Influence of Foliar Treatment with Suspensions Rich in Trichoderma Chlamydospores on Momordica charantia Physiology, Yield, and Quality
Abstract
1. Introduction
2. Materials and Methods
2.1. Biological Material
2.2. (Bio)Chemical Material
2.3. Fungal Cultivation
2.4. Morphological Analysis of T36 and Td50b Biomass by Optical Microscopy
2.5. Thermogravimetric Analysis
2.6. Experimental Treatments
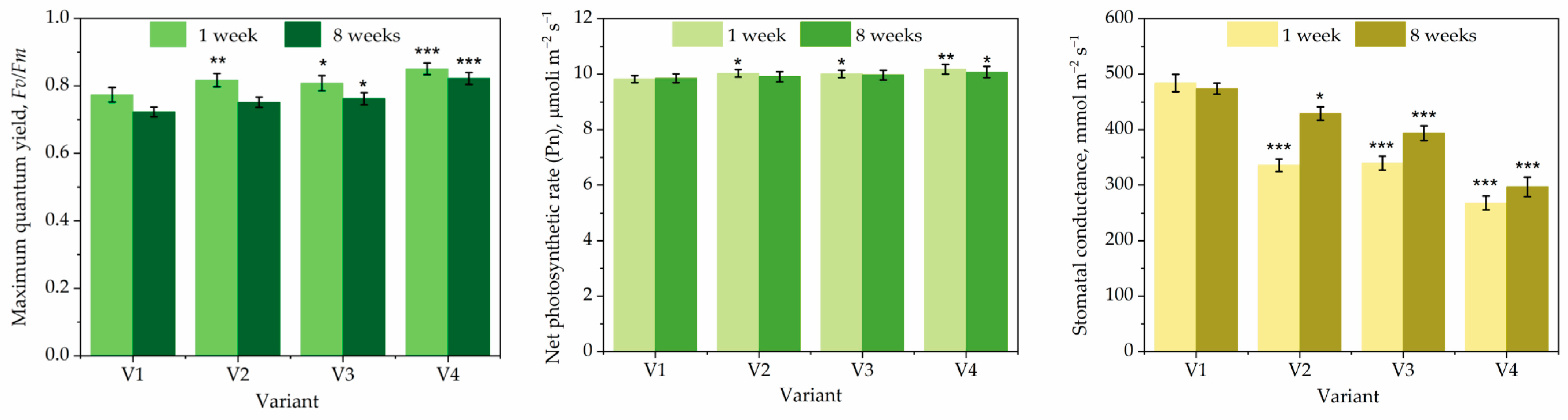
2.7. Determination of Plant Physiological Characteristics
2.8. Determination of Total Polyphenols and Total Flavonoids
2.9. Antioxidant Activity Assay
2.9.1. DPPH Scavenging Activity Assay
2.9.2. Antioxidant Capacity (TEAC) Assay
2.10. Cytocompatibility Assay
2.11. Statistical Analysis
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Abdullah, N.S.; Doni, F.; Mispan, M.S.; Saiman, M.Z.; Yusuf, Y.M.; Oke, M.A.; Suhaimi, N.S.M. Harnessing Trichoderma in Agriculture for Productivity and Sustainability. Agronomy 2021, 11, 2559. [Google Scholar] [CrossRef]
- Guzmán-Guzmán, P.; Kumar, A.; de los Santos-Villalobos, S.; Parra-Cota, F.I.; Orozco-Mosqueda, M.D.; Fadiji, A.E.; Hyder, S.; Babalola, O.O.; Santoyo, G. Trichoderma Species: Our Best Fungal Allies in the Biocontrol of Plant Diseases—A Review. Plants 2023, 12, 432. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.O.; De Mio, L.L.M.; Soccol, C.R. Trichoderma as a powerful fungal disease control agent for a more sustainable and healthy agriculture: Recent studies and molecular insights. Planta 2023, 257, 31. [Google Scholar] [CrossRef] [PubMed]
- Sharon, E.; Bar-Eyal, M.; Chet, I.; Herrera-Estrella, A.; Kleifeld, O.; Spiegel, Y. Biological control of the root-knot nematode Meloidogyne javanica by Trichoderma Harzianum. Phytopathology 2001, 91, 687–693. [Google Scholar] [CrossRef]
- Sahebani, N.; Hadavi, N. Biological control of the root-knot nematode Meloidogyne javanica by Trichoderma harzianum. Soil Biol. Biochem. 2008, 40, 2016–2020. [Google Scholar] [CrossRef]
- Rodríguez-González, A.; Casquero, P.A.; Suárez-Villanueva, V.; Carro-Huerga, G.; Alvarez-García, S.; Mayo-Prieto, S.; Lorenzana, A.; Cardoza, R.E.; Gutiérrez, S. Effect of trichodiene production by Trichoderma harzianum on Acanthoscelides obtectus. J. Stored Prod. Res. 2018, 77, 231–239. [Google Scholar] [CrossRef]
- Rodriguez-Gonzalez, A.; Guerra, M.; Ramirez-Lozano, D.; Casquero, P.A.; Gutierrez, S. Germination and Agronomic Traits of Phaseolus vulgaris L. Beans Sprayed with Trichoderma Strains and Attacked by Acanthoscelides obtectus. Agronomy 2021, 11, 2130. [Google Scholar] [CrossRef]
- López-Bucio, J.; Pelagio-Flores, R.; Herrera-Estrella, A. Trichoderma as biostimulant: Exploiting the multilevel properties of a plant beneficial fungus. Sci. Hortic. 2015, 196, 109–123. [Google Scholar] [CrossRef]
- Woo, S.L.; Hermosa, R.; Lorito, M.; Monte, E. Trichoderma: A multipurpose, plant-beneficial microorganism for eco-sustainable agriculture. Nat. Rev. Microbiol. 2023, 21, 312–326. [Google Scholar] [CrossRef]
- Viterbo, A.; Ramot, O.; Chernin, L.; Chet, I. Significance of lytic enzymes from Trichoderma spp. in the biocontrol of fungal plant pathogens. Antonie Van Leeuwenhoek 2002, 81, 549–556. [Google Scholar] [CrossRef]
- Brotman, Y.; Briff, E.; Viterbo, A.; Chet, I. Role of swollenin, an expansin-like protein from Trichoderma, in plant root colonization. Plant Physiol. 2008, 147, 779–789. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Salwan, R.; Sharma, V. Extracellular proteins of Trichoderma and their role in plant health. S. Afr. J. Bot. 2022, 147, 359–369. [Google Scholar] [CrossRef]
- Vinale, F.; Sivasithamparam, K. Beneficial effects of Trichoderma secondary metabolites on crops. Phytother. Res. 2020, 34, 2835–2842. [Google Scholar] [CrossRef] [PubMed]
- Carillo, P.; Woo, S.L.; Comite, E.; El-Nakhel, C.; Rouphael, Y.; Fusco, G.M.; Borzacchiello, A.; Lanzuise, S.; Vinale, F. Application of Trichoderma harzianum, 6-pentyl-α-pyrone and plant biopolymer formulations modulate plant metabolism and fruit quality of plum tomatoes. Plants 2020, 9, 771. [Google Scholar] [CrossRef]
- Malmierca, M.; Cardoza, R.; Alexander, N.; McCormick, S.; Hermosa, R.; Monte, E.; Gutiérrez, S. Involvement of Trichoderma trichothecenes in the biocontrol activity and induction of plant defense-related genes. Appl. Environ. Microbiol. 2012, 78, 4856–4868. [Google Scholar] [CrossRef]
- Shahriar, S.A.; Islam, M.N.; Chun, C.N.W.; Kaur, P.; Rahim, M.A.; Islam, M.M.; Uddain, J.; Siddiquee, S. Microbial metabolomics interaction and ecological challenges of Trichoderma species as biocontrol inoculant in crop rhizosphere. Agronomy 2022, 12, 900. [Google Scholar] [CrossRef]
- Elad, Y. Biological control of foliar pathogens by means of Trichoderma harzianum and potential modes of action. Crop Prot. 2000, 19, 709–714. [Google Scholar] [CrossRef]
- Coppola, M.; Cascone, P.; Lelio, I.D.; Woo, S.L.; Lorito, M.; Rao, R.; Pennacchio, F.; Guerrieri, E.; Digilio, M.C. Trichoderma atroviride P1 Colonization of Tomato Plants Enhances Both Direct and Indirect Defense Barriers Against Insects. Front. Physiol. 2019, 10, 464382. [Google Scholar] [CrossRef] [PubMed]
- Salwan, R.; Sharma, A.; Kaur, R.; Sharma, R.; Sharma, V. The riddles of Trichoderma induced plant immunity. Biol. Control 2022, 174, 105037. [Google Scholar] [CrossRef]
- Macías-Rodríguez, L.; Contreras-Cornejo, H.A.; Adame-Garnica, S.G.; del-Val, E.; Larsen, J. The interactions of Trichoderma at multiple trophic levels: Inter-kingdom communication. Microbiol. Res. 2020, 240, 126552. [Google Scholar] [CrossRef]
- Nawrocka, J.; Małolepsza, U. Diversity in plant systemic resistance induced by Trichoderma. Biol. Control 2013, 67, 149–156. [Google Scholar] [CrossRef]
- Morán-Diez, M.E.; Martínez de Alba, Á.E.; Rubio, M.B.; Hermosa, R.; Monte, E. Trichoderma and the Plant Heritable Priming Responses. J. Fungi 2021, 7, 318. [Google Scholar] [CrossRef]
- Tyśkiewicz, R.; Nowak, A.; Ozimek, E.; Jaroszuk-Ściseł, J. Trichoderma: The Current Status of Its Application in Agriculture for the Biocontrol of Fungal Phytopathogens and Stimulation of Plant Growth. Int. J. Mol. Sci. 2022, 23, 2329. [Google Scholar] [CrossRef] [PubMed]
- Pacheco-Trejo, J.; Aquino-Torres, E.; Reyes-Santamaría, M.I.; Islas-Pelcastre, M.; Pérez-Ríos, S.R.; Madariaga-Navarrete, A.; Saucedo-García, M. Plant Defensive Responses Triggered by Trichoderma spp. as Tools to Face Stressful Conditions. Horticulturae 2022, 8, 1181. [Google Scholar] [CrossRef]
- Shang, J.; Liu, B.; Xu, Z. Efficacy of Trichoderma asperellum TC01 against anthracnose and growth promotion of Camellia sinensis seedlings. Biol. Control 2020, 143, 104205. [Google Scholar] [CrossRef]
- Coppola, M.; Diretto, G.; Digilio, M.C.; Woo, S.L.; Giuliano, G.; Molisso, D.; Pennacchio, F.; Lorito, M.; Rao, R. Transcriptome and Metabolome Reprogramming in Tomato Plants by Trichoderma harzianum strain T22 Primes and Enhances Defense Responses Against Aphids. Front. Physiol. 2019, 10, 745. [Google Scholar] [CrossRef]
- Dini, I.; Pascale, M.; Staropoli, A.; Marra, R.; Vinale, F. Effect of Selected Trichoderma Strains and Metabolites on Olive Drupes. Appl. Sci. 2021, 11, 8710. [Google Scholar] [CrossRef]
- Pascale, A.; Vinale, F.; Manganiello, G.; Nigro, M.; Lanzuise, S.; Ruocco, M.; Marra, R.; Lombardi, N.; Woo, S.L.; Lorito, M. Trichoderma and its secondary metabolites improve yield and quality of grapes. Crop Prot. 2017, 92, 176–181. [Google Scholar] [CrossRef]
- Guzmán-Guzmán, P.; Porras-Troncoso, M.D.; Olmedo-Monfil, V.; Herrera-Estrella, A. Trichoderma species: Versatile plant symbionts. Phytopathology 2019, 109, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Pacheco-Ordaz, R.; Wall-Medrano, A.; Goñi, M.G.; Ramos-Clamont-Montfort, G.; Ayala-Zavala, J.F.; González-Aguilar, G. Effect of phenolic compounds on the growth of selected probiotic and pathogenic bacteria. Lett. Appl. Microbiol. 2018, 66, 25–31. [Google Scholar] [CrossRef]
- Taylor, J.T.; Harting, R.; Shalaby, S.; Kenerley, C.M.; Braus, G.H.; Horwitz, B.A. Adhesion as a Focus in Trichoderma-Root Interactions. J. Fungi 2022, 8, 372. [Google Scholar] [CrossRef] [PubMed]
- Vacher, C.; Hampe, A.; Porté, A.J.; Sauer, U.; Compant, S.; Morris, C.E. The Phyllosphere: Microbial Jungle at the Plant-Climate Interface. Annu. Rev. Ecol. Evol. Syst. 2016, 47, 1–24. [Google Scholar] [CrossRef]
- Xu, N.H.; Zhao, Q.Q.; Zhang, Z.Y.; Zhang, Q.; Wang, Y.; Qin, G.Y.; Ke, M.J.; Qiu, D.Y.; Peijnenburg, W.; Lu, T.; et al. Phyllosphere Microorganisms: Sources, Drivers, and Their Interactions with Plant Hosts. J. Agric. Food Chem. 2022, 70, 4860–4870. [Google Scholar] [CrossRef]
- Zhu, Y.G.; Xiong, C.; Wei, Z.; Chen, Q.L.; Ma, B.; Zhou, S.Y.D.; Tan, J.Q.; Zhang, L.M.; Cui, H.L.; Duan, G.L. Impacts of global change on the phyllosphere microbiome. New Phytol. 2022, 234, 1977–1986. [Google Scholar] [CrossRef] [PubMed]
- Sohrabi, R.; Paasch, B.C.; Liber, J.A.; He, S.Y. Phyllosphere Microbiome. Annu. Rev. Plant Biol. 2023, 74, 539–568. [Google Scholar] [CrossRef]
- Mandal, S.D.; Jeon, J. Phyllosphere Microbiome in Plant Health and Disease. Plants 2023, 12, 3481. [Google Scholar] [CrossRef] [PubMed]
- Oancea, F.; Raut, I.; Sesan, T.E.; Cornea, P.C. Dry Flowable Formulation of Biostimulants Trichoderma strains. Agric. Agric. Sci. Procedia 2016, 10, 494–502. [Google Scholar] [CrossRef][Green Version]
- Kamble, M.V.; Joshi, S.M.; Hadimani, S.; Jogaiah, S. Biopriming with rhizosphere Trichoderma harzianum elicit protection against grapevine downy mildew disease by triggering histopathological and biochemical defense responses. Rhizosphere 2021, 19, 100398. [Google Scholar] [CrossRef]
- Martinez, Y.; Ribera, J.; Schwarze, F.W.; De France, K. Biotechnological development of Trichoderma-based formulations for biological control. Appl. Microbiol. Biotechnol. 2023, 107, 5595–5612. [Google Scholar] [CrossRef]
- Şesan, T.E.; Oancea, A.O.; Ştefan, L.M.; Mănoiu, V.S.; Ghiurea, M.; Răut, I.; Constantinescu-Aruxandei, D.; Toma, A.; Savin, S.; Bira, A.F. Effects of foliar treatment with a Trichoderma plant biostimulant consortium on Passiflora caerulea L. yield and quality. Microorganisms 2020, 8, 123. [Google Scholar] [CrossRef]
- Sârbu, A.; Şesan, T.E.; Tănase, T.; Paraschiv, A.M.; Cîşlariu, A.G.; Oancea, F. Anatomical investigations on Momordica charantia L. plants, newly acclimated in Romania. J. Plant Dev. 2022, 29, 25–44. [Google Scholar] [CrossRef]
- Tan, S.P.; Kha, T.C.; Parks, S.E.; Roach, P.D. Bitter melon (Momordica charantia L.) bioactive composition and health benefits: A review. Food Rev. Int. 2016, 32, 181–202. [Google Scholar] [CrossRef]
- Richter, E.; Geetha, T.; Burnett, D.; Broderick, T.L.; Babu, J.R. The Effects of Momordica charantia on Type 2 Diabetes Mellitus and Alzheimer Disease. Int. J. Mol. Sci. 2023, 24, 4643. [Google Scholar] [CrossRef] [PubMed]
- Oancea, F.; Mara, G.; Sesan, T.E.; Máthé, I.; Răut, I.; Ábrahám, B.; Lányi, S. Strain of Trichoderma harzianum and Controlled Release Composition which Contains Said Strain. EU Patent 2735607A1, 28 May 2014. [Google Scholar]
- Raut, I.; Badea-Doni, M.; Calin, M.; Oancea, F.; Vasilescu, G.; Sesan, T.E.; Jecu, L. Effect of volatile and non-volatile metabolites from Trichoderma spp. against important phytopathogens. Rev. Chim. 2014, 65, 1285–1288. [Google Scholar]
- Lichtenthaler, H.; Buschmann, C.; Knapp, M. How to correctly determine the different chlorophyll fluorescence parameters and the chlorophyll fluorescence decrease ratio R Fd of leaves with the PAM fluorometer. Photosynthetica 2005, 43, 379–393. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
- Craciunescu, O.; Constantin, D.; Gaspar, A.; Toma, L.; Utoiu, E.; Moldovan, L. Evaluation of antioxidant and cytoprotective activities of Arnica montana L. and Artemisia absinthium L. ethanolic extracts. Chem. Cent. J. 2012, 6, 97. [Google Scholar] [CrossRef]
- Chang, C.-C.; Yang, M.-H.; Wen, H.-M.; Chern, J.-C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar]
- Braca, A.; De Tommasi, N.; Di Bari, L.; Pizza, C.; Politi, M.; Morelli, I. Antioxidant principles from Bauhinia tarapotensis. J. Nat. Prod. 2001, 64, 892–895. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Repetto, G.; Del Peso, A.; Zurita, J.L. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat. Protoc. 2008, 3, 1125. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-Q.; Song, K.; Li, Y.-C.; Chen, J. Statistical culture-based strategies to enhance chlamydospore production by Trichoderma harzianum SH2303 in liquid fermentation. J. Zhejiang Univ. Sci. B 2016, 17, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Girometta, C.; Dondi, D.; Baiguera, R.M.; Bracco, F.; Branciforti, D.S.; Buratti, S.; Lazzaroni, S.; Savino, E. Characterization of mycelia from wood-decay species by TGA and IR spectroscopy. Cellulose 2020, 27, 6133–6148. [Google Scholar] [CrossRef]
- Cartabia, M.; Girometta, C.E.; Milanese, C.; Baiguera, R.M.; Buratti, S.; Branciforti, D.S.; Vadivel, D.; Girella, A.; Babbini, S.; Savino, E.; et al. Collection and Characterization of Wood Decay Fungal Strains for Developing Pure Mycelium Mats. J. Fungi 2021, 7, 1008. [Google Scholar] [CrossRef] [PubMed]
- Brondi, M.; Florencio, C.; Mattoso, L.; Ribeiro, C.; Farinas, C. Encapsulation of Trichoderma harzianum with nanocellulose/carboxymethyl cellulose nanocomposite. Carbohydr. Polym. 2022, 295, 119876. [Google Scholar] [CrossRef] [PubMed]
- Adzmi, F.; Meon, S.; Musa, M.H.; Yusuf, N.A. Preparation, characterisation and viability of encapsulated Trichoderma harzianum UPM40 in alginate-montmorillonite clay. J. Microencapsul. 2012, 29, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Tiburski, J.H.; Rosenthal, A.; Guyot, S.; Perrier-Cornet, J.-M.; Gervais, P. Water distribution in bacterial spores: A key factor in heat resistance. Food Biophys. 2014, 9, 10–19. [Google Scholar] [CrossRef]
- Formisano, L.; Miras-Moreno, B.; Ciriello, M.; El-Nakhel, C.; Corrado, G.; Lucini, L.; Colla, G.; Rouphael, Y. Trichoderma and Phosphite Elicited Distinctive Secondary Metabolite Signatures in Zucchini Squash Plants. Agronomy 2021, 11, 1205. [Google Scholar] [CrossRef]
- Fiorini, L.; Guglielminetti, L.; Mariotti, L.; Curadi, M.; Picciarelli, P.; Scartazza, A.; Sarrocco, S.; Vannacci, G. Trichoderma harzianum T6776 modulates a complex metabolic network to stimulate tomato cv. Micro-Tom growth. Plant Soil 2016, 400, 351–366. [Google Scholar] [CrossRef]
- Palacios-Torres, R.E.; Bustamante-Ortiz, A.G.; Prieto-Baeza, L.A.; Hernández-Hernández, H.; Ramírez-Seañez, A.R.; Yam-Tzec, J.A.; Díaz-Félix, G. Effect of foliar application of Trichoderma on the quality of tomato fruits grown in different hydroponic substrates. Folia Hortic. 2019, 31, 355–364. [Google Scholar] [CrossRef]
- Lo, C.-T.; Nelson, E.; Harman, G. Improved biocontrol efficacy of Trichoderma harzianum 1295-22 for foliar phases of turf diseases by use of spray applications. Plant Dis. 1997, 81, 1132–1138. [Google Scholar] [CrossRef] [PubMed]
- Perazzolli, M.; Moretto, M.; Fontana, P.; Ferrarini, A.; Velasco, R.; Moser, C.; Delledonne, M.; Pertot, I. Downy mildew resistance induced by Trichoderma harzianum T39 in susceptible grapevines partially mimics transcriptional changes of resistant genotypes. BMC Genom. 2012, 13, 660. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.M.; Bae, H. An overview of stress-induced resveratrol synthesis in grapes: Perspectives for resveratrol-enriched grape products. Molecules 2017, 22, 294. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Xi, B.; Dai, H. Effects of Water Stress on Resveratrol Accumulation and Synthesis in ‘Cabernet Sauvignon’ Grape Berries. Agronomy 2023, 13, 633. [Google Scholar] [CrossRef]
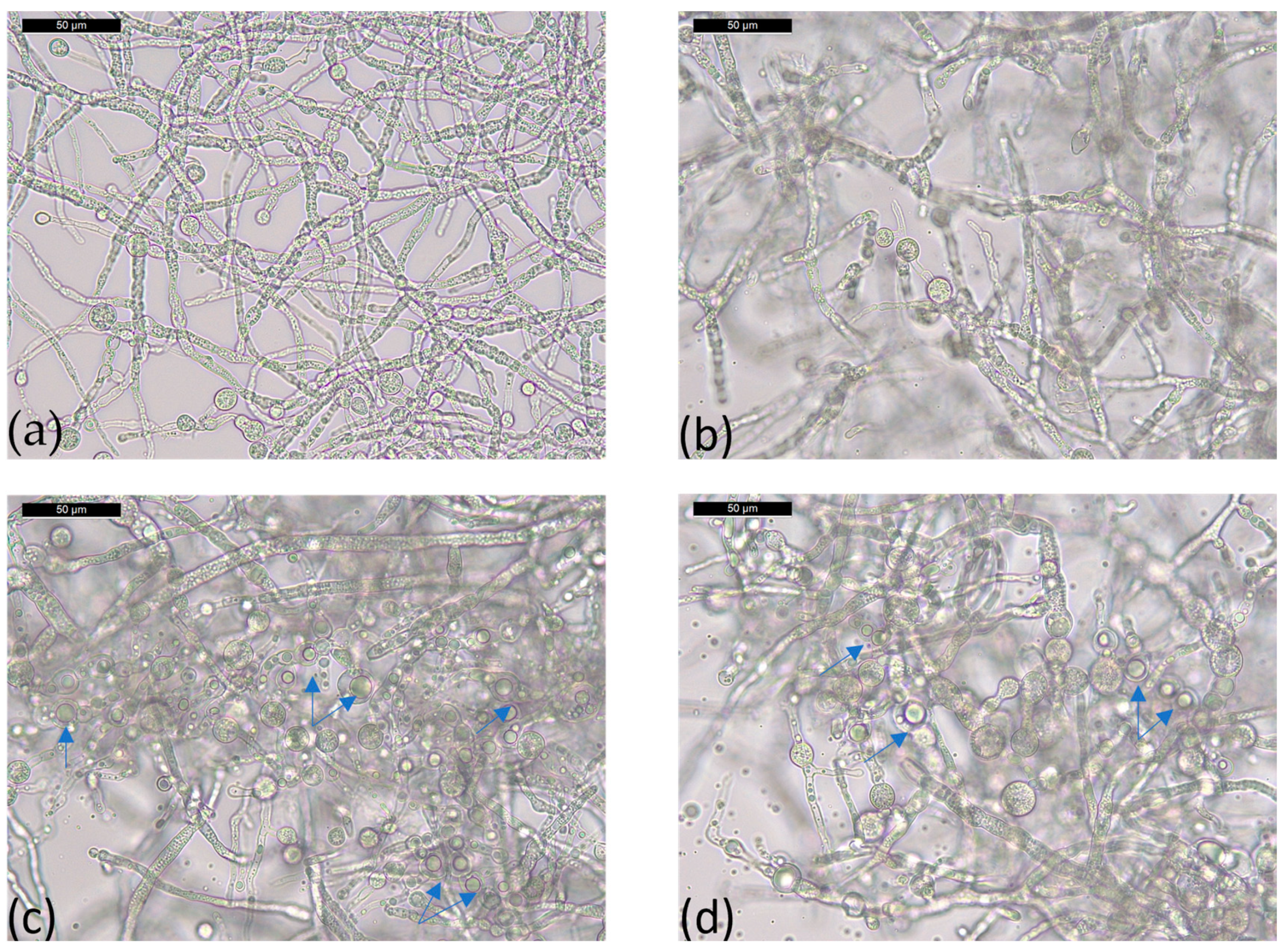
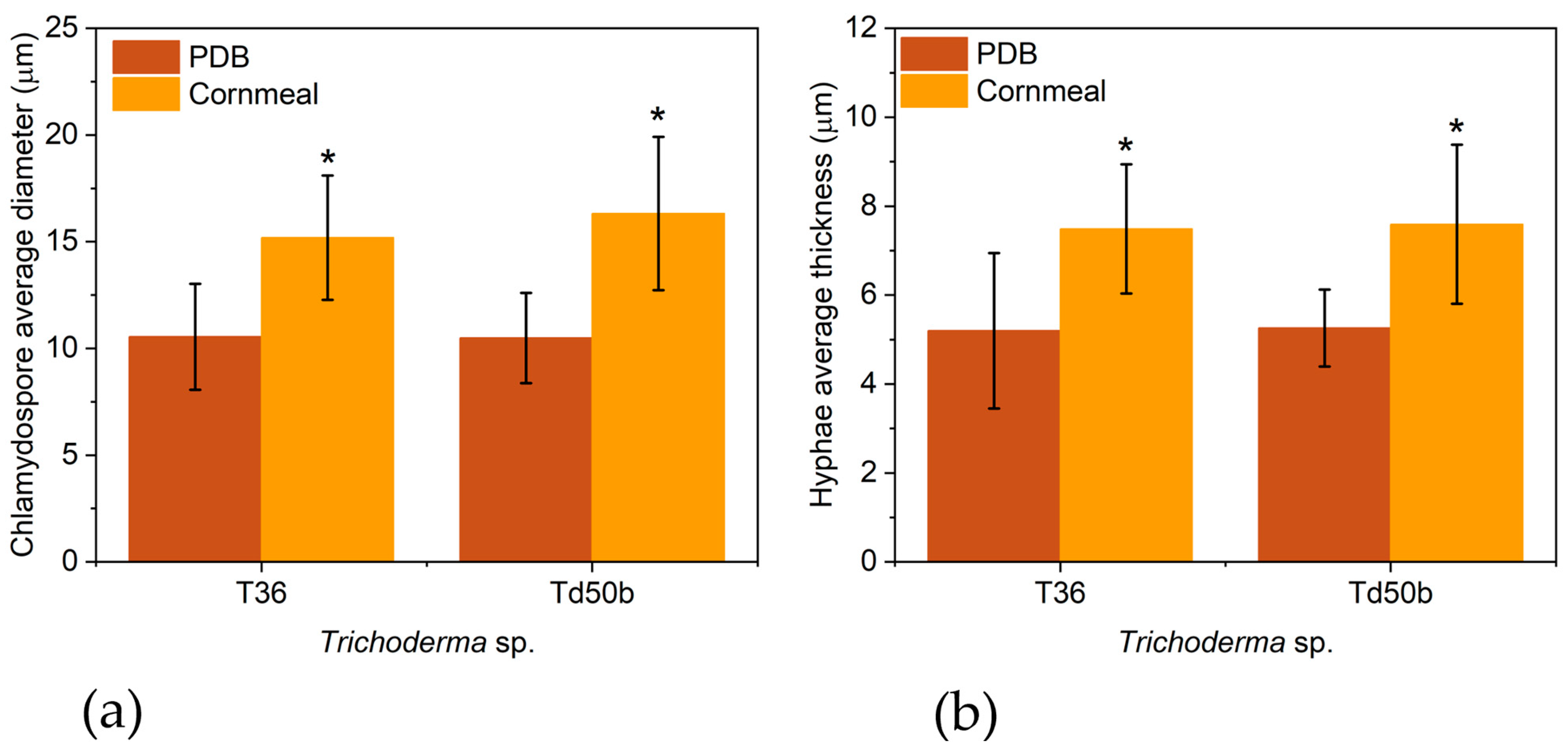
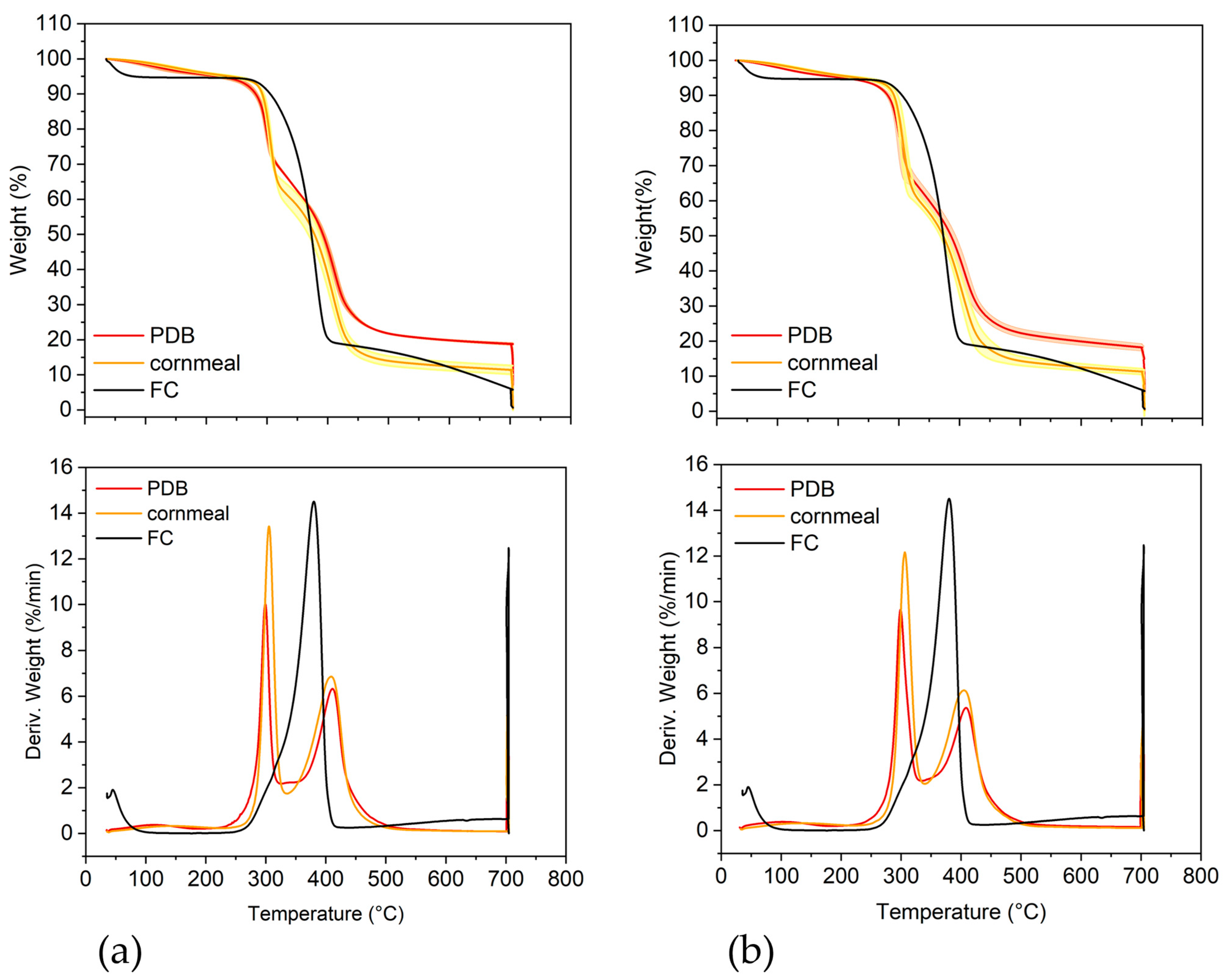
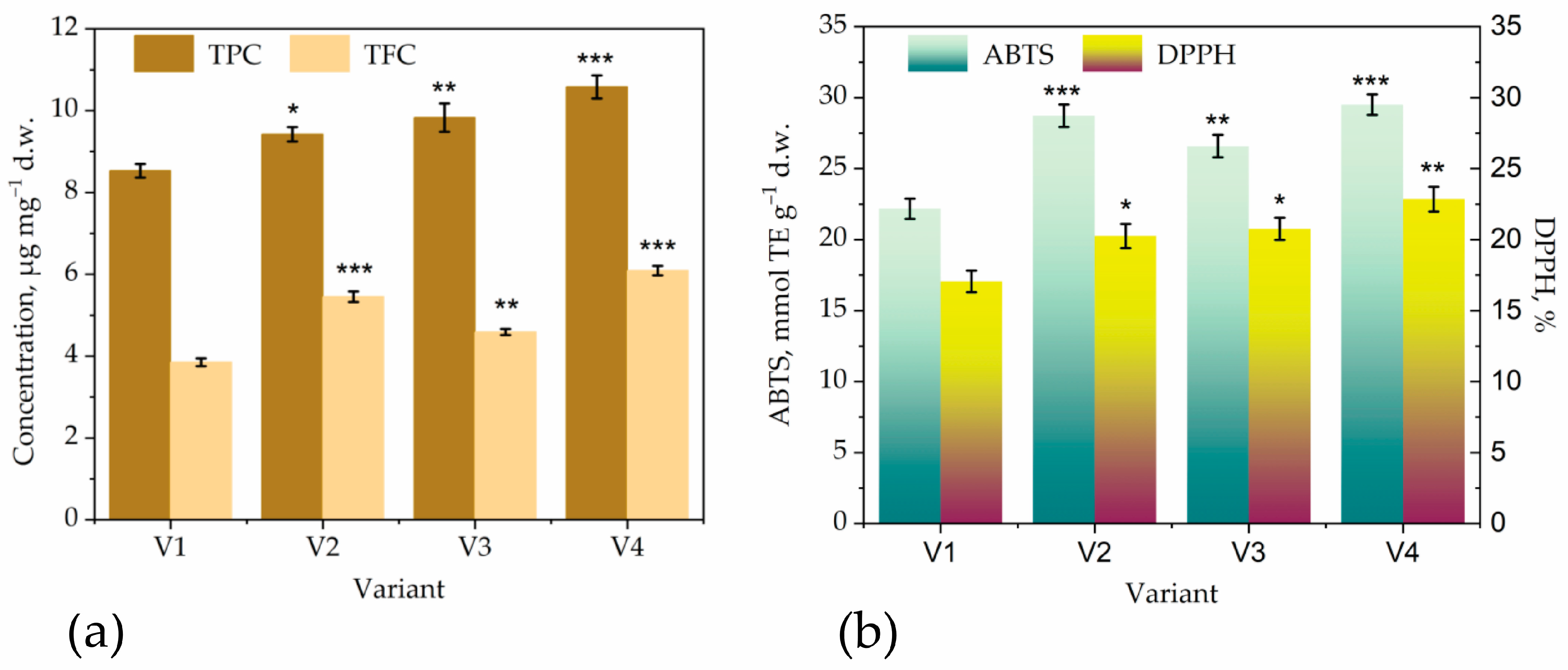
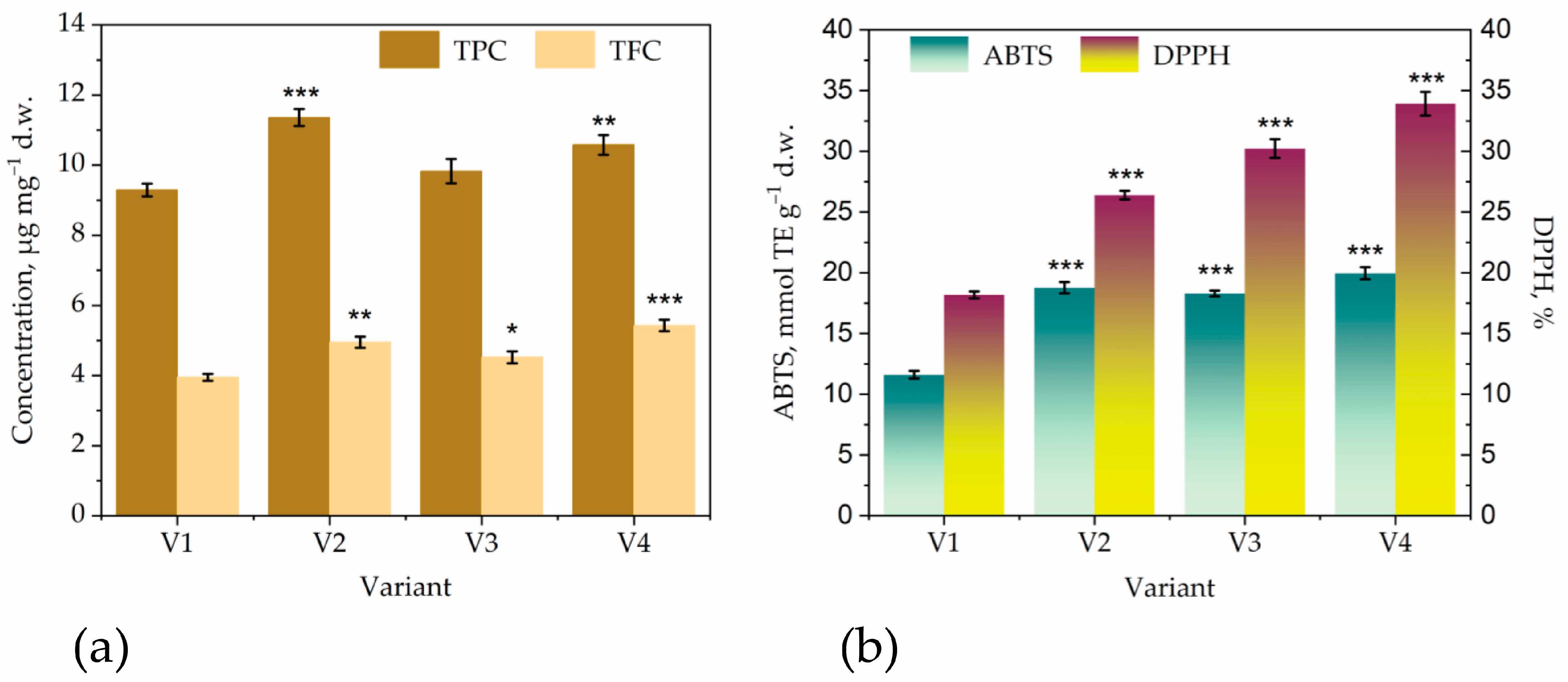
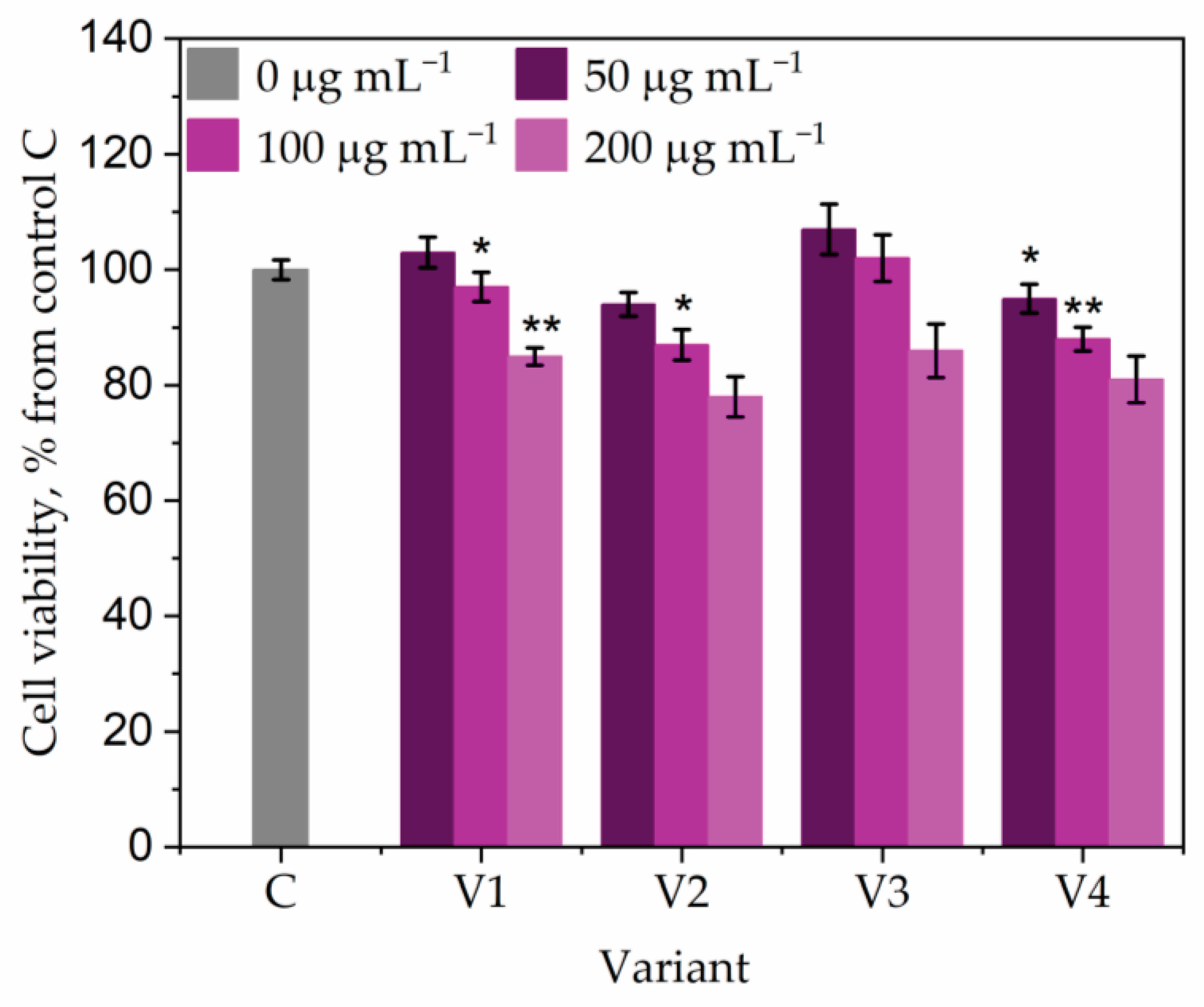
| Parameter | T36 PDB | T36 Cornmeal | Td50b PDB | Td50b Cornmeal | FC |
|---|---|---|---|---|---|
| T1 (°C) | 116.5 ± 9.5 | 142.0 ± 10.5 * | 101.6 ± 3.0 | 133.0 ± 4.6 ** | 43 |
| WL1 (%) | 4.41 ± 0.12 | 4.73 ± 0.10 | 4.48 ± 0.11 | 4.65 ± 0.14 | 5.36 |
| T2 (°C) | 299.1 ± 1.1 | 305.2 ± 1.7 * | 302.1 ± 0.4 | 307.2 ± 0.7 ** | 380.0 |
| WL2 (%) | 27.89 ± 0.47 | 34.39 ± 1.74 * | 31.58 ± 0.34 | 36.44 ± 0.66 ** | 76.14 |
| T3 (°C) | 411.0 ± 1.8 | 408.8 ± 1.5 | 409.3 ± 0.6 | 406.6 ± 0.7 * | 617.1 |
| WL3 (%) | 48.88 ± 0.72 | 49.45 ± 1.20 | 45.74 ± 0.43 | 47.58 ± 0.69 | 12.36 |
| Residue (%) # | 18.82 ± 0.13 | 11.43 ± 0.64 *** | 18.20 ± 0.51 | 11.33 ± 0.39 *** | 6.14 |
| Ash (%) ## | 7.19 ± 0.51 | 0.32 ± 0.05 ** | 4.78 ± 0.60 | 0.31 ± 0.03 ** | 0.67 |
| Residue-Ash | 11.63 ± 0.30 | 11.11 ± 0.37 | 13.42 ± 0.45 | 11.02 ± 0.23 | 5.47 |
| Nr. crt. | Treatment * | Dose | No. of Applications | Production (kg/ha) | Additional Yield | |
|---|---|---|---|---|---|---|
| kg/ha | % | |||||
| V1 | Control | - | - | 12,019 | 100 | |
| V2 | Trichoderma TCM | 2 × 1011 ufc/ha | 2 | 16,962 | 4943 *** | 141.13 |
| V3 | Trichoderma TPD | 2 × 1013 kg/ha | 2 | 15,064 | 3045 ** | 125.33 |
| V4 | Trichoderma TCM | 2 × 1013 ufc/ha | 2 | 18,398 | 6379 *** | 153.07 |
| LD 5%—1465 kg/ha | ||||||
| LD 1%—2013 kg/ha | ||||||
| LD 0.1%—3059 kg/ha | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bala, I.-A.; Șesan, T.E.; Oancea, A.; Craciunescu, O.; Ghiurea, M.; Răut, I.; Trică, B.; Nicolae, C.-A.; Constantinescu-Aruxandei, D.; Oancea, F. Influence of Foliar Treatment with Suspensions Rich in Trichoderma Chlamydospores on Momordica charantia Physiology, Yield, and Quality. Horticulturae 2024, 10, 371. https://doi.org/10.3390/horticulturae10040371
Bala I-A, Șesan TE, Oancea A, Craciunescu O, Ghiurea M, Răut I, Trică B, Nicolae C-A, Constantinescu-Aruxandei D, Oancea F. Influence of Foliar Treatment with Suspensions Rich in Trichoderma Chlamydospores on Momordica charantia Physiology, Yield, and Quality. Horticulturae. 2024; 10(4):371. https://doi.org/10.3390/horticulturae10040371
Chicago/Turabian StyleBala, Ioana-Alexandra, Tatiana Eugenia Șesan, Anca Oancea, Oana Craciunescu, Marius Ghiurea, Iuliana Răut, Bogdan Trică, Cristian-Andi Nicolae, Diana Constantinescu-Aruxandei, and Florin Oancea. 2024. "Influence of Foliar Treatment with Suspensions Rich in Trichoderma Chlamydospores on Momordica charantia Physiology, Yield, and Quality" Horticulturae 10, no. 4: 371. https://doi.org/10.3390/horticulturae10040371
APA StyleBala, I.-A., Șesan, T. E., Oancea, A., Craciunescu, O., Ghiurea, M., Răut, I., Trică, B., Nicolae, C.-A., Constantinescu-Aruxandei, D., & Oancea, F. (2024). Influence of Foliar Treatment with Suspensions Rich in Trichoderma Chlamydospores on Momordica charantia Physiology, Yield, and Quality. Horticulturae, 10(4), 371. https://doi.org/10.3390/horticulturae10040371








