Abstract
Objective: In the future, the stress of enhanced UV-B radiation on the Earth will first affect the photosynthesis of plants, including mangoes. Therefore, it is necessary to study the effects of enhanced UV-B radiation on the photosynthesis of mangoes. Methods: ‘Tainong No 1’ mango trees in the field were selected as the experimental material and divided into 2 groups: one group was shined under 96 kJ·m−2·d−1 UV-B lamps for artificially simulated treatment of enhanced UV-B radiation, and the other group was shined under sunshine directly as the control (CK). The main photosynthetic physiological indicators were measured with conventional methods, and the expression levels of the genes encoding large and small subunits of the Rubisco enzyme were measured with fluorescent qPCR. The changes in stomatal morphology and chloroplast structure were observed with scanning electron microscopy and transmission electron microscopy. Results: The content of malondialdehyde (MDA) and the relative conductivity in the leaves of the treatment tended to be significantly higher than those of the CK. The net photosynthetic rate (Pn) of the treatment tended to decrease and be lower than that of CK. The dynamics of intercellular CO2 concentration (Ci) of the treatment and CK changed differently from each other but generally tended to decrease, and that of the treatment tended to be significantly higher than that of CK. The stomatal conductance (Gs) of the treatment and CK both generally decreased, and that of the treatment was always significantly lower than that of CK. The contents of chlorophyll a, chlorophyll b and total chlorophyll and the ratio of chlorophyll a/b of the treatment were lower than those of CK, while the carotenoid content showed the opposite trend. The stomata and the surface of leaves of the treatment were sunken and damaged, respectively. The palisade tissue, spongy tissue and upper epidermis thickened more, and the total thickness significantly increased. Meanwhile, the ratio of palisade tissue to spongy tissue decreased. During treatment, the chloroplasts were swollen and shortened, the number of chloroplasts was reduced, the starch grains were degraded, and the grana lamella were distorted, loosely arranged and blurred. The expression of the genes encoding the Rubisco large subunit (rbcL) in the treatment was significantly inhibited, while that encoding the Rubisco small subunit (rbcS) decreased first and increased later. In conclusion, 96 kJ·m−2·d−1 enhanced UV-B radiation treatment caused damage to the leaf cell membrane system. This led to stomatal limitation of photosynthesis by destroying the stomatal structure and nonstomatal limitation of photosynthesis by damaging the submicrostructure of the chloroplasts and downregulating the expression of rbcL. The leaves may resist the photosynthetic damage caused by enhanced UV-B radiation by upregulating rbcS expression as much as possible.
1. Introduction
Ultraviolet (UV) radiation from solar radiation includes zone A (430−400 nm), zone B (280−320 nm) and zone C (200−280 nm) wavelengths. When passing through the stratosphere, UV-A wavelengths pass entirely through the ozone layer, and UV-C wavelengths are absorbed by the ozone layer. UV-B wavelengths are mostly absorbed by the ozone layer, but a portion passes through to the earth. However, in recent years, due to large amounts of industrial emissions and especially to the influence of chlorofluorocarbons emitted by the aviation industry, the ozone layer has attenuated, resulting in more UV-B radiation reaching the Earth’s surface [1,2]. This UV-B radiation is called “enhanced UV-B radiation”, and it has a widespread and profound effect on plant systems [3,4,5]. The UV-B radiation dose reaching the Earth’s surface still exceeds the normal range, despite some achievements since the Montreal Protocol was signed by many countries [6,7]. According to the predictions of the National Aeronautics and Space Administration National Aeronautics and Space Administration Goddard Institute for Space Studies (GISS) model, the UV-B dose in the Northern and Southern Hemispheres may be enhanced by 2% to 27% from 2040−2050 [8]. Therefore, investigating the effects of enhanced UV-B radiation on organisms has become a hot topic in ecological research.
At present, studies on the effects of enhanced UV-B radiation and its causes have been conducted mainly on algae and food crops in China and in other countries. However, fewer studies have reported on this topic in relation to tropical fruit trees [9,10,11,12]. Enhanced UV-B radiation often negatively affects plant growth morphology, photosynthetic capacity and productivity [13,14,15].
Different plant species differ in their sensitivity to UV-B radiation enhancement. Enhanced UV-B radiation may also be beneficial to plant growth and development and some physiological functions at intensities below those that cause damage, and different plants have different ranges of beneficial and harmful intensities [16]. The lucerne, snakegourd fruit and yam were treated with 3.01 w/cm2 and 8.01 w/cm2 of enhanced UV-B radiation under natural growth, with natural light as the control. The stomatal conductance (Gs) of lucerne was reduced in the 8.01 w/cm2 treatment, but the net photosynthetic rate (Pn), transpiration rate (Tr), water use efficiency (WUE) and stomatal conductance in the 3.01 w/cm2 treatment were all higher than those in the control under the same treatment. The Gs of snakegourd fruit decreased at 3.01 w/cm2, and the net photosynthetic rate, transpiration rate, water use efficiency (WUE) and stomatal conductance at 8.01 w/cm2 were higher than those of the control under both treatments. The Pn and WUE of yam were lower than those of the control, and Pn and Tr were higher than those of the control in both treatments. UV-B radiation enhancement at 3.01 w/cm2 was already detrimental to yam photosynthesis, while UV-B radiation enhancement at 8.01 w/cm2 still promoted photosynthesis in lucerne and snakegourd fruit [17]. In addition, high−intensity enhanced UV-B radiation can destroy the morphology and structure of leaf tissue and cellular chloroplasts [18]. Potted seedlings of poinsettia treated with enhanced UV-B radiation at 14.33 kJ·m−2·d−1 showed reduced leaf fenestra tissue thickness and contracted curled leaves compared to potted seedlings under natural UV-B radiation control at 11.02 kJ·m−2·d−1 [19]. The enhanced UV-B radiation damaged the microstructure of photosynthetic tissues. After treatment of the supralittoral green macroalga Prasiola crispa with enhanced UV-B radiation, the chloroplast cyst−like membrane swelled, and the number of osmiophilic droplets decreased [20]. This indicates that enhanced UV-B radiation disrupts chloroplast ultrastructural morphology. Therefore, the microscopic and ultrastructural morphological changes of the photosynthetic apparatus of mango leaves under the influence of enhanced UV-B radiation need to be observed and studied to clarify the characteristics of the effect of enhanced UV-B radiation on the microscopic morphology of the photosynthetic apparatus of mango and the mechanism of the effect on photosynthesis.
In this study, a possible future environment of enhanced UV-B radiation was artificially simulated in the field, and the theoretical mechanism of photosynthesis inhibition by enhanced UV-B radiation in the leaves of Mangifera indica cv. Tainong was prospectively explored in terms of changes in leaf photosynthetic physiological properties, ultrastructure and gene expression efficiency of key enzymes to meet the challenges of future enhanced UV-B radiation on the development of the fruit industry.
2. Materials and Methods
2.1. Plant Materials
Adult trial trees of Mangifera indica cv. ‘Tainong No 1’ was provided by the Sanya Youlong Agricultural Development Co., Ltd. in Shengchang Village, Haitang District, Sanya City, Hainan Province, China (lat. 18°25′ N, long. 109°46′ E). The area has a tropical monsoon climate with high temperatures, high humidity, long summers and no winters, with a rainy season from May to October and a dry season from November to April, and with more than 300 sunny days per year, resulting in strong solar UV radiation (average daily radiation dose of approximately 83.47 kJ·m−2·d−1). Ten 15-year-old plants with strong, uniform growth that were free of diseases and pests were selected as the test trees.
2.2. Experimental Design and Treatment Method
Based on previous research by our research group, we used artificial simulation−enhanced UV-B radiation (96 kJ·m−2·d−1) as the treatment [19,20]. Natural sunlight was used as a control (CK). The single−plant test material was plotted and replicated five times. Four UV-B lamp tubes were suspended at the top of the test trees (40 W, radiation range of 280–320 nm, and a radiation dose of 24 kJ·m−2·d−1; Beijing Electric Light Source Research Institute). A distance of 30 cm was maintained between the tops of the trees and the lamp tubes. The experimental treatments were conducted from 18 January 2022, to 17 February 2022, with lights on from sunrise to sunset every day and lights off during cloudy and rainy periods.
2.3. Sampling for Biochemical Assays
The first leaf samples were collected at the beginning of the experimental treatment and every 10 d thereafter. The measurement of photosynthetic indexes in the field was performed from 9:00−10:00 am on the day of each sampling. Five adult leaves were selected at random from the middle and outer quadrants of the crown for the determination of photosynthetic physiological indexes in the field, and after the measurement, they were quickly frozen in situ with liquid nitrogen and stored in the laboratory at −80 ℃ in an ultralow temperature refrigerator. Analysis of the dynamic changes in leaf photosynthetic rate measured in the field showed that the photosynthetic rates of the treatment leaves on 7 February and 17 February were significantly lower than those of the CK leaves. Therefore, scanning electron microscopy and transmission electron microscopy were performed on these two samples, and fluorescence quantitative PCR assays of genes encoding Rubisco major and minor subunits were performed.
2.4. Experimental Methods and Techniques
2.4.1. Determination of Leaf Physiological and Biochemical Parameters
The leaf photosynthetic rate (Pn), intercellular CO2 concentration (Ci) and stomatal conductance (Gs) were measured in the field using a Yaxin−1101 photosynthesizer according to the method described in the instrument manual. The malondialdehyde (MDA) content was determined by the thiobarbituric acid colorimetric method [21], the relative electrolyte conductance was determined by the method described by Deshmukh et al. [22], and the chlorophyll a, chlorophyll b, and carotenoid levels were determined by the modified Arnon method.
2.4.2. Observation of Leaf Anatomy and Chloroplast Ultrastructure
Segments of fresh mango leaves (2 mm × 2 mm) were sampled, taking care to avoid the leaf veins, and the samples were rinsed with phosphate buffered saline (PBS; G1102, Servicebio, Wuhan Seville Biotechnology Co., Wuhan, China), quickly placed into electron microscope fixative, fixed at room temperature for 2 h, and then transferred to 4 ℃ for storage. The fixed sample was rinsed 3 times with 0.1 M phosphoric acid buffer PB (pH 7.4) for 15 min each time. Then, the samples were transferred into 1% OsO4 in 0.1 M PB (pH 7.4) for 1−2 h at room temperature. After that, the samples were washed in 0.1 M PB (pH 7.4) 3 times for 15 min each. Subsequently, the samples were dehydrated in an increasing alcohol gradient (30%–50%–70%–80%–90%–95%–100%–100%) at room temperature for 15 min each time and finally soaked in isoamyl acetate for 15 min. After dehydration, the samples were dried with a critical pointment dryer (K850, Quorum, Lewes, UK). Then, the samples were attached to metallic stubs using carbon stickers, placed in an ion sputtering apparatus (MC1000, HITACHI, Hitachi High−Technologies Corp., Hong Kong, China) for 30 s and sputter−coated with gold. Finally, the samples were observed under a scanning electron microscope (SU8100, HITACHI, Hitachi High−Technologies Corp., China) and quantitatively analyzed using Image−Pro Plus 6.0 software (MediaCybernetics, Inc., Rockville, MD, USA).
Additional segments of fresh mango leaves (1 mm × 1 mm) were sampled, again taking care to avoid the leaf veins, and the samples were rinsed with PBS (G1102, Servicebio), quickly placed into electron microscope fixative, fixed at room temperature for 2 h, and then transferred to 4 ℃ for storage. The fixed samples were rinsed 3 times with 0.1 M phosphoric acid buffer PB (pH 7.4) for 15 min each time. Then, the samples were transferred into 1% OsO4 in 0.1 M PB (pH 7.4) at room temperature and were stored away from light for 7 h. After that, the samples were washed in 0.1 M PB (pH 7.4) 3 times for 15 min each. Subsequently, the samples were dehydrated in an increasing alcohol gradient (30%–50%–70%–80%–90%–95%–100%–100%) at room temperature for 1 h each time. After dehydration, the samples were resin−penetrated, embedded and polymerized. Then, the resin sample blocks were cut to 60−80 nm thin on an ultramicrotome (Leica UC7, Leica, Wetzlar, Germany), and the resin sample blocks were fished onto 150 mesh cuprum grids with formvar film to prepare copper mesh slices for staining (2% uranium acetate saturated alcohol solution without light staining for 8 min, rinsed in 70% ethanol 3 times and then rinsed in ultrapure water 3 times). Lead citrate (2.6%) was used to avoid CO2 staining for 8 min, and then the sections were rinsed with ultrapure water 3 times. The stained copper mesh sections were then washed using ultrapure water and placed in a copper mesh box to dry at room temperature overnight. Finally, a transmission electron microscope (HT7800, HITACHI, Hitachi High−Technologies Corp., China) was used for observation, and images were acquired for analysis.
2.4.3. Quantitative Real–Time PCR (qRT–PCR)
Total RNA was extracted from sampled mango leaves using a total RNA extraction kit (RNA Prep Pure Plant Kit, Tiangen, DP441, Beijing, China), and the remaining DNA was digested using DNase. The concentration of total RNA was then determined using a NanoDropLite spectrophotometer (Thermo Scientific, Waltham, MA, USA). cDNA was synthesized using a HiScript IIIRT SuperMix for qPCR (+gDNAwiper) kit (Vazyme, Nanjing, China). Then, the qRT–PCR solution was synthesized according to the instructions for SYBR Green Master Mix (SYBR premix EcTaqTMII, Takara, Japan). The expression levels of the Rubisco large subunit (rbcL) and small subunit (rbcS) were detected by a real−time PCR machine (qTOWER3G, Jena, Germany), and the relative gene expression was analyzed by the 2−ΔΔCt method. The internal reference gene was Actin, and the target genes and Actin primers are shown in Table 1.

Table 1.
Primers for gene expression analysis using real−time PCR.
2.5. Statistical Analysis
Statistical analysis of the experimental data was performed using SAS online software. The dynamic changes in each index were analyzed using analysis of variance (ANOVA). The t test procedure was used to test the significance of differences between the treatment and the control for each sample.
3. Results and Analysis
3.1. Effect of Enhanced UV-B Radiation on Leaves
The changes in the MDA content and the relative conductivity in the leaves are shown in Figure 1a,b. As seen from the graphs, the treatment caused cellular lipid peroxidation damage in the leaf membrane. The MDA content of the treated leaves was significantly higher than that of the CK leaves at all times, except for on 18 January, when it was not significantly different from that of the CK. The relative conductivity in leaves of both the treatment and the CK exhibited an increasing trend and was significantly higher in the treatment than in the CK.
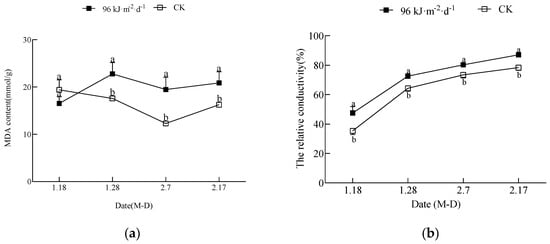
Figure 1.
The effect of enhanced UV-B radiation on MDA content and the relative conductivity in leaves: (a) content of MDA; (b) the relative conductivity. Note: the symbols with a in the figure indicate significant differences between the CK and 96 kJ·m−2·d−1 treatment for the same data type; otherwise, there was no significant difference. The same scheme applies to the figures below.
3.2. Effect of Enhanced UV-B Radiation on the Photosynthetic Properties of Leaves
The changes in leaf photosynthetic physiological indicators are shown in Figure 2, and they indicated that the treatment caused stomatal limitation and nonstomatal limitation of photosynthesis. The Pn of both the treatment and the CK decreased in general, and the CK Pn was significantly higher than that of the treatment at all times. The Gs of the treatment and the CK changed along similar single−peaked curves, and both reached the highest value on 7 February, but the Gs of the treatment was significantly lower than that of the control from the start of the experimental treatment. The Ci of both the treatment and the CK showed a decreasing trend, and the Ci of the treatment was significantly higher than that of the CK thereafter, except on 28 January, when the Ci of the treatment was significantly lower.
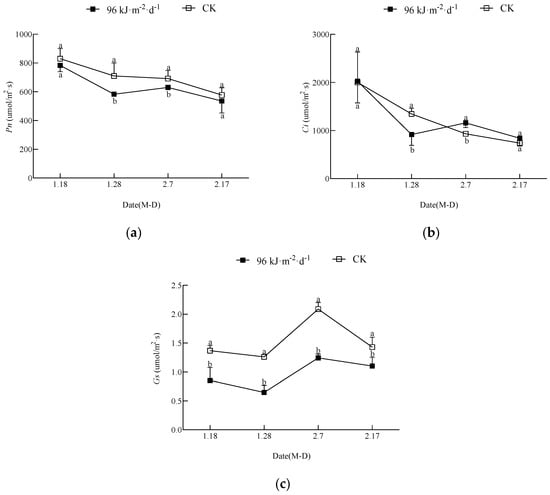
Figure 2.
The effect of enhanced UV-B radiation on the photosynthetic rate, intercellular carbon dioxide content ratio, and stomatal conductance in leaves: (a) Pn, the net photosynthetic rate; (b) Ci, the intercellular CO2 concentration; (c) Gs, the stomatal conductance. The same scheme applies to the figures below.
3.3. Enhanced UV-B Radiation Treatment Effect on the Photosynthetic Pigment Content of Leaves
As shown in Table 2, there were significant differences in the chlorophyll levels between the treatment and the CK. The chlorophyll a, chlorophyll b, and chlorophyll a + b levels of the treatment tended to increase generally and then decrease and then decrease again, and the levels in the treatment were significantly lower than those of the CK at all times. The ratio of chlorophyll a/b in both the treatment and the CK decreased, but that of the treatment was significantly lower than that of the CK during the later period. The carotenoid content of both the treatment and the CK first increased and then decreased, but that of the treatment was always significantly lower than that of the CK. This indicates that enhanced UV-B radiation damages leaves and results in a decrease in chlorophyll content, which hinders light harvesting and photophosphorylation processes. However, the increase in carotenoid content could reduce the damage produced by the stress.

Table 2.
The effect of enhanced UV-B radiation (T) on the chlorophyll content in leaves.
3.4. Effect of Enhanced UV-B Radiation on the Stomata and Epidermal Submicrostructure of Leaves
The results of the scanning electron microscopy observation are shown in Figure 3. The figure indicates that the treatment severely destroyed the leaf surface protective tissue, surface morphology and stomatal structure. The CK stomatal number was greater, the stomatal area was larger, the leaf surface was flatter and smoother, the mesophyll cells were clearer, the distribution was neater and denser, and a thin waxy layer was distributed on the surface (A1 and C1). The treatment stomata number was significantly reduced, the stomatal area was smaller, and the stomata were shrunken. Moreover, the guard cells lost water and contracted, and then the stomata sunken inward (B1 and D1). With the extension of irradiation time, the treatment leaf surface became rougher, the cell outline became unclear, and local fractures appeared; then, the leaf tissue cells were severely damaged.
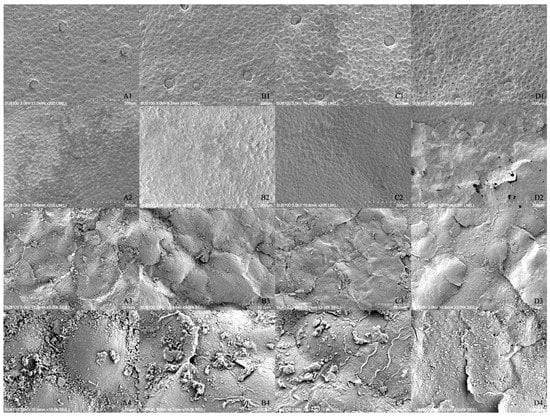
Figure 3.
The effect of enhanced UV-B radiation on stomatal and surface microstructures in leaves. Note: (A1–A4). 2.7 CK; (B1–B4). 2.7 enhanced UV-B radiation; (C1–C4). 2.17 CK; (D1–D4). 2.17 enhanced UV-B radiation. The same scheme applies to the figures below.
3.5. Effect of Enhanced UV-B Radiation on the Submicrostructure of Mesophyll Cells in Leaves
In Table 3 and Figure 4, the results reveal that the enhanced UV-B radiation obviously damaged the leaf mesophyll cells and inflicted severe damage on the photosynthetic tissue. In the treatment, both the treated palisade tissue and the spongy tissue tended to thicken and were significantly thicker than these tissues in the CK. As the treatment time increased, the CK palisade and sponge tissue remained normal, but in the treatment, the palisade and spongy tissue broke down, and the intercellular gap became larger. The palisade tissue was loosely structured and unevenly arranged. The spongy tissue crumpled, and the palisade/spongy tissue ratio in the treatment was significantly lower than that in the CK.

Table 3.
The effect of enhanced UV-B radiation on the microstructure of mesophyll cells in leaves.
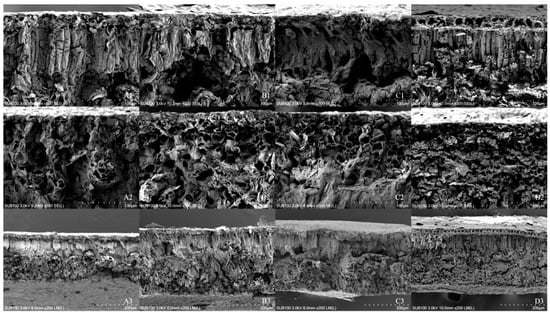
Figure 4.
The effect of enhanced UV-B radiation on the microstructure of mesophyll cells in leaves.Note: (A1–A3). 2.7 CK; (B1–B3). 2.7 enhanced UV-B radiation; (C1–C3). 2.17 CK; (D1–D3). 2.17 enhanced UV-B radiation. The same scheme applies to the figures below.
3.6. Effect of Enhanced UV-B Radiation on the Submicrostructure of Mesophyll Cells in Leaves
As shown in Figure 5, enhanced UV-B radiation severely damaged the protoplast and chloroplast submicrostructure. The chloroplasts of the CK were well structured, with a more regular oval shape and were arranged close to the cell wall, with a complete double membrane structure, and the mitochondria were distributed close to the chloroplasts. The chloroplast basal granule lamellae were closely stacked, with a clear structure and orderly arrangement, and a large number of starch granules and a small number of both plastid and osmiophilic granules were distributed throughout. Compared with the chloroplasts of the CK, those of the treatment were severely swollen and distributed in a subspherical shape, and some of the chloroplast membranes were locally disrupted, with large gaps between them and the cell walls. Mitochondria were difficult to identify, the number of starch and plastid granules was reduced, and there were almost no osmiophilic granules. The structure of the chloroplast basal granule lamellae was distorted, swollen and deformed, and some of the lamellae were blurred and disordered. Moreover, the lamellae appeared dilated.
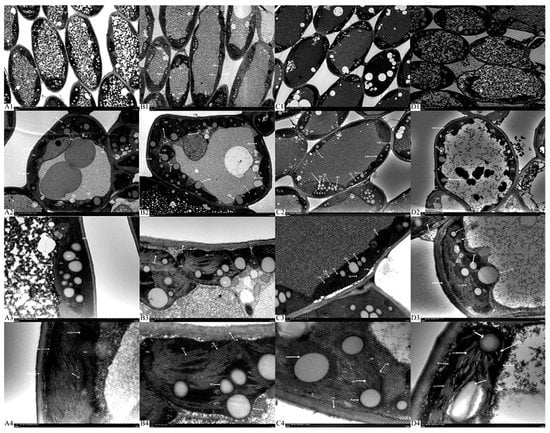
Figure 5.
The effect of enhanced UV-B radiation on the chloroplast ultrastructure of leaves. Note: Chl, chloroplast; GL, grana lamellae; OG, osmiophilic globules; SG, starch grains; P, plastid granules; CW, cell wall; CM, chloroplast membrane. (A1–A4). 2.7 CK; (B1–B4). 2.7 enhanced UV-B radiation; (C1–C4). 2.17 CK; (D1–D4). 2.17 enhanced UV-B radiation. The same scheme applies to the figures below.
3.7. Effect of Enhanced UV-B Radiation on the Gene Expression of Rubisco Major and Minor Subunits in Leaves
As shown in Figure 6, the treatment inhibited the leaf carbon dioxide fixation process by inhibiting rbcL expression. However, the inhibition of the carbon dioxide fixation process was alleviated by the promotion of rbcS expression. The expression level of rbcL in leaves increased significantly during the initial stage of the experiment and then decreased significantly. The expression level of rbcS first decreased and then increased; however, the expression level of rbcS in the treatment was significantly higher than that in the CK on 7 February.
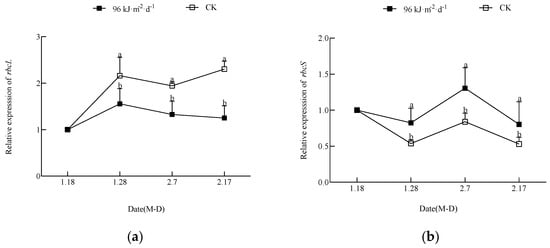
Figure 6.
Effect of enhanced UV-B radiation on the gene expression of key enzymes in the photosynthesis of leaves.Note: (a), relative expression of rbcL; (b), relative expression of rbcS.
4. Discussion
4.1. The Submicrostructure of the Choloplast is Damaged and the Expression of the rbcL Gene Is Inhibited by High−Dose Enhanced UV-B Radiation, Leading to Nonstomatal Limitation of Photosynthesis in Mango Leaves
Enhanced UV-B radiation induced lipid peroxidation and the permeability of the cell membrane increased in mango leaf membranes. As a result, it damages chloroplast microstructure and then reduces chlorophyll content. The damage became increasingly serious with the extension of the enhanced UV-B radiation treatment time. This is consistent with the findings of studies on soybean (Glycine max Merr.) [23], rice (Oryza sativa L.) [24], cotton (Gossypium hirsutum L.) [25], Mentha × piperita (Mentha canadensis Briq.) [26], and the ‘Maqiesu’ mango cultivar (Mangifera indica L.) [27]. The results of the present study showed that the chlorophyll a/b ratios decreased and that the chloroplast thylakoid structure collapsed, which was consistent with the results of the chlorophyll a/b ratio decreases previously reported by our research [28]. Usually, a decrease in the chlorophyll a/b value reflects the abnormal stacking structure of the thylakoid in the chloroplast, which is also confirmed by the results of this paper. These injuries and the inhibition of rbcL gene expression result in the formation of nonstomatal restriction in leaves, which inhibits photosynthesis [29,30,31,32,33,34,35].
4.2. Stomatal Morphology is Destroyed and Density Is Reduced by High−Dose Enhanced UV-B Radiation, Leading to Stomatal Limitation of Photosynthesis in Mango Leaves
Stomata are important channels for gas exchange and transpiration between plant leaves and the outside world, and their distribution and structure determine the execution of leaf functions. Studies have shown that enhanced UV-B radiation can decrease the leaf transpiration rate, decrease stomatal conductance, increase stomatal resistance, and subsequently affect CO2 assimilation efficiency, leading to weakened photosynthesis and decreased productivity of crops, causing stomatal restriction and inhibiting leaf photosynthesis [36,37,38,39,40]. The results of the present study confirm that enhanced UV-B radiation inhibits leaf photosynthesis by destroying stomatal morphology and reducing density, leading to a decrease in both stomatal conductance and CO2 conductance.
4.3. Passive Defense Mechanism Induced by High−Dose Enhanced UV-B Radiation in Mango Leaves
Plant leaves are important sensory organs that have strong plasticity and that can respond directly and rapidly to changes in the external environment. Changes in the microstructure of leaf cells clearly and visibly reflect the adaptation of plants to environmental changes [41]. Numerous studies have shown that leaves are usually large and thin when not under stress, and the size of the leaf area directly determines the strength of photosynthesis. A large leaf area facilitates contact with the external environment, receiving light energy and promoting photosynthesis. Thin leaves can shorten the distance between the mesophyll cells and the leaf surface, which is conducive to gas exchange and light energy absorption and can also promote photosynthesis [42]. However, under enhanced UV-B radiation stress, leaves tended to thicken. Feng et al. [43] speculated that this was an adaptation mechanism formed by plants under stress. Shi et al. showed that thickening of plant leaves could reduce the damage caused by excessive UV-B radiation absorption by the leaves. At the same time, the photosynthetic pigment content and net photosynthetic rate based on leaf area were not affected by UV-B radiation−induced photodegradation in leaf surface cells [44]. The results are consistent with those of previous studies. Enhanced UV-B radiation treatment thickens mango leaves, which in turn improves tolerance to UV-B radiation and mitigates the damage of enhanced UV-B radiation on leaf pulp cell structure, thus reducing the nonstomatal limitation of photosynthesis.
In this study, the carotenoid content increased with the extension of treatment time. This may be a protective mechanism of dispersing stress by absorbing enhanced UV-B radiation to alleviate the damage caused by radiation. Enhanced UV-B radiation−induced upregulation of rbcS may be another passive defense mechanism to suppress decreased photosynthesis as much as possible after the downregulation of rbcL expression.
5. Conclusions
Treatment of the ‘Tainong No 1’ mango cultivar in the field with artificially simulated enhanced UV-B radiation at 96 kJ·m−2·d−1 caused leaf membrane lipid peroxidation damage, disrupted leaf photosynthetic tissues and chloroplast submicroscopic structures, downregulated the expression of rbcL, and resulted in leaf nonstomatal limitation. It caused reduced stomatal conductance and disrupted leaf stomatal submicrostructure and leaf surface morphology, resulting in leaf stomatal limitation. Therefore, it can be concluded that enhanced UV-B radiation diminishes leaf photosynthesis by causing both stomatal and nonstomatal limitations. Moreover, artificially simulated 96 kJ·m−2·d−1 treatment induced a passive defense mechanism in leaves by promoting leaf carotenoid accumulation and upregulating the expression of rbcS, thereby minimizing the damage to leaf membrane lipid reactive oxygen species and the inhibition of leaf photosynthesis. This article reveals the physiological mechanism of high−dose enhanced UV-B radiation inhibition of leaf photosynthesis in ‘Tainong No 1’ mango leaves and provides a basis for further investigation of the signal transduction process induced by enhanced UV-B radiation and the molecular biology of the altered photosynthesis in leaves.
Author Contributions
Writing—original draft, T.C.; Writing—review and editing, T.C., J.P. and K.Z.; Data curation, X.S. and J.D.; Writing—original draft preparation, F.L. and M.Q. All authors have read and agreed to the published version of the manuscript.
Funding
National Natural Science Foundation of China (NSFC) (No. 31460498).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wu, J.B.; Guan, D.X.; Yuan, F.H.; Zhang, X.J. Research advances on the biological effects of elevated ultraviolet-B radiation on terrestrial plants. J. For. Res. 2009, 20, 383–390. [Google Scholar] [CrossRef]
- Madronich, S.; McKenzie, R.L.; Caldwell, M.; Bjorn, L.O. Changes in ultraviolet radiation reaching the Earth’s surface. Ambio 1995, 24, 143–152. [Google Scholar]
- Markham, K.R.; Tanner, G.J.; Caasi-Lit, M.; Whitecross, M.I.; Nayudu, M.; Mitchell, K.A. Possible protective role for 3′,4′-dihydroxyflavones induced by enhanced UV-B in a UV-tolerant rice cultivar. Phytochemistry 1998, 49, 1913–1919. [Google Scholar] [CrossRef]
- Wright, L.A.; Murphy, T.M. Short-Wave Ultraviolet Light Closes Leaf Stomata. Am. J. Bot. 1982, 69, 1196. [Google Scholar] [CrossRef]
- Wu, X.C.; Lin, W.X.; Guo, Y.C.; Ke, Y.Q.; Liang, Y.Y.; Chen, Y.F. Effect of enhancing ultraviolet-b radiation on antioxidant systems in rice seedling leaves. Fujian J. Agric. Sci. 2001, 16, 51–55. [Google Scholar]
- Bais, A.F.; Bernhard, G.; McKenzie, R.L.; Aucamp, P.J.; Young, P.J.; Ilyas, M.; Deushi, M. Ozone–climate interactions and effects on solar ultraviolet radiation. Photochem. Photobiol. Sci. 2019, 18, 602–640. [Google Scholar] [CrossRef]
- Wang, X.P.; Li, X.; Li, Z.R.; Ling, C.T.; He, Y.M.; Li, Y. Research progress on the characteristics and mechanisms of transgenerational plasticity of plants in response to enhanced UV-B radiation. Plant Physiol. J. 2022, 58, 797–805. (In Chinese) [Google Scholar]
- Taalas, P.; Kaurola, J.; Kylling, A.; Shindell, D.; Sausen, R.; Dameris, M.; Steil, B. The impact of greenhouse gases and halogenated species on future solar UV radiation doses. Geophys. Res. Lett. 2000, 27, 1127–1130. [Google Scholar] [CrossRef]
- De Bakker, N.V.J.; Van Bodegom, P.M.; Van De Poll, W.H.; Boelen, P.; Nat, E.; Rozema, J.; Aerts, R. Is UV-B radiation affecting charophycean algae in shallow freshwater systems? New Phytol. 2005, 166, 957–966. [Google Scholar] [CrossRef]
- Correia, C.M.; Torres-Pereira, M.S.; Torres-Pereira, J. Growth, photosynthesis and UV-B absorbing compounds of Portuguese Barbela wheat exposed to ultraviolet-B radiation. Environ. Pollut. Ser. A 1999, 104, 383–388. [Google Scholar] [CrossRef]
- Flores-Moya, A.; Hanelt, D.; Figueroa, F.L.; Altamirano, M.; Viñegla, B.; Salles, S. Involvement of solar UV-B radiation in recovery of inhibited photosynthesis in the brown alga Dictyota dichotoma (Hudson) Lamouroux. J. Photochem. Photobiol. B 1999, 49, 129–135. [Google Scholar] [CrossRef]
- Hu, Z.; Li, H.; Chen, S.; Yang, Y. Chlorophyll content and photosystem II efficiency in soybean exposed to supplemental ultraviolet-B radiation. Photosynthetica 2013, 51, 151–157. [Google Scholar] [CrossRef]
- Heinze, M.; Hanschen, F.S.; Wiesner-Reinhold, M.; Baldermann, S.; Gräfe, J.; Schreiner, M.; Neugart, S. Effects of Developmental Stages and Reduced UVB and Low UV Conditions on Plant Secondary Metabolite Profiles in Pak Choi (Brassica rapa subsp. chinensis). J. Agric. Food Chem. 2018, 66, 1678–1692. [Google Scholar] [CrossRef]
- Caldwell, M.M.; Teramura, A.H.; Tevini, M. The changing solar ultraviolet climate and the ecological consequences for higher plants. Trends Ecol. Evol. 1989, 4, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.W.; Zhang, X.S.; Liu, L.K.; Guo, Y.; Fan, Y.L.; Yang, X.Y.; Li, Y.X. Comparing intraspecific responses of 12 winter wheat cultivars to different doses of ultraviolet-B radiation. J. Photochem. Photobiol. B 2013, 119, 1–8. [Google Scholar] [CrossRef]
- Liu, Y.; Zhong, Z.C. Differential response of leaf gas exchange to enhanced ultraviolet-B (UV-B) radiation in three species of herbaceous climbing plants. Acta Ecol. Sin. 2009, 29, 124–129. [Google Scholar] [CrossRef]
- Kakani, V.G.; Reddy, K.R.; Zhao, D.; Sailaja, K. Field crop responses to ultraviolet-B radiation: A review. Conf. Agric. For. Meteorol. 2003, 120, 191–218. [Google Scholar] [CrossRef]
- Liu, Q.; Yao, X.Q.; Zhao, C.Z.; Cheng, X.Y. Effects of enhanced UV-B radiation on growth and photosynthetic responses of four species of seedling in subalpine forests of the eastern Tibet plateau. Environ. Exp. Bot. 2011, 74, 151–156. [Google Scholar] [CrossRef]
- Holzinger, A.; Karsten, U.; Lbtz, C.; Wiencke, C. Ultrastructure and photosynthesis in the supralittoral green macroalga Prasiola crispa from Sp itsbergen (Norway) under UV exposure. Phycologia 2006, 45, 168–172. [Google Scholar] [CrossRef]
- Robert, L.H.; Lester, P. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar]
- Deshmukh, P.S.; Sairam, R.K.; Shukla, D.S. Measurement of ionleakage as a screening technique for drought resistance in wheat genotypes. Indian J. Plant Physiol. 1991, 34, 89–91. [Google Scholar]
- Wang, H.; Guo, Y.J.; Zhu, J.J.; Yue, K.; Zhou, K.B. Characteristics of Mango Leaf Photosynthetic Inhibition by Enhanced UV-B Radiation. Horticulturae 2021, 7, 557. [Google Scholar] [CrossRef]
- Peng, Q.; Zhou, Q. Influence of lanthanum on chloroplast ultrastructure of soybean leaves under ultraviolet-B stress. J. Rare Earths. 2009, 27, 304–307. [Google Scholar] [CrossRef]
- Wu, X.C.; Lin, W.X.; Huang, Z.L. Influence of enhanced ultraviolet-B radiation on photosynthetic physiologies andultrastructure of leaves in two different resistivity rice cultivars. J. Plant Ecol. 2007, 27, 554–564. (In Chinese) [Google Scholar]
- Wang, J.; Zhang, J.; Yang, J.X.; Wang, S.M.; Tian, L.P. Effects of Enhanced UV-B Radiation on the Leaves Microstructure of Cotton. Xinjiang Agric. Sci. 2010, 47, 1619–1626. (In Chinese) [Google Scholar]
- Wu, N.B.; Ma, H.Q.; Hu, L.T.; Hong, H.; Sun, J.C.; Zhang, Y.H.; Dai, D.L. Effect of enhanced UV-B radiation on photosynthetic structure and photosynthetic characteristics of Mentha piperita. China J. Chin. Mater. Med. 2009, 34, 2995–2998. [Google Scholar] [PubMed]
- Yang, Y.J.; Guo, S.P.; Yang, S.L.; Zhang, Y.H.; Liu, H.G.; Meng, F.X.; Duan, Y.J.; Yang, Z.X.; Yang, X.Q.; Yuan, J.M.; et al. Effects of enhanced UV-B radiation on photosynthetic physiology and ultrastructure of leaves in mango (Mangifera indica L.). J. Fruit Sci. 2021, 38, 1524–1539. (In Chinese) [Google Scholar]
- Liu, P.; Zhou, K.B.; Ding, S.; Cai, H.Z. Antioxidative Response of Adult Mango(Mangifera indica L.) Leaves to Enhanced UV-radiation. J. Mount. Agric. Biotech. 2010, 29, 397–402. (In Chinese) [Google Scholar]
- He, J.M.; She, X.P.; Liu, C.; Zhao, W.M. Stomatal and nonstomatal limitations of photosynthesis in mung bean leaves under the combination of enhanced UV-B radiation and NaCl stress. J. Plant Physiol. Mol. Biol. 2004, 30, 53–58. [Google Scholar]
- Pandey, N.; Pandey-Rai, S. Modulations of physiological responses and possible involvement of defense-related secondary metabolites in acclimation of Artemisia annua L. against short-term UV-B radiation. Planta 2014, 240, 611–627. [Google Scholar] [CrossRef]
- Jordan, B.R.; Chow, W.S.; Strid, Å.; Anderson, J.M. Reduction incabandpsbA RNA transcripts in response to supplementary ultraviolet-B radiation. FEBS Lett. 1991, 284, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Mishra, V.; Srivastava, G.; Prasad, S.M.; Abraham, G. Growth, photosynthetic pigments and photosynthetic activity during seedling stage of cowpea (Vigna unguiculata) in response to UV-B and dimethoate. Pestic. Biochem. Physiol. 2008, 92, 30–37. [Google Scholar] [CrossRef]
- Grassi, G.; Magnani, F. Stomatal, mesophyll conductance and biochemical limitations to photosynthesis as affected by drought and leaf ontogeny in ash and oak trees. Plant Cell Environ. 2005, 28, 834–849. [Google Scholar] [CrossRef]
- Keiller, D.R.; Holmes, M.G. Effects of long-term exposure to elevated UV-B radiation on the photosynthetic performance of five broad-leaved tree species. Photosynth. Res. 2001, 67, 229–240. [Google Scholar] [CrossRef]
- Surabhi, G.; Reddy, K.R.; Singh, S.K. Photosynthesis, fluorescence, shoot biomass and seed weight responses of three cowpea (Vigna unguiculata (L.) Walp.) cultivars with contrasting sensitivity to UV-B radiation. Environ. Exp. Bot. 2009, 66, 160–171. [Google Scholar] [CrossRef]
- Bornman, J.F.; Teramura, A.H. Effects of Ultraviolet-B Radiation on Terrestrial Plants. In Environmental UV Photobiology; Plenum Press: New York, NY, USA, 1993; pp. 427–471. [Google Scholar]
- Musil, C.F. Differential effects of elevated ultraviolet-B radiation on the photochemical and reproductive performances of dicotyledonous and monocotyledonous arid-environment ephemerals. Plant Cell Environ. 1995, 18, 844–854. [Google Scholar] [CrossRef]
- Moody, S.A.; Coop, D.J.; Paul, N.D. Plants and UV-B: Effects of elevated UV-B radiation and elevated CO2 on heathland communities. Soc. Exp. Biol. Semin. Ser. 1997, 64, 283–304. [Google Scholar]
- Nogués, S.; Allen, D.J.; Morison, J.I.L.; Baker, N.R. Characterization of Stomatal Closure Caused by Ultraviolet-B Radiation. Plant Physiol. 1999, 121, 489–496. [Google Scholar] [CrossRef]
- Teramura, A.H.; Sullivan, J.H. Effects of UV-B radiation on photosynthesis and growth of terrestrial plants. Photosynth. Res. 1994, 39, 463–473. [Google Scholar] [CrossRef]
- Li, F.L.; Bao, W.K. Responses of the Morphological and Anatomical Structure of the Plant Leaf to Environmental Change. Chih Wu Hsueh T’ung Pao. 2005, 22, 18–127. (In Chinese) [Google Scholar]
- Kakani, V.G.; Reddy, K.R.; Zhao, D.; Mohammed, A.R. Effects of ultraviolet-B radiation on cotton (Gossypium hirsutum L.) morphology and anatomy. Ann.Bot. 2003, 91, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.Y.; An, L.Z.; Tan, L.L.; Hou, Z.D.; Wang, X.L. Effect of enhanced ultraviolet-B radiation on pollen germination and tube growth of 19 taxa in vitro. Environ. Exp. Bot. 2000, 43, 45–53. [Google Scholar] [CrossRef]
- Kofidis, G.; Bosabalidis, A.M.; Moustakas, M. Contemporary seasonal and altitudinal variations of leaf structural features in oregano (Origanum vulgare L.). Ann. Bot. 2003, 92, 635–645. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).