The Use of Wheat Starch as Gelling Agent for In Vitro Proliferation of Blackberry (Rubus fruticosus L.) Cultivars and the Evaluation of Genetic Fidelity after Repeated Subcultures
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and In Vitro Shoot Cultures
2.2. Estimation of the Costs per Liter of Culture Medium
2.3. Rheological Analyses
2.4. Genetic Fidelity Analysis Using SRAP and SCoT Markers
2.4.1. DNA Isolation
2.4.2. SCoT and SRAP Analysis
2.5. Data Collection and Statistical Analysis
3. Results
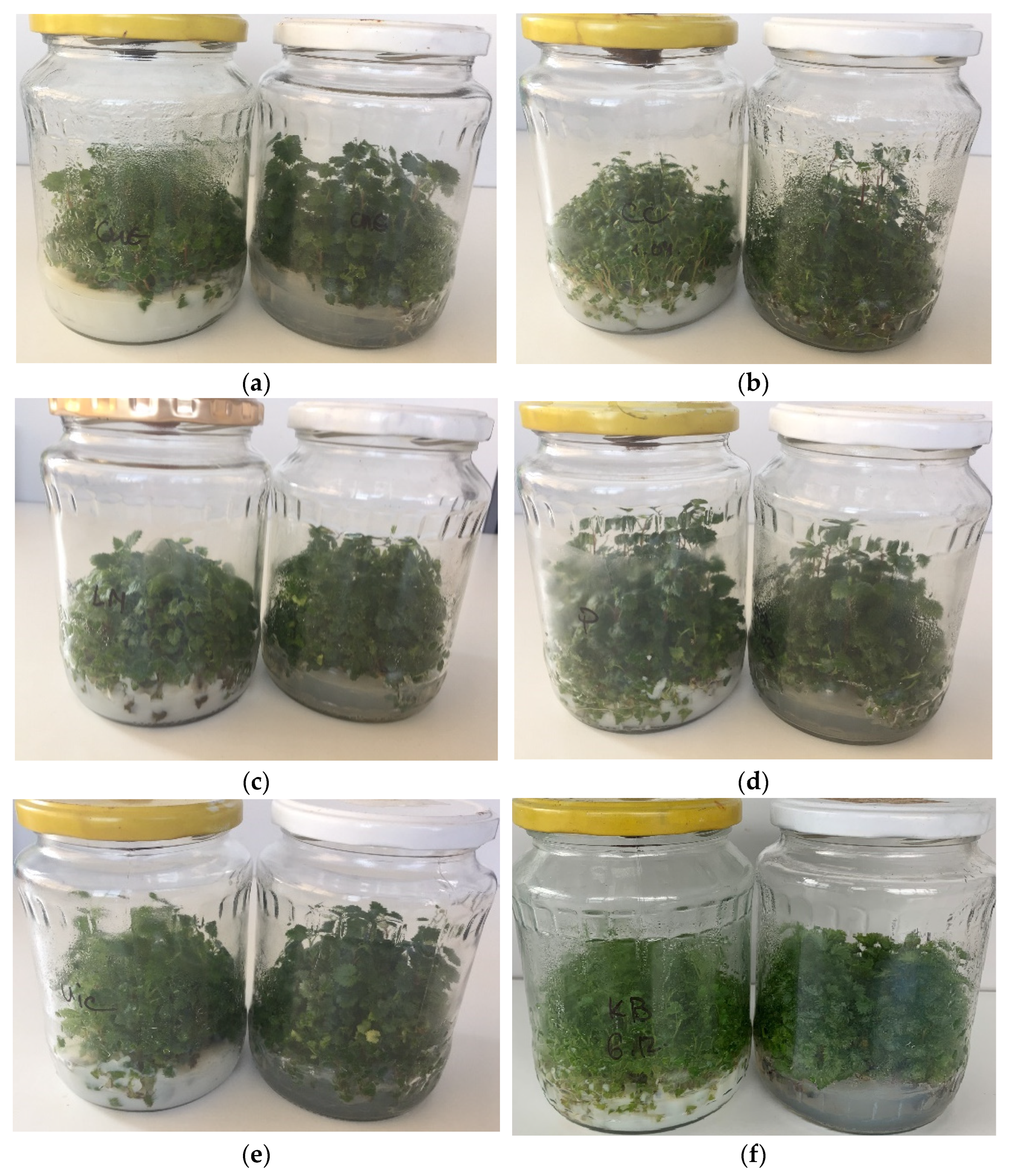
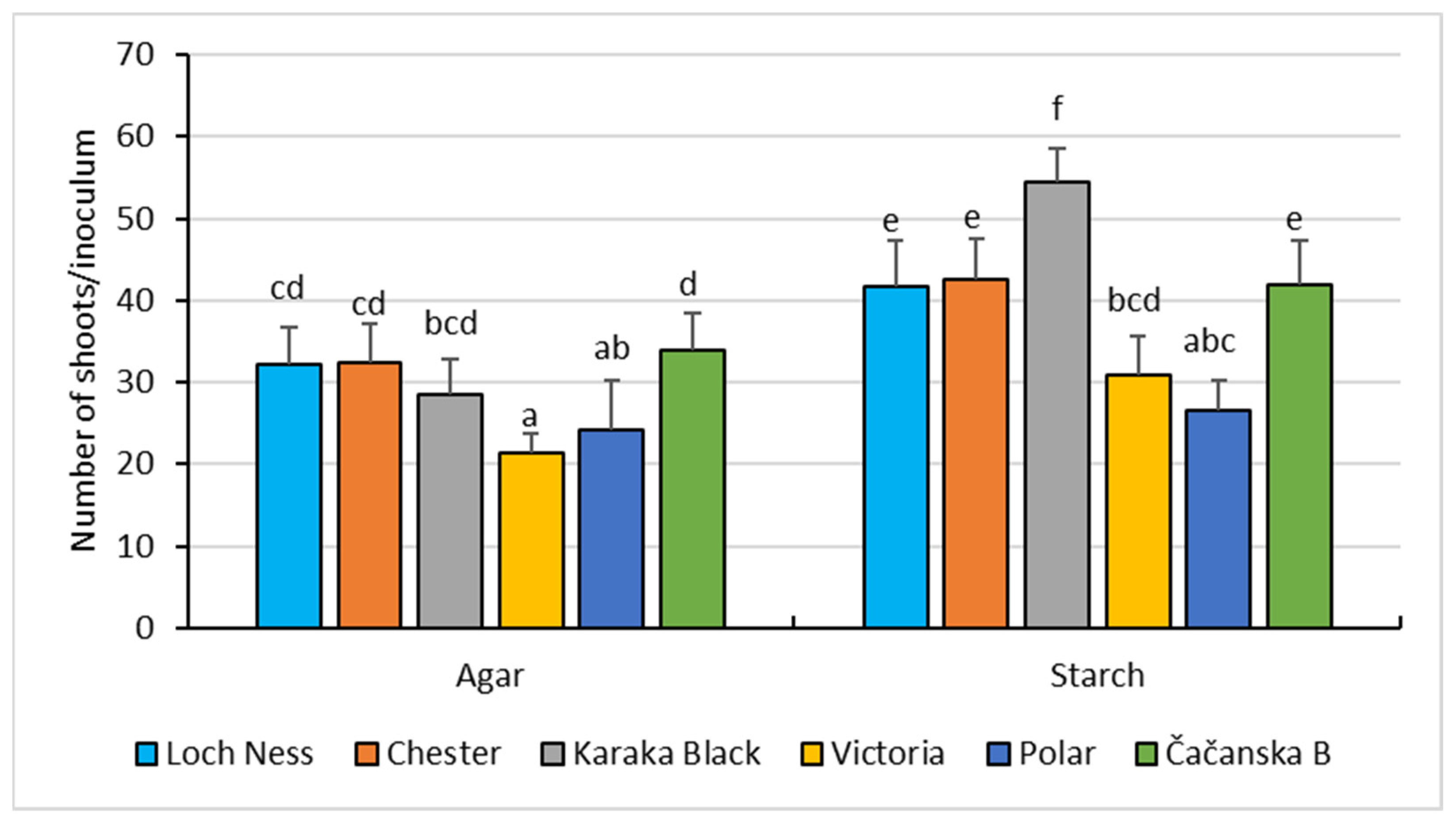
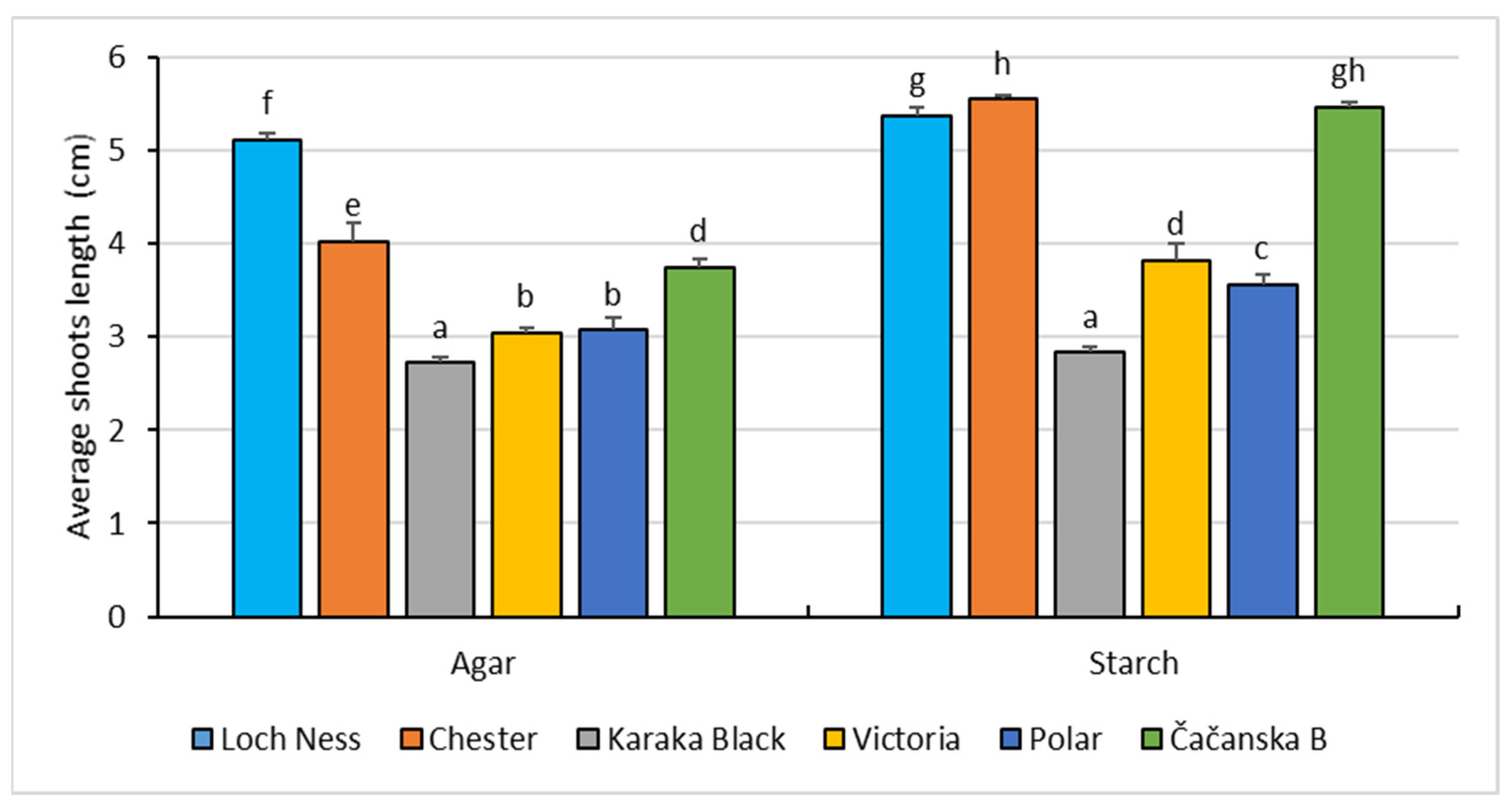
3.1. In Vitro Shoot Cultures in Starch-Gelled Culture Media and Agar-Gelled Culture Media
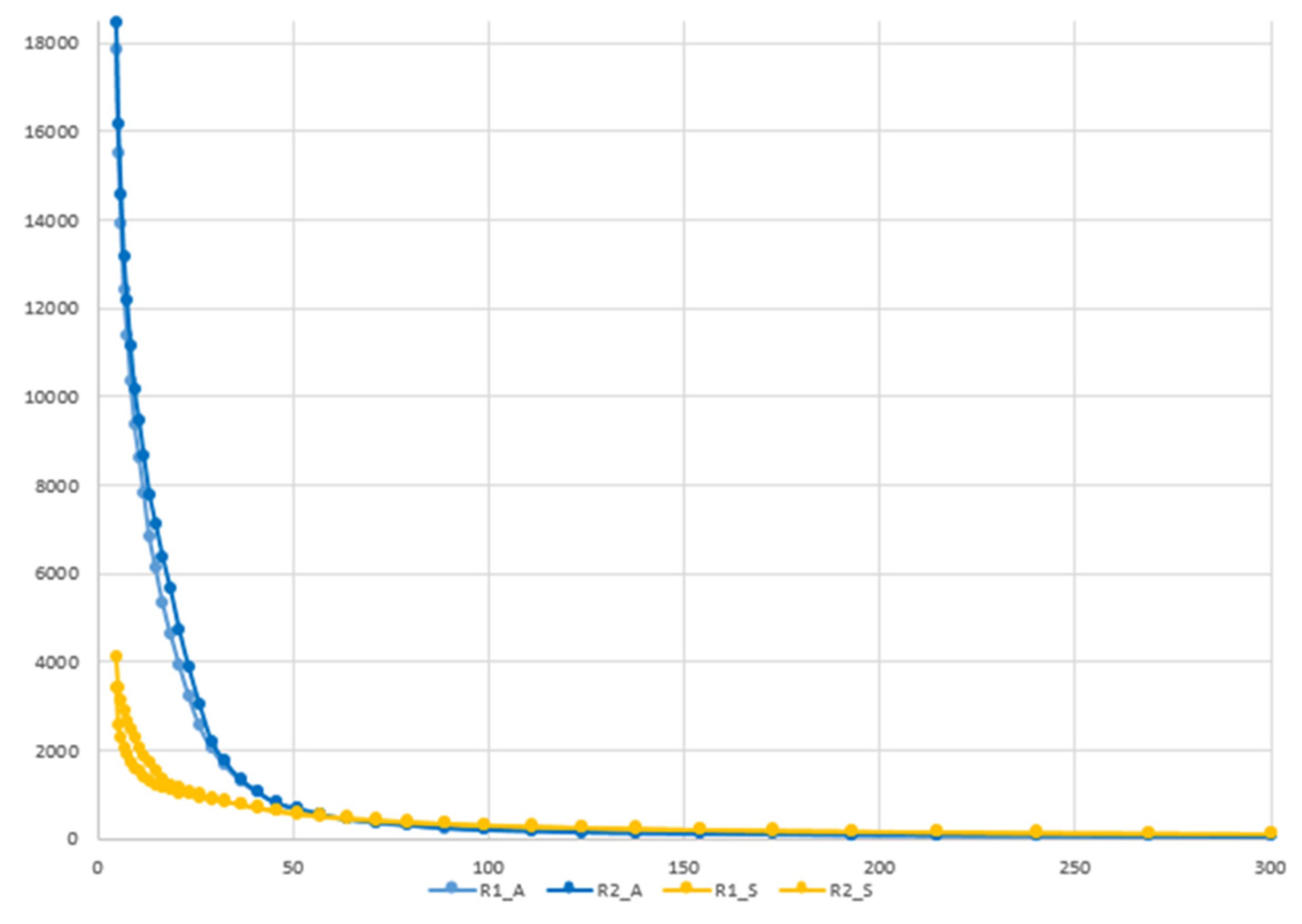
3.2. Rheological Analyses
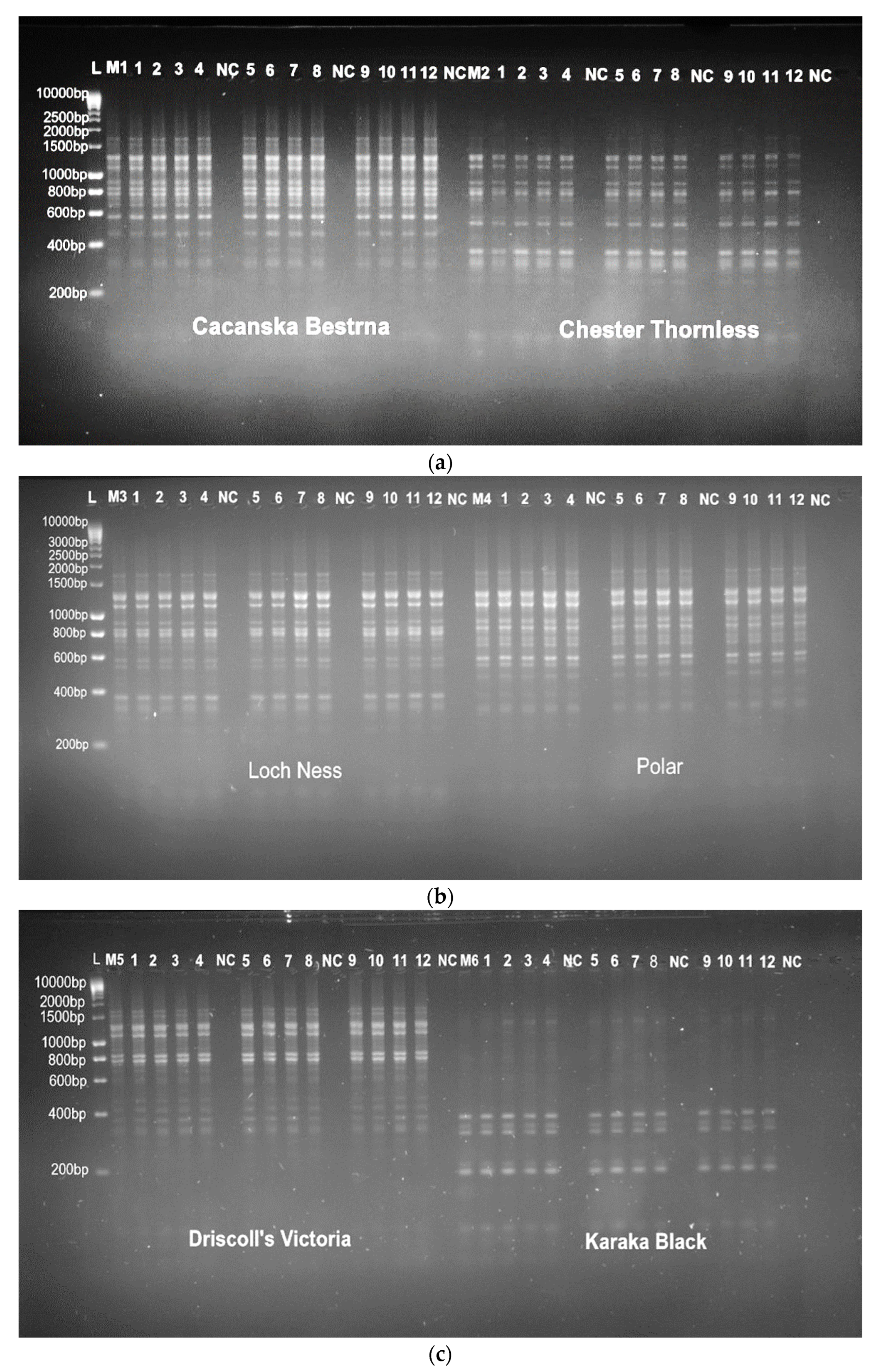
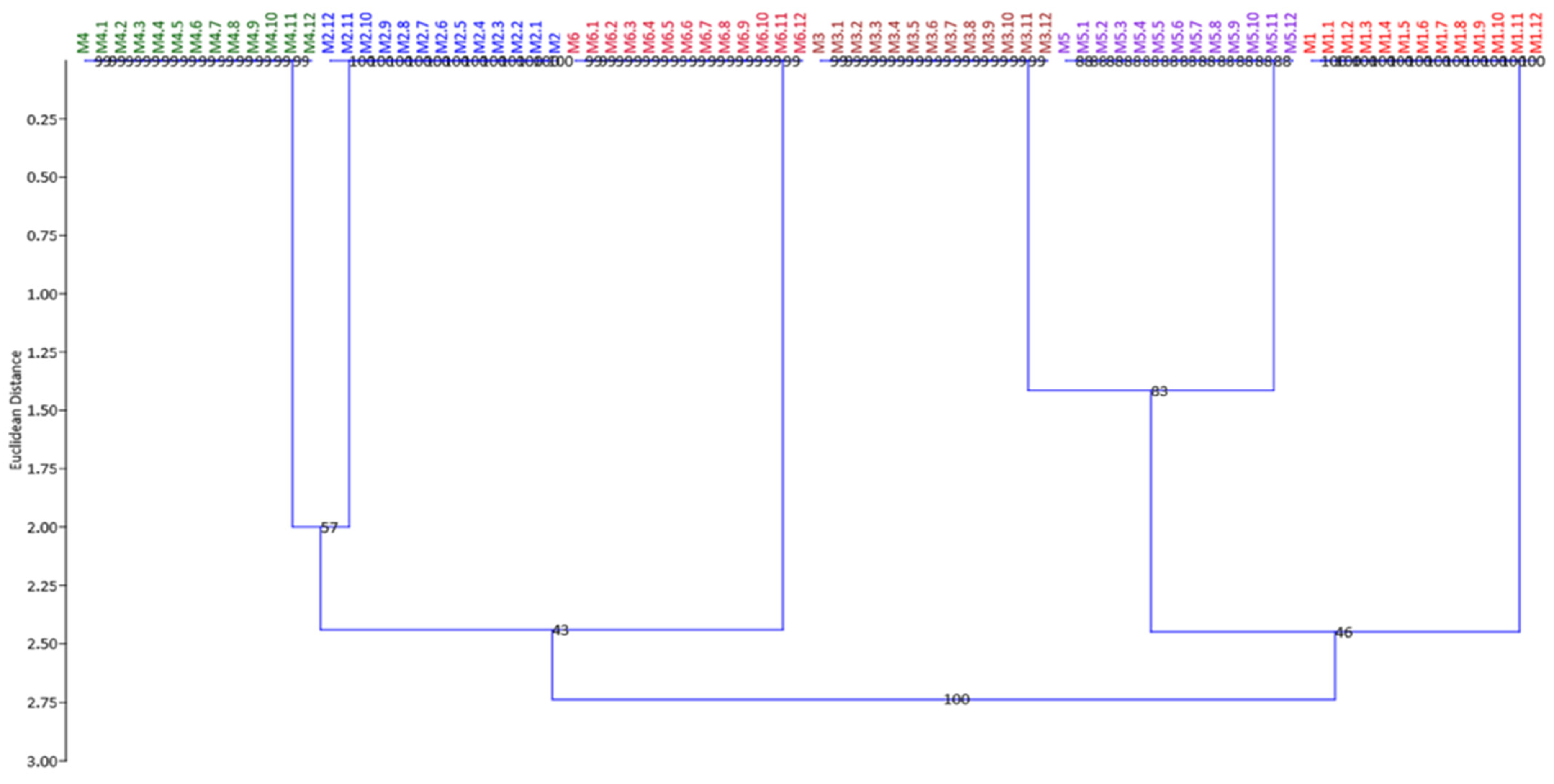
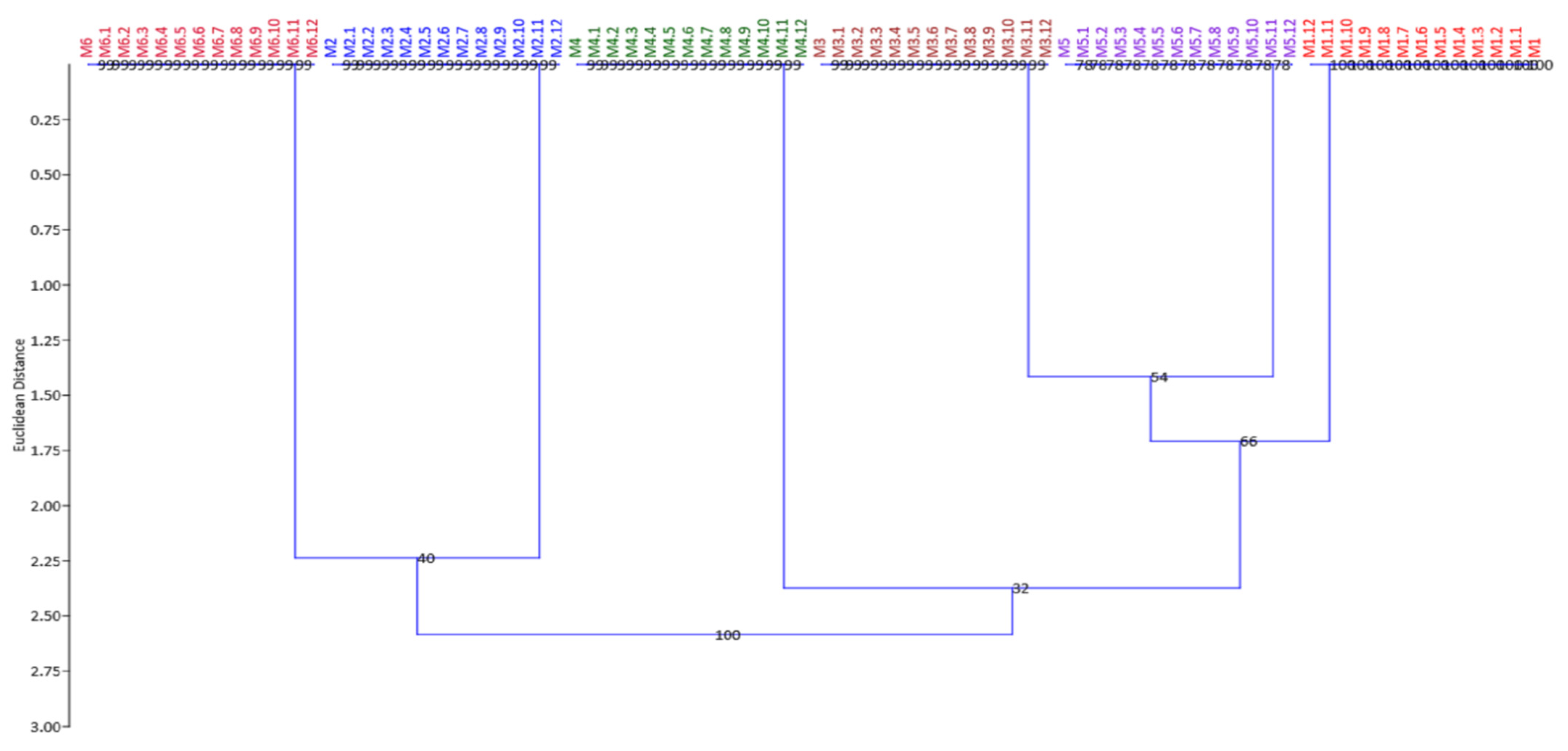
3.3. Genetic Fidelity Evaluation Using SRAP and SCoT Molecular Markers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cho, M.J.; Howard, L.R.; Prior, R.L.; Clark, J.R. Flavonol glycosides and antioxidant capacity of various blackberry and blueberry genotypes determined by high-performance liquid chromatography/mass spectrometry. J. Sci. Food Agric. 2005, 85, 2149–2158. [Google Scholar] [CrossRef]
- Zia-Ul-Haq, M.; Riaz, M.; De Feo, V.; Jaafar, H.Z.E.; Moga, M. Rubus fruticosus L.: Constituents, Biological Activities and Health Related Uses. Molecules 2014, 19, 10998–11029. [Google Scholar] [CrossRef] [PubMed]
- Oszmiański, J.; Nowicka, P.; Teleszko, M.; Wojdyło, A.; Cebulak, T.; Oklejewicz, K. Analysis of phenolic compounds and antioxidant activity in wild blackberry fruits. Int. J. Mol. Sci. 2015, 16, 14540–14553. [Google Scholar] [CrossRef] [PubMed]
- Fathy, H.M.; Abou El-Leel, O.F.; Amin, M.A. Micropropagation and Biomass Production of Rubus fruticosus L. (Blackberry) plant. Middle East J. Appl. Sci. 2018, 8, 1215–1228. [Google Scholar]
- Bray, M.; Rom, C.C.; Clark, J.R. Propagation of thornless Arkansas blackberries by hardwood cuttings. Discov. Stud. J. Dale Bump. Coll. Agric. Food Life Sci. 2003, 4, 9–13. [Google Scholar]
- Takeda, F.; Soria, J. Method for producing long-cane blackberry plants. HortTechnology 2011, 21, 563–568. [Google Scholar] [CrossRef]
- Hussain, I.; Roberto, S.R.; Colombo, R.C.; Assis, A.; Koyama, R. Cutting types collected at different seasons on blackberry multiplication. Rev. Bras. Frutic. 2017, 39. [Google Scholar] [CrossRef]
- Gomes, H.T.; Bartos, P.M.C.; Andrade, M.T.D.; Almeida, R.F.; Lacerda, L.F.D.; Scherwinski-Pereira, J.E. In vitro conservation of blackberry genotypes under minimal growth conditions and subsequent large-scale micropropagation. Pesqui. Agropecu. Bras. 2017, 52, 1286–1290. [Google Scholar] [CrossRef][Green Version]
- Broome, O.C.; Zimmerman, R.H. In vitro propagation of blackberry. Hortic. Sci. 1978, 13, 151–153. [Google Scholar]
- Najaf-Abadi, A.J.; Hamidoghli, Y. Micropropagation of thornless trailing blackberry (‘Rubus sp.’) by axillary bud explants. Aust. J. Crop Sci. 2009, 3, 191–194. [Google Scholar]
- Vujović, T.; Ružić, D.J.; Cerović, R. In vitro shoot multiplication as influenced by repeated subculturing of shoots of contemporary fruit rootstocks. Hortic. Sci. 2012, 39, 101–107. [Google Scholar] [CrossRef]
- Fira, A.; Clapa, D.; Simu, M. Studies Regarding the Micropropagation of Some Blackberry Cultivars. Bull. UASVM (H) 2014, 71, 29–37. [Google Scholar]
- Kefayeti, N.; Kafkas, E.; Ercişli, S. Micropropagation of ‘Chester thornless’ Blackberry Cultivar using Axillary Bud Explants. Not. Bot. Horti Agrobot. Cluj-Napoca 2019, 47, 162–168. [Google Scholar] [CrossRef]
- Kolarević, T.; Milinčić, D.D.; Vujović, T.; Gašić, U.M.; Prokić, L.; Kostić, A.Ž.; Cerović, R.; Stanojevic, S.P.; Tešić, Ž.L.; Pešić, M.B. Phenolic Compounds and Antioxidant Properties of Field-Grown and In vitro Leaves, and Calluses in Blackberry and Blueberry. Horticulturae 2021, 7, 420. [Google Scholar] [CrossRef]
- Murashige, T. Plant Propagation through Tissue Cultures. Annu. Rev. Plant Physiol. 1974, 25, 135–166. [Google Scholar] [CrossRef]
- Soumare, A.; Diédhiou, A.G.; Arora, N.K.; Tawfeeq Al-Ani, L.K.; Ngom, M.; Fall, S.; Hafidi, M.; Ouhdouch, Y.; Kouisni, L.; Sy, M.O. Potential role and utilization of plant growth promoting microbes in plant tissue culture. Front. Microbiol. 2021, 12, 649878. [Google Scholar] [CrossRef]
- Abdalla, N.; El-Ramady, H.; Seliem, M.K.; El-Mahrouk, M.E.; Taha, N.; Bayoumi, Y.; Shalaby, T.A.; Dobránszki, J. An Academic and Technical Overview on Plant Micropropagation Challenges. Horticulturae 2022, 8, 677. [Google Scholar] [CrossRef]
- Viswanath, M.; Ravindra Kumar, K.; Chetanchidambar, N.M.; Mahesh, S.S.N.M. Regeneration mechanisms in plant tissue culture: A. J. Pharm. Innov. 2023, 12, 2948–2952. [Google Scholar]
- Hussain, A.; Qarshi, I.A.; Nazir, H.; Ullah, I. Plant Tissue Culture: Current Status and Opportunities. In Recent Advances in Plant In Vitro Culture; IntechOpen: London, UK, 2012; pp. 2–29. [Google Scholar] [CrossRef]
- Monja-Mio, K.M.; Olvera-Casanova, D.; Herrera-Alamillo, M.Á.; Sánchez-Teyer, F.L.; Robert, M.L. Comparison of conventional and temporary immersion systems on micropropagation (multiplication phase) of Agave angustifolia Haw ‘Bacanora’. 3 Biotech 2021, 11, 77. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Bobrowski, V.L.; Mello-Farias, P.; Petters, J. Micropropagation of blackberries (Rubus sp.) cultivars. J. Agric. Sci. Technol. 1996, 2, 17–20. [Google Scholar]
- Gonzalez, M.V.; Lopez, M.; Valdes, A.E.; Ordas, R.J. Micropropagation of three berry fruit species using nodal segments from field-grown plants. Ann. Appl. Biol. 2000, 137, 73–78. [Google Scholar] [CrossRef]
- Pasa, M.D.S.; Carvalho, G.L.; Schuch, M.W.; Schmitz, J.D.; Torchelsen, M.D.M.; Nickel, G.K.; Sommer, L.R.; Lima, T.S.; Camargo, S.S. Qualidade de luz e fitorreguladores na multiplicação e enraizamento in vitro da amoreira-preta ‘Xavante’. Cienc. Rural 2012, 42, 1392–1396. [Google Scholar] [CrossRef]
- Schuchovski, C.S.; Biasi, L.A. Development of an efficient protocol for ‘Brazos’ blackberry in vitro multiplication. Acta Hortic. 2017, 1224, 157–164. [Google Scholar] [CrossRef]
- Llyod, G.; McCown, B. Commercially feasible micropropagation of mountain loure, Kalmia latifolia, by using of shoot tip culture. Comb. Proceed. Int. Plant Prop. Soc. 1980, 30, 421–427. [Google Scholar]
- Villa, F.; Fráguas, C.B.; Dutra, L.F.; Pio, L.A.S.; Pasqual, M. Multiplicacao in vitro de amoreira-preta cultivar Brazos. Ciênc. Agrotec. 2006, 30, 266–270. [Google Scholar] [CrossRef]
- Gamborg, O.L. Cells, Protoplasts and Plant Regeneration in Culture. In Manual of Industrial Microbiology and Biotechnology; Demain, A.L., Salomon, N.A., Eds.; American Society for Microbiology: Washington, DC, USA, 1986; pp. 263–273. [Google Scholar]
- Driver, J.A.; Kuniyuki, A.H. In vitro propagation of Paradox walnut rootstock. HortScience 1984, 19, 507–509. [Google Scholar] [CrossRef]
- Fira, A.; Clapa, D.; Plopa, C. Micropropagation of blackberry thornless cultivars. Fruit Grow. Res. 2009, 25, 213–221. [Google Scholar]
- Fira, A.; Clapa, D.; Plopa, C. New aspects regarding the micropropagation of blackberry cultivar ‘Thornless evergreen’. Bull. UASVM (H) 2010, 67, 106–114. [Google Scholar]
- Vescan, L.A.; Clapa, D.; Fira, A.; Pamfil, D. Micropropagation of cold resistant blackberry cultivar ‘Gazda’. Bull. USAMV Anim. Sci. Biotechnol. 2012, 69, 282–289. [Google Scholar]
- Quoirin, M.; Lepoivre, P. Improved media for in vitro culture of Prunus sp. Acta Hortic. 1977, 78, 437–442. [Google Scholar] [CrossRef]
- Borodulina, I.D.; Plaksina, T.V.; Panasenko, V.N.; Sokolova, G.G. Optimization of blackberry clonal micropropagation. Ukr. J. Ecol. 2019, 9, 339–345. [Google Scholar] [CrossRef]
- Anderson, W.C. Tissue culture propagation of red and black raspberries, Rubus idaeus and R. occidentalis. Acta Hortic. 1980, 112, 13–20. [Google Scholar] [CrossRef]
- Reed, B.M. Multiplication of Rubus germplasm in vitro: A screen of 256 accessions. Fruit Var. J. 1990, 44, 141–148. [Google Scholar]
- Murashige, T. Growth factor requirements of citrus tissue culture. Proc. First Int. Citrus Symp. 1969, 3, 1151–1161. [Google Scholar]
- Wu, J.H.; Miller, S.A.; Hall, H.K.; Mooney, P.A. Factors affecting the efficiency of micropropagation from lateral buds and shoot tips of Rubus. PCTOC 2009, 99, 17–25. [Google Scholar] [CrossRef]
- Erig, A.C.; De Rossi, A.; Fortes, G.R. 6-benzilaminopurina e ácido indolbutírico na multiplicação in vitro da amoreira-preta (Rubus idaeus L.), cv. Tupy. Ciência Rural 2002, 32, 765–770. [Google Scholar] [CrossRef]
- Ružić, D.; Lazić, T. Micropropagation as means of rapid multiplication of newly developed blackberry and black currant cultivars. Agric. Conspec. Sci. 2006, 71, 149–153. [Google Scholar]
- Lepse, L.; Laugale, V. Micropropagation, Rooting and Acclimatization of Blackberry ‘Agavam’. Acta Hortic. 2008, 839, 43–49. [Google Scholar] [CrossRef]
- Hunkova, J.; Libiakova, G.; Gajdošová, A. Shoot proliferation ability of selected cultivars of Rubus spp. as influenced by genotype and cytokinin concentration. J. Cent. Eur. Agric. 2016, 17, 252–259. [Google Scholar] [CrossRef]
- Hunková, J.; Gajdošová, A.; Szabóová, M. Effect of Mesos Components (MgSO4, CaCl2, KH2PO4) on in vitro Shoot Growth of Blackberry, Blueberry, and Saskatoon. Plants 2020, 9, 935. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Concha, D.; Quintero, J.; Ercişli, S. Media and hormones influence in micropropagation success of blackberry cv. ‘Chester’. Res. J. Biotechnol. 2021, 16, 103–108. [Google Scholar]
- Bairu, M.W.; Aremu, A.O.; Van Staden, J. Somaclonal variation in plants: Causes and detection methods. Plant Growth Regul. 2011, 63, 147–173. [Google Scholar] [CrossRef]
- Krishna, H.; Alizadeh, M.; Singh, D.; Singh, U.; Chauhan, N.; Eftekhari, M.; Sadh, R.K. Somaclonal variations and their applications in horticultural crops improvement. 3 Biotech 2016, 6, 54. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.D.S.; Rocha, A.d.J.; Nascimento, F.d.S.; Oliveira, W.D.d.S.; Soares, J.M.d.S.; Rebouças, T.A.; Morais Lino, L.S.; Haddad, F.; Ferreira, C.F.; Santos-Serejo, J.A.d.; et al. The Role of Somaclonal Variation in Plant Genetic Improvement: A Systematic Review. Agronomy 2023, 13, 730. [Google Scholar] [CrossRef]
- Smulders, M.J.M.; de Klerk, G.J. Epigenetics in plant tissue culture. Plant Growth Regul. 2011, 63, 137–146. [Google Scholar] [CrossRef]
- Moharana, A.; Das, A.; Subudhi, E.; Naik, S.K.; Barik, D.P. High frequency shoot proliferation from cotyledonary node of Lawsonia inermis L. and validation of their molecular finger printing. J. Crop Sci. Biotechnol. 2017, 20, 405–416. [Google Scholar] [CrossRef]
- Borsai, O.; Hârța, M.; Szabo, K.; Kelemen, C.D.; Andrecan, F.A.; Codrea, M.M.; Clapa, D. Evaluation of genetic fidelity of in vitro-propagated blackberry plants using RAPD and SRAP molecular markers. Hortic. Sci. 2020, 47, 21–27. [Google Scholar] [CrossRef]
- Clapa, D.; Hârța, M. Establishment of an Efficient Micropropagation System for Humulus lupulus L. cv. Cascade and Confirmation of Genetic Uniformity of the Regenerated Plants through DNA Markers. Agronomy 2021, 11, 2268. [Google Scholar] [CrossRef]
- Longchar, T.B.; Deb, C.R. Optimization of in vitro propagation protocol of Dendrobium heterocarpum Wall. ex. Lindl. and clonal genetic fidelity assessment of the regenerates: An orchid of horticultural and medicinal importance. S. Afr. J. Bot. 2022, 149, 67–78. [Google Scholar] [CrossRef]
- Rai, M.K. Start codon targeted (SCoT) polymorphism marker in plant genome analysis: Current status and prospects. Planta 2023, 257, 34. [Google Scholar] [CrossRef]
- Teleky, B.-E.; Mitrea, L.; Plamada, D.; Nemes, S.A.; Călinoiu, L.-F.; Pascuta, M.S.; Varvara, R.-A.; Szabo, K.; Vajda, P.; Szekely, C.; et al. Development of Pectin and Poly(vinyl alcohol)-Based Active Packaging Enriched with Itaconic Acid and Apple Pomace-Derived Antioxidants. Antioxidants 2022, 11, 1729. [Google Scholar] [CrossRef]
- Mitrea, L.; Teleky, B.E.; Leopold, L.F.; Nemes, S.A.; Plamada, D.; Dulf, F.V.; Pop, I.D.; Vodnar, D.C. The physicochemical properties of five vegetable oils exposed at high temperature for a short-time-interval. J. Food Compos. Anal. 2022, 106, 104305. [Google Scholar] [CrossRef]
- Lodhi, M.A.; Guang-Ning, Z.; Weeden, F.N.F.; Reisch, B.I. A simple and efficient method for DNA extraction from grapevine cultivars and Vitis species. Plant Mol. Biol. Rep. 1994, 12, 6–13. [Google Scholar] [CrossRef]
- Pop, R.; Ardelean, M.; Pamfil, D.; Gaboreanu, I.M. The efficiency of different DNA isolation and purification in ten cultivars of Vitis vinifera. Bul. USAMV CN(ZB) 2003, 59, 259–261. [Google Scholar]
- Bodea, M.; Pamfil, D.; Pop, R.; Sisea, R.C. DNA isolation from desiccated leaf material from plum tree (Prunus domestica L.) molecular analysis. Bul. UASVM CN (H) 2016, 1, 1–2. [Google Scholar] [CrossRef]
- Li, G.; Quiros, C. Sequence-related amplified polymorphism (SRAP), a new marker system based on a simple PCR reaction: Its application to mapping and gene tagging in Brassica. Theor. Appl. Genet. 2001, 103, 455–461. [Google Scholar] [CrossRef]
- Collard, B.C.Y.; Mackill, D.J. Start Codon Targeted (SCoT) Polymorphism: A Simple, Novel DNA Marker Technique for Generating Gene-Targeted Markers in Plants. Plant Mol. Biol. Rep. 2009, 27, 86–93. [Google Scholar] [CrossRef]
- ANOVA and Duncan’s Test PC Program for Variant Analyses Made for Completely Randomized Polyfactorial Experiences; Poli-Fact 2020 Version 4; University of Agricultural Sciences and Veterinary Medicine: Cluj-Napoca, Romania, 2020.
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. Past: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- AbdAlla, M.M.; Mostafa, R.A.A. In vitro Propagation of Blackberry (Rubus fruticosus L.). Assiut J. Agric. Sci. 2015, 46, 88–99. [Google Scholar]
- Fira, A.; Magyar-Tábori, K.; Hudák, I.; Clapa, D.; Dobránszky, J. Effect of gelling agents on in vitro development of Amelanchier canadensis ‘Rainbow Pillar’. Int. J. Hort. Sci. 2013, 19, 75–79. [Google Scholar] [CrossRef]
- Xu, Y.; Stokes, J.R. Soft lubrication of model shear-thinning fluids. Tribol. Int. 2020, 152, 106541. [Google Scholar] [CrossRef]
- Zhang, L.; Che, L.; Zhou, W.; Chen, X.D. Rheological behavior of agar solution in relation to the making of instant edible bird’s nest products. Int. J. Food Eng. 2012, 8. [Google Scholar] [CrossRef]
- Alam, K.; Iqbal, M.; Hasan, A.; Al-Maskari, N. Rheological characterization of biological hydrogels in aqueous state. J. Appl. Biotechnol. Rep. 2020, 7, 172–176. [Google Scholar] [CrossRef]
- You, K.M.; Murray, B.S.; Sarkar, A. Rheology and tribology of starch + κ-carrageenan mixtures. J. Texture Stud. 2021, 52, 16–24. [Google Scholar] [CrossRef]
- Iskakova, J.; Smanalieva, J.; Methner, F.J. Investigation of changes in rheological properties during processing of fermented cereal beverages. J. Food Sci. Technol. 2019, 56, 3980–3987. [Google Scholar] [CrossRef]
- Jain, N.; Babbar, S.B. Gum katira–a cheap gelling agent for plant tissue culture media. Plant Cell Tissue Organ Cult. 2002, 71, 223–229. [Google Scholar] [CrossRef]
- Amlesom, W.S.; Mehari, T.; Saleh, B.K. Evaluation of Different Starches as Gelling Agents for Micropropagation of Potato. J. Agric. Sci. 2021, 13, 144. [Google Scholar] [CrossRef]
- Ebile, P.A.; Opata, J.; Hegele, S. Evaluating suitable low-cost agar substitutes, clarity, stability, and toxicity for resource-poor countries’ tissue culture media. In Vitro Cell. Dev. Biol.-Plant 2022, 58, 989–1001. [Google Scholar] [CrossRef]
- Manish, C.; Dhawni, S.; Chandra, V.; Garima, V. Molecular markers: An important tool to assess genetic fidelity in tissue culture grown long-term cultures of economically important fruit plants. Asian J. Bio Sci. 2015, 10, 101–105. [Google Scholar]
- Abdolinejad, R.; Shekafandeh, A.; Jowkar, A.; Gharaghani, A.; Alemzadeh, A. Indirect regeneration of Ficus carica by the TCL technique and genetic fidelity evaluation of the regenerated plants using flow cytometry and ISSR. Plant Cell Tssue Organ Cult. 2020, 143, 131–144. [Google Scholar] [CrossRef]
- Tyagi, S.; Rajpurohit, D.; Sharma, A. Genetic Fidelity Studies for Testing True-to-Type Plants in Some Horticultural and Medicinal Crops Using Molecular Markers. In Agricultural Biotechnology: Latest Research and Trends; Kumar Srivastava, D., Kumar Thakur, A., Kumar, P., Eds.; Springer: Singapore, 2021; pp. 147–170. [Google Scholar]
- Kessel-Domini, A.; Pérez-Brito, D.; Guzmán-Antonio, A.; Barredo-Pool, F.A.; Mijangos-Cortés, J.O.; Iglesias-Andreu, L.G.; Cortés-Velázquez, A.; Canto-Flick, A.; Avilés-Viñas, S.A.; Rodríguez-Llanes, Y.; et al. Indirect Somatic Embryogenesis: An Efficient and Genetically Reliable Clonal Propagation System for Ananas comosus L. Merr. Hybrid “MD2”. Agriculture 2022, 12, 713. [Google Scholar] [CrossRef]
- Guo, D.; Zhang, J.; Liu, C.; Zhang, G.; Li, M.; Zhang, Q. Genetic variability and relationships between and within grape cultivated varieties and wild species based on SRAP markers. Tree Genet. Genomes 2012, 8, 789–800. [Google Scholar] [CrossRef]
- Khatab, I.; Youssef, M. Micropropagation and assessment of genetic stability of Musa sp. cv. Williams using RAPD and SRAP markers. Egypt. J. Bot. 2018, 58, 371–380. [Google Scholar] [CrossRef]
- Wójcik, D.; Trzewik, A.; Kucharska, D. Field Performance and Genetic Stability of Micropropagated Gooseberry Plants (Ribes grossularia L.). Agronomy 2021, 11, 45. [Google Scholar] [CrossRef]
- Borsai, O.; Clapa, D.; Fira, A.; Hârța, M.; Szabo, K.; Dumitraș, A.F.; Pamfil, D. In vitro propagation of Aronia melanocarpa (Michx.) Elliott. Acta Hortic. 2021, 1308, 213–222. [Google Scholar] [CrossRef]
- Bekheet, S.A.; Gabr, A.M.M.; Reda, A.A.; El Bahr, M.K. Micropropagation and assessment of genetic stability of in vitro raised jojoba (Simmondsia chinensis link.) plants using SCoT and ISSR markers. Plant Tissue Cult. Biotechnol. 2015, 25, 165–179. [Google Scholar] [CrossRef]
- Kudikala, H.; Jogam, P.; Sirikonda, A.; Mood, K.; Allini, V.R. In vitro micropropagation and genetic fidelity studies using SCoT and ISSR primers in Annona reticulata L.: An important medicinal plant. Vegetos 2020, 33, 446–457. [Google Scholar] [CrossRef]
- Thakur, M.; Sharma, V.; Chauhan, A. Genetic fidelity assessment of long term in vitro shoot cultures and regenerated plants in Japanese plum cvs Santa Rosa and Frontier through RAPD, ISSR and SCoT markers. S. Afr. J. Bot. 2021, 140, 428–433. [Google Scholar] [CrossRef]

| Gelling Agent | Price/kg (Euro) | g/L Culture Medium | Price/L Culture Media (Euro) | Price Difference (Euro) |
|---|---|---|---|---|
| Wheat starch SANOVITA (used in the present study) | 2 | 50 | 0.10 | - |
| Plant agar * (used in the present studies) | 87.4 | 5 | 0.44 | 0.34 |
| Daishin agar * | 206.5 | 7 | 1.45 | 1.35 |
| Micro agar * | 111.7 | 6 | 0.67 | 0.55 |
| Phyto agar * | 101 | 6 | 0.61 | 0.51 |
| Gelrite * | 174.4 | 3 | 0.52 | 0.42 |
| Agar Sigma A1296 ** | 438 | 5.6 | 2.45 | 2.35 |
| Primer Combinations | Size Range of Bands (bp) | No. of Scorable Monomorphic Bands | |||||
|---|---|---|---|---|---|---|---|
| Čačanska Bestrna | Chester Thornless | Loch Ness | Polar | Driscoll’s Victoria | Karaka Black | ||
| Em4-Me6 | 180–1450 | 12 | 10 | 12 | 15 | 16 | 13 |
| Em7-Me1 | 150–1350 | 14 | 15 | 11 | 10 | 10 | 11 |
| Em3-Me8 | 200–2000 | 11 | 12 | 11 | 13 | 12 | 10 |
| Em5-Me4 | 150–1800 | 10 | 11 | 8 | 10 | 12 | 8 |
| Em2-Me8 | 300–2000 | 12 | 9 | 10 | 12 | 10 | 11 |
| Em8-Me5 | 150–2000 | 15 | 15 | 13 | 15 | 16 | 14 |
| Em1-Me7 | 200–1900 | 10 | 10 | 12 | 11 | 10 | 13 |
| Em6-Me3 | 200–1700 | 11 | 11 | 9 | 12 | 12 | 10 |
| Total no. of bands/cultivar | 95 | 93 | 86 | 98 | 98 | 90 | |
| Primer Name | Size Range of Bands (bp) | No. of Scorable Monomorphic Bands | |||||
|---|---|---|---|---|---|---|---|
| Čačanska Bestrna | Chester Thornless | Loch Ness | Polar | Driscoll’s Victoria | Karaka Black | ||
| SCoT-1 | 250–1900 | 8 | 10 | 8 | 11 | 11 | 9 |
| SCoT-2 | 320–2100 | 7 | 9 | 9 | 8 | 8 | 9 |
| SCoT-3 | 210–2200 | 11 | 11 | 9 | 7 | 9 | 11 |
| SCoT-4 | 300–2300 | 8 | 9 | 11 | 9 | 8 | 12 |
| SCoT-5 | 250–1800 | 13 | 12 | 13 | 11 | 12 | 11 |
| SCoT-6 | 280–2350 | 12 | 13 | 11 | 13 | 12 | 11 |
| SCoT-9 | 220–1900 | 15 | 14 | 10 | 16 | 9 | 16 |
| Total no. of bands/cultivar | 74 | 78 | 71 | 75 | 69 | 79 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clapa, D.; Hârța, M.; Szabo, K.; Teleky, B.-E.; Pamfil, D. The Use of Wheat Starch as Gelling Agent for In Vitro Proliferation of Blackberry (Rubus fruticosus L.) Cultivars and the Evaluation of Genetic Fidelity after Repeated Subcultures. Horticulturae 2023, 9, 902. https://doi.org/10.3390/horticulturae9080902
Clapa D, Hârța M, Szabo K, Teleky B-E, Pamfil D. The Use of Wheat Starch as Gelling Agent for In Vitro Proliferation of Blackberry (Rubus fruticosus L.) Cultivars and the Evaluation of Genetic Fidelity after Repeated Subcultures. Horticulturae. 2023; 9(8):902. https://doi.org/10.3390/horticulturae9080902
Chicago/Turabian StyleClapa, Doina, Monica Hârța, Katalin Szabo, Bernadette-Emőke Teleky, and Doru Pamfil. 2023. "The Use of Wheat Starch as Gelling Agent for In Vitro Proliferation of Blackberry (Rubus fruticosus L.) Cultivars and the Evaluation of Genetic Fidelity after Repeated Subcultures" Horticulturae 9, no. 8: 902. https://doi.org/10.3390/horticulturae9080902
APA StyleClapa, D., Hârța, M., Szabo, K., Teleky, B.-E., & Pamfil, D. (2023). The Use of Wheat Starch as Gelling Agent for In Vitro Proliferation of Blackberry (Rubus fruticosus L.) Cultivars and the Evaluation of Genetic Fidelity after Repeated Subcultures. Horticulturae, 9(8), 902. https://doi.org/10.3390/horticulturae9080902












