.6 days in the growth cabinet) and was not statistically significant. Moreover, the nodal buds from the mother plants (Figure 3) grown in the climate-controlled greenhouse did not readily respond to the in vitro conditions due to both their smaller sizes and the difficulty in producing pathogen-free plants. Indeed, the plants required a long period of sterilization, which, due to their slender size, caused burning, oxidation, and arrested the development of excise materials once inoculated in the culture media chosen for multiplication. On the other hand, by reducing the sterilization time, the explants presented widespread contaminants. For this reason, the aseptic culture was initiated considering only nodal buds cut from the mother plants grown inside the growth cabinet.

Figure 2.
Mother plant in climate-controlled greenhouse, scale bar 10 cm (a); mother plant in growth cabinet, scale bar 10 cm (b).
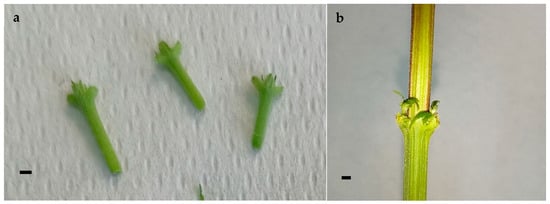
Figure 3.
Nodal segments containing axillary buds excised from mother plants grown in climate-controlled greenhouse, scale bar 2 mm (a); nodal segments containing axillary buds from mother plants grown in growth cabinet, scale bar 2 mm (b).
Table 3 shows the effects of nutrient media and different phytoregulators on in vitro shoots of ‘Finola’. A comparison between the two nutrient media tested, the Basal Medium and Driver and Kuniyuki (main effect NM), indicated no significant differences in the influence of media on the measured parameters, even if the very high presence of hyperhydrated explants (90%) grown on DKW fully limited the use of this nutrient medium. Considering the phytoregulator’s main effects, the best results for the growth and multiplication index were obtained from the shoots grown using a combination of TDZ and NAA, in terms of length, the number of nodes, and the mean multiplication index. All these data were confirmed and found to increase based on an analysis of the following interaction: BM x TDZ + NAA (3.4 cm, 3.1, and 3.0, respectively, for the length, number of nodes, and number of shoots per explant) (Figure 4). No significant increase in shoot length was obtained by adding gibberellic acid (GA3) to the nutrient media enriched with TDZ and TIBA, BAP, or MT.

Table 3.
Effect of nutrient media and different phytoregulators on growth, mean multiplication index, and hyperhidricity of ‘Finola’ shoots, measured 21 days after the beginning of in vitro culture.

Figure 4.
‘Finola’ shoot proliferation induced by TDZ and NAA, scale bar 1 cm.
3.3. Results for In Vitro Rooting and Ex Vitro Acclimatization
After 20 days, the effects of different types of light and two different concentrations of IBA on the rooting induction were evaluated (Table 4). The best results were obtained under red/blue lights using IBA at a lower concentration (Figure 5), whereas no rooting was obtained under white/pink lights. Once transplanted into the climate-controlled greenhouse, the plantlets showed a survival rate of over 90 percent (Figure 6).

Table 4.
Effect of lights and different concentrations of IBA on the induction of rooting on ‘Finola’ shoots detected after 20 days.

Figure 5.
Rooted shoot of ‘Finola’ obtained under red/blue light, using IBA at the lower concentration (0.1 mg L−1), scale bar 1 cm.

Figure 6.
Detail of rooted propagule of ‘Finola’ shoots obtained on MB × TDZ × NAA, scale bar 1 cm (a); plantlet during acclimatization phase, scale bar 1 cm (b); plant from in vitro culture, fully adapted to live conditions, scale bar 8 cm (c).
4. Discussion
Several authors underscored strong cultivar-specific responses to treatments in Cannabis tissue culture [35,36,37]. In this study, to define the most suitable protocol for the micropropagation of C. sativa L. subsp. ruderalis Janish., cv. ‘Finola’, different experimental procedures were tested. The first step of this experimental research was to select the most suitable explant to establish an aseptic culture. The next step involved drafting a functional in vitro propagation protocol while exploring the choice of nutrient substrates with different known combinations of nutrients and growth regulators, Finally, rooting trials were carried out, also evaluating the influence of different kinds and combinations of light to obtain microplants ready for transplantation in the greenhouse.
To determine the most suitable explant for micropropagation initiation, both in vitro germinated seeds and nodal explants derived from mother plants were evaluated. To ensure a successful in vitro culture, it is crucial to optimize explant sterilization protocols [38]. The comparative seed sterilization test conducted using two different sterilizing agents indicated that sodium hypochlorite was the most effective in reducing contamination values, with results of less than 10 percent. On the other hand, mercuric chloride was too harsh on the seeds, resulting in arrested germination or reduced development and slightly oxidized shoots during in vitro growth. This strong toxicity, causing irreversible damage to plants, was reported in several studies on seeds of different species [39,40,41]. Furthermore, NaClO is recommended for seed sterilization due to it is lower costs, higher availability, and decreased toxicity compared to HgCl2. Evaluating the effects of different growth regulators on inducing the multiplication of seedlings germinated under in vitro conditions yielded the best results when using metatopolin (MT) 0.5 mg L−1, although the differences were not statistically significant (Table 1). Unfortunately, 21 days after the start of the first subculture, under identical growth conditions, the explants responded by arresting growth, showing signs of necrosis of the apices, diffuse hyperhydricity, or early flowering. Starting directly from in vitro germinated seeds is not the best option for this hemp cultivar, due to the inability to differentiate the starting material based on sex and the high difficulty in cutting for subculturing, which effectively limits the possibility of mass propagation of the selected clone. This culture decline, also observed by Page et al. [35] in Cannabis sativa, limits long-term culture proliferation. Similarly, in ‘Finola’, this decline was already observed in the first subculture through hyperhydricity and/or the death of the shoots.
The results relating to the two climate-controlled environments used to grow the mother plants showed different morphological developments achieved by the plants (Table 2) that strongly affected adaptation to the aseptic culture of the nodal explants excised for in vitro micropropagation. Only the plants grown in the thermostatically controlled cabinet became suitable (Table 3).
‘Finola’ showed the pronounced presence of hyperhydrated shoots when grown on Driver and Kuniyuki (DKW). Indeed, the latter resulted in the presence of vitrescence in almost all explants (90%), causing the alteration of tissues that were found to be thickened, turgid, and glassy in texture, with leaves featuring lightened coloration, very slow or no growth, a poor multiplication index, and brittle leaves. This behavior is different from the results reported by Page et al. [35], which suggested the use of DKW nutrient medium for multiple commercial varieties of C. sativa. These divergent results could be due to the different subspecies evaluated in this study.
Based on the results shown in Table 3, most of the phytoregulators tested, alone or in combination, produced strong shortening and clustering of the shoots. Although several authors [29,30,31,32] suggest using phytoregulators like BAP and MT to improve in vitro culture of Cannabis, in the case of ‘Finola’, the results achieved using these phytoregulators have been very disappointing. Nevertheless, the addition of GA3 to the nutrient substrate to promote stem elongation, which is common in many micropropagation protocols [42,43,44], resulted only in the excessive growth of leaves, while the internodes on the stem remained very short. This factor limited the number of microcuttings that could be produced. Moreover, to overcome the apical dominance of in vitro shoots that was strongly linked to the induction of in vitro flowering in ‘Finola’, 2,3,5-triiodobenzoic acid (TIBA) was tested in combination with TDZ and GA3. TIBA is a synthetic inhibitor of auxin flux and has been widely applied in studies on in vitro shoot regeneration, as reported in Rosa hybrida [45], Cucumis sativus [46], and ‘Finola’ in combination with TDZ [22]. The results of the present study showed an increase of about 1 cm in the length of the shoots compared with the combination of BAP + GA3 or MT + GA3, albeit with a lower multiplication rate than the best mean value achieved with the combination TDZ + NAA (Figure 6a). Instead, the best results were found by adding TDZ combined with NAA. TDZ is a phenylurea-substituted compound that is highly effective in the tissue culture morphogenesis of many plant species, including C. sativa variety MX-1 [47,48,49]. Indeed, several authors added this compound in different concentrations, alone or in combination with NAA, to induce the multiplication or regeneration of shoots from different explants of Cannabis sativa [9,23,32,50], achieving high multiplication indices for each explant. In contrast to these results, ‘Finola’ yielded a much lower multiplication rate, caused by the high degree of apical dominance and low levels of branching.
To prevent or delay flowering, which represents the greatest limit in maintaining a vegetative state for day-neutral genotypes such as ‘Finola’ [21], the apical tips were regularly eliminated in each subculture to break apical dominance and promote axillary bud growth. This technique was already found to be effective in promoting the shoot regeneration of C. sativa var. Epsilon 68 [51] and Piper sarmentosum [50].
Lastly, the different LED lighting systems used in the phase of rooting induction were discussed. In recent years, several studies have been conducted to determine the best light source to improve the quality of micropropagated plants, and, at the same time, reduce costs [33,52]. In particular, Budiarto (2010) [34] reported that the in vitro root activity increased under red and blue LED lights. In this study, ‘Finola’ shoots exhibited good rooting susceptibility under red/blue lights when treated with IBA at lower concentrations. However, IBA is an auxin frequently used in the rooting induction of C. sativa [53,54,55]. This application resulted in the development of an extensive root system, in terms of both length and number of roots, which facilitated the survival of all plantlets after transplantation into the climate-controlled greenhouse (Figure 6).
5. Conclusions
‘Finola’ (C. sativa subsp. ruderalis) is an in vitro hemp recalcitrant cultivar because it is day-neutral and flowers independent of the photoperiod. For this reason, ‘Finola’ is difficult to maintain in a vegetative state. The ambitious aim of this research was to overcome this limitation and define an efficient protocol for micropropagation by evaluating many different factors and combining them. The positive results obtained in this work could contribute to developing a tissue culture system suitable for other recalcitrant hemp varieties.
In the future, the selection of in vitro elite plant materials could help to realize the super intensive production of ‘Finola’ under protected environments in very short cycles. This approach can offer a viable option for maximizing production yields while maintaining optimal conditions for in vitro plant growth.
Author Contributions
Conceptualization, C.R.; methodology, C.R. and G.N.B., validation, L.T., C.R. and G.D.M.; investigation, G.N.B.; data curation C.R. and G.N.B.; writing—original draft preparation, C.R. and G.N.B.; writing—review and editing, L.T., C.R., G.D.M. and C.P.; project administration, G.D.M.; funding acquisition, G.D.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Puglia Region, 6 June 2017, n. 21 “Promotion of the cultivation of hemp for productive and environmental purposes”. Project “Micropropagation Apulian Autoflowering”.
Data Availability Statement
Not applicable.
Acknowledgments
The support of the project leader Fattoria della Canapa and the partner Rete Etruscum for the success of the results of this research is gratefully acknowledged.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Salami, S.A.; Martinelli, F.; Giovino, A.; Bachari, A.; Arad, N.; Mantri, N. It is our turn to get cannabis high: Put cannabinoids in food and health baskets. Molecules 2020, 25, 4036. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, Y.; Wang, W.; Griffin, J.; Wang, D. High Ethanol Concentration (77 g/L) of Industrial Hemp Biomass Achieved Through Optimizing the Relationship between Ethanol Yield/Concentration and Solid Loading. ACS Omega 2020, 5, 21913–21921. [Google Scholar] [CrossRef]
- Shen, P.; Gao, Z.; Fang, B.; Rao, J.; Chen, B. Ferreting out the secrets of industrial hemp protein as emerging functional food ingredients. Trends Food Sci. Technol. 2021, 112, 1–15. [Google Scholar] [CrossRef]
- Amaducci, S.; Scordia, D.; Liu, F.H.; Zhang, Q.; Guo, H.; Testa, G.; Cosentino, S.L. Key evaluation techniques for hemp in Europe and in China. Ind. Crops Prod. 2015, 69, 2–16. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, X.; Guo, H.; Trindade, L.M.; Salentijn, E.M.J.; Guo, R.; Guo, M.; Xu, Y.; Yang, M. Latitudinal Adaptation and Genetic Insights into the Origins of Cannabis sativa L. Front. Plant Sci. 2018, 9, 1876. [Google Scholar] [CrossRef]
- Gloss, D. An overview of products and bias in research. Neurotherapeutics 2015, 12, 731–734. [Google Scholar] [CrossRef]
- McPartland, J.M.; Clarke, R.C.; Watson, D.P. Hemp Diseases and Pests: Management and Biological Control: An Advanced Treatise; CABI: Wallingford, UK, 2000. [Google Scholar]
- Dowling, C.A.; Shi, J.; Toth, J.A.; Quade, M.A.; Smart, L.B.; McCabe, P.F.; Melzer, R.; Schilling, S. A FLOWERING LOCUS T ortholog is associated with photoperiod-insensitive flowering in hemp (Cannabis sativa L.). bioRxiv 2023. preprint. [Google Scholar]
- Hillig, K.W. Genetic evidence for speciation in Cannabis (Cannabaceae). Genet. Resour. Crop Evol. 2005, 52, 161–180. [Google Scholar] [CrossRef]
- Fadel, D.; Assaad, N.; Alghazal, G.; Hamouche, Z.; Lazari, D. “Finola” Cannabis Cultivation for Cannabinoids Production in Thessaloniki-Greece. J. Agric. Sci. 2020, 12, 172–181. [Google Scholar] [CrossRef]
- Pagnani, G.; Pellegrini, M.; Galieni, A.; D’Egidio, S.; Matteucci, F.; Ricci, A.; Stagnari, F.; Sergi, M.; Lo Sterzo, C.; Pisante, M.; et al. Plant growth-promoting rhizobacteria (PGPR) in Cannabis sativa ‘Finola’ cultivation: An alternative fertilization strategy to improve plant growth and quality characteristics. Ind. Crops Prod. 2018, 123, 75–83. [Google Scholar] [CrossRef]
- George, E.F.; Hall, M.A.; De Klerk, G.J. (Eds.) Plant Propagation by Tissue Culture: Volume 1. The Background; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Cardoso, J.C.; Lee Tseng Sheng, G.; Teixeira da Silva, J.A. Micropropagation in the twenty-first century. In Plant Cell Culture Protocols; Springer: Berlin/Heidelberg, Germany, 2018; pp. 17–46. [Google Scholar]
- Boonsnongcheep, P.; Benyakan, P. Factors affecting micropropagation of Cannabis sativa L.: A review. Pharm. Sci. Asia 2020, 47, 21–29. [Google Scholar] [CrossRef]
- Adhikary, D.; Kulkarni, M.; El-Mezawy, A.; Mobini, S.; Elhiti, M.; Gjuric, R.; Ray, A.; Polowick, P.; Slaski, J.J.; Jones, M.; et al. Medical cannabis and industrial hemp tissue culture: Present status and future potential. Front. Plant Sci. 2021, 12, 627240. [Google Scholar] [CrossRef]
- Chadipiralla, K.; Gayathri, P.; Rajani, V.; Reddy, P.V.B. Plant Tissue Culture and Crop Improvement. In Sustainable Agriculture in the Era of Climate Change; Roychowdhury, R., Choudhury, S., Hasanuzzaman, M., Srivastava, S., Eds.; Springer: Cham, Switzerland, 2020; pp. 391–412. [Google Scholar]
- El-Sherif, N.A. Impact of plant tissue culture on agricultural sustainability. In Sustainability of Agricultural Environment in Egypt: Part II: Soil-Water-Plant Nexus; The Handbook of Environmental Chemistry Series; Springer: Berlin/Heidelberg, Germany, 2019; pp. 93–107. [Google Scholar]
- Lata, H.; Chandra, S.; Khan, I.A.; ElSohly, M.A. Micropropagation of Cannabis sativa L.—An update. In Cannabis sativa L.—Botany and Biotechnology; Springer: Berlin/Heidelberg, Germany, 2017; pp. 285–297. [Google Scholar]
- Monthony, A.S.; Bagheri, S.; Zheng, Y.; Jones, A.M.P. Flower power: Floral reversion as a viable alternative to nodal micropropagation in Cannabis sativa. In Vitr. Cell. Dev. Biol. Plant 2021, 57, 1018–1030. [Google Scholar] [CrossRef]
- Schilling, S.; Melzer, R.; Dowling, C.A.; Shi, J.; Muldoon, S.; McCabe, P.F. A protocol for rapid generation cycling (speed breeding) of hemp (Cannabis sativa) for research and agriculture. Plant J. 2023, 113, 437–445. [Google Scholar] [CrossRef]
- Monthony, A.S.; Page, S.R.; Hesami, M.; Jones, A.M.P. The Past, Present and Future of Cannabis sativa Tissue Culture. Plants 2021, 10, 185. [Google Scholar] [CrossRef] [PubMed]
- Dreger, M.; Szalata, M. The Effect of TIBA and NPA on Shoot Regeneration of Cannabis sativa L. Epicotyl Explants. Agronomy 2022, 12, 104. [Google Scholar] [CrossRef]
- Galán-Ávila, A.; García-Fortea, E.; Prohens, J.; Herraiz, F.J. Development of a Direct in vitro Plant Regeneration Protocol from Cannabis sativa L. Seedling Explants: Developmental Morphology of Shoot Regeneration and Ploidy Level of Regenerated Plants. Front. Plant Sci. 2020, 11, 645. [Google Scholar] [CrossRef]
- ElSohly, M.A.; Radwan, M.M.; Gul, W.; Chandra, S.; Galal, A. Phytochemistry of Cannabis sativa L. In Phytocannabinoids. Progress in the Chemistry of Organic Natural Products; Kinghorn, A., Falk, H., Gibbons, S., Kobayashi, J., Eds.; Springer: Cham, Switzerland, 2017; Volume 103, pp. 1–36. [Google Scholar]
- Ancona, S.; De Mastro, G.; Jenderek, M.M.; Ruta, C. Micropropagation Supports Reintroduction of an Apulian Artichoke Landrace in Sustainable Cropping Systems. Agronomy 2021, 11, 1169. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Nitsch, J.P.; Nitsch, C. Haploid plants for pollen grains. Science 1969, 63, 85–87. [Google Scholar] [CrossRef]
- Page, S.R.G.; Monthony, A.S.; Jones, A.M.P. DKW basal salts improve micropropagation and callogenesis compared with MS basal salts in multiple commercial cultivars of Cannabis sativa. Botany 2021, 99, 269–279. [Google Scholar] [CrossRef]
- Lata, H.; Chandra, S.; Techen, N.; Khan, I.A.; ElSohly, M.A. In vitro mass propagation of Cannabis sativa L.: A protocol refinement using novel aromatic cytokinin meta-topolin and the assessment of eco-physiological, biochemical and genetic fidelity of micropropagated plants. J. Appl. Res. Med. Aromat. Plants 2016, 3, 18–26. [Google Scholar] [CrossRef]
- Mehdi, M.; Vali-Ollah, G.-O.; Sepide, T. The effect of different concentrations of TDZ and BA on in vitro regeneration of Iranian cannabis (Cannabis sativa) using cotyledon and epicotyl explants. J. Plant Mol. Breed. 2015, 3, 20–27. [Google Scholar]
- Mestinšek Mubi, Š.; Svetik, S.; Flajšman, M.; Murovec, J. In Vitro tissue culture and genetic analysis of two high-CBD medical Cannabis (Cannabis sativa L.) breeding lines. Genetika 2020, 52, 925–941. [Google Scholar] [CrossRef]
- Chaohua, C.; Gonggu, Z.; Lining, Z.; Chunsheng, G.; Qing, T.; Jianhua, C.; Xinbo, G.; Dingxiang, P.; Jianguang, S. A rapid shoot regeneration protocol from the cotyledons of hemp (Cannabis sativa L.). Ind. Crops Prod. 2016, 83, 61–65. [Google Scholar] [CrossRef]
- Dutta Gupta, S.; Jatothu, B. Fundamentals and applications of light-emitting diodes (LEDs) in in vitro plant growth and morpho-genesis. Plant Biotechnol. Rep. 2013, 7, 211–220. [Google Scholar] [CrossRef]
- Budiarto, K. Spectral quality affects morphogenesis on Anthurium plantlet during in vitro culture. Agrivita 2010, 32, 234–240. [Google Scholar]
- Page, S.R.G.; Monthony, A.S.; Jones, A.M.P. Basal media optimiza-tion for the micropropagation and callogenesis of Cannabis sativa L. bioRxiv 2020. preprint. [Google Scholar]
- Campbell, L.G.; Naraine, S.G.U.; Dusfresne, J. Phenotypic plasticity influences the success of clonal propagation in industrial pharmaceutical Cannabis sativa. PLoS ONE 2019, 14, e0213434. [Google Scholar] [CrossRef]
- Codesido, V.; Meyer, S.; Casano, S. Influence of media composition and gen-otype for successful Cannabis sativa L. In Vitro introduction. Acta Hortic. 2020, 1285, 75–80. [Google Scholar] [CrossRef]
- Dodds, J.H.; Roberts, L.W. Experiments in Plant Tissue Culture, 2nd ed.; Cambridge University Press: Cambridge, UK, 1985; pp. 21–35. [Google Scholar]
- Barampuram, S.; Allen, G.; Krasnyanski, S. Effect of Various Sterilization Procedures on the in Vitro Germination of Cotton Seeds. Plant Cell Tissue Organ Cult. 2014, 118, 179–185. [Google Scholar] [CrossRef]
- Lu, L.M.; An, Y. Effects of Different Disinfectant on Sterilization Effect and Germination of Tobacco Seeds. Seed 2012, 31, 93–95. [Google Scholar]
- Yuan, Y. Selection and Disinfection of Tissue Culture Explants of Toona sinensis Roem. Anhui Agric. 2020, 26, 19–20. [Google Scholar]
- Camara, M.; Vandenberghe, L.; Rodríguez, C.; Oliveira, J.; Faulds, C.; Bertrand, E.; Soccol, C. Current advances in gibberellic acid (GA3) production, patented technologies and potential applications. Planta 2018, 248, 1049–1062. [Google Scholar] [CrossRef]
- Ayano, M.; Kani, T.; Kojima, M.; Sakakibara, H.; Kitaoka, T.; Kuroha, T.; Angeles-Shim, R.; Kitano, H.; Nagai, K.; Ashikari, M. Gibberellin biosynthesis and signal transduction is essential for internode elongation Subburaman in deepwater rice. Plant Cell Environ. 2014, 37, 2313–2324. [Google Scholar] [CrossRef] [PubMed]
- Geng, F.; Moran, R.; Day, M.; Halteman, W.; Zhang, D. Increasing In Vitro Shoot Elongation and Proliferation of ‘G.30’ and ‘G.41’ Apple by Chilling Explants and Plant Growth Regulators. HortScience 2016, 51, 899–904. [Google Scholar] [CrossRef]
- Sing, S.K.; Syamal, M.M. Anti-auxin enhance Rosa hybrida L. micropropagation. Biol. Plant. 2000, 43, 279–281. [Google Scholar] [CrossRef]
- Shukla, P.S.; Das, A.K.; Jha, B.; Agarwal, P.K. High-frequency in vitro shoot regeneration in Cucumis sativus by inhibition of endogenous auxin. In Vitr. Cell. Dev. Biol. Plant 2014, 50, 729–737. [Google Scholar] [CrossRef]
- Donna, I.L.; John, E.P. Thidiazuron stimulates adventitious shoot production from Hydrangea quercifolia Bartr., leaf explants. Sci. Hortic. 2004, 101, 121–126. [Google Scholar]
- Murthy, B.N.S.; Murch, S.J.; Saxena, P.K. Thidiazuron: A potent regulator of in vitro plant morphogenesis. In Vitr. Cell. Dev. Biol. Plant 1998, 34, 267–275. [Google Scholar] [CrossRef]
- Lata, H.S.; Chandra, I.K.; ElSohly, M.A. Thidiazuron-induced high-frequency direct shoot organogenesis of Cannabis sativa L. In Vitr. Cell. Dev. Biol. Plant 2009, 45, 12–19. [Google Scholar] [CrossRef]
- Stephin, S.; Gangaprasad, A.; Mathew, S.P.; Muthukrishnan, S. Enhanced In Vitro Shoot Multiplication of Piper sarmentosum by Suppression of Apical Dominance. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2020, 90, 87–94. [Google Scholar] [CrossRef]
- Wróbel, T.; Dreger, M.; Wielgus, K.; Słomski, R. Modified nodal cuttings and shoot tips protocol for rapid regeneration of Cannabis sativa L. J. Nat. Fibers 2022, 19, 536–545. [Google Scholar] [CrossRef]
- Batista, D.S.; Felipe, S.H.S.; Silva, T.D.; Motta de Castro, K.; Mamedes-Rodrigues, T.C.; Miranda, N.A.; Ríos-Ríos, A.-M.; Vidal Faria, D.; Fortini, E.A.; Chagas, K.; et al. Light quality in plant tissue culture: Does it matter? In Vitr. Cell. Dev. Biol. Plant 2018, 54, 195–215. [Google Scholar] [CrossRef]
- Lata, H.; Chandra, S.; Khan, I.; ElSohly, M.A. High frequency plant regeneration from leaf derived callus of high Δ9-tetrahydrocannabinol yielding Cannabis sativa L. Planta Med. 2010, 76, 1629–1633. [Google Scholar] [CrossRef] [PubMed]
- Stephen, C.; Zayas, V.A.; Galic, A.; Bridgen, M.P. Micropropagation of hemp (Cannabis sativa L.). HortScience 2023, 58, 307–316. [Google Scholar] [CrossRef]
- Wang, R.; He, L.S.; Xia, B.; Tong, J.F.; Li, N.; Peng, F. A micropropagation system for cloning of hemp (Cannabis sativa L.) by shoot tip culture. Pak. J. Bot. 2009, 41, 603–608. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).