Abstract
Soil Cd is absorbed by roots and accumulated in cocoa plants, which represents a problem in the commercialization of beans. In order to evaluate whether the exogenous application of macronutrients (N, N-P, N-P-K, N-P-K-S, N-P-K-S-Mg, and N-P-K-S-Mg-Ca) mitigates the absorption, translocation, and accumulation of Cd in plants, soil pH and electric conductivity, dry root and shoot biomass, leaf gas exchange, chlorophyll content, and macronutrient bioaccumulation were evaluated in two cocoa clones (CCN-51 and EET-103) grown in a greenhouse. An increase in macronutrients gradually increased the extraction capacity of Cd in both clones, with the highest Cd values being obtained with the application of N-P-K-S-Mg-Ca. Macronutrient fertilization did not affect leaf gas exchange; however, it caused significant reductions of 30, 40, and 60% in chlorophyll content, shoot, and root dry biomass, respectively. The greatest translocation of Cd from the root to the shoot was obtained with treatments that included N in clone EET-103 and Ca in clone CCN-51. Fertilization with macronutrients did not decrease the absorption and accumulation of Cd in the cocoa seedlings, because a greater removal force of Cd from the adsorption complex towards the soil solution was caused by the exogenous application of Ca and Mg and an increase in soil acidity.
1. Introduction
The presence of cadmium (Cd) in soils can be of geological origin due to the parent material from which the soils are derived (native) or caused by anthropogenic factors such as agricultural and mining activities and the use of fertilizers and pesticides; the latter are considered to be the main sources of contamination in agricultural soils, reaching critical concentrations of 130 mg Cd kg−1 of soil [1]. Cadmium is a non-essential element for plant development; however, when it is available in soil, it is absorbed by roots [2]. In the case of cocoa, Cd accumulates in the leaves, fruits, and beans, and, when consumed in excessive concentrations in cocoa-derived products, is harmful to humans [3,4,5,6].
In America, Ecuador, with a production of 365,000 t yr−1, is the world’s largest exporter of cocoa; this export may represent USD 1,001,415,000 yr−1, a potential that is due to the 90,000 farmers who dedicate themselves to this crop, most of them being small and medium-sized producers [7]. The provinces with the largest areas planted with cocoa are the departments of El Oro, Manabí, and Guayas [8], and their soils and cocoa beans may have high concentrations of Cd [9]; this would limit or even impede the commercialization of cocoa in international markets, destabilizing the active socio-economy of the country.
Worldwide, there is a growing concern to ensure food safety and reduce consumer exposure to Cd present in chocolate. In January 2019, the European Commission (EC) put into force regulation No. 488/2014, which establishes that the maximum concentrations of Cd in cocoa products should be between 0.10 and 0.80 mg kg−1. To meet this requirement, buyers have established that the permissible level of Cd in cocoa beans must be in the maximum range from 0.5 to 1.1 mg kg−1 [10]. Therefore, a high concentration of Cd in cocoa beans would be a threat to their commercialization, and there is evidence that, in Peru, Colombia, Ecuador, and Honduras, the Cd concentrations in cocoa beans exceed those required by the EC [11,12].
High Cd concentrations in plants reduce the absorption and translocation of nutrients and water, generate oxidative damage, alter metabolism, inhibit morphology and physiology, cause imbalances in chloroplast metabolism, inhibit chlorophyll synthesis, and reduce the activity of the enzymes involved in CO2 fixation, due to the chlorosis produced by Fe and P deficiencies and the reduction in Mn transport, generating an increase in the production of reactive oxygen species, ROS [13,14]. However, plants can increase the activity of their antioxidant enzymes, thereby reducing the cellular damage induced by ROS. Recently, [15] reported a conserved set of genes involved in the response to Cd stress in Arabidopsis thaliana and Oryza sativa, in which effectors in the mitochondrial protein quality control networks were significantly activated in the shoot and root under exposure to Cd, allowing the maintenance of mitochondrial homeostasis.
Additionally, a high Cd content decreases stomatal conductance (gs), photosynthetic rate (A), and transpiration rate (E) [4,16]. In seedlings of cocoa clone CCN-51 growing in soils with 0, 0.05, and 0.1 g kg−1 of Cd, Cd toxicity affected antioxidant metabolism, cellular ultrastructure, and the absorption of mineral nutrients, decreased chlorophyll content, and caused damage to photosystems I and II, therefore causing a reduction in the maximum quantum efficiency of chlorophyll a fluorescence (Fv/Fm). Biomembrane rupture was observed in root and leaf cells [17]. In Triticum aestivum grown in soils with Cd, osmotic stress was generated that caused physiological damage by reducing the leaf relative water content, gs, and E [18]. However, scant information on the effects of a high Cd concentration on physiological changes has been reported (nine articles); the main mechanisms used are alterations in dry weight (three articles), followed by alterations in A (two articles), gs (two articles), and E (two articles) [5].
Recently, the effect of the toxicity of Cd in different genotypes of cocoa in scion-rootstock combinations has been reported; the CCN 51/ BN 34 and CCN 51/PH 16 combinations showed tolerance to the toxicity of Cd in soil, mainly evidenced by the higher accumulation of Cd in the root, higher A and water use efficiency (WUE), higher activity of superoxide dismutase, ascorbate peroxidase, and catalase, and lower lipid peroxidation [4,19].
To date, no solutions have been documented about Cd accumulation in cocoa in relation to the physiological mechanisms of absorption and partitioning of Cd in different plant organs, nutrition, plant age, and cocoa clones with low Cd accumulation, i.e., genetic factors. A viable alternative would be identifying cocoa clones which, despite growing in soils with a high Cd concentration, show a low affinity for this element, thus producing low-Cd beans.
The main factors related to the uptake of soil Cd by cocoa are soil pH, soil Cd availability, genotype, geographical location, agronomic factors such as phosphate fertilizers, and Cd interaction with other minerals/metal nutrients such as Zn and Mn present in the soil. To reduce the toxicity of this metal, cocoa displays some tolerance strategies such as the uptake and transfer of Cd from the root to the shoot, molecular and biochemical changes, Cd partition between plant organs, and Cd sequestration at the cellular level [5].
Cadmium uptake in cocoa is extremely complex. This study addressed trying to decrease Cd uptake by fertilization with macronutrients while measuring a large number of physiological variables to assess more closely the complexity of the environmental situation studied. In the present study, the hypothesis was tested that macronutrient fertilization would be a possible strategy for decreasing the uptake and transport of Cd if soil with high Cd concentrations causes a negative effect on the growth and physiological performance of two cocoa clones [10], thus improving growth and physiological traits. Our main objective was evaluating the effect of fertilization with several macronutrient combinations (N, N-P, N-P-K, N-P-K-S, N-P-K-S-Mg, and N-P-K-S-Mg-Ca) on the absorption and translocation of Cd, physiological variables (leaf gas exchange and chlorophyll content), and biomass distribution in the cocoa clones CCN-51 and EET-103 under greenhouse conditions.
2. Materials and Methods
2.1. Experimental Conditions and Plant Material
The research was carried out in the greenhouse of the Department of Soil and Water Management, Pichilingue Tropical Experimental Station (EETP) of the National Institute of Agricultural Research (INIAP), Mocache canton, Los Ríos Province, Ecuador, at 1°06′ S, 79°27′ W, and 75 masl.
The cocoa rootstocks studied were sexually reproduced with assisted pollination after [18] to ensure the purity of the material. The CCN-51 and EET-103 clones originate from Ecuador: EET-103 is a national cocoa resistant to “machete disease” (Ceratocystis cacaofunesta), while CCN 51 is susceptible; they were chosen because they are highly commercial, productive genotypes and CCN 51 is currently being generalized as rootstock [20,21]. Seedlings were grown under polyshade conditions with 65% shading: mean radiation of 210 μmol m−2 s−1 and 12 h of natural light; the maximum and minimum average temperatures and relative humidity were 33.5 and 23.3 °C, and 82 and 52%, respectively.
2.2. Physical–Chemical Characteristics of the Substrate
Soil was collected from the province of El Oro (3°23′ S, 79°50′ W). The characteristics of this soil, determined using methodologies described by [22], were a pH of 6.2, nutrient contents of NH4+ 22 mg kg−1, P 23 mg kg−1, K 0.49 meq 100 mL−1, Ca 19 meq 100 mL−1, Mg 2.8 meq 100 mL−1, S 6 mg kg−1, and Zn 8.2 mg kg−1, and a clay-loam texture. Cadmium was extracted with aqua regia (1.54 mg kg−1), as described by [23]. The soil was disaggregated with a glass roller, sieved through a 2 mm mesh, and polythene bags (5″ × 8″) were filled with 700 g of this soil. The soil moisture was maintained throughout the experiment by periodic irrigation with deionized water.
2.3. Experimental Design
A randomized complete block design with split plot arrangements was used. The main plot corresponded to the two clones and the subplots were the different fertilization treatments with macronutrients (control, N, N-P, N-P-K, N-P-K-S, N-P-K-S-Mg, and N-P-K-S-Mg-Ca), which resulted in 14 treatments with 3 replicates. Each experimental unit consisted of 4 plants, resulting in a total of 168 plants.
2.4. Macronutrient Fertilization
The application and dose of macronutrients in each treatment were carried out according to the analysis of the soil and nutritional needs of the cocoa in the nursery stage. As a source of N, urea (46% N) was used; for S, ammonium sulfate (24% S, 21% N); for K, KCl (60% K2O); for P, diammonium phosphate, DAP (18% N, 46% P2 O5); for Mg, magnesium sulfate (27% Mg, 16% S); and for Ca, calcium nitrate (15% N, 26% CaO) (Table 1). The doses were divided into three parts for N and S, in two for K2O, MgO, and CaO, and there was a single application of P2O5. In total, 20 mL of nutrient solution was applied to the plants on 21, 42, and 63 days after sowing (DAS), i.e., with a frequency of every 21 days, except for P2O5, which was applied only once at 0 DAS.

Table 1.
Macronutrient application schedule for each treatment.
2.5. Physiological Variables
2.5.1. Leaf Gas Exchange
Measurements were made 40 and 80 DAS from 09:00 to 13:00 h, evaluating six different plants in each treatment (n = 6), with a CIRAS 2 portable infrared gas analyzer (PP Systems, Hitchin, UK) to determine the gs, A, E, water use efficiency (WUE = A/E), and intercellular CO2 concentration (Ci). All the measurements were performed on fully expanded and healthy adult leaves of six different plants of each clone, under the following conditions: ambient CO2 concentrations (Ca) of 415 ± 10 μmol mol−1, 21% O2, a temperature of 28 ± 1 °C, a photosynthesis-saturating photon flux density (PFD) of 1000 μmol m−2 s−1, and a leaf–air water vapor gradient (VPD) of 1.0–1.5 kPa. In Ecuador, cocoa successfully is grown in full exposure [24]; for this reason, A was evaluated at a high light intensity.
2.5.2. Chlorophyll Index (CI)
The chlorophyll content was determined using a SPAD-502 Plus chlorophyll meter (Konica Minolta, Inc., Tokyo, Japan); SPAD units are directly proportional to leaf chlorophyll content. The chlorophyll concentration was measured in the same leaves used to measure the gas exchange, performing two readings per leaf on two leaves of six different plants for each treatment (n = 6). The determinations were made between 09:00 and 13:00 h with a fortnightly frequency from 45 to 80 DAS.
2.5.3. Determination of Cd in Tissues
Dried plant tissue samples were powdered in a “Willey” type mill (IKA, model A11 basic, Wilmington, NC, USA), which, to avoid contamination prior to each grinding, was cleaned with absorbent towels, diluted aqua regia (10%) and with deionized water, and dried with absorbent paper. The Cd was extracted using nitric-perchloric mineralization (8 mL HNO3 plus 2 mL HClO4; [23]) and the readings were performed using an atomic absorption spectrophotometer (Perkin Elmer, model AAnalyst 800, Yokohama, Japan) with a graphite oven at λ = 228.8 nm.
2.5.4. Cd Content of Shoot and Root
The values of root and shoot Cd content were calculated as:
2.5.5. Cadmium Extracting Capacity
The extracting capacity of Cd was determined following the protocol of [25]; the Cd absorption efficiency (2) and Cd translocation efficiency (3) were calculated according to the following equations:
2.5.6. Macronutrient Concentration in Leaf Tissue
Nitrogen was analyzed with the Kjeldahl method (KjeltecTM 8400 TecatorTM Line Foss, Jinan, China), K, Ca, and Mg were determined with an atomic absorption spectrophotometer (AA-6800, Kyoto, Japan), and S and P were determined using the colorimetric method (Spercord 210 Plus, Jena, Germany).
2.5.7. Soil pH and Electrical Conductivity
Soil pH and electrical conductivity (EC) were quantified using 20 g of soil dissolved in deionized water at a 1:2.5 weight:volume ratio, with shaking for 5 min in a mechanical shaker, resting for 1 h, and measuring with a potentiometer (HACH model SensIONTM MM340, Dusseldort, Germany) and conductivity meter (HACH model SensIONTM EC71, Dussldort, Germany).
2.5.8. Shoot and Root Dry Biomass
To remove soil and dust particles, the plants were successively immersed in HCl (3%), distilled water, and deionized water. The shoot and root were dried separately in an oven (Memmert, Gmbh 450, Schwabach, Germany) at 65 °C for 72 h and the dry mass was determined on an analytical balance (A & D Weighing model HR200, Japan).
2.6. Statistical Analysis
A two-way analysis of variance (ANOVA) and Tukey’s post hoc test with a significance level of p < 0.05 were performed to detect the possible interaction between the clones and macronutrients. To ensure reliability in the parametric statistical tests, such as ANOVA and Pearson’s correlation, the data were subjected to a normality test using the Shapiro–Wilk Test, and the Bartlett test was applied to check the assumption of the homogeneity of variance. Minitab Software version 19.1.0 [26] was used to perform the statistical analyses. All the graphics were performed with SigmaPlot version 12 (Systat Software, San Jose, CA, USA).
3. Results
3.1. Leaf Gas Exchange
There were significant differences between the fertilization treatments in terms of WUE at 40 and 80 DAS and A at 80 DAS, while there were no differences between the clones or the interaction (clone × fertilization) (Figure 1, Table 2). On the contrary, at 40 and 80 DAS, no significant differences were found in gs, E, and Ci among the clones, fertilization, and clone × fertilization interaction. The minimum and maximum average values of A were from 2.3 ± 0.7 to 4.0 ± 0.3 μmol m−2 s−1; E ranged from 0.45 ± 0.08 to 1.32 ± 0.25 mmol m−2 s−1; the values of gs varied between 28.3 ± 2.2 and 73.4 ± 11.1 mmol m−2 s−1; Ci values of 223.1–319.11 ± 32.80 µmol mol−1 were observed; and WUE ranged from 2.52 ± 0.35 to 6.73 ± 0.50 mmol mol−1.
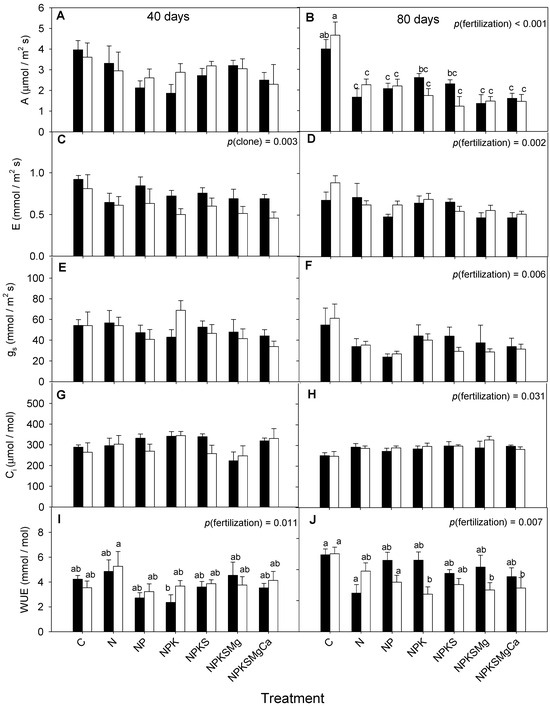
Figure 1.
Changes with fertilization treatments of cocoa clones CCN-51 (filled bars) and EET-103 (empty bars) in: (A,B) photosynthetic rate; (C,D) transpiration rate; (E,F) stomatal conductance; (G,H) intercellular CO2 concentration; and (I,J) water use efficiency. Values of p indicate the effect of the factors on the dependent variables after a two-way ANOVA (clone × fertilization). The vertical bars represent the mean ± SE (n = 6). Different letters above bars show statistical differences between treatments after Tukey’s test (p < 0.05). The panels in which no letters appear above the bar are due to the fact that there were no significant differences.

Table 2.
Results of the ANOVAs on the effect of clone, fertilization, and the interaction (clone × fertilization) on the different physiological traits studied. Values indicate statistically significant effect of the factor at p < 0.05.
3.2. Chlorophyll Index
The values of SPAD at 45, 60, 75, and 90 DAS were higher in clone CCN-51. At 90 DAS, significant effects were obtained in the fertilization factor with macronutrients and interaction (clone × fertilization), finding that the fertilized plants reduced their chlorophyll concentration by up to 30% (Table 3).

Table 3.
Response of chlorophyll index of cocoa clones to the application of macronutrients. Values are the mean ± SE (n = 6).
3.3. Cadmium Accumulation and Extraction Capacity
Cadmium absorption, translocation, and shoot and root content showed significant differences among the clones and fertilization treatments (Figure 2, Table 2), whereas no significant effects were observed for the interaction (clone × treatment) in Cd absorption. Cadmium uptake increased in each clone with the addition of a macronutrient; the highest absorptions of Cd were obtained with the application of Ca (Figure 2A).
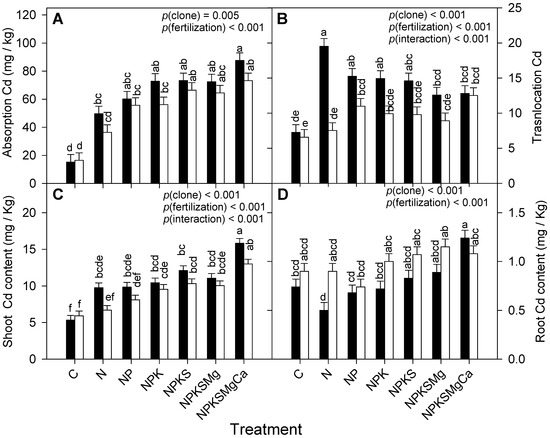
Figure 2.
Changes with exogenous nutrient application to cocoa clones CCN-51 (filled bars) and EET-103 (empty bars) on: (A,B) Cd absorption and translocation, and (C,D) shoot and root Cd contents. Values of p indicate the effect of factors on dependent variables after a two-way ANOVA (clone × fertilization). The vertical bars represent the mean ± SE (n = 12). Different letters above bars denote significant differences among treatments after Tukey’s test (p < 0.05).
Between the clones, Cd translocation showed significant differences among the macronutrients, where N was the most influential on Cd transport from the root to the shoot of CCN-51, while, in EET-103 fertilized with Ca, the highest Cd transport to the shoot was obtained (Figure 2B, Table 2).
Significant differences were detected in the shoot for the interaction, especially when the clones were treated with Ca, where Cd contents were the highest (Figure 2C, Table 2). Conversely, the interaction did not cause significant effects on root Cd content; however, with the application of Mg and Ca, the highest Cd contents were obtained in EET-103 (1.15 ± 0.14 mg kg−1) and CCN-51 (1.24 ± 0.10 mg kg−1) (Figure 2D). Less Cd was observed in the shoot and more in the root of the EET-103 clone; in CCN-51, the opposite response was observed.
3.4. Nutrient Application
The fertilized treatments had higher foliar concentrations of N, P, S, Mg, and Ca. Significant effects were also observed between the clones (p = 0.0001); clone CCN-51 presented the highest concentrations of N and P, while clone EET-103 showed the highest S concentration. In the interaction, statistical differences were detected in the concentrations of N, P, S, and Ca (Figure 3A–D, Table 2).
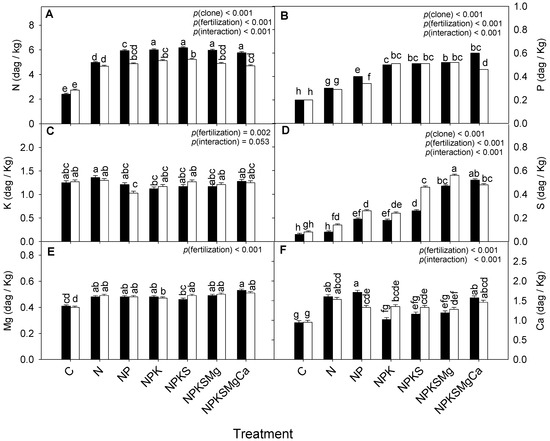
Figure 3.
Response to macronutrient application to clones CCN-51 (filled bars) and EET-103 (empty bars) on leaf content of: (A) nitrogen; (B) phosphorus; (C) potassium; (D) sulfur; (E) magnesium; and (F) calcium. Values of p indicate the effect of the factors on the dependent variables after a two-way ANOVA (clone × fertilization). The vertical bars represent the mean ± SE (n = 12). Different letters above bars show statistical differences between treatments after Tukey’s test (p < 0.05).
3.5. Soil pH and Electrical Conductivity
There were statistically significant differences in the pH and EC of the soil, independent of each simple factor and their interaction (clone × fertilization) (Figure 4, Table 2). The soil where clone EET-103 was grown reached the highest pH (5.7 ± 0.0) and EC (3.46 ± 0.07 dS m−1) values. However, in the fertilization treatments of both cocoa clones, pH decreases and EC increases were found. The minimum and maximum pH values went from 4.9 ± 0.1 to 7.0 ± 0.1 (EET-103) and 5.0 ± 0.1 to 6.8 ± 0.3 (CCN-51), respectively. Low and high values of electrical conductivity were found: 0.34 ± 0.01 to 4.67 ± 0.20 dS m−1 (EET-103) and 0.43 ± 0.01 to 4.49 ± 0.16 dS m−1 (CCN-51). The low values of electrical conductivity corresponded to the control plants, where no fertilization was applied.
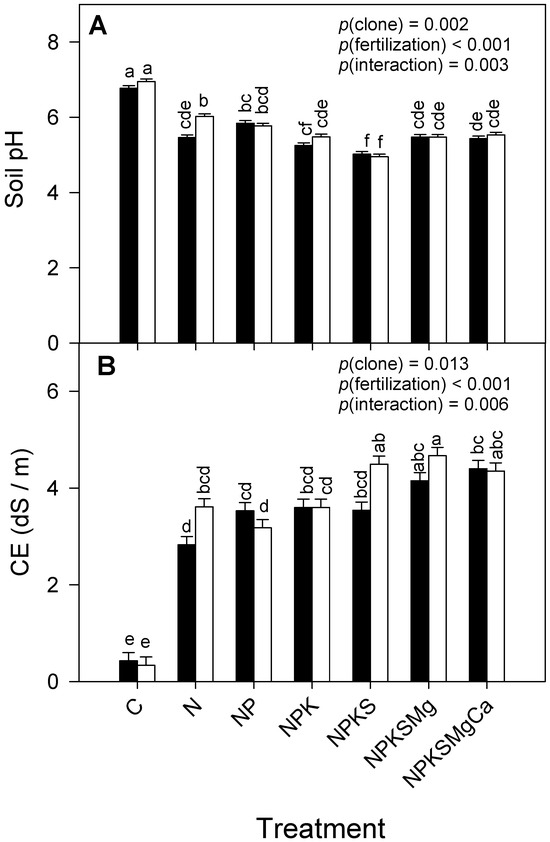
Figure 4.
Effects of fertilization with macronutrients in clones CCN-51 (filled bars) and EET-103 (open bars) on: (A) soil pH and (B) electric soil conductivity. Values of p indicate the effect of factors on dependent variables after a two-way ANOVA (clone × fertilization). The vertical bars represent the mean ± SE (n = 12). Different letters above bars indicate significant differences among treatments after Tukey’s test (p < 0.05).
3.6. Shoot and Root Dry Biomass
Regardless of the clone, fertilization with macronutrients had significant effects on the production of shoot and root dry mass. Between the clones, significant differences were only found for shoot dry mass, detecting the highest mean in EET-103, with 1.74 ± 0.03 g plant−1 (Figure 5, Table 2). On the contrary, in the interaction (clone × fertilization), there were no significant differences in dry mass production; however, it was observed that the fertilized treatments caused 40 and 60% reductions in the dry masses of the shoot and root, respectively, in both clones.
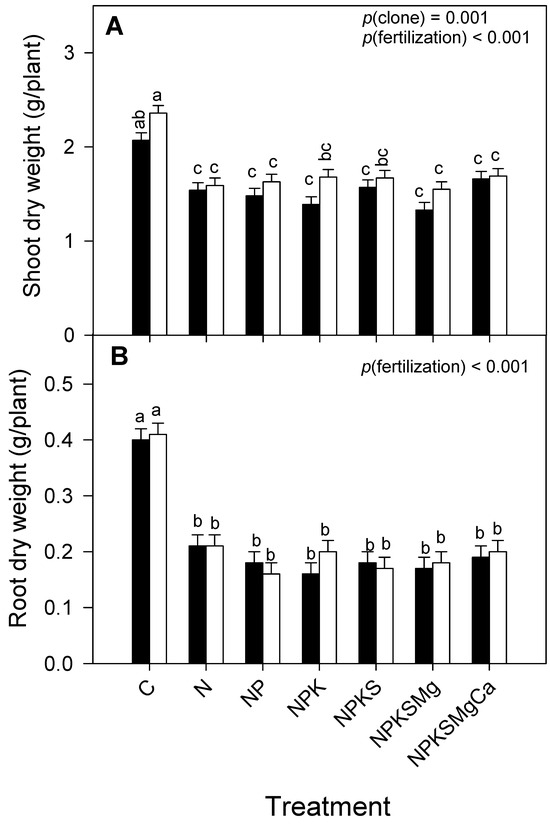
Figure 5.
Response to fertilization with exogenous macronutrients of clones CCN-51 (filled bars) and EET-103 (empty bars) on: (A) shoot dry mass and (B) root dry mass. Values of p indicate the effect of factors on dependent variables after a two-way ANOVA (clone × fertilization). The vertical bars represent the mean ± SE (n = 12). Different letters above bars indicate significant differences among treatments after Tukey’s test (p < 0.05).
3.7. Pearson’s Correlation Matrix
Only the correlation coefficients (r2) with a significance level of p < 0.001 were considered (Table 4). Thus, root dry mass had positive relationships with shoot dry mass, soil pH, and A at 80 DAS, but had inversely proportional effects with soil EC, Cd uptake efficiency, Cd translocation efficiency, and all macronutrient contents. Likewise, shoot dry mass showed positive associations with A at 80 DAS and soil pH, but negative associations with Cd extraction efficiency, soil EC, N, P, and leaf Mg.

Table 4.
Pearson’s correlation matrix for each variable analyzed. Highly significant correlations (p < 0.001) are shown in Bold. (n = 42). NS = not significant.
While the shoot and plant Cd content showed directly proportional relationships with the efficiency of Cd absorption, the EC of the soil and leaf concentrations of N, P, S, and Mg also presented a significant negative relationship with the soil pH.
There were negative and strong associations among the soil pH and leaf concentrations of N, P, S, and Mg. On the contrary, EC had directly proportional relationships with the leaf concentrations of N, P, S, Mg, and Ca, but also exhibited negative associations with A at 80 DAS. The values of A at 80 DAS showed negative correlations with the concentrations of N, P, S, Mg, Ca, and soil EC; however, it had positive associations with the soil pH and E at 40 DAS.
3.8. Nutrient Interactions with Cd
No unique effect was observed in the interaction with nutrient fertilization and its effect on shoot and root Cd uptake (Table 5). Regardless of the species, in some studies, a positive effect was found, observing that fertilization with nutrients caused a reduction in Cd absorption, while in other studies, the effect was negative, i.e., the application of some nutrients increased Cd accumulation.

Table 5.
Plant species, purpose of the research, nutrient interactions with Cd, and references of bibliographic information used by us for data discussion, in which assessed the interactions between macro and micronutrients with Cd uptake of different species.
4. Discussion
The increase in a macronutrient in fertilization gradually increased the extracting capacity of Cd in both cocoa clones, causing significant reductions of 30, 40, and 60% in chlorophyll content, shoot, and root dry mass, respectively, without significantly affecting leaf gas exchange, although the reduction in chlorophyll content might partially explain the decrease in A at the end of the experiment. However, the highest transport of Cd from the root to the shoot was obtained with the application of N and N-P-K-S-Mg-Ca in both clones. Our results reject the hypothesis that fertilization would reduce the absorption and accumulation of Cd in cocoa. The application of Ca and Mg ions caused the desorption of Cd towards the soil solution, increasing the availability with a greater extraction of Cd. A desorption phenomenon was also obtained by the supply of ammonium that releases hydrogen and removes Cd from the adsorption complex towards the aqueous phase of the soil.
The chemical processes of N metabolism release protons, especially when nitrogen fertilizers such as urea, ammonium sulfate, ammonium nitrate, and diaommonium phosphate are applied, which increases soil acidity [26]. While calcium nitrate, magnesium sulfate, and potassium nitrate are fertilizers, they tend to increase soil EC [36]. Ionic stress produces harmful effects that induce a loss of turgidity and the inhibition of cell division processes [37,38], a physiological disorder that, at the plant level, is reflected in a loss pf dry matter production [39]. This agronomic response was corroborated by [26], who reported that extreme EC values resulting from fertilization decrease plant dry mass production. Similarly, ref. [40] observed in coffee seedlings that exogenous applications of K, Mg, and Ca through the supply of conventional fertilization significantly elevated EC and caused a reduction in plant mass.
Mg and Ca, being divalent ions, have a high ionic potential to compete with Cd for cation exchange sites in the solid phase of soil [41,42], displacing other ions from the adsorption complex and significantly increasing the availability of Cd in the soil solution [43,44], thus allowing for a greater extraction of Cd by the root system.
Clone CCN-51 reached the highest chlorophyll indices from 45 to 90 DAS. In this regard, it has been documented that its pigment contents vary according to the varieties, plant ages, environmental conditions, and even among the analytical methods used in their determination [45]. However, [46] found, under field conditions, similar chlorophyll contents in CCN-51 and EET-103. In the same cocoa clones, ref. [23] observed identical photochemical activities; these clones showed the same values of electron transport rate, relative quantum efficiency of PSII, and coefficient of photochemical extinction.
However, at 90 DAS, a reduction in chlorophyll was found, this being more severe with the applications of S, Mg, and Ca. In this sense, it has been found that the ionic stress produced by the high presence of exchangeable bases affects the functioning of PSI and PSII, an alteration in electron transfer, causing reductions in ATP and NADPH, and the degradation of chlorophyll biosynthesis [47,48]. In the photorespiration process, when there is low energy synthesis, greater oxygen intoxication is generated, having a stronger effect in tropical climates, where rubisco increases its preference for oxygen with an increase in temperature [49,50].
The cocoa clones fertilized with macronutrients reflected values of A, E, and gs twice as low as those found for cocoa seedlings under greenhouse conditions [51] and young plants established in the field [52]. This effect could have been due to the plants being treated with macronutrients presenting a type of oxidative stress combined between the accumulation of Cd in their tissues and the elevated EC in the soil. The photosynthetic capacity in clone CCN-51 was affected when growing it in excessive Cd concentrations, i.e., ≥0.4 mmol kg−1 of soil and ≥1.2 Zn mmol kg−1 of soil [30]. Similarly, in juvenile cacao plants, Cd promoted physiological and biochemical changes in the scion–rootstock interaction [4]. When cocoa plants are subjected to some type of stress due to abiotic factors, their values of WUE tend to decrease, which negatively impacts gs, and hence photosynthetic efficiency [53].
We found that the macronutrients increased the Cd absorption and transport from the root to the shoot, a result that contrasts with those reported by [54]; these authors sustained that Ca, P, and K are the main nutrients with antagonistic effects on the transfer of Cd from the roots to the shoots. Similarly, our results differ from what was indicated by [31], who reported that the exogenous application of Ca decreases the transport of Cd in plants of Brassica napus, because Ca ions interrupt the entry of the Cd cation through the plasma membrane, thus preventing Cd from reaching the conductive tissues. Ref. [32] found, in rice plants, that nitrate had a synergistic effect on the translocation of Cd; this same phenomenon was found in CCN-51 with N, where the greatest internal transfer of Cd was reflected. Likewise, synergistic effects have been found between K with Cd in sunflower, rice, wheat, and cabbage [33,34]. Ref. [55] noted, in tobacco plants, that sulfur favored the transport of Cd from the roots to the shoots. However, this result contrasts with those obtained in rice by [35], who observed that S reduced the transport of Cd from the underground system toward the shoot. The results found in the present research agree with a report by [56], who argued that fertilization is not a strategy that allows for mitigating Cd transfer in plants.
Regarding the Cd contents at the organ level, the highest accumulation of Cd in the shoot was found in both cocoa clones, in a similar way to a report on cocoa seedlings obtained from two progenies, CCN-10 × SCA-6 and Catongo × Catongo [28]. However, these results differ from what was evidenced by [30], who detected that the root accumulated more Cd than the shoot in seedlings of clone CCN-51. In adult plants established in the field, a difference was also detected between the accumulation of Cd in the aerial and underground organs; for example, ref. [57] reported the highest accumulation of Cd in the roots (1.68 mg kg−1), followed by the stems (0.75 mg kg−1) and leaves (0.51 mg kg−1). In contrast, [58] found, in cocoa plants growing in alluvial and residual soil, that the accumulation of Cd in the production stage followed the descending order of branches > leaves > root > beans > shells, whereas [59] reported the highest general means of Cd concentration in rootstocks (2.9 mg kg−1), followed by stems (2.4 mg kg−1), leaves (2.4 mg kg−1), and beans (2.5 mg kg−1). These antecedents show that cocoa plants do not always show the same tendency to distribute or accumulate Cd in their organs, with concentration changing according to the phenological stage of the crop, clone, climatic conditions, and physical–chemical factors of the soil [59].
In Ecuador, several studies have been carried out on cocoa farms related to the different Cd contents of cocoa clones [1,60], but these authors did not show specific genetic effects on the absorption and accumulation of Cd, because the clones that were evaluated did not grow in the same edapho-climatic conditions and were not grafted on the same pattern with homogeneous genetic characteristics. Therefore, to date, our work in Ecuador is the first to corroborate intraspecific variations in cocoa patterns in the accumulation of Cd among genetic materials. Specifically, clone EET-103, considered to be resistant to machete disease, had the lowest ability to extract and accumulate Cd in its shoots [20]. Therefore, this gives us the certainty to propose clone EET-103 as a cocoa standard to reduce the absorption of Cd.
The nutrient concentrations in the leaf tissues of both cocoa clones increased with fertilization. It has been found that, when cocoa seedlings grow in a soil with critical concentrations of Cd (28 mg kg−1) and physical–chemical factors (pH and EC) are in favorable conditions for Cd availability, the uptake and accumulation of Mg and Ca decrease [27]. Similarly, [28,30] observed that cocoa seedlings accumulate more Cd when there are critical concentrations of this metal in the soil and limitations of essential divalent cations (Zn, Fe, Cu, and Mn), because cocoa is a crop with a high affinity for Cd and Cd is transferred from the soil to the different organs by the same nutritional cation transporter proteins [61].
Clone CCN-51 reached higher concentrations of N and P in a response that has been reported by [62,63], who observed that, this clone, unlike other commercial clones, has a higher ability to capture these elements.
Fertilization is an agricultural activity that induces changes in the chemical properties of soil [64]. In this work, it was found that the exogenous application of macronutrients through fertilization reduced the soil pH to 4.8 and increased the EC to as high as 4.67 dS m−1. When cocoa plants grow in extremely acidic soil (pH < 5.5), they suffer physiological disorders and present damage to their morphological structures [65], and acidity increases the bioavailability of Cd in the soil solution. Additionally, soils with an EC of > 2 dS m−1 represent saline soils [66], and this causes water deficit, decreases water absorption, produces ionic imbalance, and reduces respiration and photosynthetic activity. In addition, it is indicative of high cation contents and excess chloride, sodium, and magnesium that increase the availability of Cd in the soil [38]. These antecedents suggest that, when soils contaminated by Cd are enriched with nutrient solutions, periodic monitoring of their chemical properties should be performed, since the changes may favor the availability of Cd and its absorption by plants, affecting growth and development.
In general, the variables that showed positive and significant associations (p < 0.001) among them were the leaf concentrations of N, P, S Mg, and Cd, absorption efficiency of Cd, and soil EC, and these variables showed strong and negative relationships with photosynthetic activity at 40 and 80 DAS, root dry mass, shoot dry mass, and soil pH. All of this apparently occurred as a consequence of the decrease in pH and increase in EC, which caused a greater transfer of N, P, S Mg, and Cd to the shoot, causing damage to the photosynthetic machinery and production of dry matter. Moreover, these results corroborate that, when the plant captures macronutrients, it also absorbs Cd. However, the toxicity caused by Cd in cocoa to date has only been observed in seedlings, a phenomenon that was reported in the experiments carried out by [28,30,67,68], who found harmful effects caused by Cd when seedlings grew in soils enriched with this metal in concentrations of ≥ 5 mg kg−1. In the foliar tissues, Cd concentrations lay in the severe to critical range (7.5 to 18.5 mg kg−1); thus, the concentrations detected in this work fell within the range reported by these authors, and the negative effects on photosynthetic capacity were due not only to the presence of Cd, but also to ionic stress (EC). Furthermore, ref. [27] observed a positive and strong relationship between N and Cd. Likewise, ref. [69] observed a linear regression between the increase in S with Cd concentrations in cocoa leaf tissues. In other Cd accumulator plants such as rice, ref. [29] evidenced positive relationships in foliar tissues between Fe, Zn, Cu, and Cd.
There was a negative correlation between Ci and A at 40 DAS, because of a reduction in photosynthetic activity, as previously reported [70], that affects the production of dry matter [45]. A direct dependence was found between A at 80 DAS and the dry mass of the stem and root.
The acidity of soil facilitates a greater absorption and accumulation of Cd in cocoa leaf tissues, as observed by [1,71,72], who pointed out that, among the soil factors, pH has the greatest influence on the availability and absorption of Cd.
5. Conclusions
Fertilization with macronutrients did not represent a viable strategy to allow for mitigating the absorption and accumulation of Cd in cocoa plants, because the application of nitrogen in the form of ammonium releases hydrogen and removes Cd from the soil particles into the aqueous phase, thus increasing Cd availability. Also, when Ca and Mg are applied, there is a greater desorption of Cd from the fixation sites into the soil solution, increasing its availability and allowing for a greater extraction of Cd. Cocoa plants absorb and accumulate macronutrients at the same time that they absorb Cd from the soil and transport it to their aerial tissues. Specifically, it was found that the nutrients that most increase the transport of Cd from the roots to the shoots are N and Ca in clones CCN-51 and EET-103, respectively. The high accumulation of Cd in the foliar tissues and the EC of the soil caused damage to the photosynthetic capacity of the cocoa plants, causing a reduction in the production of dry matter. The genetic cocoa material EET-103 concentrates less Cd in the aerial tissues than CCN-51, resulting in a good alternative for use as a rootstock for use in soils with high Cd concentrations, because of its lower affinity for Cd. Our results contribute to the achievement of knowledge for the genetic improvement of cacao, regarding toxicity tolerance strategies and a reduction in soil Cd uptake.
Author Contributions
Conceptualization, M.D.C.-Z.; methodology, J.J.R.-P., M.D.C.-Z., K.P.-S. and W.T.; software, W.T., V.R., J.J.R.-P. and R.A.P.-G.; validation, W.T., V.R., M.D.C.-Z. and R.A.P.-G.; formal analysis, J.J.R.-P., M.D.C.-Z. and R.A.P.-G.; investigation, R.A.P.-G., M.D.C.-Z., K.P.-S., V.R. and W.T.; resources, M.D.C.-Z., J.J.R.-P. and W.T.; data curation, M.D.C.-Z. and R.A.P.-G.; writing—original draft preparation, R.A.P.-G. and W.T.; writing—review and editing, M.D.C.-Z., W.T. and J.J.R.-P.; supervision, M.D.C.-Z., J.J.R.-P. and W.T.; project administration, M.D.C.-Z., J.J.R.-P. and W.T.; funding acquisition, M.D.C.-Z., J.J.R.-P. and W.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the German Society for International Cooperation (GIZ) and partially the National Science and Technology Fund (FONACIT; project 2019000026).
Data Availability Statement
The data analyzed and presented in this study are available upon request from the Corresponding author.
Acknowledgments
The authors thank the technical support provided by Mayra Macías.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chavez, E.; He, Z.L.; Stoffella, P.J.; Mylavarapub, R.S.; Li, Y.C.; Moyanod, B.; Baligar, V.C. Concentration of cadmium in cacao beans and its relationship with soil cadmium in southern Ecuador. Sci. Total Environ. 2015, 533, 205–214. [Google Scholar] [CrossRef]
- Hamid, Y.; Tang, L.; Hussain, B.; Usman, M.; Liu, L.; Cao, X.; Ulhassan, Z.; Bilal, M.; Yang, X. Cadmium mobility in three contaminated soils amended with different additives as evaluated by dynamic flow-through experiments. Chemosphere 2020, 261, 127763. [Google Scholar] [CrossRef]
- Santander Ruiz, W.; Garay Montesa, R.; Verde Girbaub, C.; Mendieta Taboadaa, O. Determinación del contenido de cadmio en suelos, frutos, granos fermentados y secos, licor de cacao y chocolate en zonas productoras de la región San Martín. Rev. Soc. Quím. Per. 2021, 87, 39–49. [Google Scholar] [CrossRef]
- De Almeida, N.M.; de Almeida, A.-A.F.; de Almeida Santos, N.; do Nascimento, J.L.; de Carvalho Neto, C.H.; Pirovani, C.P.; Ahnert, D.; Baligar, V.C. Scion-rootstock interaction and tolerance to cadmium toxicity in juvenile Theobroma cacao plants. Sci. Hortic. 2022, 300, 111086. [Google Scholar] [CrossRef]
- Li, X.; Yu, H.; Sun, X.; Yang, J.; Wang, D.; Shen, L.; Zhao, Y. Effects of sulfur application on cadmium bioaccumulation in tobacco and its possible mechanisms of rhizospheric microorganisms. J. Hazard. Mater. 2019, 368, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, B.R.M.; de Almeida, A.-A.F.; de Almeida Santos, N.; Pirovani, C.P. Tolerance strategies and factors that influence the cadmium uptake by cacao tree. Sci. Hortic. 2022, 293, 110733. [Google Scholar] [CrossRef]
- ICCO. Production of Cocoa Beans. Quarterly Bulletin of Cocoa Statistics. Cocoa Year 455 2020/21, 2022, (Vol. XLVI). Available online: https://www.icco.org/about-us/icco-news/398-456quarterly-bulletin-of-cocoa-statistics-november-2018.htm (accessed on 17 September 2022).
- Instituto Nacional de Estadística y Censos (INEC). Encuesta Sobre la Superficie y la Continua Producción Agrícola 2020; Government of Ecuador: Quito, Ecuador, 2021; 36p.
- Mite, F.; Carrillo, M.; Durango, W. Advances in monitoring the presence of cadmium in cocoa beans, soils, and water in Ecuador. In Proceedings of the 2010, XII Ecuadorian Congress of Soil Science, Brisbane, Australia, 1–6 August 2010. [Google Scholar]
- Meter, A.; Atkinson, R.J.; Laliberte, B. Cadmio en el Cacao de América Latina y el Caribe—Análisis de la Investigación y Soluciones Potenciales para la Mitigación; Bioversity International: Roma, Italy, 2019; ISBN 978-92-9255-136-0. [Google Scholar]
- Argüello, D.; Chavez, E.; Lauryssen, F.; Vanderschueren, R.; Smolders, E.; Montalvo, D. Soil properties and agronomic factors affecting cadmium concentrations in cocoa beans: A nationwide survey in Ecuador. Sci. Total Environ. 2019, 649, 120–127. [Google Scholar] [CrossRef]
- Gramlich, A.; Tandy, S.; Gauggel, C.; López, M.; Perla, D.; Gonzalez, V.; Schulin, R. Soil cadmium uptake by cocoa in Honduras. Sci. Total Environ. 2018, 612, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, B.; Basu, S. Regulation of boron, iron, and cadmium transporters in maintaining proper balance between their deficiency and excess in plants (Chapter 12). In Metal and Nutrient Transporters in Abiotic Stress; Roychoudhury, A., Kumar, D., Deshmuk, R., Eds.; Academic Press: London, UK, 2021; pp. 237–250. ISBN 978-0-12-817955-0. [Google Scholar]
- Maksymiec, W.; Wojcik, M.; Krupa, Z. Variation in oxidative stress and photochemical activity in Arabidopsis thaliana leaves subjected to cadmium and excess copper in the presence or absence of jasmonate and ascorbate. Chemosphere 2007, 66, 421–427. [Google Scholar] [CrossRef]
- Liu, M.; Huang, Z.; Xie, K.; Guo, C.; Wang, Y.; Wang, X. Mitostasis is the central biological hub underlying the response of plants to cadmium stress. J. Hazard. Mater. 2023, 41, 129930. [Google Scholar] [CrossRef]
- Younis, U.; Malik, S.A.; Rizwan, M.; Qayyum, M.F.; Ok, Y.S.; Shah, M.H.R.; Rehman, R.A.; Ahmad, N. Biochar enhances the cadmium tolerance in spinach (Spinacia oleracea) through modification of Cd uptake and physiological and biochemical attributes. Environ. Sci. Pollut. Res. 2016, 23, 21385–21394. [Google Scholar] [CrossRef]
- Pereira de Araújo, R.; Furtado de Almeida, A.A.; Silva Pereira, L.; Mangabeira, P.A.O.; Olimpio Souza, J.; Pirovani, C.P.; Ahnert, D.; Baligar, V.C. Photosynthetic, antioxidant, molecular and ultrastructural responses of young cocoa plants to Cd toxicity in the soil. Ecotoxicol. Environ. Saf. 2017, 144, 148–157. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Hussain, A.; Ali, Q.; Shakoor, M.B.; Zia-Ur-Rehman, M.; Farid, M.; Asma, M. Effect of zinc-lysine on growth, yield and cadmium uptake in wheat (Triticum aestivum L.) and health risk assessment. Chemosphere 2017, 187, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Vera-Chang, J.; Cabrera-Verdezoto, R.; Morán-Morán, J.; Neira-Rengifo, K.; Haz-Burgos, R.; Vera-Barahona, J.; Molina-Triviño, H.; Moncayo-Carreño, O.; Díaz-Ocampo, E.; Cabrera-Verdesoto, C. Evaluación de tres métodos de polinización artificial en clones de cacao (Theobroma cacao L.) CCN-51. Idesia 2016, 34, 35–40. [Google Scholar] [CrossRef]
- Paladines-Rezabala, A.; Moreira-Morrillo, A.A.; Mieles, A.E.; Garcés-Fiallos, F.R. Avances en la comprensión de la interacción entre Ceratocystis cacaofunesta y Xyleborus ferrugineus (Coleoptera: Curculionidae: Scolytinae) en árboles de cacao. Sci. Agropecu. 2022, 13, 43–52. [Google Scholar] [CrossRef]
- Vera Barahona, J.; Suárez Capello, C.; Mogrovejo, E. Technical Description of Some Cocoa Hybrids and Clones Recommended by the National Institute of Agricultural Research (INIAP); INIAP, Pichilingue Tropical Experimental Station, Cocoa Program: Quevedo, Ecuador, 1984; (Technical Communication No. 12). Available online: https://repositorio.iniap.gob.ec/handle/41000/1602 (accessed on 7 May 2022).
- Quezada Crespo, C.J.; Carrillo Zenteno, M.D.; Morales Intriago, F.L.; Carrillo Alvarado, R.A. Nutrient critical levels and availability in soils cultivated with peach palm (Bactris gasipaes Kunth.) in Santo Domingo de Los Tsáchilas, Ecuador. Acta Agron. 2017, 66, 235–240. [Google Scholar] [CrossRef]
- Carrillo, M. Characterization of the Forms of Heavy Metals, Their Bioavailability and Their Adsorption and Mobility Dynamics in Ecuador Alone. Doctoral Thesis, Federal University of Viçosa, Minas Gerais, Brazil, 2003. [Google Scholar]
- Tezara, W.; De Almeida, J.; Valencia, E.; Cortes, J.; Bolaños, M. Actividad fotoquímica de clones élites de cacao Theobroma cacao L, ecuatoriano en el norte de la provincia Esmeraldas. Rev. Cient. Inter. Investig. Sab. 2015, 4, 37–52. Available online: http://revistasdigitales.utelvt.edu.ec/revista/index.php/investigacion_y_saberes/article/view/90 (accessed on 3 July 2022).
- Wang, F.Y.; Lin, X.G.; Yin, R. Inoculation with arbuscular mycorrhizal fungus Acaulospora mellea decreases Cu phytoextraction by maize from Cu-contaminated soil. Pedobiologia 2007, 51, 99–109. [Google Scholar] [CrossRef]
- Minitab. Userߣs Guide: Statistical Software; Version 19; Minitab Inc.: State College, PA, USA, 2021. [Google Scholar]
- Engbersen, N.; Gramlich, A.; Lopez, M.; Schwarz, G.; Hattendorf, B.; Gutierrez, O.; Schulin, R. Cadmium accumulation and allocation in different cacao cultivars. Sci. Total Environ. 2019, 678, 660–670. [Google Scholar] [CrossRef]
- Zhang, D.; Du, G.; Chen, D.; Shi, G.; Rao, W.; Li, X.; Jianga, Y.; Liua, S.; Wang, D. Effect of elemental sulfur and gypsum application on the bioavailability and redistribution of cadmium during rice growth. Sci. Total Environ. 2019, 657, 1460–1467. [Google Scholar] [CrossRef]
- García, G.; García, M.; Ramírez, H. Comportamiento de siete cultivares de Allium cepa L. ante diferentes niveles de estrés salino. Bioagro 2015, 27, 93–102. Available online: http://www.redalyc.org/articulo.oa?id=85741585005 (accessed on 27 May 2022).
- García Lozano, J.; Moreno Fonseca, L.P. Respuestas fisiológicas de Theobroma cacao L. en etapa de vivero a la disponibilidad de agua en el suelo. Acta Agron. 2016, 65, 44–50. Available online: https://www.redalyc.org/articulo.oa?id=169943143008 (accessed on 7 June 2022). [CrossRef]
- Almeida, A.A.-F.; Valle, R. Ecophysiology of the cacao tree. Braz. J. Plant Physiol. 2007, 19, 425–448. [Google Scholar] [CrossRef]
- Kabata-Pendias, A.; Pendias, H. Trace Elements in Soils and Plants, 3rd ed.; CRC Press: Boca de Raton, FL, USA, 2001; p. 331. ISBN 0-8493-1575-1. [Google Scholar]
- Wan, G.; Najeeb, U.; Jilani, G.; Naeem, M.S.; Zhou, W. Calcium invigorates the cadmium-stressed Brassica napus L. plants by strengthening their photosynthetic system. Environ. Sci. Pollut. Res. 2011, 18, 1478–1486. [Google Scholar] [CrossRef] [PubMed]
- Jalloh, M.A.; Chen, J.; Zhen, F.; Zhang, G. Effect of different N fertilizer forms on anti-oxidant capacity and grain yield of rice growing under Cd stress. J. Hazard. Mater. 2009, 162, 1081–1085. [Google Scholar] [CrossRef]
- Wang, K.; Fu, G.; Yu, Y.; Wan, Y.; Liu, Z.; Wang, Q.; Li, H. Effects of different potassium fertilizers on cadmium uptake by three crops. Environ. Sci. Pollut. Res. 2019, 26, 27014–27022. [Google Scholar] [CrossRef]
- Toledo, M. Management of Acid Soils in the Highlands of Honduras: Concepts and Methods; Inter-American Institute for Cooperation on Agriculture (IICA): Tegucigalpa, Honduras, 2016; 151p, ISBN 978-99979-55-01-2. [Google Scholar]
- Torres, D.; Mendoza, B.; Meru, M.L.; Gómez, C. Riesgos de salinización y sodificación por el uso de abonos orgánicos en la depresión de Quíbor-Venezuela. Multiciencias 2016, 16, 133–142. [Google Scholar]
- Martínez-Villavicencio, N.; López-Alonzo, C.V.; Pérez-Leal, R.; Basurto-Sotelo, M. Efectos por salinidad en el desarrollo vegetativo: Effects of salinity on vegetative growth. Tecnociencia Chihuahua 2011, 5, 156–161. [Google Scholar] [CrossRef]
- Nawaz, K.; Husain, K.; Majeed, A.; Khan, F.; Afghan, S.; Ali, K. Fatality of salt stress to plants: Morphological, physiological and biochemical aspects. Afr. J. Biotechnol. 2010, 9, 5475–5480. [Google Scholar]
- Rodríguez-Pérez, L. Implicaciones fisiológicas de la osmorregulación en plantas. Agron. Colomb. 2006, 24, 28–37. Available online: https://www.redalyc.org/articulo.oa?id=180316238004 (accessed on 3 October 2022).
- Sadeghian Khalajabadi, S.; Zapata, R.D. Crecimiento de café (Coffea arabica L.) durante la etapa de almácigo en respuesta a la salinidad generada por fertilizantes. Revis Cienc. Agríc. 2014, 31, 40–50. [Google Scholar] [CrossRef][Green Version]
- Cai, W.; Navarro, D.A.; Du, J.; Ying, G.; Yang, B.; McLaughlin, M.J.; Kookana, R.S. Increasing ionic strength and valency of cations enhance sorption through hydrophobic interactions of PFAS with soil surfaces. Sci. Total Environ. 2022, 817, 152975. [Google Scholar] [CrossRef]
- Cardero Llopiz, Y.; Garcell Puyáns, L.R. Efecto del potencial iónico sobre la adsorción específica de cationes en suspensiones de laterita y de cieno carbonatado. Tecnología Química 2010, 30, 78–86. Available online: https://www.redalyc.org/articulo.oa?id=445543771009 (accessed on 7 March 2023).
- Carbonel Ramos, D. Adsorción de Cadmio, Cobre y Plomo en Bentonita, Caolinita y Zeolita Naturales y Modificadas: Una Revisión de los Parámetros de Operación, Isotermas y Cinética. Ingeniería 2018, 23, 252–273. [Google Scholar] [CrossRef]
- He, L.; Dai, Z.; Liu, X.; Tang, C.; Xu, J. Effect of alkaline lignin on immobilization of cadmium and lead in soils and the associated mechanisms. Chemosphere 2021, 281, 130969. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Huang, D.; Liu, X.; Meng, J.; Tang, C.; Xu, J. Remediation of As(III) and Cd(II) co-contamination and its mechanism in aqueous systems by a novel calcium-based magnetic biochar. J. Hazard. Mater. 2018, 348, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Ávila-Lovera, E.; Coronel, I.; Jaimez, R.; Urich, R.; Pereyra, G.; Araque, O.; Tezara, W. Ecophysiological traits of adult trees of Criollo cocoa cultivars (Theobroma cocoa L.) from a germplasm bank in Venezuela. Exp. Agric. 2016, 52, 137–153. [Google Scholar] [CrossRef]
- Héctor Ardisana, E.F.; Torres García, A.; Fosado Téllez, O.; Álava Álava, J.; Sancán Pin, G.T.; León Aguilar, R. Contenido de clorofilas totales en doce clones de cacao (Theobroma cacao L.). Rev. Agroc. 2018, 20, 11–18. [Google Scholar] [CrossRef]
- Ahmad, A. Salinity in soil increased cadmium uptake and accumulation potential of two terrestrial plants. Int. J. Biol. Sci. 2017, 10, 132–142. [Google Scholar]
- Akhter, S.M.; Noreen, S.; Mahmood, S.; Athar, H.-R.; Ashraf, M.; Abdullah Alsahli, A.; Ahmad, P. Influence of salinity stress on PSII in barley (Hordeum vulgare L.) Genotypes, probed by chlorophyll-a fluorescence. J. King Saud Univ. Sci. 2021, 33, 101239. [Google Scholar] [CrossRef]
- Gonçalves, A.Z.; Latansio, S.; Detmann, K.C.; Marabesi, M.A.; Neto, A.A.C.; Aidar, M.P.M.; Mercier, H. What does the RuBisCO activity tell us about a C3-CAM plant? Plant Physiol. Biochem. 2020, 147, 172–180. [Google Scholar] [CrossRef]
- Sage, R.F.; Stata, M. Photosynthetic diversity meets biodiversity: The C4 plant example. J. Plant Physiol. 2015, 172, 104–119. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, J.; Tezara, W.; Herrera, A. Physiological responses to drought and experimental water deficit and waterlogging of four clones of cocoa (Theobroma cacao L.) selected for cultivation in Venezuela. Agric. Water Manag. 2016, 171, 80–88. [Google Scholar] [CrossRef]
- Souza dos Santos, M.L.; de Almeida, A.-A.F.; da Silva, N.M.; Machado, B.; Oliveira, B.R.M.; Silva, J.V.S.; Souza, J.O., Jr.; Ahnert, D.; Baligar, V.C. Mitigation of cadmium toxicity by zinc in juvenile cacao: Physiological, biochemical, molecular and micromorphological responses. Environ. Exp. Bot. 2020, 179, 104201. [Google Scholar] [CrossRef]
- Hao, X.Z.; Zhou, D.M.; Li, D.D.; Jiang, P. Cadmium and Zinc Accumulation of Ornamental Sunflower (Helianthus annuus L.) in Contaminated Soil with Different Amendments. Pedosphere 2012, 22, 631–639. [Google Scholar] [CrossRef]
- Lyčka, M.; Barták, M.; Helia, O.; Kopriva, S.; Moravcová, D.; Hajek, J.; Fojt, L.; Cmelíl, R.; Fajkus, J.; Fojtová, M. Sulfate supplementation affects nutrient and photosynthetic status of Arabidopsis thaliana and Nicotiana tabacum differently under prolonged exposure to cadmium. J. Hazard. Mater. 2023, 445, 130527. [Google Scholar] [CrossRef]
- Haider, F.U.; Liqun, C.; Coulter, J.A.; Cheema, S.A.; Wu, J.; Zhang, R.; Wenjun, M.; Farooq, M. Cadmium toxicity in plants: Impacts and remediation strategies. Ecotoxicol. Environ. Saf. 2021, 211, 111887. [Google Scholar] [CrossRef]
- Castro, A.V.; de Almeida, A.-A.F.; Pirovani, C.P.; Reis, G.S.M.; Almeida, N.M.; Mangabeira, P.A.O. Morphological, biochemical, molecular and ultrastructural changes induced by Cd toxicity in seedlings of Theobroma cacao L. Ecotoxicol. Environ. Saf. 2015, 115, 174–186. [Google Scholar] [CrossRef]
- Llatance, W.; Gonza, C.; Guzmán, W.; Pariente, E. Bioacumulación de cadmio en el cacao (Theobroma cacao) en la comunidad nativa de Pakun, Perú. Rev. For. Perú. 2018, 33, 63–75. [Google Scholar] [CrossRef]
- Tantalean Pedraza, E.; Huauya Rojas, M.A. Distribución del contenido de cadmio en los diferentes órganos del cacao CCN-51 en suelo aluvial y residual en las localidades de Jacintillo y Ramal de Aspuzana. Rev. Inv. Agrop. Sust. 2017, 1, 69–78. [Google Scholar] [CrossRef]
- Barraza, F.; Schreck, E.; Uzu, G.; Lévêque, T.; Zouiten, C.; Boidot, M.; Maurice, L. Beyond cadmium accumulation: Distribution of other trace elements in soils and cacao beans in Ecuador. Environ. Res. 2021, 192, 110241. [Google Scholar] [CrossRef] [PubMed]
- Arévalo-Hernández, C.O.; Arévalo-Gardini, E.; Barraza, F.; Farfán, A.; He, Z.; Baligar, V.C. Growth and nutritional responses of wild and domesticated cocoa clones to soil cd stress. Sci. Total Environ. 2021, 763, 144021. [Google Scholar] [CrossRef]
- Ullah, I.; Wang, Y.; Eide, D.J.; Dunwell, J.M. Evolution, and functional analysis of Natural Resistance-Associated Macrophage Proteins (NRAMPs) from Theobroma cocoa and their role in cadmium accumulation. Sci. Rep. 2018, 8, 14412. [Google Scholar] [CrossRef] [PubMed]
- Puentes-Páramo, Y.; Menjivar-Flores, J.; Aranzazu-Hernández, F. Eficiencias en el uso de nitrógeno, fósforo y potasio en clones de cacao (Theobroma cacao L.). Bioagro 2014, 26, 99–106. Available online: https://www.redalyc.org/articulo.oa?id=85731100004 (accessed on 22 March 2023).
- Rosas-Patiño, G.; Puentes-Páramo, Y.J.; Menjivar-Flores, J.C. Efecto del pH sobre la concentración de nutrientes en cacao (Theobroma cacao L.) en la Amazonia Colombiana. Rev. U.D.C.A Act. Div. Cient. 2021, 24, 1–10. [Google Scholar] [CrossRef]
- López, M.; López de Rojas, I.; España, M.; Izquierdo, A.; Herrera, L. Efecto de la fertilización inorgánica sobre la disponibilidad de nutrimentos en el suelo, nivel nutricional de la planta y hongos micorrícicos arbusculares en plantaciones de Theobroma cacao. Agronomía Trop. 2007, 57, 31–43. Available online: http://ve.scielo.org/scielo.php?script=sci_arttext&pid=S0002192X2007000100005&lng=es&tlng=es (accessed on 21 June 2022).
- Quinteiro, M.; Furtado de Almeida, A.-A.; Schramm, M.; Pinto Gomes, F.; Viana Pires, M.; Baligar, V.C. Aluminum effects on growth photosynthesis and mineral nutrition of cocoa clones. J. Plant Nutr. 2013, 36, 1161–1179. [Google Scholar] [CrossRef]
- Correa, J.E.; Ramírez, R.; Ruíz, O.; Leiva, E.I. Effect of soil characteristics on cadmium absorption and plant growth of Theobroma cacao L. seedlings. J. Sci. Food Agric. 2021, 101, 5437–5445. [Google Scholar] [CrossRef]
- Liu, J.G.; Liang, J.S.; Li, K.Q.; Zhang, Z.J.; Yu, B.Y.; Lu, X.L.; Yang, J.C.; Zhu, Q.S. Correlations between cadmium and mineral nutrients in absorption and accumulation in various clones of rice under cadmium stress. Chemosphere 2003, 52, 1467–1473. [Google Scholar] [CrossRef]
- Tezara, W.; Coronel, I.; Dzib, G.; Calvo Irabien, L.M. Relación entre la capacidad fotosintética y el contenido de aceites esenciales de Lippia graveolens (Verbenaceae) en dos localidades con diferencias en precipitación anual. Interciencia 2013, 38, 669–675. Available online: https://www.redalyc.org/articulo.oa?id=33929480010 (accessed on 4 May 2022).
- Oliva, M.; Rubio, K.; Epquin, M.; Marlo, G.; Leiva, S. Cadmium Uptake in Native Cacao Trees in Agricultural Lands of Bagua, Peru. Agronomy 2020, 10, 1551. [Google Scholar] [CrossRef]
- Scaccabarozzi, D.; Castillo, L.; Aromatisi, A.; Milne, L.; Búllon Castillo, A.; Muñoz-Rojas, M. Soil, Site, and Management Factors Affecting Cadmium Concentrations in Cacao-Growing Soils. Agronomy 2020, 10, 806. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).