The Use of Two Locally Sourced Bio-Inocula to Improve Nitrogen and Phosphorus Cycling in Soils and Increase Macro and Micronutrient Nutrient Concentration in Edamame (Glycine max. L.) and Pumpkin (Cucurbita maxima)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Bio-Inocula: LEM or F-LEM
2.3. Composting Process
2.4. Experimental Design and Treatments
2.5. Soil Sampling
2.6. Microbial Community Analysis
2.7. Soil Respiration (CO2) and Ammonia (NH3) Volatilization
2.8. Soil Analysis
2.9. Edamame and Pumpkin Productivity
2.10. Nutritional Value of Edamame and Pumpkin
2.11. Statistical Analysis
3. Results and Discussion
3.1. Microbial Communities in Liquid Bio-Inocula and Compost Inoculated with Liquid Bio-Inocula
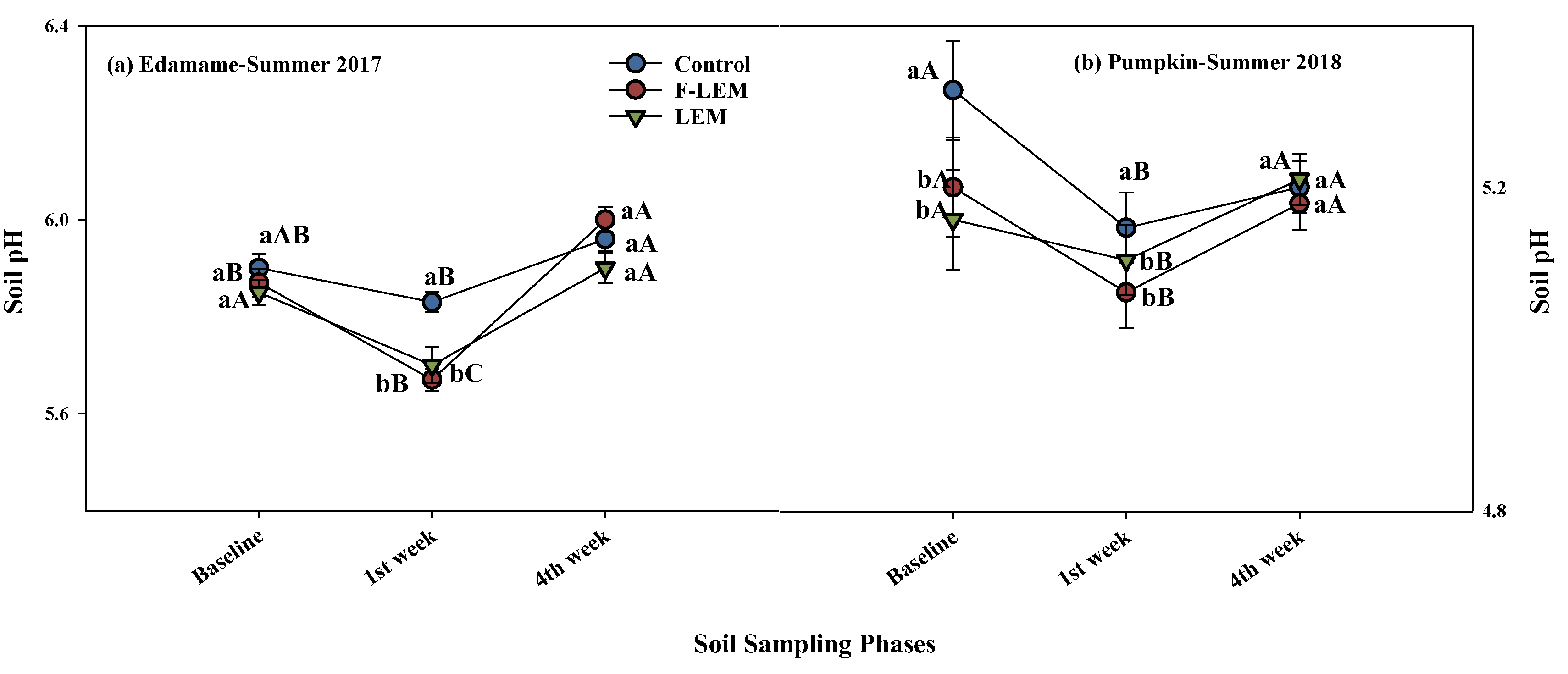
3.2. Soil pH, NH3 Volatilization, and CO2 Respiration from the Soil in Edamame, Summer 2017, and Pumpkin, Summer 2018
3.3. Soil-N Fractions
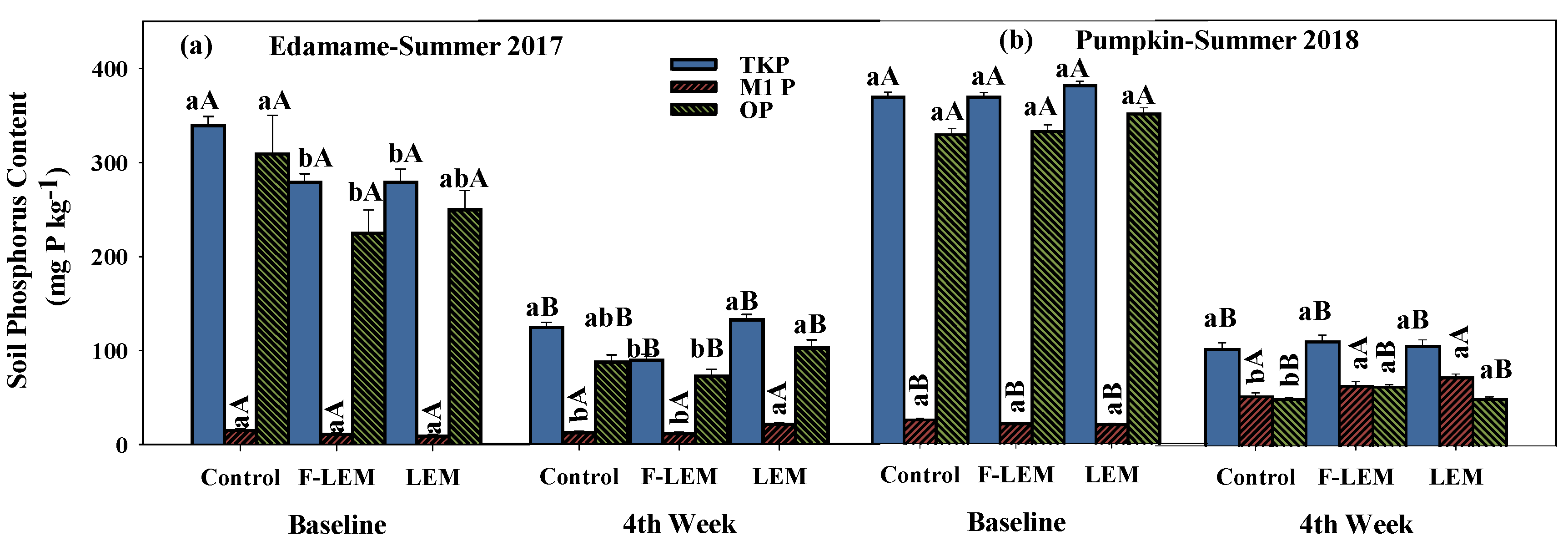
3.4. Phosphorous Fractions of Soil
3.5. Edamame and Pumpkin Productivity; Nutritional Value of Butterbean Edamame and Pumpkin
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Franklin, D.; Bender-Özenç, D.; Özenç, N.; Cabrera, M. Nitrogen mineralization and phosphorus release from composts and soil conditioners found in the Southeastern United States. Soil Sci. Soc. Am. J. 2015, 79, 1386–1395. [Google Scholar] [CrossRef]
- Mahmud, K.; Makaju, S.; Ibrahim, R.; Missaoui, A.J.P. Current Progress in Nitrogen Fixing Plants and Microbiome Research. Plants 2020, 9, 97. [Google Scholar] [CrossRef]
- Hamim, A.; Boukeskasse, A.; Ouhdouch, Y.; Farrouki, A.; Barrijal, S.; Miché, L.; Mrabet, R.; Duponnois, R.; Hafidi, M.J.B. Phosphate solubilizing and PGR activities of ericaceous shrubs microorganisms isolated from Mediterranean forest soil. Biocatal. Agric. Biotechnol. 2019, 19, 101128. [Google Scholar] [CrossRef]
- Zhu, J.; Li, M.; Whelan, M. Phosphorus activators contribute to legacy phosphorus availability in agricultural soils: A review. Sci. Total Environ. 2018, 612, 522–537. [Google Scholar] [CrossRef]
- Wyngaard, N.; Cabrera, M.L.; Jarosch, K.A.; Bünemann, E.K. Phosphorus in the coarse soil fraction is related to soil organic phosphorus mineralization measured by isotopic dilution. Soil Biol. Biochem. 2016, 96, 107–118. [Google Scholar] [CrossRef]
- Kruse, J.; Abraham, M.; Amelung, W.; Baum, C.; Bol, R.; Kühn, O.; Lewandowski, H.; Niederberger, J.; Oelmann, Y.; Rüger, C.; et al. Innovative methods in soil phosphorus research: A review. J. Plant Nutr. Soil Sci. 2015, 178, 43–88. [Google Scholar] [CrossRef]
- Davis, D.R. Declining fruit and vegetable nutrient composition: What is the evidence? HortScience 2009, 44, 15–19. [Google Scholar] [CrossRef]
- Marles, R.J. Mineral nutrient composition of vegetables, fruits and grains: The context of reports of apparent historical declines. J. Food Compos. Anal. 2017, 56, 93–103. [Google Scholar] [CrossRef]
- Horton, S.; Alderman, H.; Rivera, J.A. The challenge of hunger and malnutrition. In Copenhagen Consensus; Cambridge University Press: Cambridge, UK, 2008; pp. 3–4. [Google Scholar]
- Black, R.E.; Allen, L.H.; Bhutta, Z.A.; Caulfield, L.E.; De Onis, M.; Ezzati, M.; Mathers, C.; Rivera, J.; Maternal and Child Undernutrition Study Group. Maternal and child undernutrition: Global and regional exposures and health consequences. Lancet 2008, 371, 243–260. [Google Scholar] [CrossRef] [PubMed]
- Wakeel, A.; Farooq, M.; Bashir, K.; Ozturk, L. Micronutrient malnutrition and biofortification: Recent advances and future perspectives. In Plant Micronutrient Use Efficiency; Academic Press: Cambridge, MA, USA, 2018; pp. 225–243. [Google Scholar]
- Niang, A.; Becker, M.; Ewert, F.; Tanaka, A.; Dieng, I.; Saito, K. Yield variation of rainfed rice as affected by field water availability and N fertilizer use in central Benin. Nutr. Cycl. Agroecosyst. 2018, 110, 293–305. [Google Scholar] [CrossRef]
- Salim, N.; Raza, A. Nutrient use efficiency (NUE) for sustainable wheat production: A review. J. Plant Nutr. 2020, 43, 297–315. [Google Scholar] [CrossRef]
- Alloway, B.J. Micronutrients and crop production: An introduction. In Micronutrient Deficiencies in Global Crop Production; Springer: Berlin/Heidelberg, Germany, 2008; pp. 1–39. [Google Scholar]
- Saquee, F.S.; Diakite, S.; Kavhiza, N.J.; Pakina, E.; Zargar, M. The Efficacy of Micronutrient Fertilizers on the Yield Formulation and Quality of Wheat Grains. Agronomy 2023, 13, 566. [Google Scholar] [CrossRef]
- Drewnowski, A.; Burton-Freeman, B. A new category-specific nutrient rich food (NRF9f. 3) score adds flavonoids to assess nutrient density of fruit. Food Funct. 2020, 11, 123–130. [Google Scholar] [CrossRef]
- Barker, A.V.; Stratton, M.L. Nutrient density of fruit crops as a function of soil fertility. In Fruit Crops; Elsevier: Amsterdam, The Netherlands, 2020; pp. 13–31. [Google Scholar]
- Lugtenberg, B.; Kamilova, F. Plant-growth-promoting rhizobacteria. Annu. Rev. Microbiol. 2009, 63, 541–556. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhao, Z.; Yin, X.-A.; Zhu, Y. Impacts of biochars on bacterial community shifts and biodegradation of antibiotics in an agricultural soil during short-term incubation. Sci. Total Environ. 2021, 771, 144751. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Wang, J.; He, Y.; Yu, X.; Chen, S.; Penttinen, P.; Liu, S.; Yang, Y.; Zhao, K.; Zou, L. Organic fertilizers shape soil microbial communities and increase soil amino acid metabolites content in a blueberry orchard. Microb. Ecol. 2023, 85, 232–246. [Google Scholar] [CrossRef]
- Rocchi, L.; Boggia, A.; Paolotti, L. Sustainable agricultural systems: A bibliometrics analysis of ecological modernization approach. Sustainability 2020, 12, 9635. [Google Scholar] [CrossRef]
- Diacono, M.; Persiani, A.; Testani, E.; Montemurro, F.; Ciaccia, C. Recycling agricultural wastes and by-products in organic farming: Biofertilizer production, yield performance and carbon footprint analysis. Sustainability 2019, 11, 3824. [Google Scholar] [CrossRef]
- Mahmud, K.; Missaoui, A.; Lee, K.; Ghimire, B.; Presley, H.W.; Makaju, S. Rhizosphere microbiome manipulation for sustainable crop production. Curr. Plant Biol. 2021, 27, 100210. [Google Scholar] [CrossRef]
- Fu, W.; Tunney, H.; Zhang, C. Spatial variation of soil nutrients in a dairy farm and its implications for site-specific fertilizer application. Soil Tillage Res. 2010, 106, 185–193. [Google Scholar] [CrossRef]
- Sun, B.; Zhang, L.; Yang, L.; Zhang, F.; Norse, D.; Zhu, Z. Agricultural non-point source pollution in China: Causes and mitigation measures. AMBIO 2012, 41, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Kloepper, J.W. Plant growth-promoting rhizobacteria on radishes. In Proceedings of the 4th Internet. Conf. on Plant Pathogenic Bacter, Station de Pathologie Vegetale et Phytobacteriologie, INRA, Angers, France, 1978; pp. 879–882. [Google Scholar]
- Bonkowski, M.; Villenave, C.; Griffiths, B. Rhizosphere fauna: The functional and structural diversity of intimate interactions of soil fauna with plant roots. Plant Soil 2009, 321, 213–233. [Google Scholar] [CrossRef]
- Buée, M.; De Boer, W.; Martin, F.; Van Overbeek, L.; Jurkevitch, E. The rhizosphere zoo: An overview of plant-associated communities of microorganisms, including phages, bacteria, archaea, and fungi, and of some of their structuring factors. Plant Soil 2009, 321, 189–212. [Google Scholar] [CrossRef]
- Lynch, J.; Whipps, J. Substrate flow in the rhizosphere. Plant Soil 1990, 129, 1–10. [Google Scholar] [CrossRef]
- Mendes, R.; Garbeva, P.; Raaijmakers, J.M. The rhizosphere microbiome: Significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS Microbiol. Rev. 2013, 37, 634–663. [Google Scholar] [CrossRef] [PubMed]
- Metting, F.B. Soil Microbial Ecology, Applications in Agriculture and Environmental Management; Citeseer; Marcel Dekker, Inc.: New City, NY, USA, 1993. [Google Scholar]
- Raaijmakers, J.M.; Paulitz, T.C.; Steinberg, C.; Alabouvette, C.; Moënne-Loccoz, Y. The rhizosphere: A playground and battlefield for soilborne pathogens and beneficial microorganisms. Plant Soil 2009, 321, 341–361. [Google Scholar] [CrossRef]
- Van Vliet, P.; Bloem, J.; De Goede, R.J. Microbial diversity, nitrogen loss and grass production after addition of Effective Micro-organisms®(EM) to slurry manure. Appl. Soil Ecol. 2006, 32, 188–198. [Google Scholar] [CrossRef]
- Mupondi, L.; Mnkeni, P.; Brutsch, M. The effects of goat manure, sewage sludge and effective microorganisms on the composting of pine bark. Compos. Sci. Util. 2006, 14, 201–210. [Google Scholar] [CrossRef]
- Hu, C.; Qi, Y. Long-term effective microorganisms application promote growth and increase yields and nutrition of wheat in China. Eur. J. Agron. 2013, 46, 63–67. [Google Scholar] [CrossRef]
- Mayer, J.; Scheid, S.; Widmer, F.; Fließbach, A.; Oberholzer, H.-R. How effective are ‘Effective microorganisms®(EM)’? Results from a field study in temperate climate. Appl. Soil Ecol. 2010, 46, 230–239. [Google Scholar] [CrossRef]
- Meyer, G.; Maurhofer, M.; Frossard, E.; Gamper, H.A.; Mäder, P.; Mészáros, É.; Schönholzer-Mauclaire, L.; Symanczik, S.; Oberson, A. Pseudomonas protegens CHA0 does not increase phosphorus uptake from 33P labeled synthetic hydroxyapatite by wheat grown on calcareous soil. Soil Biol. Biochem. 2019, 131, 217–228. [Google Scholar] [CrossRef]
- Ney, L.; Franklin, D.; Mahmud, K.; Cabrera, M.; Hancock, D.; Habteselassie, M.; Newcomer, Q. Examining trophic-level nematode community structure and nitrogen mineralization to assess local effective microorganisms’ role in nitrogen availability of swine effluent to forage crops. Appl. Soil Ecol. 2018, 130, 209–218. [Google Scholar] [CrossRef]
- Luo, Y.; Zhou, X. Soil Respiration and the Environment; Elsevier: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Bremner, J.M. Nitrogen-total. In Methods of Soil Analysis: Part 3 Chemical Methods; John Wiley & Sons: Hoboken, NJ, USA, 1996; Volume 5, pp. 1085–1121. [Google Scholar]
- Maynard, D.; Kalra, Y.; Crumbaugh, J. Nitrate and exchangeable ammonium nitrogen. Soil Sampl. Methods Anal. 1993, 1, 25–38. [Google Scholar]
- Kempers, A.; Zweers, A. Ammonium determination in soil extracts by the salicylate method. Commun. Soil Sci. Plant Anal. 1986, 17, 715–723. [Google Scholar] [CrossRef]
- Zhu, X.; Burger, M.; Doane, T.A.; Horwath, W.R. Ammonia oxidation pathways and nitrifier denitrification are significant sources of N2O and NO under low oxygen availability. Proc. Natl. Acad. Sci. USA 2013, 110, 6328–6333. [Google Scholar] [CrossRef]
- Taylor, M. Determination of total phosphorus in soil using simple Kjeldahl digestion. Commun. Soil Sci. Plant Anal. 2000, 31, 2665–2670. [Google Scholar] [CrossRef]
- Mehlich, A. Determination of P, Ca, Mg, K, Na and NH4 by North Carolina soil testing laboratories; University of North Carolina: Raleigh, NC, USA, 1953. [Google Scholar]
- Sparks, D.L.; Page, A.L.; Helmke, P.A.; Loeppert, R.H. Methods of Soil Analysis, Part 3: Chemical Methods; John Wiley & Sons: Hoboken, NJ, USA, 2020; Volume 14. [Google Scholar]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Marsalis, M.; Angadi, S.; Contreras-Govea, F. Dry matter yield and nutritive value of corn, forage sorghum, and BMR forage sorghum at different plant populations and nitrogen rates. Field Crops Res. 2010, 116, 52–57. [Google Scholar] [CrossRef]
- SAS Institute Inc. Base SAS 9.4 Procedures Guide: Statistical Procedures; SAS Institute Inc.: Cary, NC, USA, 2013. [Google Scholar]
- Alori, E.T.; Glick, B.R.; Babalola, O.O. Microbial phosphorus solubilization and its potential for use in sustainable agriculture. Front. Microbiol. 2017, 8, 971. [Google Scholar] [CrossRef]
- Liang, J.-L.; Liu, J.; Jia, P.; Yang, T.-T.; Zeng, Q.-W.; Zhang, S.-C.; Liao, B.; Shu, W.-S.; Li, J.-T. Novel phosphate-solubilizing bacteria enhance soil phosphorus cycling following ecological restoration of land degraded by mining. ISME J. 2020, 14, 1600–1613. [Google Scholar] [CrossRef]
- Yu, J.; Whalen, J.K. A new perspective on functional redundancy and phylogenetic niche conservatism in soil microbial communities. Pedosphere 2020, 30, 18–24. [Google Scholar]
- Oteino, N.; Lally, R.D.; Kiwanuka, S.; Lloyd, A.; Ryan, D.; Germaine, K.J.; Dowling, D.N. Plant growth promotion induced by phosphate solubilizing endophytic Pseudomonas isolates. Front. Microbiol. 2015, 6, 745. [Google Scholar] [CrossRef]
- Allison, S.D.; Martiny, J.B. Resistance, resilience, and redundancy in microbial communities. Proc. Natl. Acad. Sci. USA 2008, 105 (Suppl. S1), 11512–11519. [Google Scholar] [CrossRef] [PubMed]
- Grządziel, J. Functional redundancy of soil microbiota—Does more always mean better? Pol. J. Soil Sci. 2017, 50, 75. [Google Scholar] [CrossRef]
- Sánchez, Ó.J.; Ospina, D.A.; Montoya, S. Compost supplementation with nutrients and microorganisms in composting process. Waste Manag. 2017, 69, 136–153. [Google Scholar] [CrossRef]
- Wickramatilake, A.R.P.; Munehiro, R.; Nagaoka, T.; Wasaki, J.; Kouno, K. Compost amendment enhances population and composition of phosphate solubilizing bacteria and improves phosphorus availability in granitic regosols. Soil Sci. Plant Nutr. 2011, 57, 529–540. [Google Scholar] [CrossRef]
- Rochette, P.; Angers, D.A.; Chantigny, M.H.; MacDonald, J.D.; Bissonnette, N.; Bertrand, N. Ammonia volatilization following surface application of urea to tilled and no-till soils: A laboratory comparison. Soil Tillage Res. 2009, 103, 310–315. [Google Scholar] [CrossRef]
- Pfennig, N.; Trüper, H.G. The family chromatiaceae. In The Prokaryotes: A Handbook on the Biology of Bacteria: Ecophysiology, Isolation, Identification, Applications; Springer: Berlin/Heidelberg, Germany, 1992; pp. 3200–3221. [Google Scholar]
- Bast, E. Utilization of nitrogen compounds and ammonia assimilation by Chromatiaceae. Arch. Microbiol. 1977, 113, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Paillat, L.; Cannavo, P.; Barraud, F.; Huché-Thélier, L.; Guénon, R. Growing medium type affects organic fertilizer mineralization and CNPS microbial enzyme activities. Agronomy 2020, 10, 1955. [Google Scholar] [CrossRef]
- Grzyb, A.; Wolna-Maruwka, A.; Niewiadomska, A. Environmental Factors Affecting the Mineralization of Crop Residues. Agronomy 2020, 10, 1951. [Google Scholar] [CrossRef]
- Curtin, D.; Beare, M.H. Temperature and moisture effects on microbial biomass and soil organic matter mineralization. Soil Sci. Soc. Am. J. 2012, 76, 2055. [Google Scholar] [CrossRef]
- Ney, L.; Franklin, D.; Mahmud, K.; Cabrera, M.; Hancock, D.; Habteselassie, M.; Newcomer, Q.; Fatzinger, B. Rebuilding soil ecosystems for improved productivity in biosolarized soils. Int. J. Agron. 2019, 2019, 5827585. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y.; Di Mattia, E.; El-Nakhel, C.; Cardarelli, M. Co-inoculation of Glomus intraradices and Trichoderma atroviride acts as a biostimulant to promote growth, yield and nutrient uptake of vegetable crops. J. Sci. Food Agric. 2015, 95, 1706–1715. [Google Scholar] [CrossRef]
- Lamont, J.R.; Wilkins, O.; Bywater-Ekegärd, M.; Smith, D.L. From yogurt to yield: Potential applications of lactic acid bacteria in plant production. Soil Biol. Biochem. 2017, 111, 1–9. [Google Scholar] [CrossRef]
- Rokhbakhsh-Zamin, F.; Sachdev, D.; Kazemi-Pour, N.; Engineer, A.; Pardesi, K.R.; Zinjarde, S.; Dhakephalkar, P.K.; Chopade, B.A. Characterization of plant-growth-promoting traits of Acinetobacter species isolated from rhizosphere of Pennisetum glaucum. J. Microbiol. Biotechnol. 2011, 21, 556–566. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, A.H. Bioprocessing of rock phosphate ore: Essential technical considerations for the development of a successful commercial technology. In Proceedings of the 4th International Fertilizer Association Technical Conference, IFA, Paris, France, 1–4 October 2000. [Google Scholar]
- Giassi, V.; Kiritani, C.; Kupper, K.C. Bacteria as growth-promoting agents for citrus rootstocks. Microbiol. Res. 2016, 190, 46–54. [Google Scholar] [CrossRef]
- Shrestha, A.; Kim, B.S.; Park, D.H. Biological control of bacterial spot disease and plant growth-promoting effects of lactic acid bacteria on pepper. Biocontrol Sci. Technol. 2014, 24, 763–779. [Google Scholar] [CrossRef]
- Li, H.-P.; Han, Q.-Q.; Liu, Q.-M.; Gan, Y.-N.; Rensing, C.; Rivera, W.L.; Zhao, Q.; Zhang, J.-L. Roles of phosphate-solubilizing bacteria in mediating soil legacy phosphorus availability. Microbiol. Res. 2023, 272, 127375. [Google Scholar] [CrossRef]
- Sharpley, A.N. Dependence of Runoff Phosphorus on Extractable Soil Phosphorus; Wiley Online Library: Hoboken, NJ, USA, 1995. [Google Scholar]
- Zhang, J.; Guo, T.; Tao, Z.; Wang, P.; Tian, H. Transcriptome profiling of genes involved in nutrient uptake regulated by phosphate-solubilizing bacteria in pepper (Capsicum annuum L.). Plant Physiol. Biochem. 2020, 156, 611–626. [Google Scholar] [CrossRef]
- Myers, S.S.; Zanobetti, A.; Kloog, I.; Huybers, P.; Leakey, A.D.; Bloom, A.J.; Carlisle, E.; Dietterich, L.H.; Fitzgerald, G.; Hasegawa, T. Increasing CO2 threatens human nutrition. Nature 2014, 510, 139–142. [Google Scholar] [CrossRef]


| Summer 2017 (kg ha−1 from Liquid Bio-Inocula) | Summer 2018 (kg ha−1 from Compost) | |||||||
|---|---|---|---|---|---|---|---|---|
| N | P | N | P | Ca | Mg | K | Zn | |
| Control | ND | ND | 80 | 50 | 62 | 24 | 112 | 2 |
| F-LEM | ND | ND | 74 | 50 | 72 | 28 | 132 | 2 |
| LEM | ND | ND | 76 | 50 | 68 | 28 | 126 | 2 |
| Edamame—Summer 2017 | Pumpkin—Summer 2018 | ||
|---|---|---|---|
| Month | Activities | Month | |
| 25 June 2017 | Baseline soil sampling | Baseline soil sampling | 30 June 2018 |
| 28 June 2017 | Plot preparation | Plot preparation and compost application | 5 July 2018 |
| 8 July 2017 | Edamame planting | Pumpkin planting | 5 July 2018 |
| 3 August 2017 | Liquid bio-inocula application | Liquid bio-inocula application | 18 July 2018 |
| 11 August 2017 and 5 September 2017 | Post-application soil sampling (“1st and 4th”week after application) | Post-application soil sampling (“1st and 4th” week after application) | 26 July 2018 and 25 August 2018 |
| 21 September 2017 | Plant harvested | Plant harvested | 4, 11, 20, and 29 September 2018 |
| Phylum | Class | Order | Family | F-LEM (2017) | LEM (2017) | Control Compost (2018) | F-LEM Compost (2018) | LEM Compost (2018) |
|---|---|---|---|---|---|---|---|---|
| Proteobacteria | α-Proteobacteria | Rhizobiales | Hyphomicrobiaceae (Pht) | Abs | 41 (1) | 16 (33) | 100 (0.2) | 100 (0.6) |
| Bradyrhizobiaceae (N-fix) | Abs | 59 (2) | Abs | Abs | Abs | |||
| Rhodospirillales | Rhodospirillaceae (N-fix) | Abs | 43 (0.5) | 100 (5) | 100 (2) | 100 (1) | ||
| Acetobacteraceae (N-fix) (PSB) | Abs | 57 (0.7) | Abs | Abs | Abs | |||
| β-Proteobacteria | Burkholderiales | Burkholderiaceae (PSB) | Abs | 100 (1) | Abs | Abs | Abs | |
| γ-Proteobacteria | Pseudomonadales | Pseudomonadaceae (PSB) (BC) | Abs | Abs | 75 (4) | 100 (60) | 100 (53) | |
| Chromatiaceae (PrSlfB) (AOB) | Abs | Abs | 28 (2) | 65 (0.2) | 72 (0.4) | |||
| Firmicutes | Bacilli | Lactobacillales | Lactobacillaceae (PSB) | 100 (99) | 99 (97) | Abs | Abs | Abs |
| Bacillales | Bacillalaceae (PSB) (BC) | Abs | 95 (95) | 47 (26) | 60 (21) | 60 (20) |
| Treatment | Edamame Biomass [kg ha−1] | Edamame Beans [kg ha−1] | Cumulative Pumpkin Yield [kg ha−1] |
|---|---|---|---|
| Control | 3395 a [166] | 4568 a [1573] | 2455 a [274] |
| F-LEM | 3052 a [209] | 5381 a [1769] | 2035 a [336] |
| LEM | 2838 a [517] | 5006 a [1650] | 2026 a [243] |
| Edamame Beans [mg/100 g] | Pumpkin Pulp [mg/100 g] (Cumulative of 4 Weeks) | Pumpkin Seeds [mg/100 g] (Cumulative of 4 Weeks) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P | Ca | Mg | K | Zn | P | Ca | Mg | K | Zn | P | Ca | Mg | K | Zn | ||
| Control | 289 a [13] | 44 b [3] | 156 b [2] | 562 b [9] | 5 b [0.043] | 13 b [1] | 140 b [7] | 650 ab [38] | 692 ab [41] | 2 b [1] | 273 b [2] | 462 b [38] | 683 ab [55] | 828 ab [595] | 15 b [1] | |
| F-LEM | 283 a [11] | 47 ab [2] | 157 b [1] | 525 c [5] | 5 b [0.070] | 9 b [3] | 139 b [9] | 494 b [81] | 563 b [94] | 2 b [3] | 270 b [21] | 543 b [155] | 535 b [84] | 591 b [822] | 18 b [1] | |
| LEM | 266 a [17] | 54 a [2] | 161 a [1] | 591 a [5] | 6 a [0.067] | 25 a [5] | 193 a [18] | 856 a [60] | 820 a [45] | 3 a [2] | 342 a [21] | 700 a [204] | 854 a [60] | 1077 a [82] | 27 a [3] | |
| C to L(%) | −8 | +23 | +3 | +265 | +20 | +92 | +38 | +32 | +18 | +50 | +25 | +52 | +25 | +30 | +80 | |
| F to L(%) | −6 | +15 | +3 | +13 | +20 | +178 | +39 | +73 | +46 | +50 | +27 | +29 | +60 | +82 | +50 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahmud, K.; Franklin, D.; Cabrera, M.; Ney, L.; Dahal, S.; Subedi, A. The Use of Two Locally Sourced Bio-Inocula to Improve Nitrogen and Phosphorus Cycling in Soils and Increase Macro and Micronutrient Nutrient Concentration in Edamame (Glycine max. L.) and Pumpkin (Cucurbita maxima). Horticulturae 2023, 9, 1200. https://doi.org/10.3390/horticulturae9111200
Mahmud K, Franklin D, Cabrera M, Ney L, Dahal S, Subedi A. The Use of Two Locally Sourced Bio-Inocula to Improve Nitrogen and Phosphorus Cycling in Soils and Increase Macro and Micronutrient Nutrient Concentration in Edamame (Glycine max. L.) and Pumpkin (Cucurbita maxima). Horticulturae. 2023; 9(11):1200. https://doi.org/10.3390/horticulturae9111200
Chicago/Turabian StyleMahmud, Kishan, Dorcas Franklin, Miguel Cabrera, Laura Ney, Subash Dahal, and Anish Subedi. 2023. "The Use of Two Locally Sourced Bio-Inocula to Improve Nitrogen and Phosphorus Cycling in Soils and Increase Macro and Micronutrient Nutrient Concentration in Edamame (Glycine max. L.) and Pumpkin (Cucurbita maxima)" Horticulturae 9, no. 11: 1200. https://doi.org/10.3390/horticulturae9111200
APA StyleMahmud, K., Franklin, D., Cabrera, M., Ney, L., Dahal, S., & Subedi, A. (2023). The Use of Two Locally Sourced Bio-Inocula to Improve Nitrogen and Phosphorus Cycling in Soils and Increase Macro and Micronutrient Nutrient Concentration in Edamame (Glycine max. L.) and Pumpkin (Cucurbita maxima). Horticulturae, 9(11), 1200. https://doi.org/10.3390/horticulturae9111200







