Abstract
Several Xylella fastidiosa subsp. fastidiosa (ST1) strains that cause Pierce’s disease were isolated from grapevine in Spain. In this study, we applied an approach to assess PD susceptibility among 24 different well-known Vitis vinifera subsp. vinifera cultivars and five rootstocks belonging to different species of the genus Vitis. Both were commonly commercialized, representing about 75% of the cultivated area in Spain. This method incorporated disease severity, disease progression, and water potential from the stem xylem. The trials were carried out under field and greenhouse conditions. The virulence of the Xff strain XYL 2055/17 was significantly higher than that of strain XYL 2177/18. However, while this difference in strain virulence did not seem to modify the susceptibility profiles of the cultivars, disease severity could be climate dependent. This work established two significantly different groups of European cultivars of grapevine characterized by high and low susceptibility to Xff ST1: cultivars with high susceptibility, including reference cultivars such as Tempranillo and Tempranillo Blanco, and cultivars with high resistance, such as Hondarrabi Zuri and Cabernet Sauvignon. Cultivar susceptibility was independent of the rootstock on which they were grafted. No conclusive data were found regarding the potential of water loss as an early detection test prior to symptom onset. This study provides a framework with which to advance cultivar susceptibility studies under different environmental conditions.
1. Introduction
Xylella fastidiosa (Xf) [1] is a vascular plant pathogen with an extremely large host range [2]. To date, this species has been found to infect 690 plant species classified under 306 genera and 88 families (EFSA, 2023; Sicard, 2018; Trkulja, 2022) [2,3,4]. Major plant hosts (https://gd.eppo.int/taxon/XYLEFA/hosts (accessed on 8 October 2023)) include two ornamental species (Nerium oleander and Polygala myrtifolia) and six major crops: citrus, coffee, almond, peach, olive, and grapevine (Vitis vinifera). The diverse diseases caused by Xf have significant economic impacts and agricultural management consequences [5]. Since 2019, Xf has been included as a Priority Pest under European Regulations (Regulation (EU) 2019/1702). One of the characteristics that makes Xf a very dangerous pathogen, besides its very high genetic plasticity, is its ability to be transmitted by likely all species of the two main xylem-feeder insect groups: leafhoppers (Cicadellidae subfamily Cicadellinae) and spittlebugs (Cercopoidea families, Aphrophoridae, Cercopidae, and Clastopteridae) [3].
From a taxonomic point of view, Xf is a complex species with several subspecies and sequence types (ST). X. fastidiosa subsp. fastidiosa (Xff), which belongs to ST1, causes Pierce’s disease (PD) and diverse syndromes in several plant hosts [6,7]. PD was first described in California in 1892, where it remains a significant problem for the grape industry. A devastating PD outbreak in the Temecula Valley in Southern California during the late 1990s was associated with the establishment of a very efficient vector in the area, which prompted an intense investigation of the pathogen and the development of programs to manage PD and the vectors involved [3]. Control of this disease is difficult and, despite significant projected savings of around USD 200 million under the PD Control Program [8], a corresponding increase in efficacy will require the deployment of diverse improved strategies that should include the management of water stress and appropriate plant resistance [6,9].
The main factors causing PD symptoms appear to be related to water stress and the blockage of xylem vessels by the bacterial biofilm, as well as the resulting production of tyloses and gums by the plant, which causes hydraulic dysfunctions, leading to desiccation and plant death within a few years [10,11]. The development of symptoms may not be entirely due to the vessel’s occlusion. Instead, the pathogenesis may be more complex and occur starting from the earliest stages of infection, before the colonization of vessels [12,13]. The time period between inoculation and the appearance of symptoms in plants varies depending on the plant species and age and occurs between three and four months, extending beyond a year after the initial infection [13], with longer periods observed in woody plants compared to herbaceous plants [14]. The symptoms typically begin when environmental conditions are generally hot and dry and plants are subjected to water stress and other physiological responses, which vary in different varieties [15,16].
Several techniques are available to assess the water status of plants via physiological indicators [17,18]. Leaf water potential is recognized as one of the most important indexes to evaluate the water status of plants, providing high theoretical value and important information for multiple applications to quantify critical physiological processes, including drought responses [19,20,21]. Predawn leaf water potential (PLWP) and stem water potential (SWP) were found to be simple and precise indicators for assessing the grapevine water status [22,23,24,25]. Drought tolerance is known to be determined in part by the factors related to water transport within the plant, and there is a direct relationship between hydraulic conductivity and the xylem physiological status [26]. The data provided by this technology suggest early infection indicators that may be related to PD resistance factors. Moreover, the water status data obtained via destructive measurement were correlated with the data provided by other nondestructive and portable devices applied in the open field for grapevine [27].
PD resistance has only been identified in several wild grape species endemic to the Americas, including V. arizonica/candicans and Muscadinia rotundifolia, whereas all assayed European V. vinifera genotypes are susceptible to this disease [28,29,30,31,32]. Recent research has compared PD tolerance/resistance within the three major Eurasian pedigree lineages and provides a benchmark for PD susceptibility levels for some of the most widespread table and wine grape cultivars [33]. The complexity of the genomic architecture of resistance to the bacterium and the role of climate in shaping this resistance [32] have also required the use of additional research efforts to evaluate a larger number of V. vinifera cultivars from different genetic pools under different environmental conditions. These studies obtained information on the vascular and anatomical characteristics of these grapevine cultivars to develop tools for assessing and predicting the PD susceptibility [10].
After the first outbreak in 2013 in olive trees [34], on the Balearic Islands of Spain, Xff was first reported in October 2016 to cause PD and also infect other hosts [35]. Since the first occurrence of PD in Europe, the presence and spread of this bacterium in Mediterranean crops and plant species in the natural and urban landscapes have become a major risk. For this reason, Italy, France, and Spain are now taking specific phytosanitary measures aimed at the eradication or containment of this disease. These measures are based on exhaustive surveys and monitoring PD symptoms to achieve a quick removal of problematic vines.
The recent outbreaks of PD in Europe have driven the pursuit of new and more effective control strategies for both PD and other diseases caused by Xf, resulting in the proposal of promising and innovating methods that could facilitate crop sustainability with little to no economic, social, or environmental risks [9]. These methods include techniques conferring systemic resistance in vines among others [36,37,38]. Nevertheless, to achieve the appropriate management of PD in Europe, it will be necessary to evaluate both the aggressiveness of the different Xff isolates and the resistance responses of the numerous clones and cultivars of Vitis planted in this continent. Furthermore, given the expected variability in PD epidemiology dependent on agro-climatic factors and the expression of pathogenicity factors [33,39], these studies should be conducted under standardized climatic conditions and correlated with the agro-climatic variability of viticultural areas.
The main objective of this work is to describe the severity of PD using a local inoculum and grapevine cultivars and rootstocks; evaluate disease progression under different climatic conditions; and establish the relationship between symptomatology, xylem pressure changes, and the detection of the bacterium using a standardized molecular methodology. Cultivars and rootstocks were chosen to provide data that could allow the implementation of clonal selection processes. The disease severity, water potential, and time course of PCR detection were studied under four different weather environments, including a climate-controlled greenhouse and open field conditions.
2. Materials and Methods
2.1. Terminology and General Overview of the Trials
Grapevine plants for new plantations are normally marketed as grafted. The same variety can be grafted onto different rootstocks to achieve better adaptation to the soil, phenological development, or resistance to endemic pests. In this study, European grapevine varieties (Vitis vinifera subsp. vinifera) grafted onto different rootstocks were evaluated. To avoid discrepancies in the terminology, a variety grafted onto the rootstock is referred to as the “Cultivar”. Plants corresponding to varieties used as rootstock and belonging to different species of the genus Vitis were also studied; the term used in this work to designate such species is “Rootstock”.
The pathogen and handling conditions are worth to be considered as set out in the current European regulations. Xf is in the category of “Priority quarantine pathogen” (Regulation (EU) 2016/2031 and Commission Delegated Regulation (EU) 2019/829), which means that we are dealing with one of the 20 most dangerous pathogens for European wine-growing areas as well as for other agricultural areas with major crops. Consequently, its handling, including for scientific purposes, has been extremely restricted even under regional rules (limited to areas where the disease has been widely detected or in accredited facilities (such as high biosafety greenhouses) to avoid any risk of pathogen spread.
The varietal selection and the way the trials were conducted correspond to the following chronology: The first trial in 2019 was carried out in the Mallorca, Balearic Islands, under field conditions. The area for testing had to be restricted and carried out with local Xf-isolated strains (XYL 2055/17 and XYL 2177/18). Until then (at the time the trials started), there was no reference to the strains’ virulence in different cultivars or rootstocks. A total of 28 Cultivars and Rootstocks were evaluated. That amount (28) was selected due to their vast degree of establishment (approx. 75%) in the most important wine-growing areas of Spain. Once the results of the first trial were analyzed, and according to the space availability restrictions, a second trial was carried out in the following year (2020). It was conducted during the vegetative period of the grapevine, in the same location and under the same operational conditions. In this year, due to the above-mentioned space availability restrictions, the number of cultivars to be evaluated was reduced to 22 in total vs. 28. Due to the results obtained in the previous year regarding the strain virulence, the susceptibility was assessed just against the most virulent strain (XYL 2055/17). Considering the results obtained in these two trials carried out under field conditions (2019 and 2020), it was decided to carry out a new trial during a new growing season in 2021. In this case, the objective of this third trial was evaluating the influence of climate conditions on disease progression. This is the reason behind the restricted number of plants in this trial (five Cultivars and one Rootstock). The assay was carried out in parallel under field conditions (the same location in the Balearic Islands) and also in a greenhouse located in the Basque Country. This particular study under the greenhouse condition (in the Basque Country) was located in a free-disease area of Spain and in a facility accredited for handling infected plant material with this harmful pathogen. The 2021 trials were carried out with the only strain that we were authorized to import for this type of experimental trials (IVIA 5770). The cultivars evaluated each year as well as the inoculated strains are detailed in Table 1, included in the results section.

Table 1.
Maximum Severity Index value (max. SI) recorded in the plants of each Cultivar and Rootstock evaluated in all of the trials carried out in this study at 8 and 16 WPI (max. SI-8/max. SI-16). The first line and the columns below it shows the year in which the trials were carried out, the second line shows where the trials were conducted, and the third line corresponds to the Xff strain artificially inoculated (XYL2055/17, XYL2177/18, and IVIA5770) in 24 grapevine Cultivars and five Rootstocks. The Rootstock column indicates the rootstock on which each cultivar was grafted.
2.2. Plant Material and Facilities
A total of 24 Cultivars and five Rootstocks [40,41] were selected based on their representativeness in the Spanish Protected Designations of Origin (DOs) or Protected Geographical Indications (PGIs) [28] to evaluate their resistance and disease progression.
During the years 2019, 2020, and 2021, three trials were carried out in an open field (see the climate variables in Figure 1a). One additional trial in a greenhouse was carried out in 2021. These plants under controlled conditions were grown with 70% relative humidity; a day/night temperature of 25 °C ± 2 °C and 18 °C ± 2 °C, respectively; and the absence of additional artificial lighting. All trials were conducted during the leaf development from May (BBCH 11-First leaf unfolded and spread away from shoot) to September (BBCH 19- nine or more leaves unfolded), ensuring a minimum daily light period of 16 h during the first four months after inoculation. After each trial, the plants were destroyed, so each annual test was implemented with new healthy plants.
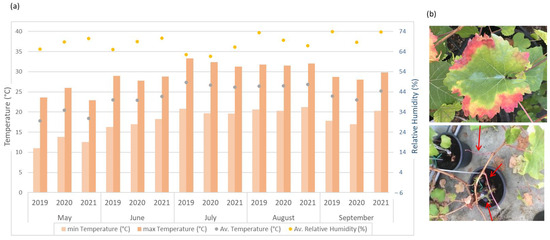
Figure 1.
(a) Monthly average, maximum, and minimum temperature (°C), and monthly average of relative humidity (%) for the three years and the evaluation period (from June to September) in which the field trials were conducted to evaluate the Cultivar’s susceptibility to Xff. (b) Characteristic symptoms of PD recorded in the field and greenhouse trials. Early symptoms after inoculation: reddish spots surrounded by a yellowish chlorotic halo (top) and defoliation indicated by red arrows as late symptoms (bottom).
Pre-grafted and rooted plants (one-bud cuttings) from the nursery were kept at 4 °C before planting. The plants were potted (6 L pots) into a mixture of peat and siliceous sand (3:1). The peat (pH 6.0 and 90% organic dry matter) was supplemented with calcium carbonate (7 g/L) and NPK14-10-18 fertilizer (1.5 g/L). The plants were selected for the trial if they showed the same phenotypic profile after a period of 20–30 days. Irrigation was applied as necessary to maintain field capacity and no phytosanitary treatment was applied.
The greenhouse was accredited as a temporal confinement greenhouse for Xff tasks; NEIKER′s facilities are located in this area on the northern peninsula of Spain (42°51′10.2″ N 2°37′29.7″ W). The trials using an open field were conducted in Palma de Mallorca, Spain, at the Balearic Governmental Center for Agricultural Improvement “SEMILLA” (39°35′23.5″ N 2°39′51.1″ E). The plants were randomly distributed in 12-plant rows along an insect-proof net tunnel and exposed to environmental temperatures.
2.3. In Planta Evaluation of PD Susceptibility
Inoculations were performed as follows: Xff ST1 strains (IVIA5770, XYL 2055/17 and XYL 2177/18) were isolated from Vitis vinifera on the Balearic Islands. These strains were previously characterized for several scientific grapevine tests [7,29,42,43]. The bacteria were cultured on a buffered charcoal yeast extract (BCYE) solid medium at 28 °C for 7–10 days. A bacterial suspension was prepared in phosphate-buffered saline (PBS) and adjusted to OD600 = 0.3, resulting in a final concentration of approximately 1 × 108 CFU.mL−1. Next, 10 μL of that suspension was used to inoculate the plants following a general procedure consisting of the pin-prick method [44,45]. In total, 9 out of 12 plants per genotype were inoculated, while the remaining three plants were mechanically injured in the same way as those prepared in PBS, but without an inoculum.
The Severity Index (SI) was assessed on a scale of 0 to 5, as previously described by Su et al. in 2013 [46], based on the number of affected and symptomatic leaves (Figure 1b) as follows: asymptomatic leaves (0), 1–2 symptomatic leaves (1), 3–4 symptomatic leaves (2), 5–7 symptomatic leaves (3), 8–10 symptomatic leaves (4), and more than 10 symptomatic leaves (5). The SI data were recorded every 15 days during the period between 8 weeks post-inoculation (WPI) and 16 WPI. Based on these data, it was possible to calculate the disease progression expressed as the absolute area under the disease progression curve (AUDPC) and transformed by the relative AUDPC (rAUDPC) The AUDPC units, as indicators of resistance or susceptibility, are not easily interpretable. In an effort to standardize the AUDPC, same researchers often use rAUDPC. The rAUDPC is calculated by dividing the AUDPC by the “maximum potential AUDPC”. The maximum potential AUDPC is simply the AUDPC a cultivar would have if it had had 100% infection all days during the reading period. Considering that the period in which AUDPC was evaluated in our experiments was 56 days (from 8 to 16 WPI) and the maximum SI value is 5, the maximum value of AUDPC would be 280 square units [47].
2.4. Detection of Xf
Each leaf was taken between three and five nodes above the inoculation point. Only petioles and veins were used for DNA extraction, amounting to a minimum of 0.5 g fresh tissue. The CTAB protocol for DNA extraction was applied [48]. Real-time PCR (RTi-PCR) for Xf detection was carried out using the primers XF-F: 5′-CAC GGC TGG TAA CGG AAG A-3′, XF-R: 5′-GGG TTG CGT GGT GAA ATC AAG-3′, and XF-P: 5′-FAM-TCG CAT CCC GTG GCT CAG TCC-BHQ-1-3′ [48,49], using TaqMan Universal Master Mix (Applied Biosystems®, Thermo Fisher Scientific Inc., Waltham, MA, USA). The final optimized reaction conditions were as follows: RTi-PCR reactions were performed in 20 μL reaction volumes containing 10 μL of 2X TaqMan universal master mix (Applied Biosystems), 300 nM Xf sense (XF-F) and antisense (XF-R) primers, 100 nM 6′FAM/BHQ-labeled XF-P probe, ultra-pure bovine serum albumin (BSA) at 300 ng/μL (Invitrogen), and 2 μL of total DNA template. The optimal thermocycling conditions were as follows: 50 °C for 2 min and 94 °C for 10 min, then 40 cycles for 10 s and 62 °C for 40 s, using Quant Studio TM 5 model QS5STD. All samples were amplified in duplicate. Threshold values were applied automatically using the QuantStudio™ Design and Analysis Software (Applied Biosystems). Positive amplification was determined using a crossing threshold (Ct value < 38 cycles). Bacterial re-isolation was accomplished from the same tissue sample RTi-PCR analyzed. The recovered colonies were scraped from the BCYE growth medium and resuspended in a potassium phosphate buffer, prior to being assessed via RTi-PCR.
2.5. Water Potential (Ψ) Assessment
Ψ was assessed only in the greenhouse trial using the Scholander pressure chamber (PMS Instrument Company, Albany, NY, USA). The equipment was set up using a pressure of 3 bar in the N2 gas cylinder and a gas flow rate of 5 bar in the equipment. Measurements were performed systematically between 9:30 and 11:30 am, two hours after sunrise, as recommended by Knipfer et al. (2020) [50]. Leaves were cut from positions 2, 3, or 4 above the inoculation point, depending on phenological development and using a scalpel blade. Immediately after excision, the petiole was placed in the Scholander chamber and the pressure equilibrium technique described by Castander et al. (2020) [51] was used to measure Ψ in MPa. The Ψ data were recorded during the period between 8 WPI and 21 WPI.
2.6. Statistical Analysis
rAUDPC and water potential (Ψ) were analyzed in each trial to assess the effect of cultivars and rootstocks. The rAUDPC data were normalized via arcsine of the square root of the proportion and then subjected to analysis of two-way ANOVA (Cultivar × strain, Cultivar × year, or Rootstock × Strain for each Cultivar), followed by Fisher’s Least Significant Difference (LSD) test (R software, version 4.2.2) in the case of no significant interactions. Differences at p < 0.05 were considered significant. Additional descriptive data in boxplots, comparisons based on estimated marginal means by Tukey′s tests for comparing two means, and Pearson correlation coefficients using average trait values were also obtained via the free Jamovi software version 2.3.28.
3. Results
3.1. Pierce’s Disease Susceptibility in Vitis Vinifera Cultivars and Rootstocks
During 2019, we evaluated the susceptibility of 24 different grapevine Cultivars and five Rootstocks to two different strains of Xff (XYL 2055/17 and XYL 2177/18) that were previously isolated in the same geographical area where the trial was conducted (Balearic Islands). The rAUDPC data were recorded during the period between 8 WPI and 16 WPI, which corresponded to a maximum AUDPC of 280 u2 for calculating the rAUDPC. Inoculated plants in which the bacterium was not detected via RT-PCR were not considered for an rAUDPC assessment.
We observed significant differences in the susceptibility of Cultivars and Rootstocks to each of the two strains of Xff without significative interaction (Figure 2). The average rAUDPC for all Cultivars combined was significantly higher for strain XLY 2055/17 (0.58) than for strain XYL 2177/18 (0.46), indicating the higher virulence of strain XLY 2055/17 (Figure 2a). In this case, the correlation between the rAUDPC values of both Xff strains was high, with a significant coefficient of r = 0.903 (p < 0.05). The significant difference between Xff strains indicates that one of the factors involved in the cultivar resistance was determined using the bacterial genotype. This relation could be established under a linear regression (R2 = 0.8156) expressed as:
rAUDPC XYL 2177/18 = 0.8437 rAUDPC XYL 2055/17 − 0.0266.
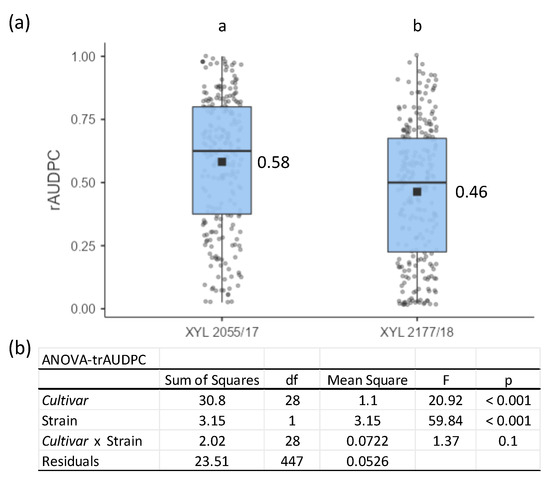
Figure 2.
Descriptive and statistical parameters for the evaluation of grapevine Cultivars’ susceptibility to Xff strains based on an estimation of the rAUDPC recorded from 8 WPI to 16 WPI for 24 Cultivars and five Rootstocks artificially inoculated with XYL 2055/17 and XYL 2177/18. (a) Each boxplot reports the second and third quartiles, with median (line) and mean values (dot) indicated as squares and points showing all values considered. The Xff strains followed by different letters indicate significant differences among the mean rAUDPC values based on Fisher’s Least Significant Difference (LSD) test (p < 0.05). (b) Statistical parameters after two-way ANOVA analysis of rAUDPC previously transformed using arcsine of the square root of the proportion (trAUDPC).
The two Cultivars studied were grafted onto three different Rootstocks. However, this grafting did not appear to produce any variation in the intrinsic susceptibility of the grafted Cultivar. Disease progression, expressed as the rAUDPC values for Tempranillo and Garnacha Tintorera, was independent of the rootstock upon which the plants were grafted (Figure 3). Therefore, hereafter, we refer to each Cultivar independently from the Cultivars that were grafted.
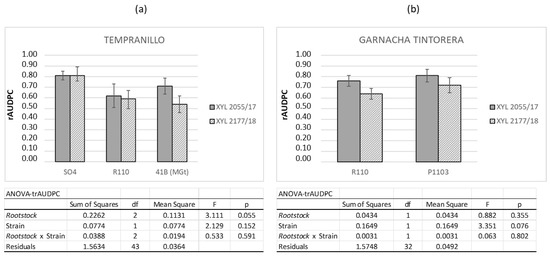
Figure 3.
Mean rAUDPC values obtained for Tempranillo (a) and Garnacha Tintorera (b) Cultivars grafted onto different Rootstocks (SO4, R110, 41 B, and P1103). The plants were artificially inoculated with two Xff strains: XYL 2055/17 (solid grey) and XYL 2177/18 (grey grid). The data were recorded over one year (2019) in open field over 8 weeks, from 8 to 16 WPI. Error bars represent the standard error of the mean. Statistical parameters after two-way ANOVA analysis of rAUDPC previously transformed using arcsine of the square root of the proportion (trAUDPC) shown in the table conclude no significant differences among Rootstocks, Strains, and their interaction (Rootstock*Strain).
To establish the resistance levels to Xff, a new trial was repeated under the same conditions the following year (2020) via inoculation with only the most virulent strain, XYL 2055/17, according to the results obtained the year prior (Figure 2). We observed significant differences among Cultivars in their individual susceptibility to the pathogen and also significant differences between the years (Figure 4). This statistical result indicates that the factors involved in grapevine Cultivars’ resistance could be dependent on climate (or environmental), but not for all varieties or with the same determination attending to the significant interaction between both factors (Cultivar and year) (Figure 4a). The Cultivars Tempranillo Blanco, Tempranillo RJ43, Tempranillo RF78, Tempranillo, Malvasía, Monastrel, Mencía, Garnacha Tintorera, and Macabeo yielded the highest rAUDPC values, with no significant differences among them. Conversely, Pedro Ximenez, Albariño, Verdejo, Bobal, Pinot Noir, Hondarrabi Zuri, H. Beltza, and Cabernet Souvignon presented the highest resistance to Xff (Figure 4b).
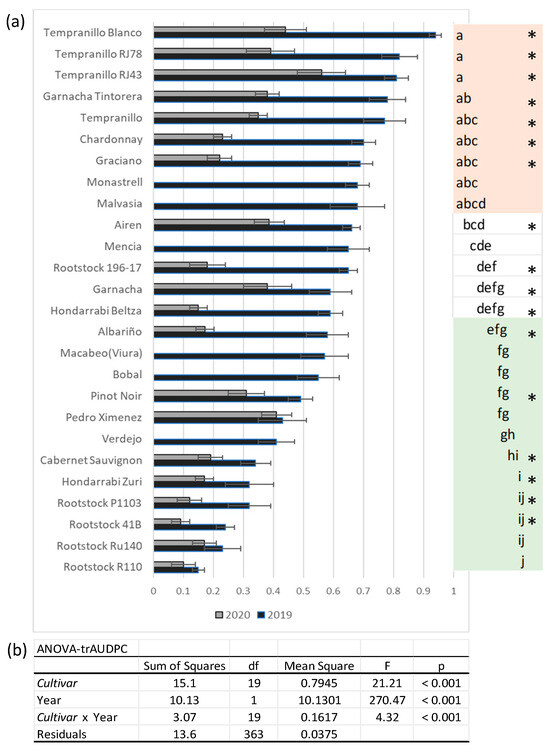
Figure 4.
(a) Mean rAUDPC values obtained for Cultivars and Rootstocks artificially inoculated with Xff ST1 XYL 2055/17, recorded over two consecutive years (2019 and 2020) for 8 weeks, from 2 months (8 WPI) to 4 months (16 WPI). Error bars represent the standard error of the mean. Cultivars followed by the same letter indicate non-significant differences among rAUDPC values based on Fisher’s LSD test (p < 0.05) for just the 2019 trial. The most susceptible cultivars with the highest rAUDPC values are marked in red, while cultivars with the lowest values are marked in green. The number of inoculated plants per Cultivar considered to obtain the mean rADPC varied from seven to nine. (b) Statistical parameters after two-way ANOVA analysis of rAUDPC, previously transformed using arcsine of the square root of the proportion (trAUDPC), shown in the table conclude significant differences among Cultivars, year of trial conduction, and significative interaction between both factors (Rootstock*Strain). The asterisks indicate significant differences (p < 0.05) by Tukey′s test between rAUDPC registered in 2019 and 2020 at each Cultivar.
In this study, the five most common Rootstocks upon which European varieties are grafted were shown to be less susceptible to Xff than most commercial Cultivars of V. vinifera. This result was essentially confirmed under our conditions because all Rootstocks (R110, 41B-MGt, Ru140, P1103, and 196-17 Cl) presented very low susceptibility to the pathogen, with R110 yielding the lowest rAUDPC for Xff (Figure 4a).
3.2. Disease Severity and Disease Progression (rAUDPC) under Open Field and Controlled Greenhouse Conditions
To better estimate and establish the degree of susceptibility, it is necessary to evaluate plants during several campaigns or under different climate conditions. Accordingly, we repeated the assays for one additional year. However, due to operational limitations, we decided to perform the inoculations with only five Cultivars that showed different levels of susceptibility to Xff in previous field trials together with one Rootstock. In this case, we used the Xff ST1 strain IVIA 5770, the only strain that current regulatory restrictions allow to be manipulated outside of the demarcated areas in Spain.
During 2021, two new trials were carried out in parallel: one in the same location as the two previous trials (Balearic Islands), and the other under controlled conditions in a greenhouse located in a different climatic area of northern Spain. Special care was taken to ensure that the plants for both trials belonged to the same grafted lot and were maintained without fertilizer or water restrictions.
The results obtained in this new trial carried out in 2021 using a restricted number of Cultivars and Rootstocks confirm those obtained in the two previous years. All the Cultivars and Rootstocks were susceptible to Xff, in this case, against the strain Xff IVIA 5770, with clear pathogen resistance depending on climatic conditions. The rAUDPC values were significantly higher in four of the five Cultivars tested: Tempranillo, Garnacha, Pinot Noir, and Chardonay (Figure 5). The Cultivars in which the rAUDPC values were not significatively different between the field and greenhouse were Hondarrabi Zuri and Rootstock R110 (Figure 5). Both Cultivars belonged to the group with the lowest rAUDPC values recorded in previous field trials, as shown in Figure 4a.
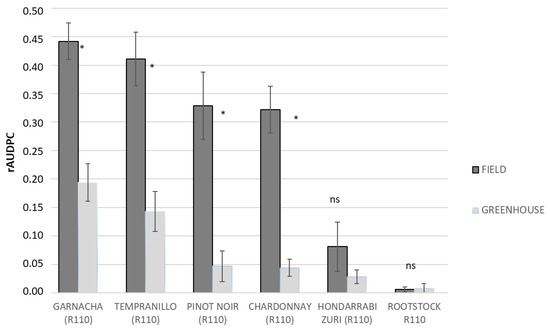
Figure 5.
Mean rAUDPC values obtained for five Cultivars and one Rootstock artificially inoculated with Xff IVIA 5770, recorded over one year (2021) in two locations under both an open field (FIELD) and controlled conditions (GREENHOUSE). The rAUDPC was calculated based on the SI progress for 8 weeks, from 8 to 16 WPI. Error bars represent the standard error of the mean. The number of inoculated plants per Cultivar considered to obtain the mean rADPC was nine. Asterisks indicate the level of significance: *, p < 0.05; ns, not significant) based on Tukey-test between the means values obtained in Field (dark grey) and Greenhouse (light grey) for each Cultivar.
Four months (16 WPI) after inoculation, the SI recorded under field conditions exceeded level 3. However, under greenhouse conditions, this level was not exceeded in any of the Cultivars studied (Figure 6). In both the greenhouse and the field, it was possible to observe significant differences in the level of Cultivar susceptibility to Xff (rAUDPC). However, in the greenhouse, the average of this indicator did not clearly discriminate between the resistance levels of the Cultivars studied. Only Tempranillo yielded a significantly different rAUDPC than that recorded in Rootstock R110 (Figure 6).
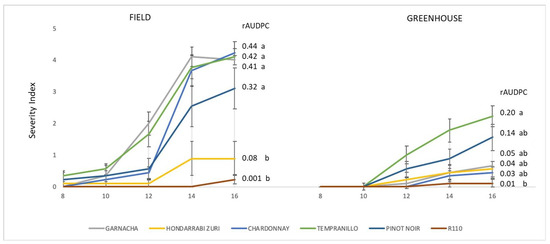
Figure 6.
Disease progression in an open field (Field) and greenhouse-controlled conditions (Greenhouse) from 8 to 16 WPI. The plants were artificially inoculated with the Xff IVIA 5770 strain. Values are the means of 13 replicates, and error bars represent the standard error of the Severity Index (SI). The rAUDPC derivates from the time progression of the SI as shown in columns. Cultivars followed by the same letter indicate non-significant differences between the rAUDPC values based on Fisher’s LSD test (p < 0.05).
Importantly, after inoculation, the asymptomatic period was shorter in the open field than that in the greenhouse. An asymptomatic to symptomatic transition occurred between 10 and 12 WPI in the greenhouse and after 8 WPI in the open field (Figure 6).
Table 1 shows the maximum SI recorded in the inoculated plants, in both the field and the greenhouse. Two months after inoculation (16 WPI), all Cultivars of Vitis vinifera tested in the field recorded the maximum value of SI (5). Only two of the five evaluated Rootstocks (41 B-MGt and R110) did not obtain the same maximum SI value. In the greenhouse, no Cultivar yielded the maximum SI value (5), and only Tempranillo reached level 4 (Table 1).
3.3. Water Potential Progression during Xff Infection and Disease Development
To associate PD symptomatology and bacterial detection with the effects of infection on the water status of the plants, the Ψ differences between treatments (control and inoculated) were also analyzed for 19 weeks, from 2 WPI to 21 WPI, in plants growing under controlled conditions in the greenhouse. Ψ varied as the phenological stage of the plants progressed, but significant differences between inoculated and non-inoculated plants were observed from 12 WPI onwards (Figure 7). However, this result was not clearly established in all tested Cultivars. In two of the five cultivars (Garnacha and Pinot Noir), significant differences were not found, even at 21 WPI (Figure 8a). In the Rootstock R110 and Hondarrabi Zuri, which both presented the lowest severity index in the greenhouse (Figure 5), it was possible to find significant differences in Ψ between inoculated and healthy plants (Figure 8a). These results suggest no relationship between the severity of the disease and variation in water potential, at least in some Cultivars. On the other hand, at 12 WPI, the first period with significant differences in Ψ between inoculated and control plants, 41% of inoculated plants already indicated positive bacterial detection, as shown in Figure 8b.
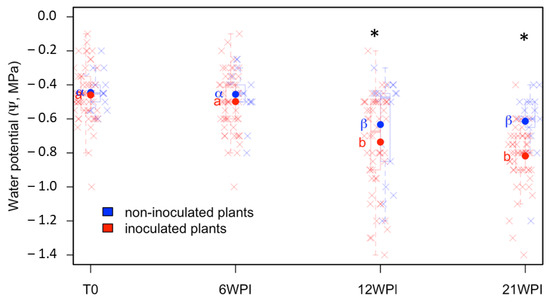
Figure 7.
Water potential (Ψ) variation over time under greenhouse-controlled conditions. Latin letters correspond to average Ψ of the inoculated plants, while Greek letters represent the non-inoculated plants. The same letter indicates non-significant differences among mean Ψ values based on Fisher’s LSD test (p < 0.05). The asterisks indicate significant differences (p < 0.05) by Tukey′s test between inoculated and non-inoculated plants at each time point: T0 (before inoculation), 6, 12, and 21 WPI. Individual measurements for each plant are shown with “×” signs. The plants were artificially inoculated with Xff IVIA 5770.
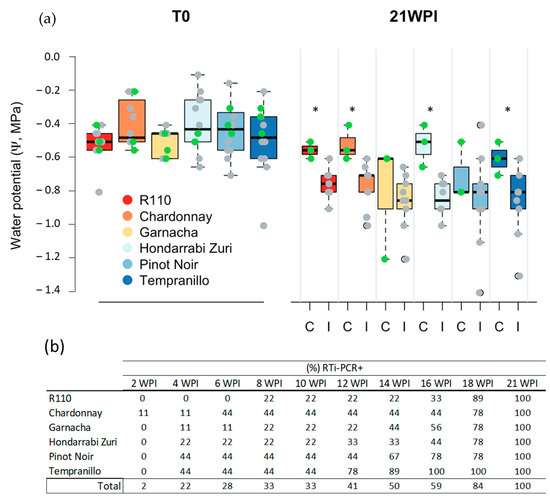
Figure 8.
Progression of the water potential (Ψ) and RT-PCR detection of Xff IVIA 5770 artificially inoculated in grapevine plants. The trial was carried out under controlled conditions in a greenhouse. (a) Ψ recorded before (T0) and after 21 WPI for artificial inoculation with Xff IVIA 5770 in five grapevine Cultivars and one Rootstock (R110) and 21 WPI under greenhouse-controlled conditions. Each boxplot reports the second and third quartiles, with median values (line) in the square and points showing outliers (green for non-inoculated (C) and grey for inoculated plants (I)). The asterisks indicate significant differences (p < 0.05) by Tukey′s test between I and C plants at 21 WPI. (b) Percentage of artificially inoculated plants in which the bacterium was detected via RTi-PCR from 2 to 21 WPI.
4. Discussion
As previously described, strains of Xff ST1, isolated for the first time in the Balearic Islands, were able to cause disease in some of the main grapevine varieties planted in different Spanish regions [29]. The 24 Cultivars studied in this work were found to be susceptible. However, it was also possible to clearly establish different levels of resistance among cultivars. The results obtained agree with those previously published, showing different degrees of resistance in certain grapevine cultivars, most likely influenced by their different pedigrees (linked to their shared centers of domestication) and xylem anatomical features [33], or an engineered innate immune defense in grapevines [52].
Notably, the two determining factors of grapevine cultivar resistance to Xff in this study were the bacterial genotype (Xff strain) and environmental climate. The virulence of the Xff strain XYL 2055/17 was significantly higher than that of strain XYL 2177/18.
Plant–pathogen interactions are multifaceted processes mediated by the pathogen- and plant-derived molecules. Thus, the differential virulence between strains is well known. In this work, we detected this condition in two strains of Xff isolated independently in the same geographical area over consecutive years, which could corroborate the results of the genetic variability of Xff isolated in the Balearic Islands recently presented by Dr. Landa’s research team in 2022 [7]. Despite the differences in virulence found between the two strains in this study, resistance was highly correlated between cultivars. Thus, strain virulence does not appear to modify the susceptibility profiles.
In the absence of a greater number of trials under different agroclimatic conditions, this work established, for the first time, two significantly different groups of European cultivars of Vitis vinifera, characterized as having high and low susceptibility to Xff. The results indicate that Tempranillo and Tempranillo Blanco can be considered as a reference cultivar (variety) (among others) with high susceptibility compared to Hodarrabi Zuri and Cabernet Sauvignon with lower susceptibility.
The results obtained in this work demonstrate that the Rootstock commonly used to graft commercial varieties of grapevine are also susceptible to PD caused by Xff European isolates. However, the level of susceptibility was significantly lower than that of most commercial Cultivars. Several studies showed that rootstocks affect scion responses in many different ways. Rootstocks, for example, can influence scion vigor and phenology and confer differential tolerance to drought and disease in various crops [53,54]. Recent relevant studies [55] proposed that a low root mass may incite resource-limiting conditions to activate carbohydrate metabolic pathways, which reciprocally interact with plant immune system genes to elicit differential levels of cultivar susceptibility in bacterial pathogen–plant interaction. However, the low susceptibility found in the Rootstocks studied was not found to confer lower susceptibility among the cultivars. Our results indicate that the susceptibility of one grapevine cultivar to PD could be independent of the rootstock on which the cultivars were grafted.
To ensure higher control over environmental variables, it is common to evaluate cultivars for their resistance to pathogens in growth chambers or greenhouses before the evaluations under field conditions. However, the susceptibility ratings under these two conditions were previously found to show either a significant correlation [56,57] or no correlation [58]. Additionally, in certain cases, greenhouse evaluations tend to overestimate susceptibility [59]. In the end, screening for resistance to pathogens could be more accurate when conducted in open fields or greenhouses, depending on the expression of plant defense-related genes after bacterial inoculation or the presence of climatic conditions more conducive to bacterial growth [60]. Therefore, since inoculation with a quarantine pathogen such as Xff is more practical in controlled facilities, we evaluated the correlation between plant susceptibility to Xff among five cultivars and one Rootstock in a greenhouse and in the field. The results showed that disease progression was significantly slower in the greenhouse than in the open field. Consequently, the rAUPC found in grapevines grown in this facility was no greater than 0.25 units from 2 to 4 months after inoculation. This result is not surprising since in other pathogenicity models of Xff in greenhouses, disease severity did not reach the levels found in the open field, as in the case of Xff in southern highbush blueberry (Vaccinium sp.) [61] and almond [43].
Under the current disease scenario, investigators can handle tasks with Xf-infected plant material only under confined, approved conditions to avoid the spread of the disease, denoted as temporal confinement stations for quarantine pathogens (Regulation (EU) 2016/2031). Biosafety greenhouses are the only facilities that can be used for this purpose. Taking into account our results, it would be possible to discriminate between highly and moderately susceptible cultivars with reference to Tempranillo Cultivars. Tempranillo grafted onto Rootstock R110 was able to reach a severity index of 4 after 16 WPI, as shown by our results. Together with Tempranillo, Pinot Noir (both grafted onto R110) presented the earliest symptoms. The first symptoms were recorded at 12 WPI. This result confirms our selection of Chardonnay and Pinot Noir varieties as indicator plants in the pathogenicity tests and suggests the inclusion of the Tempranillo and Garnacha varieties in standards for the Diagnostic Protocol of Xff.
The differences in varietal susceptibility levels highlighted in this work under open field conditions, together with previous work showing that disease incidence and severity may be related to agricultural management, suggest that PD in Europe could be, in the future, managed as a chronic disease as it is now managed in American winegrowing areas [32]. Several scientific results support this theory, including crop management (Moralejo, 2019 [39]), which found positive results when using innovative synthesized chemical treatments [37,42], the efficacy of foliar-applied biological treatments [62], insect vector deterrence [63], and others [9]. We should also consider the wide variety of biostimulants appearing on the fertilizer and agro-sanitary markets whose contributions to vineyard health in different agroclimatic grapevine areas remain to be studied [64,65].
It is well known that PD in grapevine is related to a decrease in water conductance, water potential, and hydraulic conductance [10,11,66]. Compared to healthy leaves developed under the same conditions, Xf-infected plants showed a reduction in available water and nutrients, resulting in wilting and death due to stress. This result was corroborated using the Scholander test in woody plants [12]. In this study, we were able to associate this physiological effect with the onset of symptomatology and Xff infection. However, based on our results, it is not easy to consider this parameter as an early detection test for Xff infection prior to the symptomatic period. The differences in water potential started to become significantly distinct between healthy and infected plants once the bacteria were detectable via standard RTi-PCR. In addition, the results obtained show differences among cultivars; thus, it was not possible to establish a significant relationship between infection and potential water in all cultivars. This alternative technology would be discarded as an alternative diagnostic measure unless further research is carried out to discriminate its potential use for different cultivars, evaluation times, and agroclimatic conditions. We cannot ignore these new and non-destructive technologies as they create the possibility to survey, and potentially geo-reference, large numbers of plants in real time.
Undoubtably, grapevine agro-management in novel environments coupled with Cultivar resistance will play a determining role in PD control in Europe. Indeed, such methods are already being prioritized in other crops [67]. Considering the data presented in this work, several agro-climatic parameters should be tested to enhance breeding programs and establish a discriminative threshold for cultivar selection. At the same time, we should explore new technologies for the early detection of differential physiological responses and phenotyping of foliar disease severity under controlled conditions [68,69].
5. Conclusions
The 24 European Vitis vinifera cultivars studied in this work, representing more than 70% of the cultivated area in Spain, are susceptible to the PD caused by current Xff ST1 strains detected in Europe. After carrying out trials over three consecutive years, we obtained consistent results indicating two significantly different groups of resistance which may not be influenced by the rootstock upon which the plant is grafted. Importantly, Cultivar susceptibility is clearly influenced by the environmental conditions under which the plants are grown. The results of cultivar resistance evaluations may differ between the field and the greenhouse conditions.
Tempranillo could be included as references in breeding programs for Xff as indicators of highly susceptible Cultivars, since under field and greenhouse conditions, these variety presented the largest SI and rAUDPC values four months after inoculation.
Author Contributions
Conceptualization, A.O.-B. and M.L.; methodology, S.M., A.A. and A.H.; formal analysis, J.B.R.; investigation, S.M.; resources, A.O.-B. and J.B.R.; data curation, S.M.; writing—original draft preparation, S.M. and A.O.-B.; writing—review and editing, A.O.-B. and S.M.; supervision, M.L. and A.O.-B.; funding acquisition, A.O.-B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially funded by AEI-INIA Spain, through a NEIKER-Girona University agreement (Laboratory of Plant Pathology, Institute of Food and Agricultural Technology-CIDSAV-XaRTA): Grant number E-RTA2017-00004-C06-03; Basque Government through Grupo de Investigación del Sistema Universitario Vasco: Grant numbers T1682-22 and 00039-IDA2021-45; S.M. was a recipient of a PhD research grant from Department of Economic Development, Sustainability and Environment of the Basque Government (2018–2022): Grant reference SARA MARTINEZ 2018.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
All data are contained in the article.
Acknowledgments
We thank Ander Castander and Diego Llamazares for their assistance with the Scholander pressure chamber methodology.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; the analyses or interpretation of the data; the writing of the manuscript; or the decision to publish the results.
References
- Wells, J.M.; Raju, B.C.; Hung, H.-Y.; Weisburg, W.G.; Mandelco-Paul, L.; Brenner, D.J. Xylella fastidiosa gen. nov., sp. nov: Gram-negative, xylem-limited, fastidious plant bacteria related to Xanthomonas spp. Int. J. Syst. Bacteriol. 1987, 37, 136–143. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA); Gibin, D.; Pasinato, L.; Delbianco, A. Update of the Xylella Spp. Host Plant Database—Systematic Literature Search up to 31 December 2022. EFSA J. 2023, 21, 8061. [Google Scholar] [CrossRef]
- Sicard, A.; Zeilinger, A.R.; Vanhove, M.; Schartel, T.E.; Beal, D.J.; Daugherty, M.P.; Almeida, R.P.P. Xylella fastidiosa: Insights into an emerging plant pathogen. Annu. Rev. Phytopathol. 2018, 56, 181–202. [Google Scholar] [CrossRef]
- Trkulja, V.; Tomić, A.; Iličić, R.; Nožinić, M.; Milovanović, T.P. Xylella fastidiosa in Europe: From the introduction to the current status. Plant Pathol. J. 2022, 38, 551–571. [Google Scholar] [CrossRef]
- Frem, M.; Fucilli, V.; Nigro, F.; El Moujabber, M.; Abou Kubaa, R.; La Notte, P.; Bozzo, F.; Choueiri, E. The potential direct economic impact and private management costs of an invasive alien species: Xylella fastidiosa on Lebanese wine grapes. NeoBiota 2021, 70, 43–67. [Google Scholar] [CrossRef]
- Hopkins, D.L.; Purcell, A.H. Xylella fastidiosa: Cause of Pierce’s Disease of grapevine and other emergent Diseases. Plant Dis. 2002, 86, 1056–1066. [Google Scholar] [CrossRef]
- Velasco-Amo, M.P.; Arias-Giraldo, L.F.; Olivares-García, C.; Denancé, N.; Jacques, M.-A.; Landa, B.B. Use of traC Gene to Type the Incidence and Distribution of pXFAS_Plasmid-Bearing Strains of Xylella fastidiosa subsp. fastidiosa ST1 in Spain. Plants 2022, 11, 1562. [Google Scholar] [CrossRef] [PubMed]
- Alston, J.M.; Fuller, K.; Kaplan, J.D.; Tumber, K. The Costs and Benefits of Pierce’s Disease Research in the California Winegrape Industry. In Proceedings of the Agricultural and Applied Economics Association (AAEA) Conferences, 2013, Annual Meeting, Washington, DC, USA, 4–6 August 2013; p. 37. [CrossRef]
- Kyrkou, I.; Pusa, T.; Ellegaard-Jensen, L.; Sagot, M.-F.; Hansen, L.H. Pierce’s Disease of grapevines: A review of control strategies and an outline of an epidemiological model. Front. Microbiol. 2018, 9, 2141. [Google Scholar] [CrossRef]
- Chatelet, D.S.; Wistrom, C.M.; Purcell, A.H.; Rost, T.L.; Matthews, M.A. Xylem structure of four grape varieties and alternative hosts to the xylem-limited bacterium Xylella fastidious. Ann. Bot. 2011, 108, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Donoso, A.G.; Lenhof, J.J.; Pinney, K.; Labavitch, J.M. Vessel Embolism and tyloses in early stages of Pierce’s Disease: Embolism and tyloses in Pierce’s Disease. Aust. J. Grape Wine Res. 2016, 22, 81–86. [Google Scholar] [CrossRef]
- Raicavoli, J.; Ingel, B.; Blanco-Ulate, B.; Cantu, D.; Roper, C. Xylella fastidiosa: An examination of a re-emerging plant pathogen: Xylella fastidiosa. Mol. Plant Pathol. 2018, 19, 786–800. [Google Scholar] [CrossRef]
- Carluccio, G.; Greco, D.; Sabella, E.; Vergine, M.; De Bellis, L.; Luvisi, A. Xylem embolism and pathogens: Can the vessel anatomy of woody plants contribute to X. fastidiosa resistance? Pathogens 2023, 12, 825. [Google Scholar] [CrossRef]
- Lopes, S.A.; Teixeira, D.C.; Fernandes, N.G.; Ayres, A.J.; Torres, S.C.Z.; Barbosa, J.C.; Li, W.B. An experimental inoculation system to study citrus- Xylella fastidiosa interactions. Plant Dis. 2005, 89, 250–254. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Almeida, R.P.P.; Nunney, L. How Do plant diseases caused by Xylella fastidiosa emerge? Plant Dis. 2015, 99, 1457–1467. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, P.H.; DeVay, J.E.; Meredith, C.P. Physiological responses of Vitis vinifera Cv. “Chardonnay” to infection by the Pierce’s Disease bacterium. Physiol. Mol. Plant Pathol. 1988, 32, 17–32. [Google Scholar] [CrossRef]
- Fernández, J. Plant-Based methods for irrigation scheduling of woody crops. Horticulturae 2017, 3, 35. [Google Scholar] [CrossRef]
- Ihuoma, S.O.; Madramootoo, C.A. Recent Advances in crop water stress detection. Comput. Electron. Agric. 2017, 141, 267–275. [Google Scholar] [CrossRef]
- Virnodkar, S.S.; Pachghare, V.K.; Patil, V.C.; Jha, S.K. Remote sensing and machine learning for crop water stress determination in various crops: A critical review. Precis. Agric. 2020, 21, 1121–1155. [Google Scholar] [CrossRef]
- Rodriguez-Dominguez, C.M.; Forner, A.; Martorell, S.; Choat, B.; Lopez, R.; Peters, J.M.R.; Pfautsch, S.; Mayr, S.; Carins-Murphy, M.R.; McAdam, S.A.M.; et al. Leaf water potential measurements using the pressure chamber: Synthetic testing of assumptions towards best practices for precision and accuracy. Plant Cell Environ. 2022, 45, 2037–2061. [Google Scholar] [CrossRef]
- Gálvez Pavez, R.; Callejas Rodríguez, R.; Reginato Meza, G. Comparación de la cámara de presión tipo scholander modelo pump-up respecto a la cámara de presión tradicional en vides de mesa. Idesia 2011, 29, 175–179. [Google Scholar] [CrossRef]
- Choné, X. Stem water potential is a sensitive indicator of grapevine water status. Ann. Bot. 2001, 87, 477–483. [Google Scholar] [CrossRef]
- Williams, L.E.; Araujo, F.J. Correlations among predawn leaf, midday leaf, and midday stem water potential and their correlations with other measures of soil and plant water status in Vitis vinifera. J. Am. Soc. Hortic. Sci. 2002, 127, 448–454. [Google Scholar] [CrossRef]
- Santesteban, L.G.; Miranda, C.; Marín, D.; Sesma, B.; Intrigliolo, D.S.; Mirás-Avalos, J.M.; Escalona, J.M.; Montoro, A.; De Herralde, F.; Baeza, P.; et al. Discrimination ability of leaf and stem water potential at different times of the day through a meta-analysis in grapevine (Vitis vinifera L.). Agric. Water Manag. 2019, 221, 202–210. [Google Scholar] [CrossRef]
- Suter, B.; Triolo, R.; Pernet, D.; Dai, Z.; Van Leeuwen, C. Modeling stem water potential by separating the effects of soil water availability and climatic conditions on water status in grapevine (Vitis vinifera L.). Front. Plant Sci. 2019, 10, 1485. [Google Scholar] [CrossRef]
- Tyree, M.T.; Zimmermann, M.H. Hydraulic architecture of whole plants and plant performance. In Xylem Structure and the Ascent of Sap; Springer Series in Wood Science; Springer: Berlin/Heidelberg, Germany, 2022; pp. 175–214. [Google Scholar] [CrossRef]
- Diago, M.P.; Fernández-Novales, J.; Gutiérrez, S.; Marañón, M.; Tardaguila, J. Development and validation of a new methodology to assess the vineyard water status by on-the-go near infrared spectroscopy. Front. Plant Sci. 2018, 9, 59. [Google Scholar] [CrossRef]
- OIV–International Organization of Vine and Wine. Distribution of the world’s grapevine varieties. In Focus OIV 2017; OIV–International Organization of Vine and Wine: Dijon, France, 2017; p. 53. ISBN 979-10-91799-89-8. [Google Scholar]
- Giménez-Romero, A.; Galván, J.; Montesinos, M.; Bauzà, J.; Godefroid, M.; Fereres, A.; Ramasco, J.J.; Matías, M.A.; Moralejo, E. Global predictions for the risk of establishment of Pierce’s Disease of grapevines. Commun. Biol. 2022, 5, 1389. [Google Scholar] [CrossRef]
- Fritschi, F.B.; Lin, H.; Walker, M.A. Xylella fastidiosa population dynamics in grapevine genotypes differing in susceptibility to Pierce’s Disease. Ann. J. Enol. Vitic. 2007, 58, 326–332. [Google Scholar] [CrossRef]
- Riaz, S.; Huerta-Acosta, K.; Tenscher, A.C.; Walker, M.A. Genetic characterization of Vitis germplasm collected from the southwestern us and Mexico to expedite Pierce’s Disease-resistance breeding. Theor. Appl. Genet. 2018, 131, 1589–1602. [Google Scholar] [CrossRef]
- Morales-Cruz, A.; Aguirre-Liguori, J.; Massonnet, M.; Minio, A.; Zaccheo, M.; Cochetel, N.; Walker, A.; Riaz, S.; Zhou, Y.; Cantu, D.; et al. Multigenic resistance to Xylella fastidiosa in wild grapes (Vitis spp.) and its implications within a changing climate. Commun. Biol. 2023, 6, 580. [Google Scholar] [CrossRef]
- Deyett, E.; Pouzoulet, J.; Yang, J.-I.; Ashworth, V.E.; Castro, C.; Roper, M.C.; Rolshausen, P.E. Assessment of Pierce’s Disease susceptibility in Vitis vinifera cultivars with different pedigrees. Plant Pathol. 2019, 68, 1079–1087. [Google Scholar] [CrossRef]
- Schneider, K.; Van Der Werf, W.; Cendoya, M.; Mourits, M.; Navas-Cortés, J.A.; Vicent, A.; Oude Lansink, A. Impact of Xylella fastidiosa subspecies pauca in European olives. Proc. Natl. Acad. Sci. USA 2020, 117, 9250–9259. [Google Scholar] [CrossRef] [PubMed]
- Latest Developments of Xylella Fastidiosa in the EU Territory. Available online: https://ec.europa.eu/food/plants/plant-healthand-biosecurity/legislation/control-measures/xylella-fastidiosa/latest-developments-xylella-fastidiosa-eu-territory_en (accessed on 6 October 2023).
- Castro, C.; Massonnet, M.; Her, N.; DiSalvo, B.; Jablonska, B.; Jeske, D.R.; Cantu, D.; Roper, M.C. Priming grapevine with lipopolysaccharide confers systemic resistance to Pierce’s Disease and identifies a peroxidase linked to defense priming. New Phytol. 2023, 239, 687–704. [Google Scholar] [CrossRef] [PubMed]
- Badosa, E.; Planas, M.; Feliu, L.; Montesinos, L.; Bonaterra, A.; Montesinos, E. Synthetic peptides against plant pathogenic bacteria. Microorganisms 2022, 10, 1784. [Google Scholar] [CrossRef]
- Avosani, S.; Nieri, R.; Mazzoni, V.; Anfora, G.; Hamouche, Z.; Zippari, C.; Vitale, M.L.; Verrastro, V.; Tarasco, E.; D’Isita, I.; et al. Intruding into a conversation: How behavioral manipulation could support management of Xylella fastidiosa and its insect vectors. J. Pest Sci. 2023, 1, 1–17. [Google Scholar] [CrossRef]
- Moralejo, E.; Borràs, D.; Gomila, M.; Montesinos, M.; Adrover, F.; Juan, A.; Nieto, A.; Olmo, D.; Seguí, G.; Landa, B.B. Insights into the epidemiology of Pierce’s Disease in vineyards of Mallorca, Spain. Plant Pathol. 2019, 68, 1458–1471. [Google Scholar] [CrossRef]
- Maul Team. Vitis International Variety Catalogue. 2023. Available online: www.vivc.de (accessed on 6 October 2023).
- Ollat, N.; Bordenave, L.; Tandonnet, J.P.; Boursiquot, J.M.; Marguerit, E. Grapevine rootstocks: Origins and perspectives. In Proceedings of the I International Symposium on Grapevine Roots, Rauscedo, Italy, 16–17 October 2014; pp. 11–22. [Google Scholar] [CrossRef]
- Moll, L.; Badosa, E.; Planas, M.; Feliu, L.; Montesinos, E.; Bonaterra, A. Antimicrobial peptides with antibiofilm activity against Xylella fastidiosa. Front. Microbiol. 2021, 12, 753874. [Google Scholar] [CrossRef]
- Baró, A.; Montesinos, L.; Badosa, E.; Montesinos, E. Aggressiveness of Spanish Isolates of Xylella fastidiosa to almond plants of different cultivars under greenhouse conditions. Phytopathology 2021, 111, 1994–2001. [Google Scholar] [CrossRef] [PubMed]
- Hill, B.L.; Purcell, A.H. Multiplication and Movement of Xylella fastidiosa within Grapevine and Four Other Plants. Phytopathology 1995, 85, 1368–1372. [Google Scholar] [CrossRef]
- Almeida, R.P.P.; Pereira, E.F.; Purcell, A.H.; Lopes, J.R.S. Multiplication and movement of a citrus strain of Xylella fastidiosa within sweet orange. Plant Dis. 2001, 85, 382–386. [Google Scholar] [CrossRef]
- Su, C.; Chang, C.J.; Chang, C.-M.; Shih, H.-T.; Tzeng, K.-C.; Jan, F.-J.; Kao, C.-W.; Deng, W.-L. Pierce’s Disease of grapevines in Taiwan: Isolation, cultivation and pathogenicity of Xylella fastidiosa. J. Phytopathol. 2013, 161, 389–396. [Google Scholar] [CrossRef]
- Simko, I.; Piepho, H.-P. The area under the disease progress stairs: Calculation, advantage, and application. Phytopathology 2012, 102, 381–389. [Google Scholar] [CrossRef] [PubMed]
- OEPP/EPPO. PM 7/24 (4) Xylella fastidiosa. EPPO Bull. 2019, 49, 175–227. [Google Scholar] [CrossRef]
- Harper, S.J.; Ward, L.I.; Clover, G.R.G. Development of LAMP and Real-Time PCR methods for the rapid detection of Xylella fastidiosa for quarantine and field applications. Phytopathology 2010, 100, 1282–1288. [Google Scholar] [CrossRef] [PubMed]
- Knipfer, T.; Bambach, N.; Hernandez, M.I.; Bartlett, M.K.; Sinclair, G.; Duong, F.; Kluepfel, D.A.; McElrone, A.J. Predicting stomatal closure and turgor loss in woody plants using predawn and midday water potential. Plant Physiol. 2020, 184, 881–894. [Google Scholar] [CrossRef]
- Castander-Olarieta, A.; Moncaleán, P.; Pereira, C.; Pěnčík, A.; Petřík, I.; Pavlović, I.; Novák, O.; Strnad, M.; Goicoa, T.; Ugarte, M.D.; et al. Cytokinins are involved in drought tolerance of Pinus radiata plants originating from embryonal masses induced at high temperatures. Tree Physiol. 2021, 41, 912–926. [Google Scholar] [CrossRef]
- Dandekar, A.M.; Gouran, H.; Ibáñez, A.M.; Uratsu, S.L.; Agüero, C.B.; McFarland, S.; Borhani, Y.; Feldstein, P.A.; Bruening, G.; Nascimento, R.; et al. An engineered innate immune defense protects grapevines from Pierce′s Disease. Proc. Natl. Acad. Sci. USA 2012, 109, 3721–3725. [Google Scholar] [CrossRef]
- Warschefsky, E.J.; Klein, L.L.; Frank, M.H.; Chitwood, D.H.; Londo, J.P.; von Wettberg, E.J.B.; Miller, A.J. Rootstocks: Diversity, domestication, and impacts on shoot phenotypes. Trends Plant Sci. 2016, 21, 418–437. [Google Scholar] [CrossRef]
- Kumar, P.; Rouphael, Y.; Cardarelli, M.; Colla, G. Vegetable grafting as a tool to improve drought resistance and water use efficiency. Front. Plant Sci. 2017, 8, 1130. [Google Scholar] [CrossRef]
- Singh, J.; Fabrizio, J.; Desnoues, E.; Pereira Silva, J.; Busch, W.; Khan, A. Root system traits impact early fire blight susceptibility in apple (Malus × domestica). BMC Plant Biol. 2019, 19, 579–593. [Google Scholar] [CrossRef]
- Heaton, J.B.; Dullahide, S.R.; Tancred, S.J.; Zeppa, A.G.; McWaters, A.D. Comparison of orchard and glasshouse tests in screening of apple breeding progeny for resistance to Venturia inaequalis in Queensland from 1985 to 1994. Australas. Plant Pathol. 1995, 24, 243–248. [Google Scholar] [CrossRef]
- Martinez-Bilbao, A.; Ortiz-Barredo, A.; Montesinos, E.; Murillo, J. Venturia inaequalis resistance in local Spanish cider apple germplasm under controlled and field conditions. Euphytica 2012, 188, 273–283. [Google Scholar] [CrossRef][Green Version]
- MacHardy, W.E. Apple Scab: Biology, Epidemiology and Management; APS Press: St. Paul, MN, USA, 1996. [Google Scholar]
- Martínez-Bilbao, A.; Ortiz-Barredo, A.; Montesinos, E.; Murillo, J. Evaluation of a cider apple germplasm collection of local cultivars from Spain for resistance to fire blight (Erwinia amylovora) using a combination of inoculation assays on leaves and shoots. HortScience 2009, 44, 1223–1227. [Google Scholar] [CrossRef]
- Fred, A.K.; Kiswara, G.; Yi, G.; Kim, K.-M. Screening Rice Cultivars for Resistance to Bacterial Leaf Blight. J. Microbiol. Biotechnol. 2016, 26, 938–945. [Google Scholar] [CrossRef]
- Oliver, J.E.; Cobine, P.A.; De La Fuente, L. Xylella fastidiosa isolates from both subsp. multiplex and fastidiosa cause disease on southern highbush blueberry (Vaccinium sp.) under greenhouse conditions. Phytopathology 2015, 105, 855–862. [Google Scholar] [CrossRef]
- Das, M.; Bhowmick, T.S.; Ahern, S.J.; Young, R.; Gonzalez, C.F. Control of Pierce’s Disease by phage. PLoS ONE 2015, 10, e0128902. [Google Scholar] [CrossRef] [PubMed]
- Rashed, A.; Kwan, J.; Baraff, B.; Ling, D.; Daugherty, M.P.; Killiny, N.; Almeida, R.P.P. Relative susceptibility of Vitis vinifera cultivars to vector-borne Xylella fastidiosa through time. PLoS ONE 2013, 8, e55326. [Google Scholar] [CrossRef][Green Version]
- Irani, H.; ValizadehKaji, B.; Naeini, M.R. Biostimulant-induced drought tolerance in grapevine is associated with physiological and biochemical changes. Chem. Biol. Technol. Agric. 2021, 8, 5. [Google Scholar] [CrossRef]
- Monteiro, E.; Gonçalves, B.; Cortez, I.; Castro, I. The role of biostimulants as alleviators of biotic and abiotic stresses in grapevine: A review. Plants 2022, 11, 396. [Google Scholar] [CrossRef]
- Sarcina, L.; Macchia, E.; Loconsole, G.; D’Attoma, G.; Bollella, P.; Catacchio, M.; Leonetti, F.; Di Franco, C.; Elicio, V.; Scamarcio, G.; et al. Fast and reliable electronic assay of a Xylella fastidiosa single bacterium in infected plants sap. Adv. Sci. 2022, 9, 2203900. [Google Scholar] [CrossRef]
- León, L.; De La Rosa, R.; Arriaza, M. Prioritization of olive breeding objectives in Spain: Analysis of a producers and researchers survey. Span. J. Agric. Res. 2021, 19, e0701. [Google Scholar] [CrossRef]
- Surano, A.; Abou Kubaa, R.; Nigro, F.; Altamura, G.; Losciale, P.; Saponari, M.; Saldarelli, P. Susceptible and resistant olive cultivars show differential physiological response to Xylella fastidiosa infections. Front. Plant. Sci. 2022, 13, 968934. [Google Scholar] [CrossRef] [PubMed]
- Alves, K.S.; Guimarães, M.; Ascari, J.P.; Queiroz, M.F.; Alfenas, R.F.; Mizubuti, E.S.G.; Del Ponte, E.M. RGB-Based Phenotyping of foliar disease severity under controlled conditions. Trop. Plant Pathol. 2022, 47, 105–117. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).