Differences in Physiological Characteristics of Green Prickly Ash Germplasm Resources in Response to Low-Temperature Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Overview of the Test Site
2.2. Testing Material
2.3. Test Method
2.3.1. Experimental Design and Sampling
2.3.2. Determination of Physiological and Biochemical Indexes
2.4. Data Statistics and Analysis
2.5. Principal Component Analysis
2.6. Cluster Analysis
3. Results
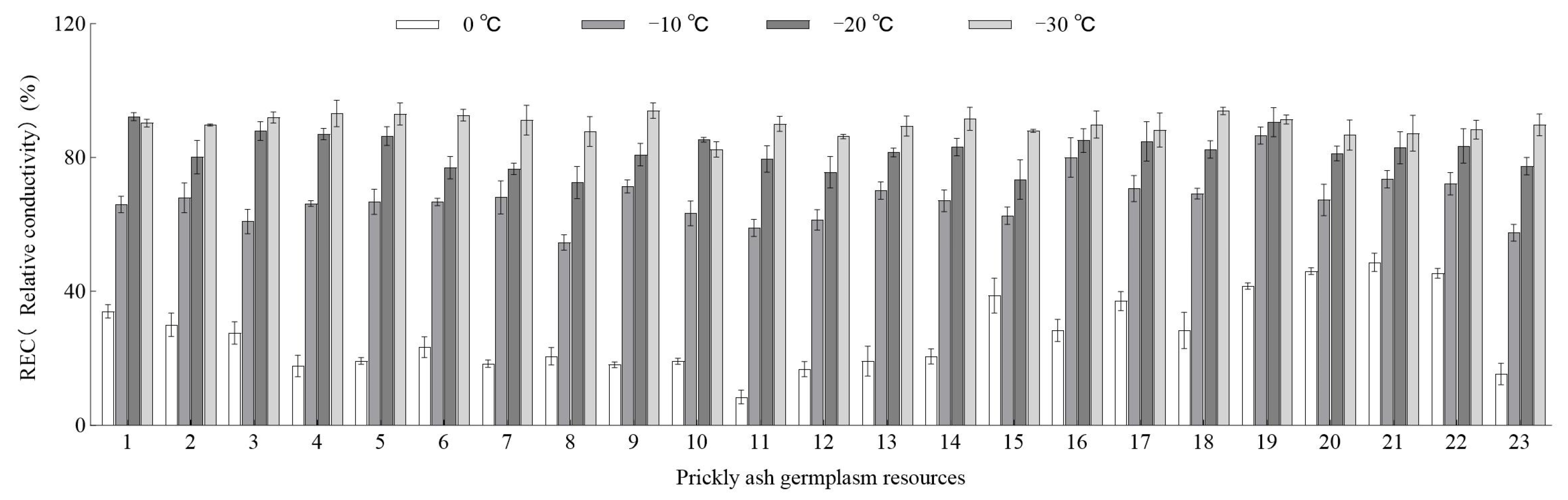
3.1. REC and LT50 of Green Prickly Ash Germplasm under Low-Temperature Stress
3.2. Effect of Low-Temperature Stress on Osmotic Adjustment Substances of Green Prickly Ash
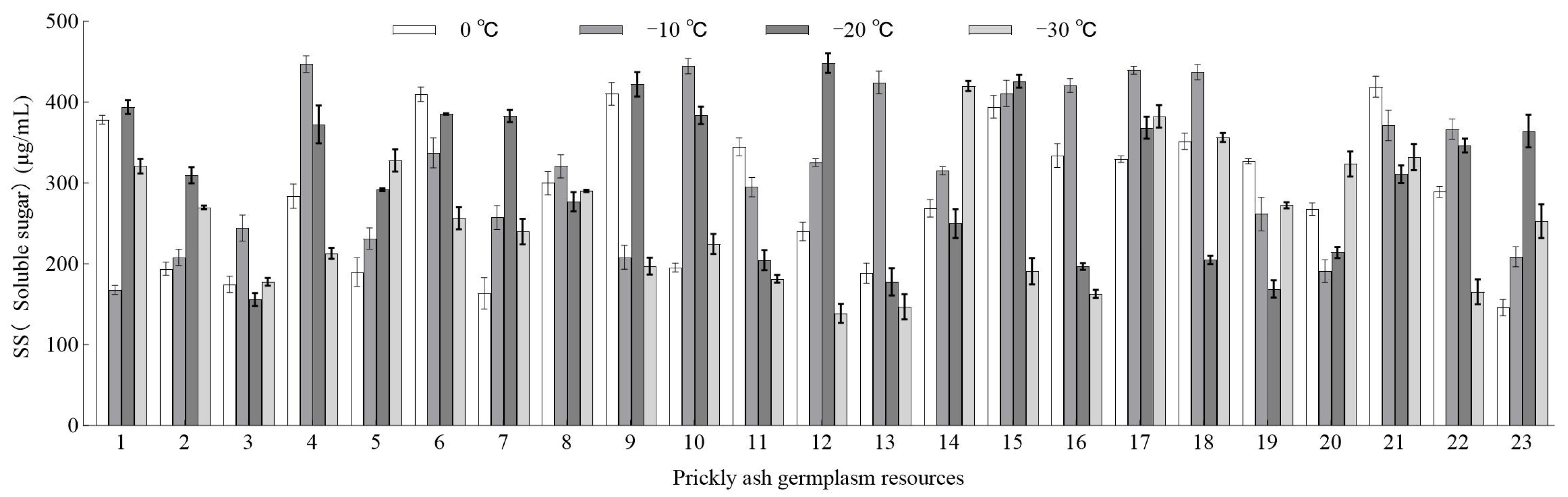
3.2.1. Effect of Low-Temperature Stress on the SS Content of Green Prickly Ash
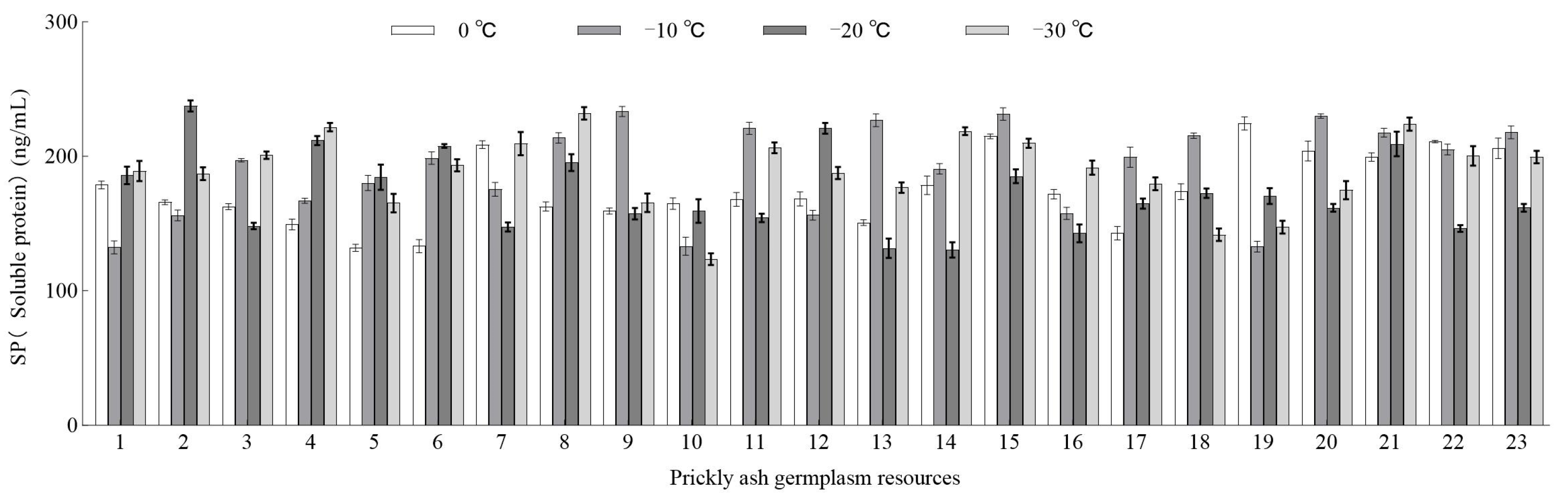
3.2.2. Effect of Low-Temperature Stress on the SP Content of Green Prickly Ash
3.2.3. Effect of Low-Temperature Stress on the PRO Content of Green Prickly Ash
3.3. Effect of Low-Temperature Stress on Antioxidant Enzyme System of Green Prickly Ash
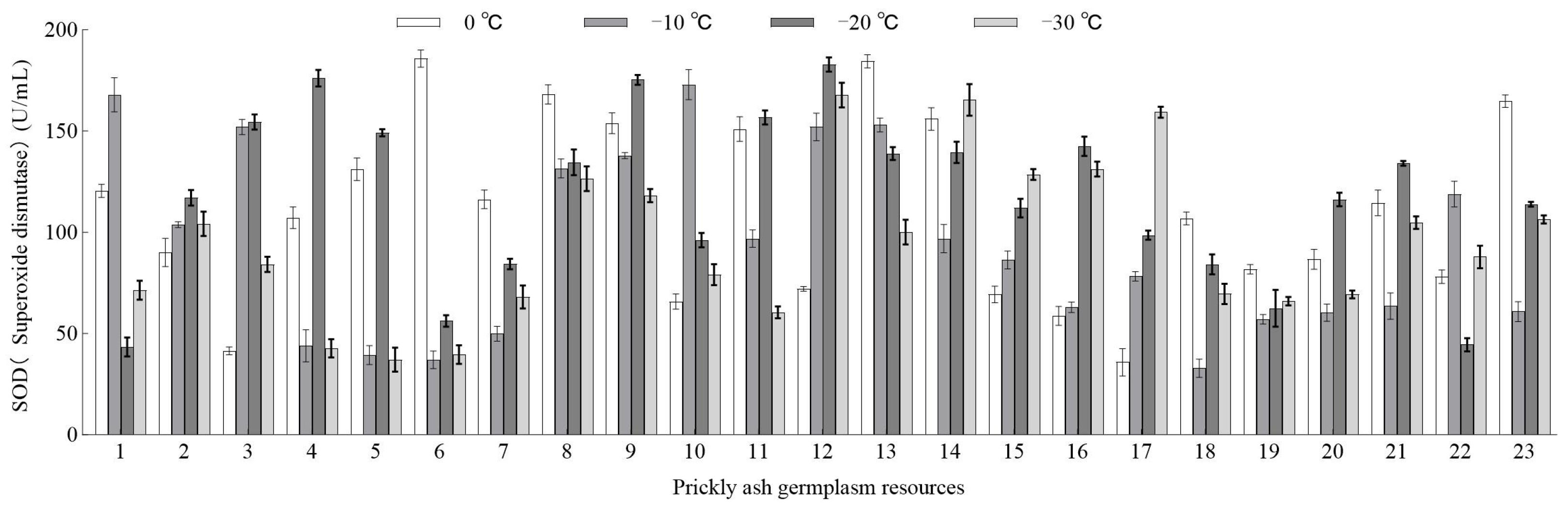
3.3.1. Effect of Low-Temperature Stress on SOD Activity of Green Prickly Ash
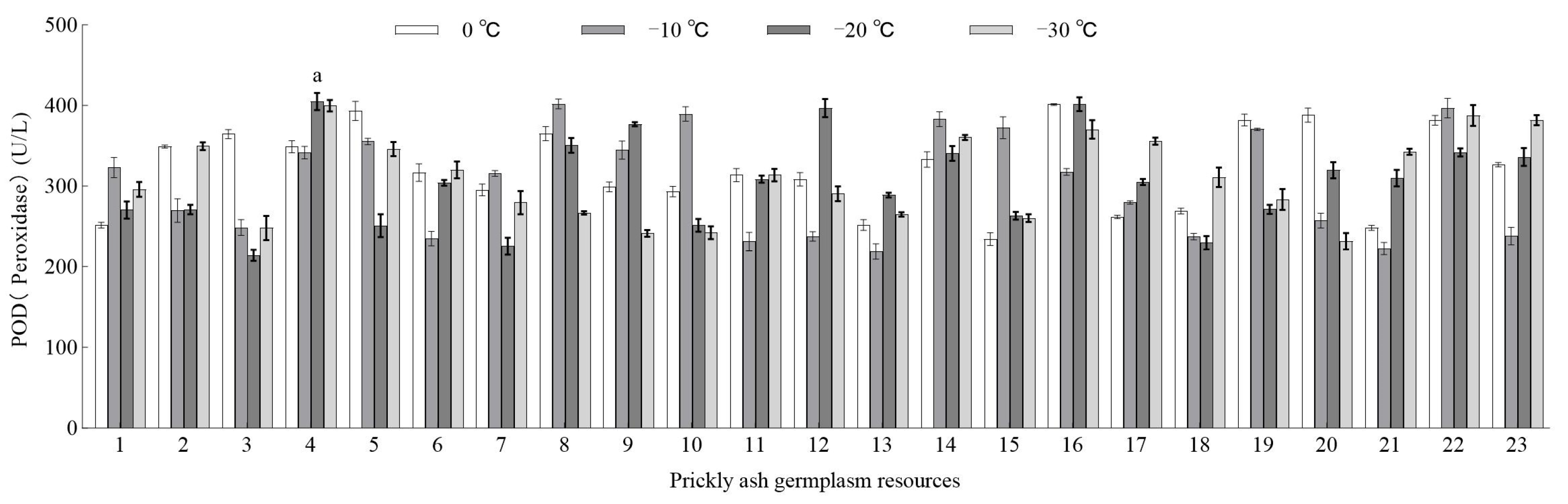
3.3.2. Effect of Low-Temperature Stress on the POD Activity of Green Prickly Ash
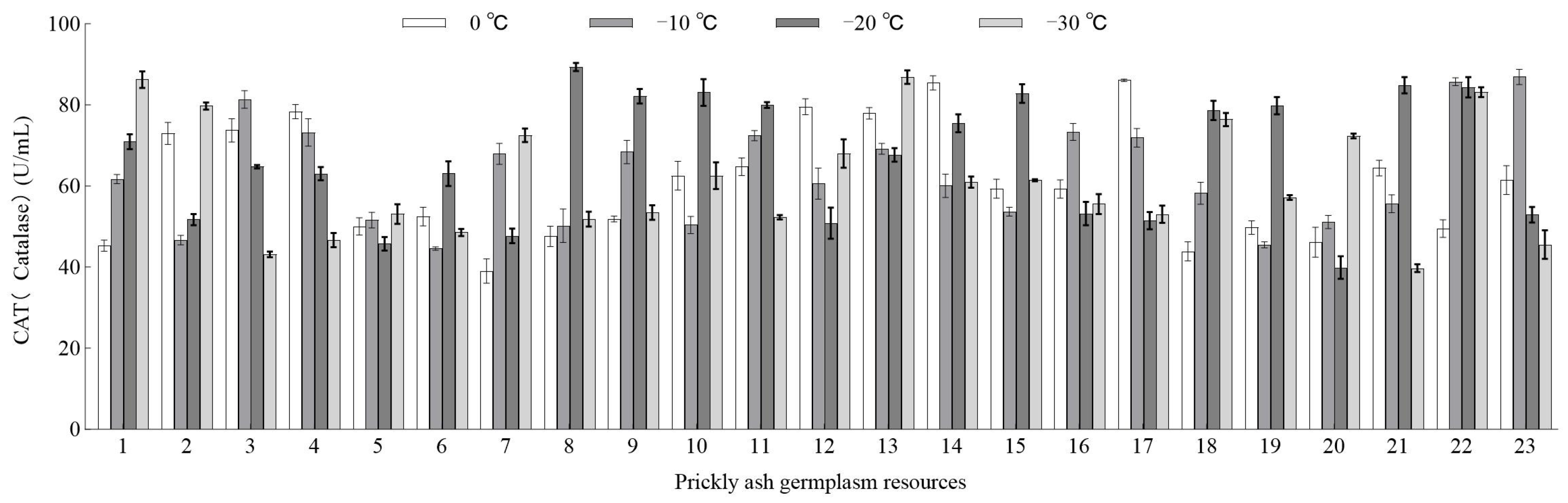
3.3.3. Effect of Low-Temperature Stress on CAT Activity of Green Prickly Ash
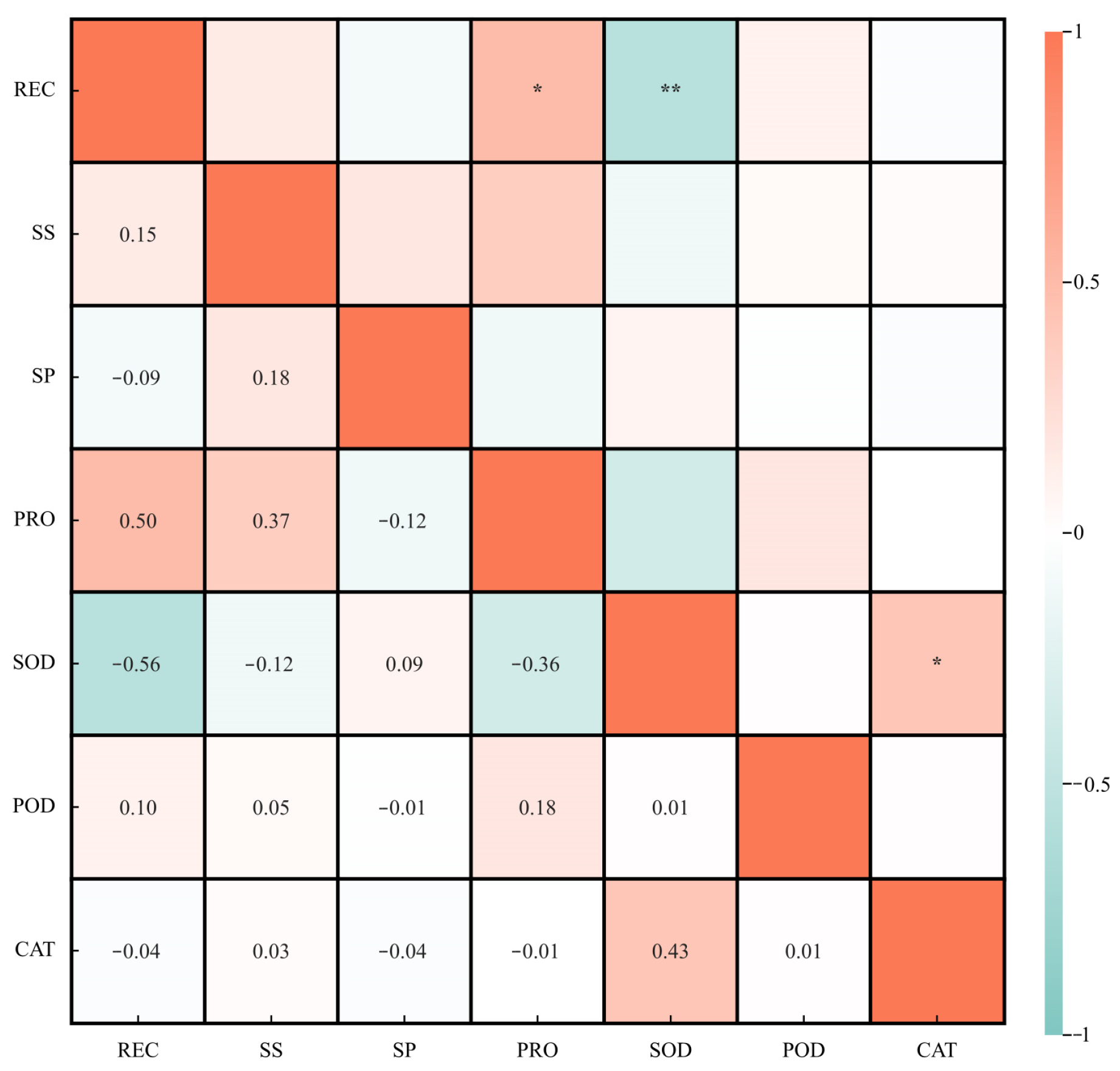
3.4. Correlation Analysis of the Subordinate Function Values of Cold Resistance Indexes of Green Prickly Ash
3.5. Comprehensive Evaluation of Cold Resistance of Green Prickly Ash Germplasm Resources
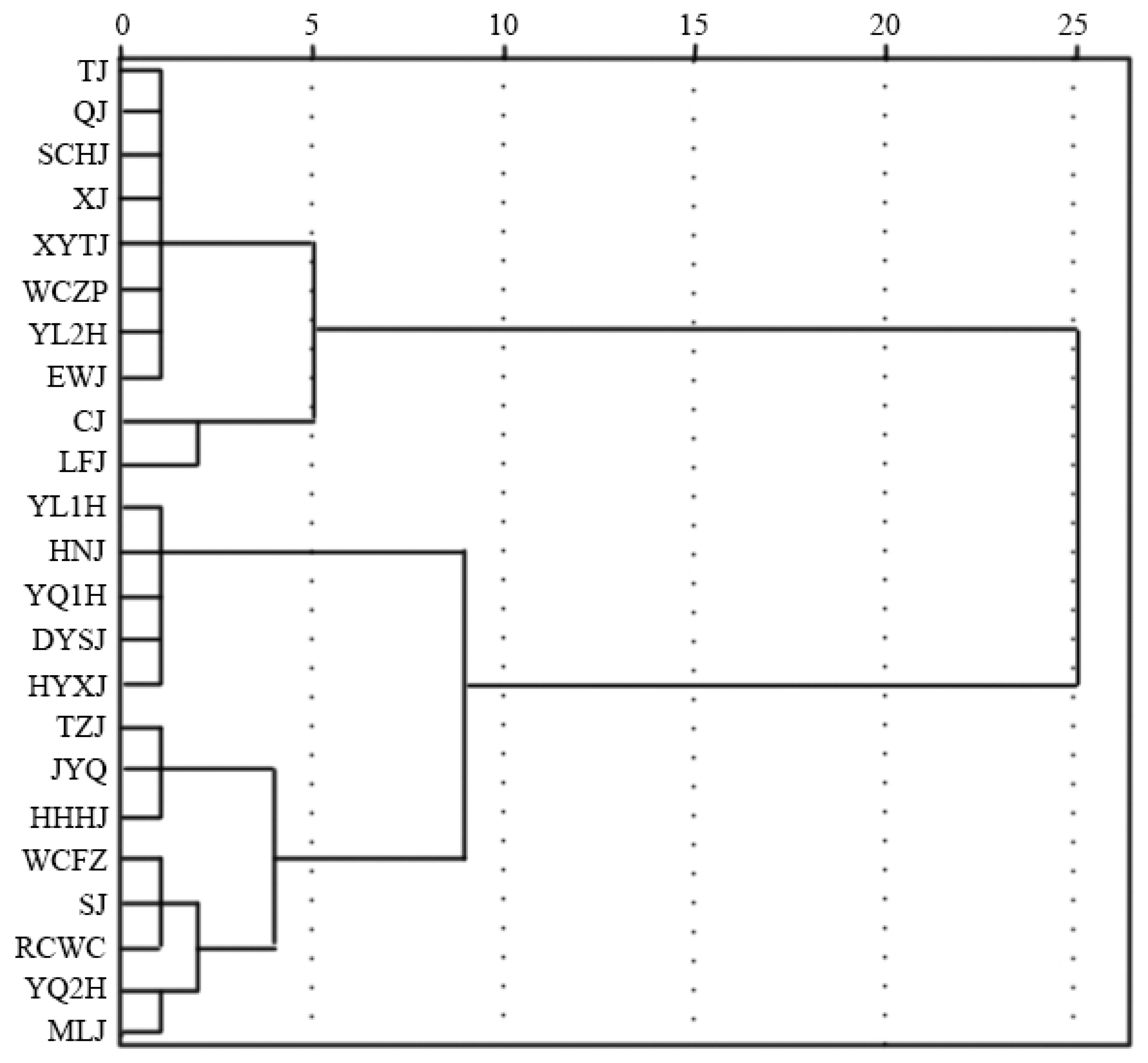
3.6. Cluster Analysis of the Physiological and Biochemical Parameters of Different Green Prickly Ash Varieties
4. Discussion
4.1. Response of Green Prickly Ash to Low-Temperature Stress
4.2. Evaluation of Cold Resistance of Green Prickly Ash Germplasm Resources
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| REC | Relative conductivity |
| LT50 | Lethal dose-50 temperature |
| SS | Soluble sugar |
| SP | Soluble protein |
| PRO | Free protein |
| SOD | Superoxide dismutase |
| POD | Peroxidase |
| CAT | Catalase |
References
- Tian, J.Y.; Ma, Y.; Tian, L.; Huang, C.; Chen, M.; Wei, A.Z. Comparative physiology and transcriptome response patterns in cold-tolerant and cold-sensitive varieties of Zanthoxylum bungeanum Maxim. Ind. Crops Prod. 2021, 167, 113562. [Google Scholar] [CrossRef]
- Tian, J.Y.; Ma, Y.; Chen, Y.B.; Chen, X.; Wei, A.Z. Plant hormone response to low-temperature stress in cold-tolerant and cold-sensitive varieties of Zanthoxylum bungeanum Maxim. Front. Plant Sci. 2022, 13, 847202. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.S.; Xu, Z.P.; Fan, R.; Wang, G.Y.; Wang, F.Q.; Qin, X.W.; Yan, L.; Ji, X.Z.; Meng, M.H.; Sim, S.L.; et al. The complex genome and adaptive evolution of polyploid Chinese pepper (Zanthoxylum armatum and Zanthoxylum bungeanum). Plant Biotechnol. J. 2023, 21, 78–96. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.J.; Liu, Z.S.; Chen, L.; Hou, N.; Yang, T.X.; Wei, A.Z. Phylogenetic relationships among cultivated Zanthoxylum species in China based on cpDNA markers. Tree Genet. Genomes 2016, 12, 451–459. [Google Scholar] [CrossRef]
- Huang, Q.Q.; Liu, X.; Sun, C.; Liu, H.W.; Zhou, H.L.; Huang, F.T.; Liu, H.; Chen, Z.X. The complete chloroplast genome of Zanthoxylum stenophyllum Hemsl. (Rutaceae), a traditional Chinese medicinal plant. Mitochondrial DNA Part B 2022, 7, 1642–1644. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.L.; Yang, S.H. Surviving and thriving: How plants perceive and respond to temperature stress. Dev. Cell 2022, 57, 947–958. [Google Scholar] [CrossRef]
- Aslam, M.; Fakher, B.; Ashraf, M.A.; Cheng, Y.; Wang, B.R.; Qin, Y. Plant low-temperature stress: Signaling and response. Agronomy 2022, 12, 702. [Google Scholar] [CrossRef]
- Song, J.S.; Lv, J.H.; Wang, J.; Wu, Y.H.; Ou, L.J. Research progress on low temperature tolerance mechanism of plants. Hunan Agric. Sci. 2019, 9, 107–113. [Google Scholar]
- Lin, Z.H.; Zhong, Q.S.; You, X.M.; Chen, Z.H.; Chen, C.S.; Shan, R.Y.; Ruan, Q.C. Effects of low temperature stress on antioxidant enzyme activities of tea plants. Tea Sci. 2018, 38, 363–371. [Google Scholar]
- Yuan, J. Effect of Organic Molecules on the Surface of Biochar on Cold Resistance of Rice Seedlings. Doctor’s Thesis, Shenyang Agricultural University, Shenyang, China, 2018. [Google Scholar]
- Wang, X.X.; Wang, C.; Zhan, Y.Y. Effects of Low Temperature Stress on Physiological Characteristics of Leaves of Solanaceae in Qinghai. J. Qinghai Univ. 2023, 41, 53–57. [Google Scholar]
- Sun, C.X.; Zhang, R.N.; Yuan, Z.Y.; Cao, H.X.; Martin, J.J. Physiology response and resistance evaluation of twenty coconut germplasm resources under low temperature stress. Horticulturae 2021, 7, 234. [Google Scholar] [CrossRef]
- Liu, D.L.; Zhang, B.Y.; Sun, H.M.; Peng, S.B.; Zhu, H.L. Comprehensive evaluation on cold resistance of early fruiting walnut cultivars. Acta Hortic. Sin. 2015, 42, 545–553. [Google Scholar]
- Cao, H.X.; Sun, C.X.; Shao, H.B.; Lei, X.T. Effects of low temperature and drought on the physiological and growth changes in oil palm seedlings. Afr. J. Biotechnol. 2011, 10, 2630–2637. [Google Scholar]
- Liu, B.; Wang, Y.K. Determination of Low Semi—Lethal Temperature of Pepper Branches by Logistic Equation. J. Gansu Agric. Univ. 2005, 4, 475–479. [Google Scholar]
- Lv, X.J.; Yang, T.X.; He, X.H.; Wei, A.Z.; Feng, S.J.; Wang, Y.; Qi, J.H. Influence of low temperature stress on the cold-resistance physiological indexes of Zanthoxylum bungeanum in winter. Acta Agric. Boreali-Occident. Sin. 2013, 22, 143–148. [Google Scholar]
- Lu, Y.Z.; Hu, Y.G.; Li, P.P. Consistency of electrical and physiological properties of tea leaves on indicating critical cold temperature. Biosyst. Eng. 2017, 159, 89–96. [Google Scholar] [CrossRef]
- Liu, X.M.; Xu, Q.L.; Li, Q.Q.; Zhang, H.; Xiao, J.X. Physiological responses of the two blueberry cultivars to inoculation with an arbuscular mycorrhizal fungus under low-temperature stress. J. Plant Nutr. 2017, 40, 2562–2570. [Google Scholar] [CrossRef]
- Huang, Z.; He, J.; Xia, D.; Zhong, X.J.; Li, X.; Sun, L.X.; Cai, S.Z. Evaluation of physiological responses and tolerance to low-temperature stress of four Iceland poppy (Papaver nudicaule) varieties. J. Plant Interact. 2016, 11, 117–123. [Google Scholar] [CrossRef][Green Version]
- Ding, Y.L.; Shi, Y.T.; Yang, S.H. Advances and challenges in uncovering cold tolerance regulatory mechanisms in plants. New Phytol. 2019, 222, 1690–1704. [Google Scholar] [CrossRef]
- Wang, Y.X.; Hu, Y.; Chen, B.H.; Zhu, Y.F.; Dawuda, M.M.; Svetia, S. Physiological mechanisms of resistance to cold stress associated with 10 elite apple rootstocks. J. Integr. Agric. 2018, 17, 857–866. [Google Scholar] [CrossRef]
- Chen, X.; Tian, L.; Tian, J.Y.; Wang, G.; Gong, X.; Feng, S.J.; Wei, A.Z. Extensive sampling provides new insights into phylogenetic relationships between wild and domesticated Zanthoxylum species in China. Horticulturae 2022, 8, 440. [Google Scholar]
- Pan, C.P.; Xie, H.J.; Wang, Y.Q.; Zhang, H.; Deng, Q.X.; Yang, Z.W.; Wen, L.; He, S.S. Physiological responses and evaluation of cold resistance under cold stress for six varieties of Eriobotrya japonica (Thunb.). Chin. J. Trop. Crops 2019, 40, 2369–2374. [Google Scholar]
- Ramazan, S.; Qazi, H.A.; Dar, Z.A.; John, R. Low temperature elicits differential biochemical and antioxidant responses in maize (Zea mays) genotypes with different susceptibility to low temperature stress. Physiol. Mol. Biol. Plants 2021, 27, 1395–1412. [Google Scholar] [PubMed]
- Zhong, P.; Liu, J.; Wang, J.L.; Chang, B.W. Physiological responses and cold resistance evaluation of peanut under low-temperature stress. J. Nucl. Agric. Sci. 2018, 32, 1195–1202. [Google Scholar]
- Hou, R.P.; Jiang, J.L.; Yang, N.; Li, L.; Deng, J.R.; Ding, D.K.; Sun, W.; Dong, Y.X. Physiological response and cold resistance evaluation of Eight species of Citrus under low temperature stress. Mol. Plant Breed. 2021, 19, 2013–2022. [Google Scholar]
- Xue, S.; Rao, L.S.; Zuo, D.D.; Wang, F.L.; Lin, S.Z. Research progress on the response mechanism of plants to low temperature stress. J. Anhui Agric. Sci. 2016, 44, 17–19, 48. [Google Scholar]
- Yang, T.X.; Wei, A.Z.; Li, X.; Wei, Y. Changes of cold resistance and tissue water and osmoregulation substances of Zanthoxylum bungeanum Maxim. during winter. Plant Physiol. Commun. 2010, 46, 579–582. [Google Scholar]
- Yang, Y.F. Cold Resistance of Zanthoxylum bungeanum Shoots. Master’s Thesis, Gansu Agricultural University, Lanzhou, China, 2009. [Google Scholar]
- Tajvar, Y.; Ghazvini, R.F.; Hamidoghli, Y.; Sajedi, R.H. Antioxidant changes of Thomson navel orange (Citrus sinensis) on three rootstocks under low temperature stress. Hortic. Environ. Biotechnol. 2011, 52, 576–580. [Google Scholar] [CrossRef]
- Radynk, M.S.; Domanskaya, I.N.; Shcherbakov, R.A.; Shalygo, N.V. Effect of low above-zero temperature on the content of low-molecular antioxidants and activities of antioxidant enzymes in green barley leaves. Russ. J. Plant Physiol. 2009, 56, 175–180. [Google Scholar]
- Ma, H.; Zhang, G.Z.; Zhang, X.J. Effect of low temperature stress on protective enzyme system of pepper at flowering stage. Pract. For. Technol. 2012, 12, 11–13. [Google Scholar]
- Liu, B.B.; Chen, L.N.; Niu, J.; Li, H.X.; Zhang, J.; Cao, S.Y. Selection of methods for evaluation on cold tolerance of six pomegranate varieties. J. Fruit Sci. 2018, 35, 66–73. [Google Scholar]
- Xiang, K.; Zhang, M.Y.; Xu, Y.; Wang, X.F.; Yue, L.X. Cold-tolerance of walnut cultivars: A comprehensive evaluation. Chin. J. Appl. Ecol. 2011, 22, 2325–2330. [Google Scholar]
- Liu, S.Y.; Zhang, M.Z.; Wang, H.T.; Liu, X.M.; Zhan, Z.C. Evaluation of the cold resistance capability of three different cultivars of Zanthoxylum bungeanum. J. For. Environ. 2021, 41, 388–395. [Google Scholar]
- Liu, S.Y.; Zhang, C.M.; Xu, P.; Wang, H.T.; Liu, X.M. Evaluation on cold resistance of Zanthoxylum bungeanum cultivars based on hydraulic conductivity. Shandong Acad. Agric. Sci. 2021, 53, 58–63. [Google Scholar]
- Liu, B. Cold Resistance Characteristics of Zanthoxylum bungeanum. Master’s Thesis, Gansu Agricultural University, Lanzhou, China, 2005. [Google Scholar]
- Liu, L. Research on Cold and Drought Resistance of Zanthoxylum bungeanum. Master’s Thesis, Northwest A&F University, Xianyang, China, 2009. [Google Scholar]


| No. | Variety | Sampling Locality | No. | Variety | Sampling Locality |
|---|---|---|---|---|---|
| 1 | RCWC | Rongchang, Chongqing | 13 | HHHJ | Yongshan, Yunnan |
| 2 | WCFZ | Yongchuan, Chongqing, | 14 | LFJ | Yongshan, Yunnan |
| 3 | YL1H | Kunming, Yunnan | 15 | TZJ | Jiangjin, Chongqing |
| 4 | YL2H | Kunming, Yunnan | 16 | JYQ | Jiangjin, Chongqing |
| 5 | YQ1H | Zhaotong, Yunnan | 17 | XJ | Yaan, Sichuan |
| 6 | YQ2H | Zhaotong, Yunnan | 18 | SJ | Jiangjin, Chongqing |
| 7 | HNJ | Honghe, Yunnan | 19 | HYXJ | Qujing, Yunnan |
| 8 | CJ | Yueyang, Hunan | 20 | DYSJ | Honghe, Yunnan |
| 9 | XYTJ | Jiangjin, Chongqing | 21 | QJ | Hanyuan, Sichuan |
| 10 | MLJ | Yingshan, Sichuan | 22 | EWJ | Jiangjin, Chongqing |
| 11 | TJ | Hongya, Sichuan | 23 | SCHJ | Qujing, Yunnan |
| 12 | WCZP | Yongchuan, Chongqing |
| Variety | Logistic Equation | LT50 (°C) | Degree of Fitting |
|---|---|---|---|
| RCWC | y = 100/(1 + 1.49e0.11x) | −3.78 | 0.98 * |
| WCFZ | y = 100/(1 + 1.80e0.10x) | −6.04 | 0.98 * |
| YL1H | y = 100/(1 + 2.20e0.12x) | −6.69 | 0.99 ** |
| YL2H | y = 100/(1 + 3.12e0.14x) | −8.31 | 0.98 * |
| YQ1H | y = 100/(1 + 2.88e0.13x) | −7.99 | 0.98 * |
| YQ2H | y = 100/(1 + 2.56e0.12x) | −8.04 | 0.97 * |
| HNJ | y = 100/(1 + 2.96e0.12x) | −9.12 | 0.95 * |
| CJ | y = 100/(1 + 3.20e0.11x) | −10.84 | 0.99 * |
| XYTJ | y = 100/(1 + 3.00e0.13x) | −8.24 | 0.96 * |
| MLJ | y = 100/(1 + 2.52e0.10x) | −9.07 | 0.94 * |
| TJ | y = 100/(1 + 6.20e0.15x) | −12.37 | 0.96 * |
| WCZP | y = 100/(1 + 3.28e0.11x) | −10.81 | 0.96 * |
| HHHJ | y = 100/(1 + 2.57e0.11x) | −8.31 | 0.95 * |
| LFJ | y = 100/(1 + 2.66e0.12x) | −8.08 | 0.97 * |
| TZJ | y = 100/(1 + 1.51e0.08x) | −5.22 | 0.99 ** |
| JYQ | y = 100/(1 + 1.43e0.10x) | −3.67 | 0.92 * |
| XJ | y = 100/(1 + 1.28e0.09x) | −2.92 | 0.97 * |
| SJ | y = 100/(1 + 2.05e0.12x) | −6.11 | 0.98 * |
| HYXJ | y = 100/(1 + 0.76e0.09x) | 3.16 | 0.92 * |
| DYSJ | y = 100/(1 + 0.87e0.07x) | 2.24 | 0.97 * |
| QJ | y = 100/(1 + 1.05e0.07x) | −0.72 | 0.99 ** |
| EWJ | y = 100/(1 + 0.99e0.07x) | 0.13 | 0.98 * |
| SCHJ | y = 100/(1 + 4.00e0.13x) | −11.02 | 0.98 * |
| Ingredient | Principal Component Eigenvalues | ||
|---|---|---|---|
| Characteristic Value λ | Proportion of Factors % | Total Percentage % | |
| 1 | 2.16 | 30.90 | 30.90 |
| 2 | 1.27 | 18.18 | 49.08 |
| 3 | 1.14 | 16.31 | 65.38 |
| Physiological Index | Weight Coefficient of Each Index under Each Principal Component | ||
|---|---|---|---|
| W1 | W2 | W3 | |
| REC | 0.80 | 0.02 | −0.16 |
| SS | 0.41 | 0.56 | 0.45 |
| SP | 0.13 | 0.23 | 0.83 |
| PRO | 0.77 | 0.34 | −0.13 |
| SOD | −0.79 | 0.43 | −0.10 |
| POD | 0.19 | 0.37 | −0.24 |
| CAT | −0.31 | 0.69 | −0.39 |
| Variety | Subordinate Function Values | Ranking | |||||||
|---|---|---|---|---|---|---|---|---|---|
| REC | SS | SP | PRO | SOD | POD | CAT | Average | ||
| RCWC | 0.37 | 0.66 | 0.36 | 0.33 | 0.43 | 0.24 | 0.62 | 0.43 | 16 |
| WCFZ | 0.57 | 0.30 | 0.57 | 0.11 | 0.47 | 0.44 | 0.49 | 0.42 | 17 |
| YL1H | 0.56 | 0.00 | 0.44 | 0.26 | 0.52 | 0.11 | 0.61 | 0.36 | 20 |
| YL2H | 0.62 | 0.73 | 0.58 | 0.37 | 0.32 | 0.97 | 0.59 | 0.60 | 5 |
| YQ1H | 0.60 | 0.38 | 0.28 | 0.15 | 0.28 | 0.66 | 0.00 | 0.34 | 22 |
| YQ2H | 0.68 | 0.83 | 0.53 | 0.73 | 0.16 | 0.31 | 0.08 | 0.47 | 14 |
| HNJ | 0.75 | 0.38 | 0.55 | 0.31 | 0.16 | 0.19 | 0.26 | 0.37 | 19 |
| CJ | 1.00 | 0.57 | 0.77 | 0.13 | 0.92 | 0.75 | 0.38 | 0.65 | 2 |
| XYTJ | 0.61 | 0.63 | 0.47 | 0.43 | 1.00 | 0.49 | 0.54 | 0.60 | 6 |
| MLJ | 0.80 | 0.65 | 0.00 | 0.35 | 0.46 | 0.31 | 0.57 | 0.45 | 15 |
| TJ | 0.98 | 0.36 | 0.58 | 0.43 | 0.62 | 0.30 | 0.67 | 0.56 | 9 |
| WCZP | 0.94 | 0.52 | 0.53 | 0.25 | 0.97 | 0.43 | 0.57 | 0.60 | 4 |
| HHHJ | 0.67 | 0.24 | 0.36 | 0.21 | 0.97 | 0.00 | 0.99 | 0.49 | 13 |
| LFJ | 0.64 | 0.65 | 0.48 | 0.51 | 0.91 | 0.81 | 0.80 | 0.69 | 1 |
| TZJ | 0.64 | 0.87 | 0.90 | 0.00 | 0.41 | 0.22 | 0.55 | 0.51 | 11 |
| JYQ | 0.36 | 0.47 | 0.29 | 0.68 | 0.40 | 0.96 | 0.40 | 0.51 | 12 |
| XJ | 0.39 | 1.00 | 0.37 | 1.00 | 0.33 | 0.37 | 0.61 | 0.58 | 7 |
| SJ | 0.49 | 0.78 | 0.42 | 0.52 | 0.08 | 0.05 | 0.55 | 0.41 | 18 |
| HYXJ | 0.00 | 0.36 | 0.33 | 0.73 | 0.00 | 0.58 | 0.31 | 0.33 | 23 |
| DYSJ | 0.39 | 0.32 | 0.66 | 0.46 | 0.21 | 0.36 | 0.09 | 0.35 | 21 |
| QJ | 0.24 | 0.89 | 1.00 | 0.69 | 0.47 | 0.20 | 0.43 | 0.56 | 10 |
| EWJ | 0.28 | 0.54 | 0.63 | 0.67 | 0.20 | 1.00 | 1.00 | 0.62 | 3 |
| SCHJ | 0.94 | 0.29 | 0.71 | 0.54 | 0.56 | 0.53 | 0.45 | 0.57 | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, L.; Dong, X.; Fu, H.; Chai, X.; Bao, S.; Ren, Y.; Hu, K.; Li, Q.; Chen, Z. Differences in Physiological Characteristics of Green Prickly Ash Germplasm Resources in Response to Low-Temperature Stress. Horticulturae 2023, 9, 1242. https://doi.org/10.3390/horticulturae9111242
Shi L, Dong X, Fu H, Chai X, Bao S, Ren Y, Hu K, Li Q, Chen Z. Differences in Physiological Characteristics of Green Prickly Ash Germplasm Resources in Response to Low-Temperature Stress. Horticulturae. 2023; 9(11):1242. https://doi.org/10.3390/horticulturae9111242
Chicago/Turabian StyleShi, Lin, Xixi Dong, Hao Fu, Xingying Chai, Shuqin Bao, Yun Ren, Kai Hu, Qiang Li, and Zexiong Chen. 2023. "Differences in Physiological Characteristics of Green Prickly Ash Germplasm Resources in Response to Low-Temperature Stress" Horticulturae 9, no. 11: 1242. https://doi.org/10.3390/horticulturae9111242
APA StyleShi, L., Dong, X., Fu, H., Chai, X., Bao, S., Ren, Y., Hu, K., Li, Q., & Chen, Z. (2023). Differences in Physiological Characteristics of Green Prickly Ash Germplasm Resources in Response to Low-Temperature Stress. Horticulturae, 9(11), 1242. https://doi.org/10.3390/horticulturae9111242






