Novel Approach to Organic Mulching from Natural-Based Solutions to Enhance Soil Health and Functional Value of Calafate Fruit
Abstract
:1. Introduction
2. Materials and Methods
2.1. Agronomic Management of the Orchard and Soil and Climatic Conditions
2.2. Experimental Setup
2.3. Weeds, Soil Moisture, Temperature of Mulch, and Leaves
2.4. Microbiological and Chemical Soil Analysis
2.5. Plant Physiological Measurements
2.6. Yield and Physical Fruit Measurements
2.7. Soluble Solids and Antioxidant Properties of the Fruit
2.8. Statistical Analysis
3. Results
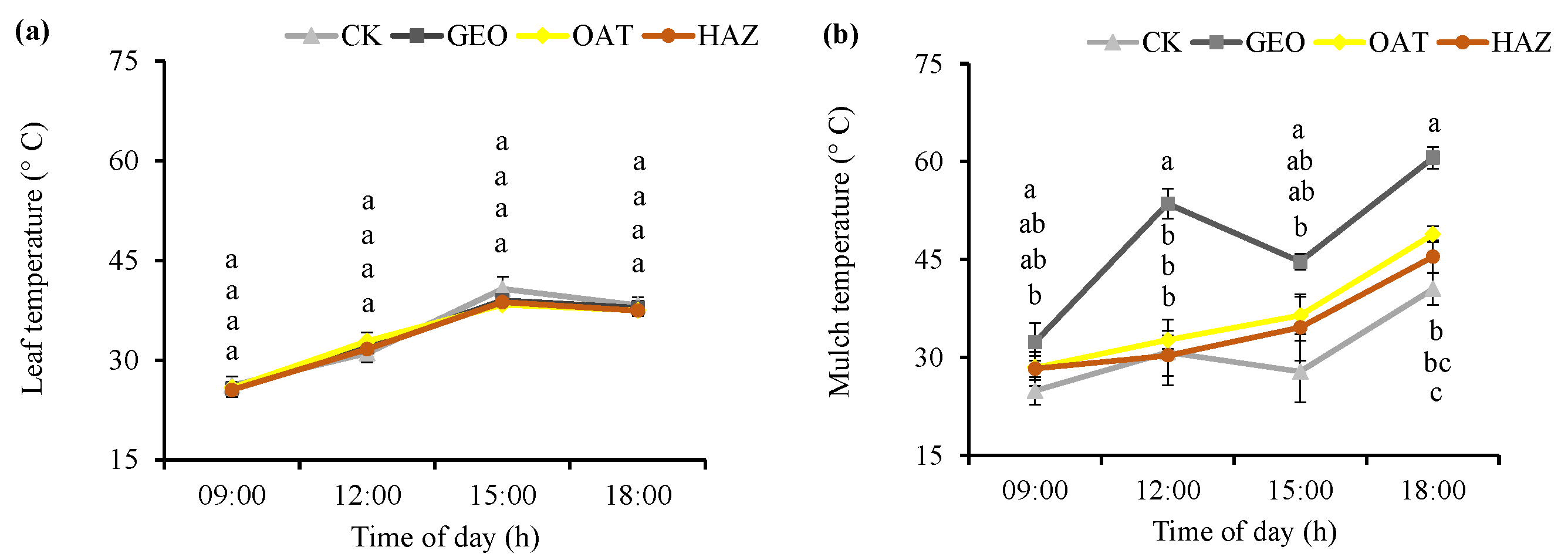
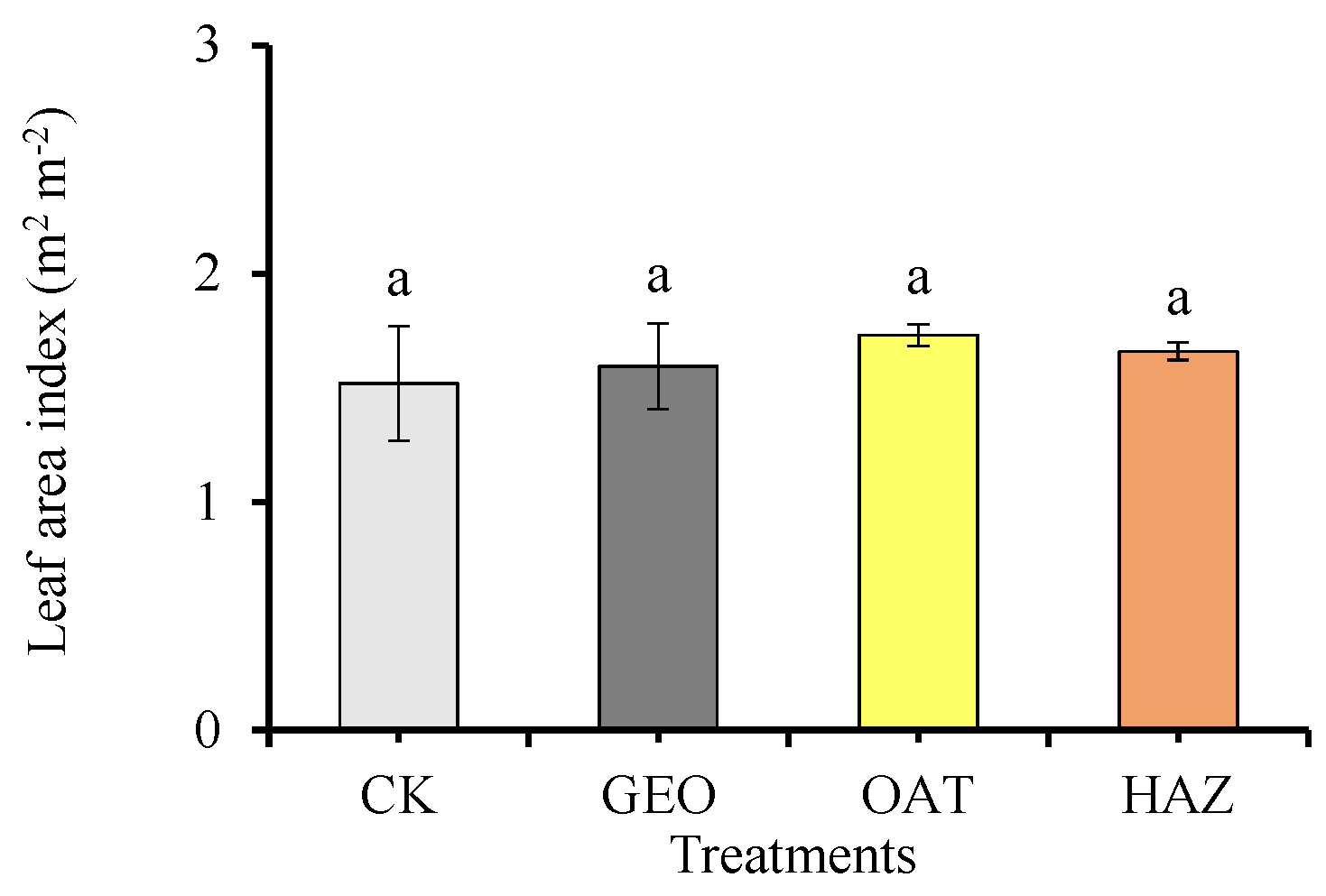
3.1. Weeds, Soil Moisture and Temperature, and Leaf Temperature
3.2. Soil Microbiological and Chemical Parameters
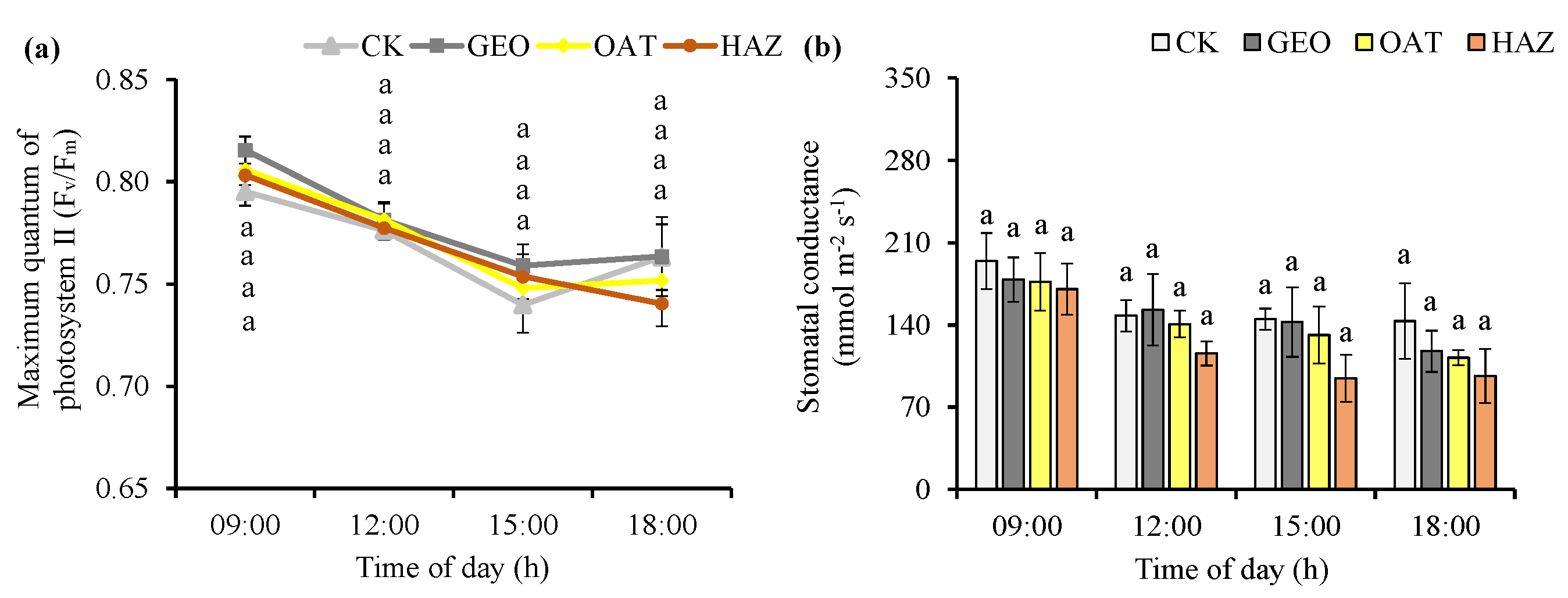
3.3. Plant Physiological Parameters
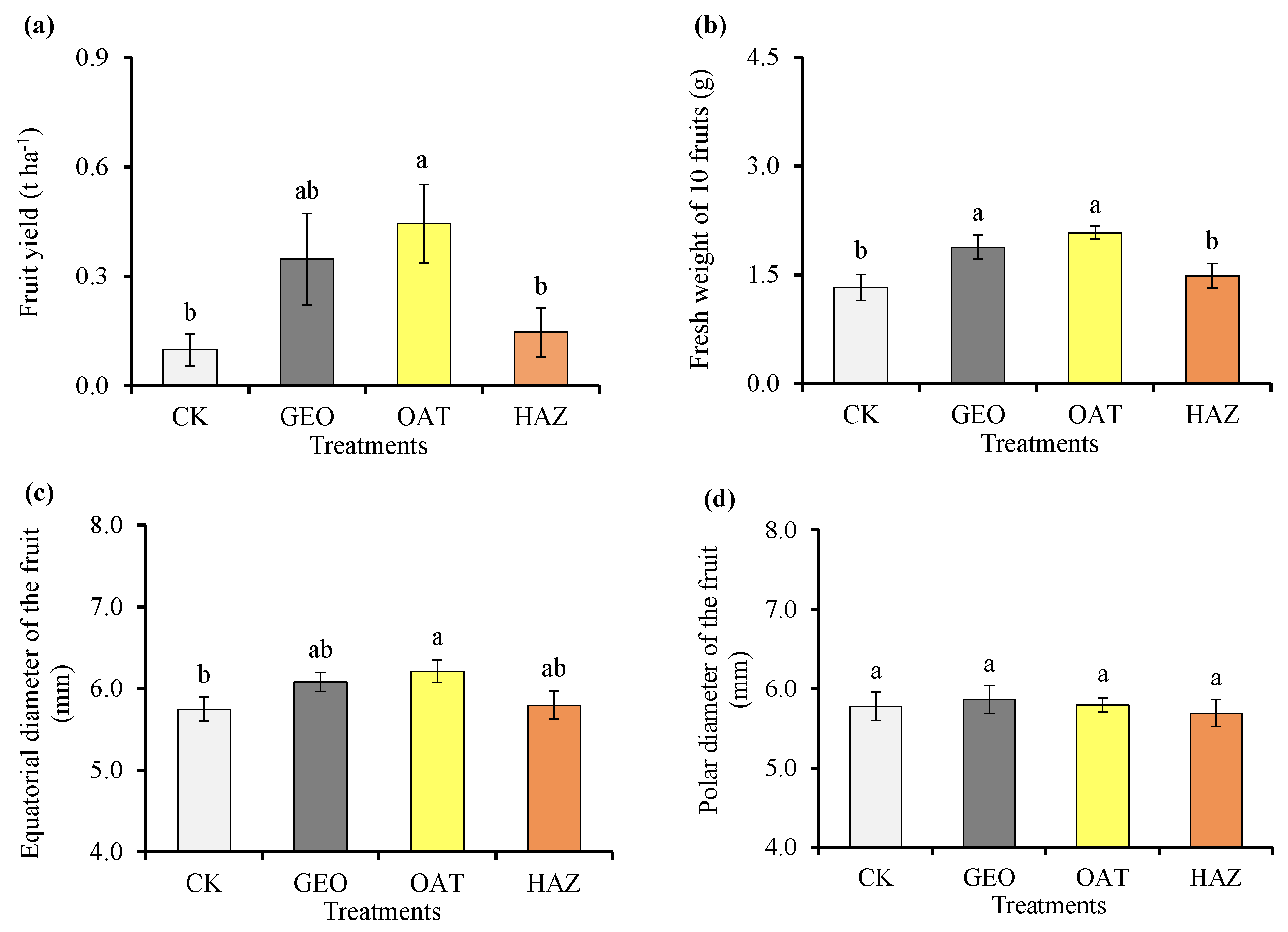
3.4. Yield and Physical Parameters of the Fruit
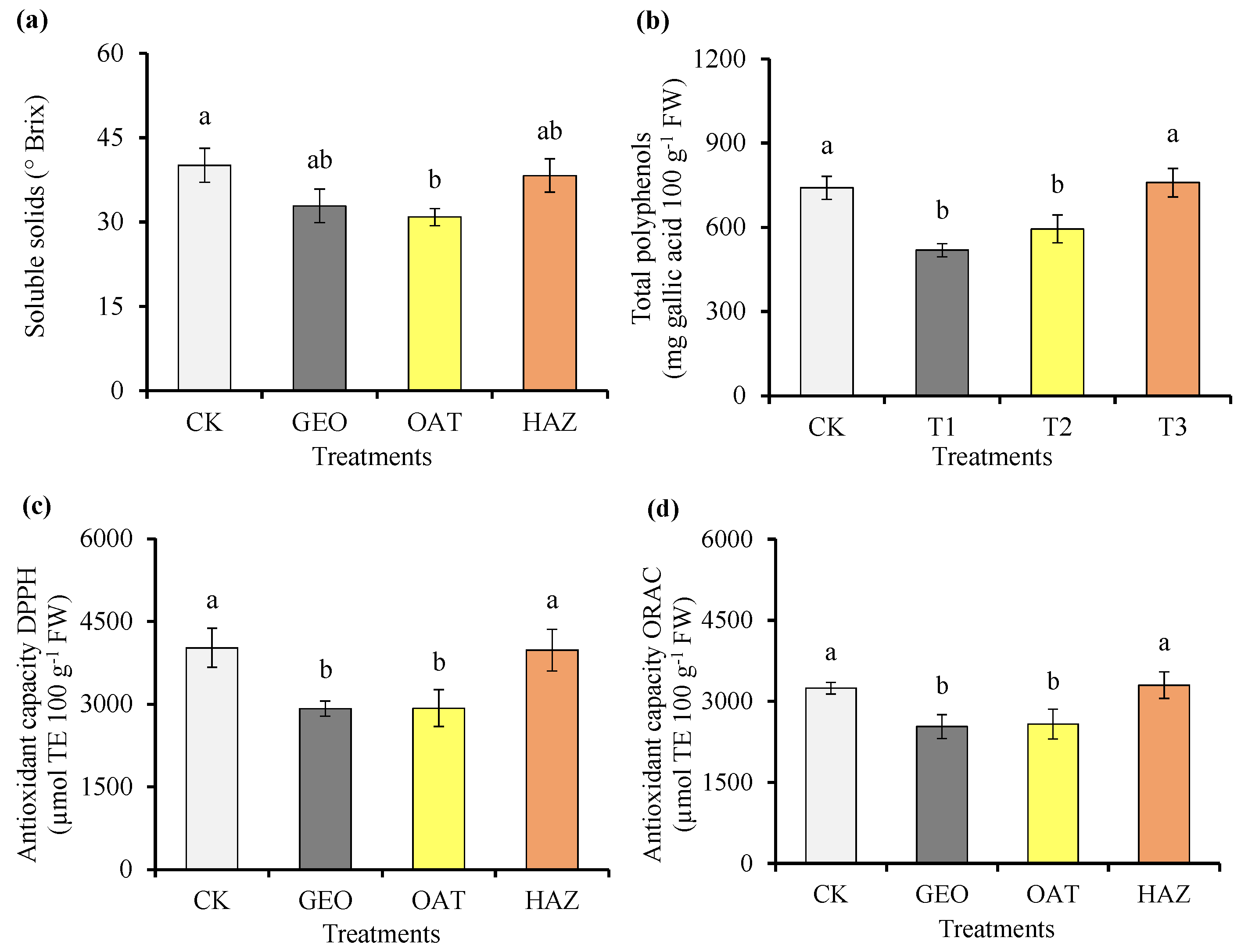
3.5. Soluble Solids and Antioxidant Parameters of the Fruit
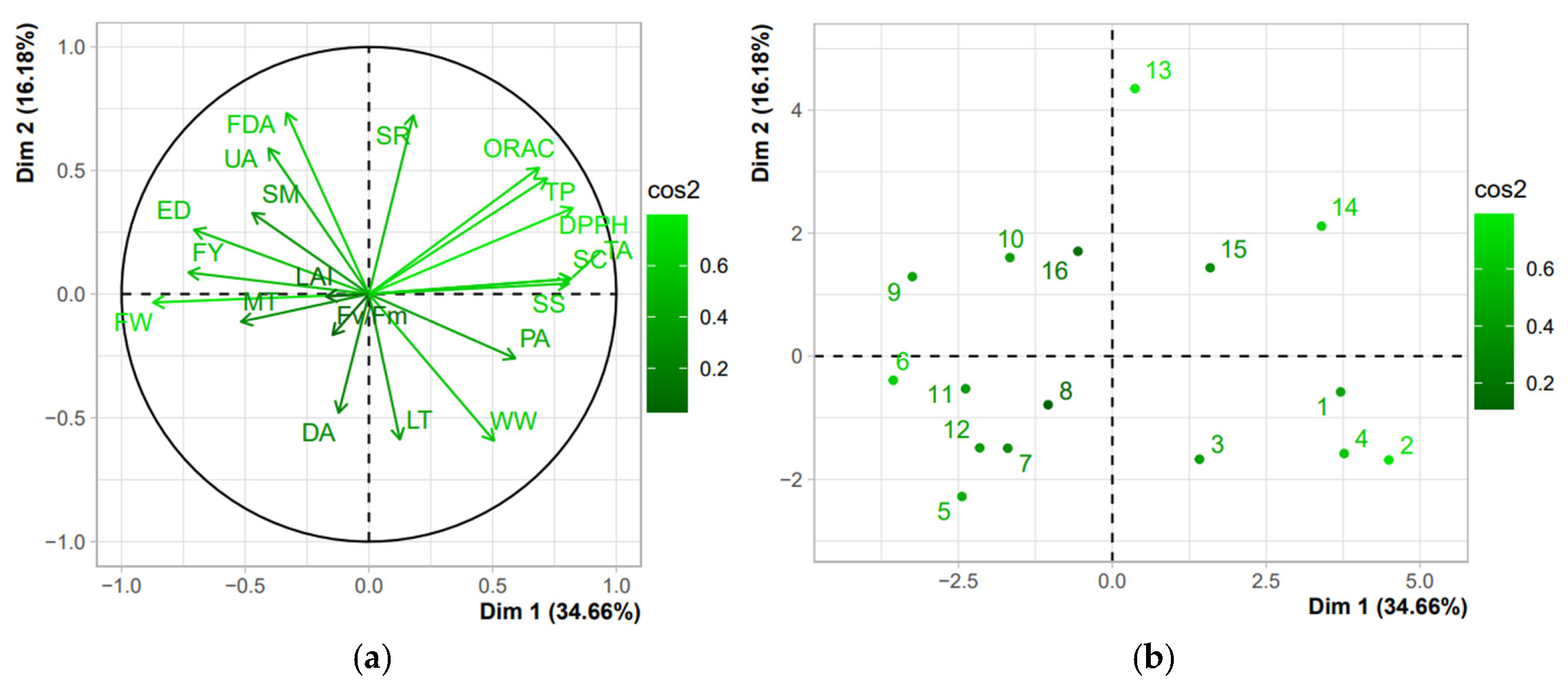
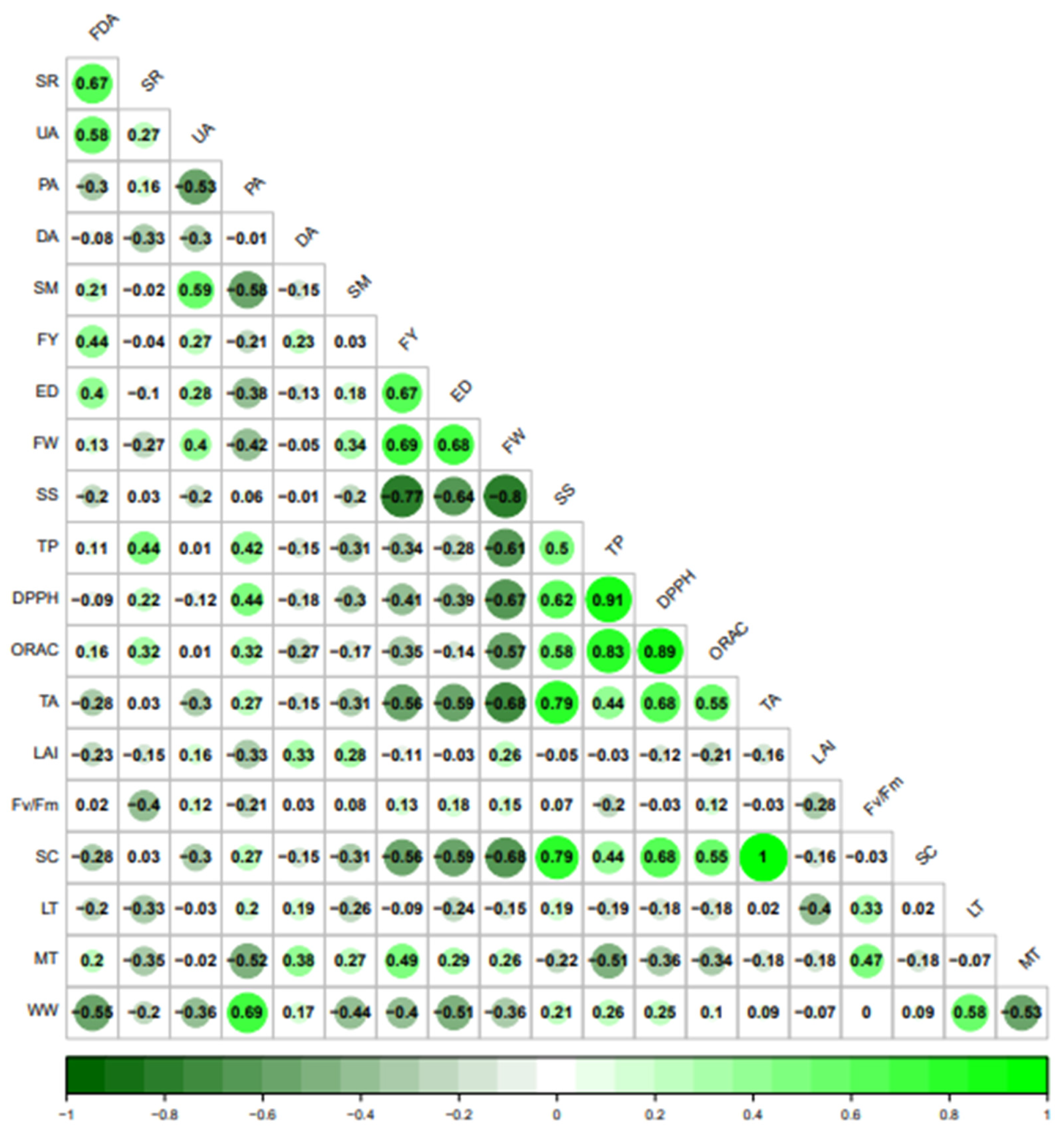
3.6. Soil–Plant–Fruit Parameter Interaction
4. Discussion
4.1. Weeds, Soil Moisture and Temperature, and Leaf Temperature
4.2. Soil Microbiological and Chemical Parameters
4.3. Plant Physiological Parameters
4.4. Physicochemical Parameters of Fruits
4.5. Soluble Solids and Antioxidant Parameters of the Fruit
4.6. Soil–Plant–Fruit Parameter Interaction
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tian, G.; Li, Y. Lignocellulose Mulch Increases the Economic Benefit of Chinese Chestnut by Suppressing Weed and Ameliorating Soil Properties. Sci. Hortic. 2022, 291, 110576. [Google Scholar] [CrossRef]
- Paušič, A.; Tojnko, S.; Lešnik, M. Permanent, Undisturbed, in-Row Living Mulch: A Realistic Option to Replace Glyphosate-Dominated Chemical Weed Control in Intensive Pear Orchards. Agric. Ecosyst. Environ. 2021, 318, 107502. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Y.; Zhao, L.; Zhang, W.; Liu, L. Change of Soil Microbial Community under Long-Term Fertilization in a Reclaimed Sandy Agricultural Ecosystem. PeerJ 2019, 7, e6497. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, J.; Dong, Q.; Feng, H.; Siddique, K.H.M. Plastic Mulching Significantly Improves Soil Enzyme and Microbial Activities without Mitigating Gaseous N Emissions in Winter Wheat-Summer Maize Rotations. Field Crops Res. 2022, 286, 108630. [Google Scholar] [CrossRef]
- Cerdà, A.; Terol, E.; Daliakopoulos, I.N. Weed Cover Controls Soil and Water Losses in Rainfed Olive Groves in Sierra de Enguera, Eastern Iberian Peninsula. J. Environ. Manag. 2021, 290, 112516. [Google Scholar] [CrossRef]
- Frey, S.D.; Drijber, R.; Smith, H.; Melillo, J. Microbial Biomass, Functional Capacity, and Community Structure after 12 Years of Soil Warming. Soil Biol. Biochem. 2008, 40, 2904–2907. [Google Scholar] [CrossRef]
- Raffa, D.W.; Antichi, D.; Carlesi, S.; Puig-Sirera, À.; Rallo, G.; Bàrberi, P. Ground Vegetation Covers Increase Grape Yield and Must Quality in Mediterranean Organic Vineyards despite Variable Effects on Vine Water Deficit and Nitrogen Status. Eur. J. Agron. 2022, 136, 126483. [Google Scholar] [CrossRef]
- Zhang, H.; Miles, C.; Gerdeman, B.; LaHue, D.G.; DeVetter, L. Plastic Mulch Use in Perennial Fruit Cropping Systems—A Review. Sci. Hortic. 2021, 281, 109975. [Google Scholar] [CrossRef]
- Taparauskienė, L.; Miseckaitė, O. Effect of Mulch on Soil Moisture Depletion and Strawberry Yield in Sub-Humid Area. Pol. J. Environ. Stud. 2014, 23, 475–482. [Google Scholar]
- Zhang, Q.; Wang, S.; Li, L.; Inoue, M.; Xiang, J.; Qiu, G.; Jin, W. Effects of Mulching and Sub-Surface Irrigation on Vine Growth, Berry Sugar Content and Water Use of Grapevines. Agric. Water Manag. 2014, 143, 1–8. [Google Scholar] [CrossRef]
- Webber, S.M.; Bailey, A.P.; Huxley, T.; Potts, S.G.; Lukac, M. Traditional and Cover Crop-Derived Mulches Enhance Soil Ecosystem Services in Apple Orchards. Appl. Soil Ecol. 2022, 178, 104569. [Google Scholar] [CrossRef]
- Chen, Y.; Wen, X.; Sun, Y.; Zhang, J.; Wu, W.; Liao, Y. Mulching Practices Altered Soil Bacterial Community Structure and Improved Orchard Productivity and Apple Quality after Five Growing Seasons. Sci. Hortic. 2014, 172, 248–257. [Google Scholar] [CrossRef]
- Xie, B.; Chen, Y.; Cheng, C.; Ma, R.; Zhao, D.; Li, Z.; Li, Y.; An, X.; Yang, X. Long-Term Soil Management Practices Influence the Rhizosphere Microbial Community Structure and Bacterial Function of Hilly Apple Orchard Soil. Appl. Soil Ecol. 2022, 180, 104627. [Google Scholar] [CrossRef]
- Huang, Z.; Xu, Z.; Chen, C. Effect of Mulching on Labile Soil Organic Matter Pools, Microbial Community Functional Diversity and Nitrogen Transformations in Two Hardwood Plantations of Subtropical Australia. Appl. Soil Ecol. 2008, 40, 229–239. [Google Scholar] [CrossRef]
- Larrañaga, P.; Osores, M.; Escobar, C.; Henriquez, G.; Beltrán, P.; Mendieta, B.; Peña, J.; Woywood, C.; Palomino, M.; Avendaño, A.; et al. Catastro Fruticola Ñuble. Available online: https://www.odepa.gob.cl/estadisticas-del-sector/catastros-fruticolas/catastro-fruticola-ciren-odepa (accessed on 21 August 2023).
- Boeri, P.; Piñuel, L.; Dalzotto, D.; Monasterio, R.; Fontana, A.; Sharry, S.; Barrio, D.A.; Carrillo, W. Argentine Patagonia Barberry Chemical Composition and Evaluation of Its Antioxidant Capacity. J. Food Biochem. 2020, 44, 13254. [Google Scholar] [CrossRef]
- Rodoni, L.M.; Feuring, V.; Zaro, M.J.; Sozzi, G.O.; Vicente, A.R.; Arena, M.E. Ethylene Responses and Quality of Antioxidant-Rich Stored Barberry Fruit (Berberis microphylla). Sci. Hortic. 2014, 179, 233–238. [Google Scholar] [CrossRef]
- Manach, C.; Mazur, A.; Scalbert, A. Polyphenols and Prevention of Cardiovascular Diseases. Curr. Opin. Lipidol. 2005, 16, 77–84. [Google Scholar] [CrossRef]
- Arena, M.E.; Pastur, G.M.; Lencinas, M.V.; Soler, R.; Bustamante, G. Changes in the Leaf Nutrient and Pigment Contents of Berberis microphylla, G. Forst. in Relation to Irradiance and Fertilization. Heliyon 2020, 6, e03264. [Google Scholar] [CrossRef]
- Pinto-Morales, F.; Retamal-Salgado, J.; Lopéz, M.D.; Zapata, N.; Vergara-Retamales, R.; Pinto-Poblete, A. The Use of Compost Increases Bioactive Compounds and Fruit Yield in Calafate Grown in the Central South of Chile. Agriculture 2022, 12, 98. [Google Scholar] [CrossRef]
- Betancur, M.; Retamal-Salgado, J.; López, M.D.; Vergara-Retamales, R.; Schoebitz, M. Plant Performance and Soil Microbial Responses to Irrigation Management: A Novel Study in a Calafate Orchard. Horticulturae 2022, 8, 1138. [Google Scholar] [CrossRef]
- USDA Natural Resources Conservation Service Keys to Soil Taxonomy, 13th Edition. Available online: https://www.nrcs.usda.gov/sites/default/files/2022-09/Keys-to-Soil-Taxonomy.pdf (accessed on 9 July 2023).
- Alef, K.; Nannipieri, P. 5—Estimation of Microbial Activities. In Methods in Applied Soil Microbiology and Biochemistry; Academic Press: London, UK, 1995; pp. 193–270. ISBN 978-0-12-513840-6. [Google Scholar]
- Joergensen, R.G. 8—Microbial Biomass. In Methods in Applied Soil Microbiology and Biochemistry; Alef, K., Nannipieri, P., Eds.; Academic Press: London, UK, 1995; pp. 375–417. ISBN 978-0-12-513840-6. [Google Scholar]
- Nannipieri, P.; Ceccanti, B.; Cervelli, S.; Matarese, E. Extraction of Phosphatase, Urease, Proteases, Organic Carbon, and Nitrogen from Soil. Soil Sci. Soc. Am. J. 1980, 44, 1011–1016. [Google Scholar] [CrossRef]
- Garcia, C.; Hernandez, T.; Costa, F. Potential Use of Dehydrogenase Activity as an Index of Microbial Activity in Degraded Soils. Commun. Soil Sci. Plant Anal. 1997, 28, 123–134. [Google Scholar] [CrossRef]
- Tabatabai, M.A.; Bremner, J.M. Use of P-Nitrophenyl Phosphate for Assay of Soil Phosphatase Activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Retamal-Salgado, J.; Vásquez, R.; Fischer, S.; Hirzel, J.; Zapata, N. Decrease in Artificial Radiation with Netting Reduces Stress and Improves Rabbit-Eye Blueberry (Vaccinium virgatum Aiton) Ochlockonee Productivity. Chil. J. Agric. Res. 2017, 77, 226–233. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll Fluorescence—A Practical Guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144. [Google Scholar] [CrossRef]
- Radice, S.; Arena, M.E. Environmental Effect on the Leaf Morphology and Anatomy of Berberis microphylla G. Forst. IJPB 2015, 6, 5677. [Google Scholar] [CrossRef]
- Romero Román, M.; Noriega Vásquez, F.; Farías Villagra, M.; Jara Zapata, P.; Vera Flores, B.; López Belchi, M. Nuevas fuentes de antioxidantes naturales: Caracterización de compuestos bioactivos en cinco frutos nativos de Chile. Perf 2020, 2, 34–41. [Google Scholar] [CrossRef]
- Romero-Román, M.E.; Schoebitz, M.; Bastías, R.M.; Fernández, P.S.; García-Viguera, C.; López-Belchi, M.D. Native Species Facing Climate Changes: Response of Calafate Berries to Low Temperature and UV Radiation. Foods 2021, 10, 196. [Google Scholar] [CrossRef]
- Mena, P.; García-Viguera, C.; Navarro-Rico, J.; Moreno, D.A.; Bartual, J.; Saura, D.; Martí, N. Phytochemical Characterisation for Industrial Use of Pomegranate ( Punica granatum L.) Cultivars Grown in Spain: Selection of Pomegranates for Juices. J. Sci. Food Agric. 2011, 91, 1893–1906. [Google Scholar] [CrossRef]
- Allaire, J.J. RStudio: Integrated Development Environment for R. Available online: http://www.rstudio.org/ (accessed on 4 August 2023).
- Kahle, D.J.; Wickham, H. Ggmap: Spatial Visualization with Ggplot2. R J. 2013, 5, 144. [Google Scholar] [CrossRef]
- Faras, B.M.U.P. Kau Efficacy of Mulches for Weed Management in Okra (Abelmoschus esculentus (L.) Moench.); Kerala Agricultural University: Kerala, India, 2015. [Google Scholar]
- Lamont, W.J. 3—Plastic Mulches for the Production of Vegetable Crops. In A Guide to the Manufacture, Performance, and Potential of Plastics in Agriculture; Orzolek, M.D., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 45–60. ISBN 978-0-08-102170-5. [Google Scholar]
- Busscher, W.J.; Novak, J.M.; Ahmedna, M. Physical Effects of Organic Matter Amendment of a Southeastern US Coastal Loamy Sand. Soil Sci. 2011, 176, 661–667. [Google Scholar] [CrossRef]
- Giese, G.; Velasco-Cruz, C.; Roberts, L.; Heitman, J.; Wolf, T.K. Complete Vineyard Floor Cover Crops Favorably Limit Grapevine Vegetative Growth. Sci. Hortic. 2014, 170, 256–266. [Google Scholar] [CrossRef]
- Retamal-Salgado, J.; Adaos, G.; Cedeño-García, G.; Ospino-Olivella, S.C.; Vergara-Retamales, R.; Lopéz, M.D.; Olivares, R.; Hirzel, J.; Olivares-Soto, H.; Betancur, M. Preharvest Applications of Oxalic Acid and Salicylic Acid Increase Fruit Firmness and Polyphenolic Content in Blueberry (Vaccinium corymbosum L.). Horticulturae 2023, 9, 639. [Google Scholar] [CrossRef]
- Chauhan, S.; Basnet, B.; Adhikari, S.K.; Budhathoki, P.; Shrestha, A.K. Innovative Farming Techniques for Superior Okra Yield in Chitwan, Nepal: The Benefits of Plastic Film Mulch and Pest Exclusion Net on Soil Properties, Growth, Quality and Profitability. Acta Ecol. Sin. 2023, in press. [CrossRef]
- El-Beltagi, H.S.; Basit, A.; Mohamed, H.I.; Ali, I.; Ullah, S.; Kamel, E.A.R.; Shalaby, T.A.; Ramadan, K.M.A.; Alkhateeb, A.A.; Ghazzawy, H.S. Mulching as a Sustainable Water and Soil Saving Practice in Agriculture: A Review. Agronomy 2022, 12, 1881. [Google Scholar] [CrossRef]
- Sharma, R.; Chand Sharma, J.; Singh, U.; Kumar, V. Peach (Prunus persica L. Batsch) Perfection: Boosting Yields with Mulching. Waste Manag. Bull. 2023, 1, 114–134, S2949750723000342. [Google Scholar] [CrossRef]
- Mairata, A.; Labarga, D.; Puelles, M.; Huete, J.; Portu, J.; Rivacoba, L.; Pou, A. The Organic Mulches in Vineyards Exerted an Influence on Spontaneous Weed Cover and Plant Biodiversity. Eur. J. Agron. 2023, 151, 126997. [Google Scholar] [CrossRef]
- Holland, T.C.; Reynolds, A.G.; Bowen, P.A.; Bogdanoff, C.P.; Marciniak, M.; Brown, R.B.; Hart, M.M. The Response of Soil Biota to Water Availability in Vineyards. Pedobiologia 2013, 56, 9–14. [Google Scholar] [CrossRef]
- Duan, C.; Chen, J.; Li, J.; Feng, H.; Wu, S.; Meng, Q.; Siddique, K.H.M. Effects of Organic Amendments and Ridge–Furrow Mulching System on Soil Properties and Economic Benefits of Wolfberry Orchards on the Tibetan Plateau. Sci. Total Environ. 2022, 827, 154317. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L. Phenol Oxidase, Peroxidase and Organic Matter Dynamics of Soil. Soil Biol. Biochem. 2010, 42, 391–404. [Google Scholar] [CrossRef]
- Nannipieri, P.; Giagnoni, L.; Renella, G.; Puglisi, E.; Ceccanti, B.; Masciandaro, G.; Fornasier, F.; Moscatelli, M.C.; Marinari, S. Soil Enzymology: Classical and Molecular Approaches. Biol. Fertil. Soils 2012, 48, 743–762. [Google Scholar] [CrossRef]
- McCarty, G.W.; Shogren, D.R.; Bremner, J.M. Regulation of Urease Production in Soil by Microbial Assimilation of Nitrogen. Biol. Fertil. Soils 1992, 12, 261–264. [Google Scholar] [CrossRef]
- Sun, D.; Li, K.; Bi, Q.; Zhu, J.; Zhang, Q.; Jin, C.; Lu, L.; Lin, X. Effects of Organic Amendment on Soil Aggregation and Microbial Community Composition during Drying-Rewetting Alternation. Sci. Total Environ. 2017, 574, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Ouni, Y.; Lakhdar, A.; Scelza, R.; Scotti, R.; Abdelly, C.; Barhoumi, Z.; Rao, M.A. Effects of Two Composts and Two Grasses on Microbial Biomass and Biological Activity in a Salt-Affected Soil. Ecol. Eng. 2013, 60, 363–369. [Google Scholar] [CrossRef]
- Wang, J.; Niu, W.; Zhang, M.; Li, Y. Effect of Alternate Partial Root-Zone Drip Irrigation on Soil Bacterial Communities and Tomato Yield. Appl. Soil Ecol. 2017, 119, 250–259. [Google Scholar] [CrossRef]
- Chen, C.R.; Xu, Z.H.; Mathers, N.J. Soil Carbon Pools in Adjacent Natural and Plantation Forests of Subtropical Australia. Soil Sci. Soc. Am. J. 2004, 68, 282–291. [Google Scholar] [CrossRef]
- Von Lützow, M.; Kögel-Knabner, I. Temperature Sensitivity of Soil Organic Matter Decomposition—What Do We Know? Biol. Fertil. Soils 2009, 46, 1–15. [Google Scholar] [CrossRef]
- Micallef, S.A.; Callahan, M.T.; McEgan, R.; Martinez, L. Soil Microclimate and Persistence of Foodborne Pathogens Escherichia coli O157:H7, Listeria Monocytogenes, and Salmonella Enterica Newport in Soil Affected by Mulch Type. J. Food Prot. 2023, 86, 100159. [Google Scholar] [CrossRef]
- Wu, Y.; Sun, M.; Liu, S. Mulching Broad Ridges with a Woven Polypropylene Fabric Increases the Growth and Yield of Young Pear Trees ‘Yuluxiang’ in the North China Plain. Hortic. Plant J. 2023, 9, 414–424. [Google Scholar] [CrossRef]
- Kiczorowski, P.; Kopacki, M.; Kiczorowska, B. The Response of Šampion Trees Growing on Different Rootstocks to Applied Organic Mulches and Mycorrhizal Substrate in the Orchard. Sci. Hortic. 2018, 241, 267–274. [Google Scholar] [CrossRef]
- Gholami, R.; Fahadi Hoveizeh, N.; Zahedi, S.M.; Arji, I. Effect of Organic and Synthetic Mulches on Some Morpho-Physiological and Yield Parameters of ‘Zard’ Olive Cultivar Subjected to Three Irrigation Levels in Field Conditions. S. Afr. J. Bot. 2023, 162, 749–760. [Google Scholar] [CrossRef]
- Radice, S.; Alonso, M.; Arena, M.E. Berberis Microphylla: A Species with Phenotypic Plasticity in Different Climatic Conditions. Int. J. Agric. Biol. 2018, 20, 10. [Google Scholar]
- Guo, X.; Li, S.; Wang, D.; Huang, Z.; Sarwar, N.; Mubeen, K.; Shakeel, M.; Hussain, M. Effects of Water and Fertilizer Coupling on the Physiological Characteristics and Growth of Rabbiteye Blueberry. PLoS ONE 2021, 16, e0254013. [Google Scholar] [CrossRef]
- Ojeda, A.; Hirzel, J.; Pino, M.T.; Leod, C.M.; Águila, K. Composición y Evolución Nutricional del Calafate en la Región de Magallanes. INFORMATIVO N° 68. 2017; pp. 1–8. Available online: https://hdl.handle.net/20.500.14001/4680 (accessed on 4 August 2023).
- Sharma, S.; Basnet, B.; Bhattarai, K.; Sedhai, A.; Khanal, K. The Influence of Different Mulching Materials on Tomato’s Vegetative, Reproductive, and Yield in Dhankuta, Nepal. J. Agric. Food Res. 2023, 11, 100463. [Google Scholar] [CrossRef]
- Suo, G.-D.; Xie, Y.-S.; Zhang, Y.; Luo, H. Long-Term Effects of Different Surface Mulching Techniques on Soil Water and Fruit Yield in an Apple Orchard on the Loess Plateau of China. Sci. Hortic. 2019, 246, 643–651. [Google Scholar] [CrossRef]
- Pinto-Morales, F.; Retamal-Salgado, J.; López, M.D.; Zapata, N.; Vergara-Retamales, R.; Palma, D. Variation in Physical-Chemical Parameters and Phenolic Compounds in Fruits of Four Calafate Clones. Agronomy 2022, 12, 2146. [Google Scholar] [CrossRef]
- DeVetter, L.W.; Zhang, H.; Ghimire, S.; Watkinson, S.; Miles, C.A. Plastic Biodegradable Mulches Reduce Weeds and Promote Crop Growth in Day-Neutral Strawberry in Western Washington. HortScience 2017, 52, 1700–1706. [Google Scholar] [CrossRef]
- Torres, N.; Yu, R.; Martinez-Luscher, J.; Girardello, R.C.; Kostaki, E.; Oberholster, A.; Kaan Kurtural, S. Shifts in the Phenolic Composition and Aromatic Profiles of Cabernet Sauvignon (Vitis vinifera L.) Wines Are Driven by Different Irrigation Amounts in a Hot Climate. Food Chem. 2022, 371, 131163. [Google Scholar] [CrossRef]
- Cho, M.J.; Howard, L.R.; Prior, R.L.; Clark, J.R. Flavonoid Glycosides and Antioxidant Capacity of Various Blackberry, Blueberry and Red Grape Genotypes Determined by High-Performance Liquid Chromatography/Mass Spectrometry. J. Sci. Food Agric. 2004, 84, 1771–1782. [Google Scholar] [CrossRef]
- Watson, R.R.; Schönlau, F. Nutraceutical and Antioxidant Effects of a Delphinidin-Rich Maqui Berry Extract Delphinol®: A Review. Minerva Cardioangiol. 2015, 63, 1–12. [Google Scholar] [PubMed]


| Treatments | Microbial Activity | Soil Basal Respiration | Urease Activity | Dehydrogenase Activity | Acid Phosphatase Activity |
|---|---|---|---|---|---|
| (µg FDA g−1) | (μg CO2 g−1 h−1) | (μmol NH4+ g−1 h−1) | (μg INTF g−1) | (μmol PNP g−1 h−1) | |
| CK | 33.38 ± 3.20 b | 1.32 ± 0.10 bc | 1.25 ± 0.04 c | 36.36 ± 6.10 a | 27.29 ± 2.36 a |
| GEO | 41.42 ± 7.31 ab | 0.98 ± 0.16 c | 1.34 ± 0.01 bc | 38.96 ± 3.95 a | 17.57 ± 2.03 b |
| OAT | 49.75 ± 3.71 ab | 1.46 ± 0.15 b | 1.44 ± 0.04 ab | 32.36 ± 2.60 a | 17.28 ± 1.89 b |
| HAZ | 55.75 ± 7.99 a | 1.90 ± 0.15 a | 1.48 ± 0.05 a | 29.16 ± 1.39 a | 19.66 ± 2.10 b |
| Anova p values | 0.0095 | 0.0053 | 0.0063 | 0.3462 | 0.0181 |
| Anthocyanins | Treatments | Anova p Values | |||
|---|---|---|---|---|---|
| CK | GEO | OAT | HAZ | ||
| Delphinidin 3,3-dihexoside | 2.4 ± 0.6 a | 1.8 ± 0.4 a | 2.2 ± 0.2 a | 2.3 ± 0.4 a | 0.8147 |
| Petunidin 3,5-dihexoside | 9.4 ± 0.4 a | 9.8 ± 2.1 a | 10.4 ± 3.0 a | 8.6 ± 1.5 a | 0.9253 |
| Malvidin 3,5-dihexoside | 5.8 ± 0.2 b | 8.7 ± 1.1ab | 8.2 ± 2.3 ab | 11.5 ± 0.8 a | 0.0446 |
| Delphinidin 3-glucoside | 190.6 ± 28.8 a | 109.3 ± 14.9 b | 105.6 ± 20.5 b | 137.3 ± 11.9 ab | 0.0395 |
| Delphinidin 3-rutinoside | 1.7 ± 0.3 a | 1.5 ± 0.3 ab | 1.0 ± 0.2 b | 1.7 ± 0.3 ab | 0.0371 |
| Cyanidin 3-glucoside | 29.7 ± 1.3 a | 20.5 ± 2.7 a | 20.5 ± 3.3 a | 22.4 ± 4.6 a | 0.1939 |
| Petunidin 3-glucoside | 133.9 ± 12.6 a | 99.3 ± 8.2 bc | 92.0 ± 12.0 c | 124.6 ± 6.9 ab | 0.0380 |
| Petunidin 3-rutinoside | 3.1 ± 0.2 a | 2.1 ± 0.6 ab | 1.1 ± 0.1 b | 2.1 ± 0.4 ab | 0.0239 |
| Peonidin 3-glucoside | 11.9 ± 0.7 a | 20.9 ± 3.7 a | 10.2 ± 1.2 a | 13.2 ± 1.7 a | 0.2730 |
| Malvidin 3-glucoside | 72.7 ± 3.8 a | 79.4 ± 18.8 a | 67.8 ± 1.7 a | 98.5 ± 9.9 a | 0.2553 |
| Total anthocyanins | 461.2 ± 46.0 a | 353.3 ± 27.8 ab | 319.1 ± 39.0 b | 422.2 ± 28.3 ab | 0.0439 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Betancur, M.; Retamal-Salgado, J.; López, M.D.; Vergara-Retamales, R.; Schoebitz, M. Novel Approach to Organic Mulching from Natural-Based Solutions to Enhance Soil Health and Functional Value of Calafate Fruit. Horticulturae 2023, 9, 1202. https://doi.org/10.3390/horticulturae9111202
Betancur M, Retamal-Salgado J, López MD, Vergara-Retamales R, Schoebitz M. Novel Approach to Organic Mulching from Natural-Based Solutions to Enhance Soil Health and Functional Value of Calafate Fruit. Horticulturae. 2023; 9(11):1202. https://doi.org/10.3390/horticulturae9111202
Chicago/Turabian StyleBetancur, Matías, Jorge Retamal-Salgado, María Dolores López, Rosa Vergara-Retamales, and Mauricio Schoebitz. 2023. "Novel Approach to Organic Mulching from Natural-Based Solutions to Enhance Soil Health and Functional Value of Calafate Fruit" Horticulturae 9, no. 11: 1202. https://doi.org/10.3390/horticulturae9111202
APA StyleBetancur, M., Retamal-Salgado, J., López, M. D., Vergara-Retamales, R., & Schoebitz, M. (2023). Novel Approach to Organic Mulching from Natural-Based Solutions to Enhance Soil Health and Functional Value of Calafate Fruit. Horticulturae, 9(11), 1202. https://doi.org/10.3390/horticulturae9111202








