Seed Priming with Exogenous Amino Acids Improves Germination Rates and Enhances Photosynthetic Pigments of Onion Seedlings (Allium cepa L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Treatments
2.2. Data Collection and Calculation
2.3. Statistical Analyses
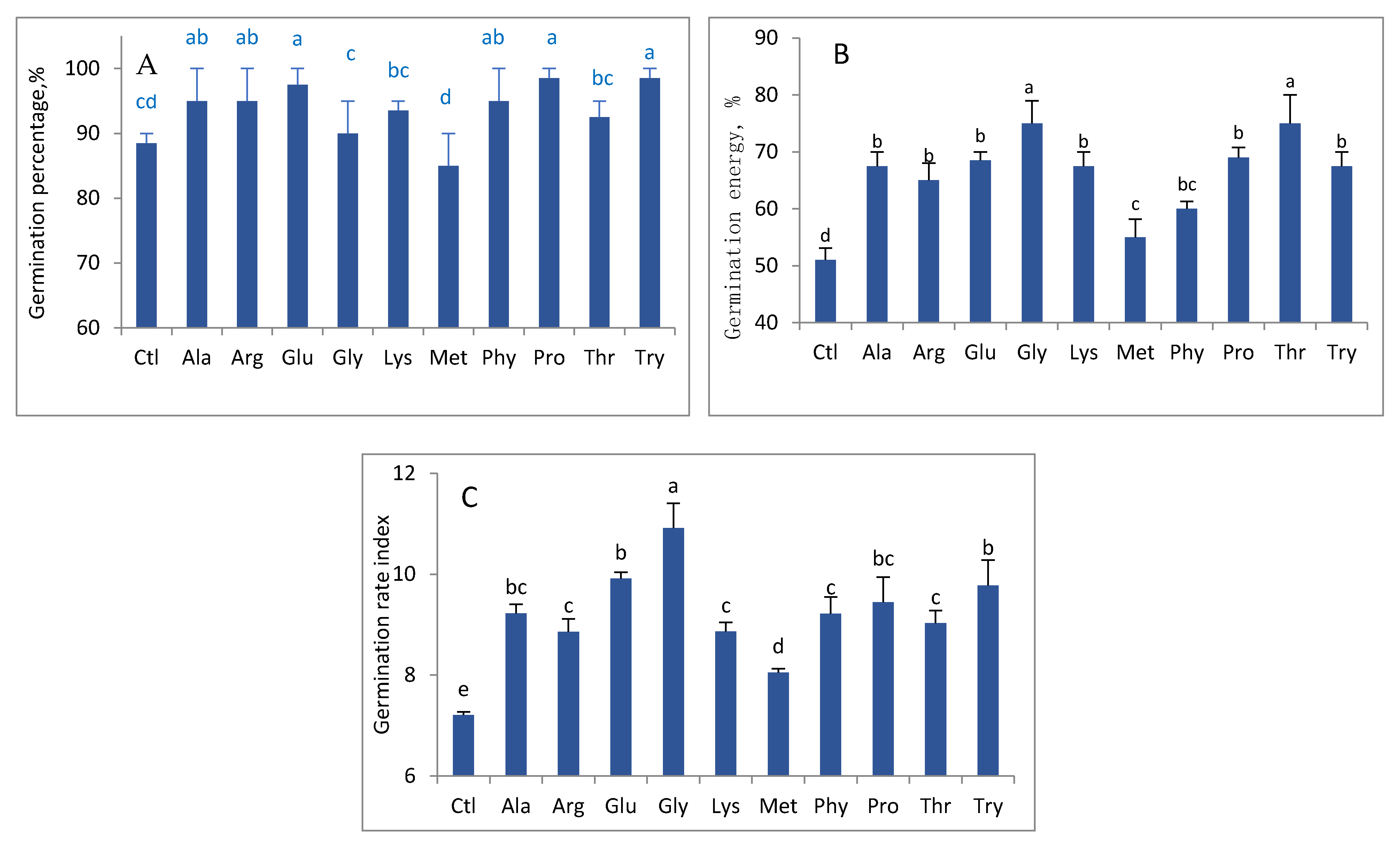
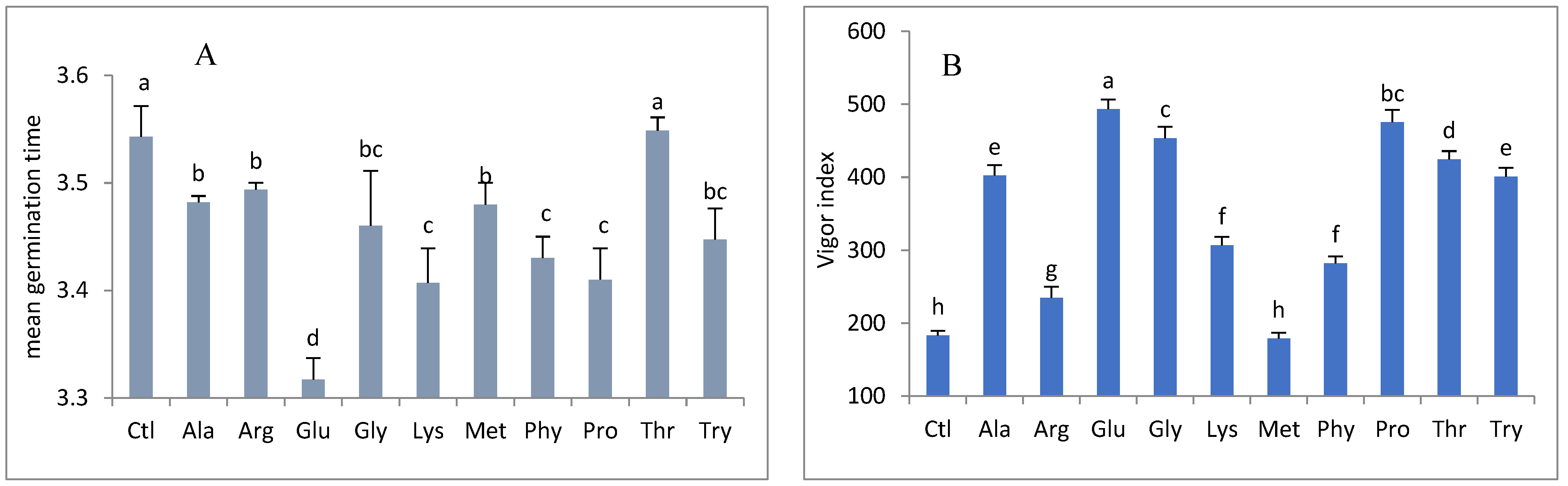
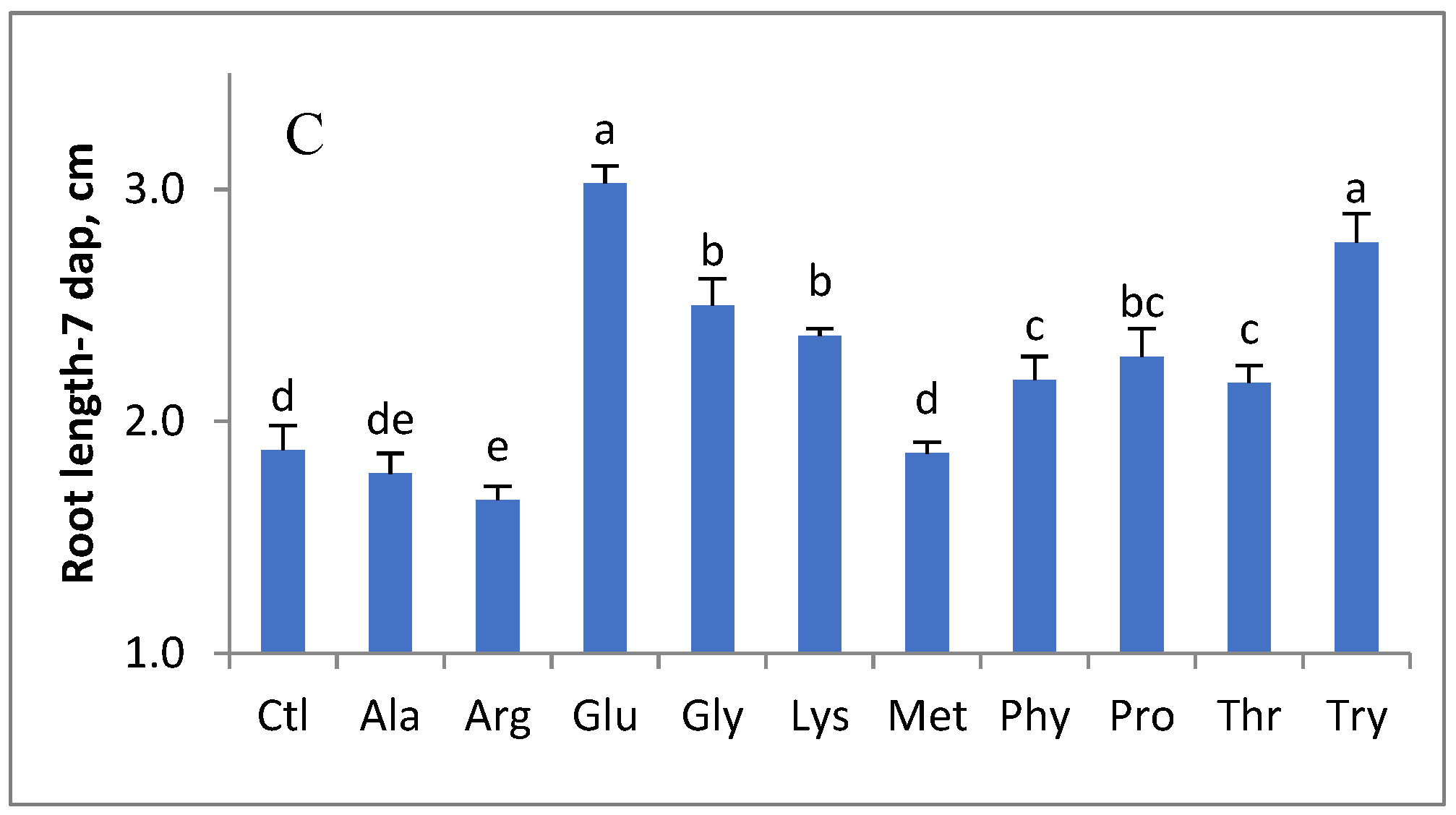
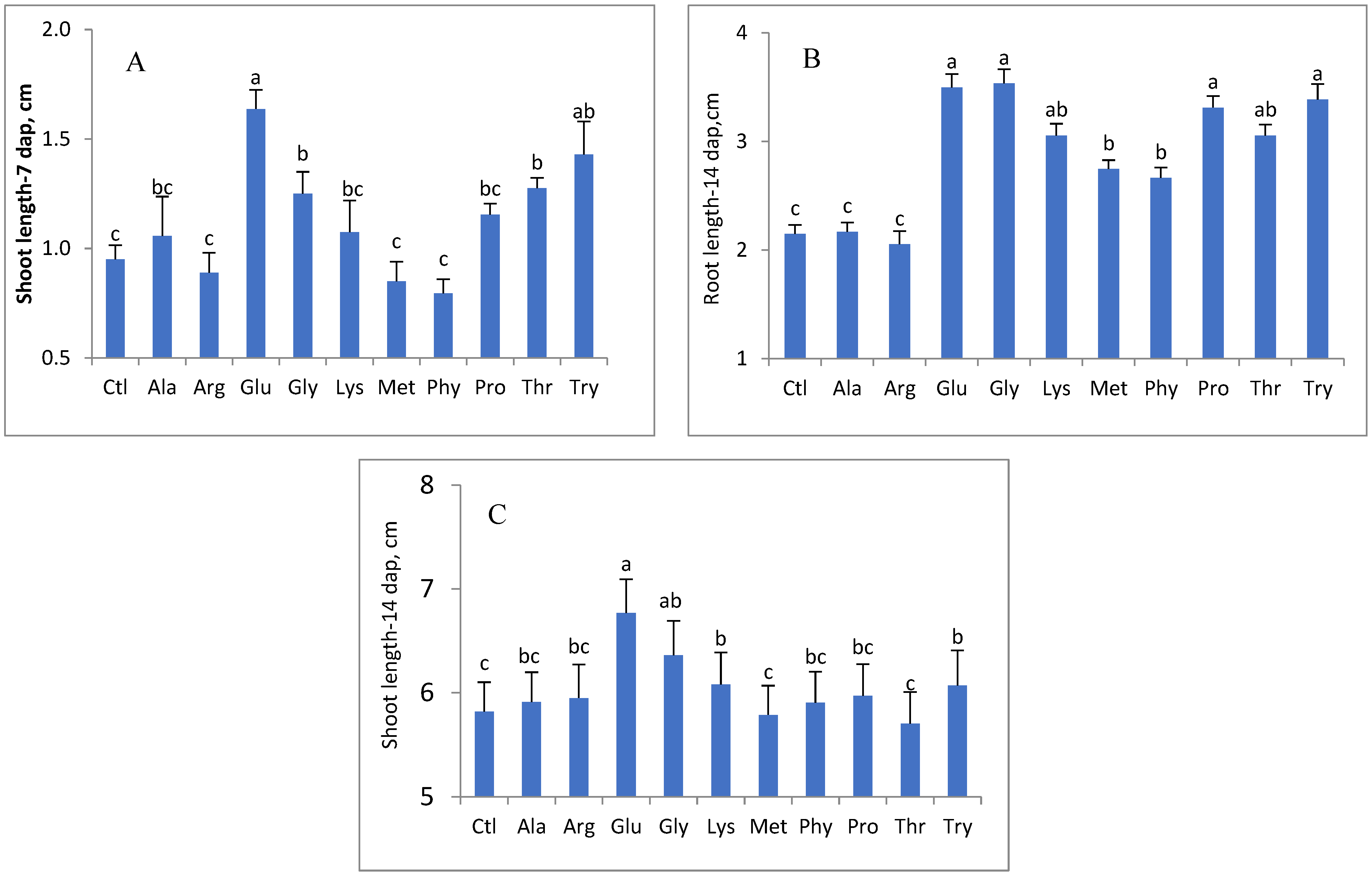
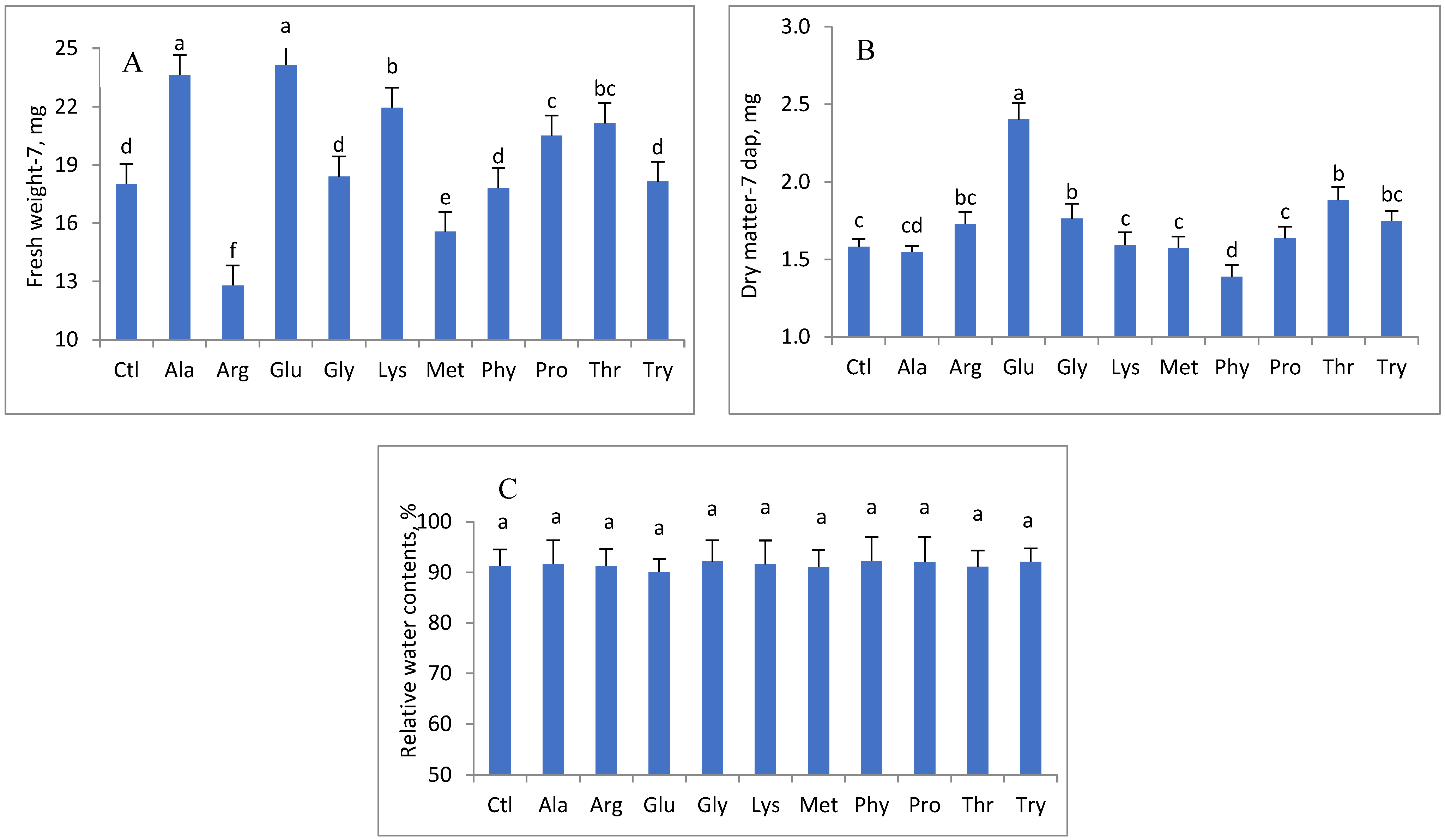
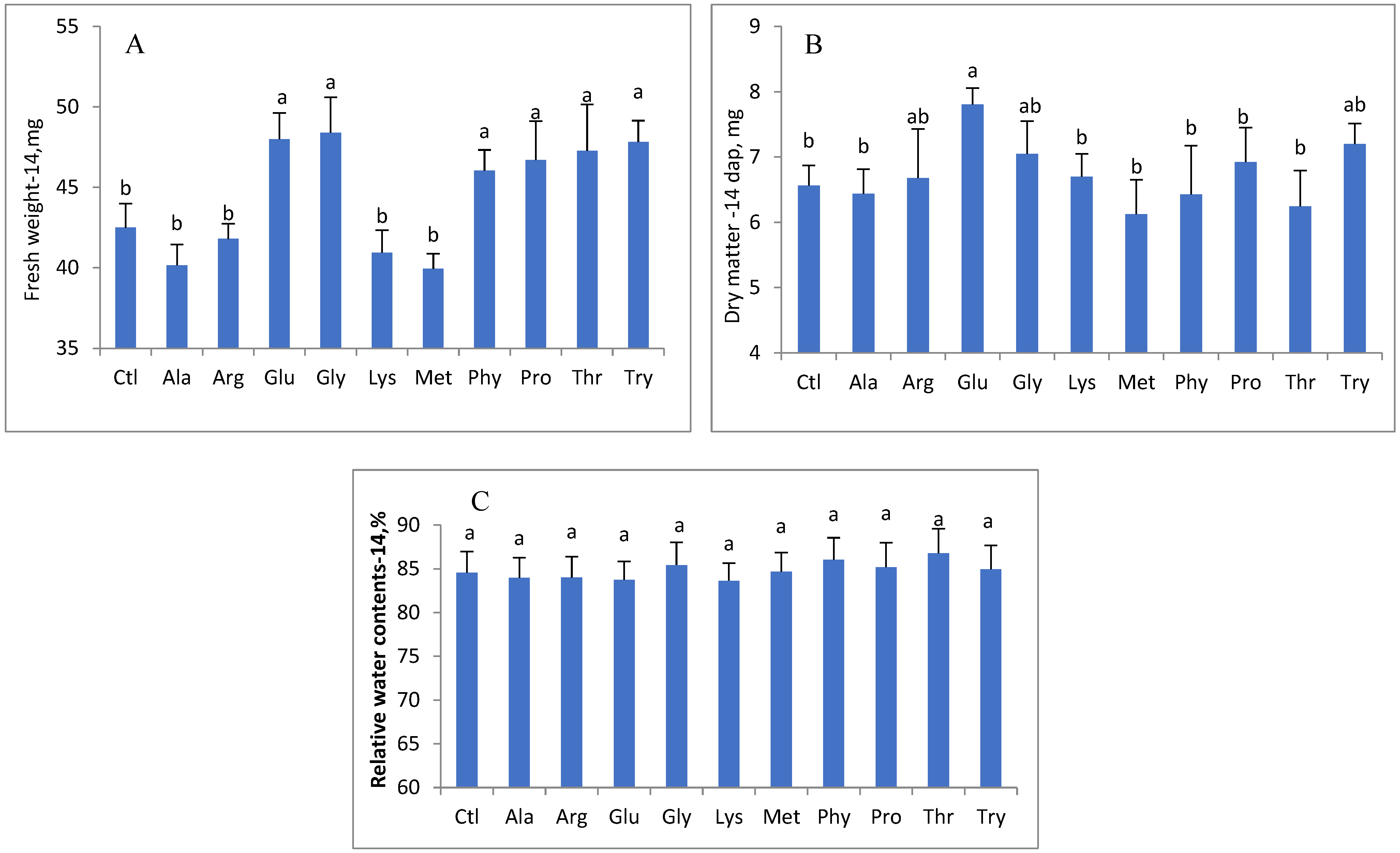
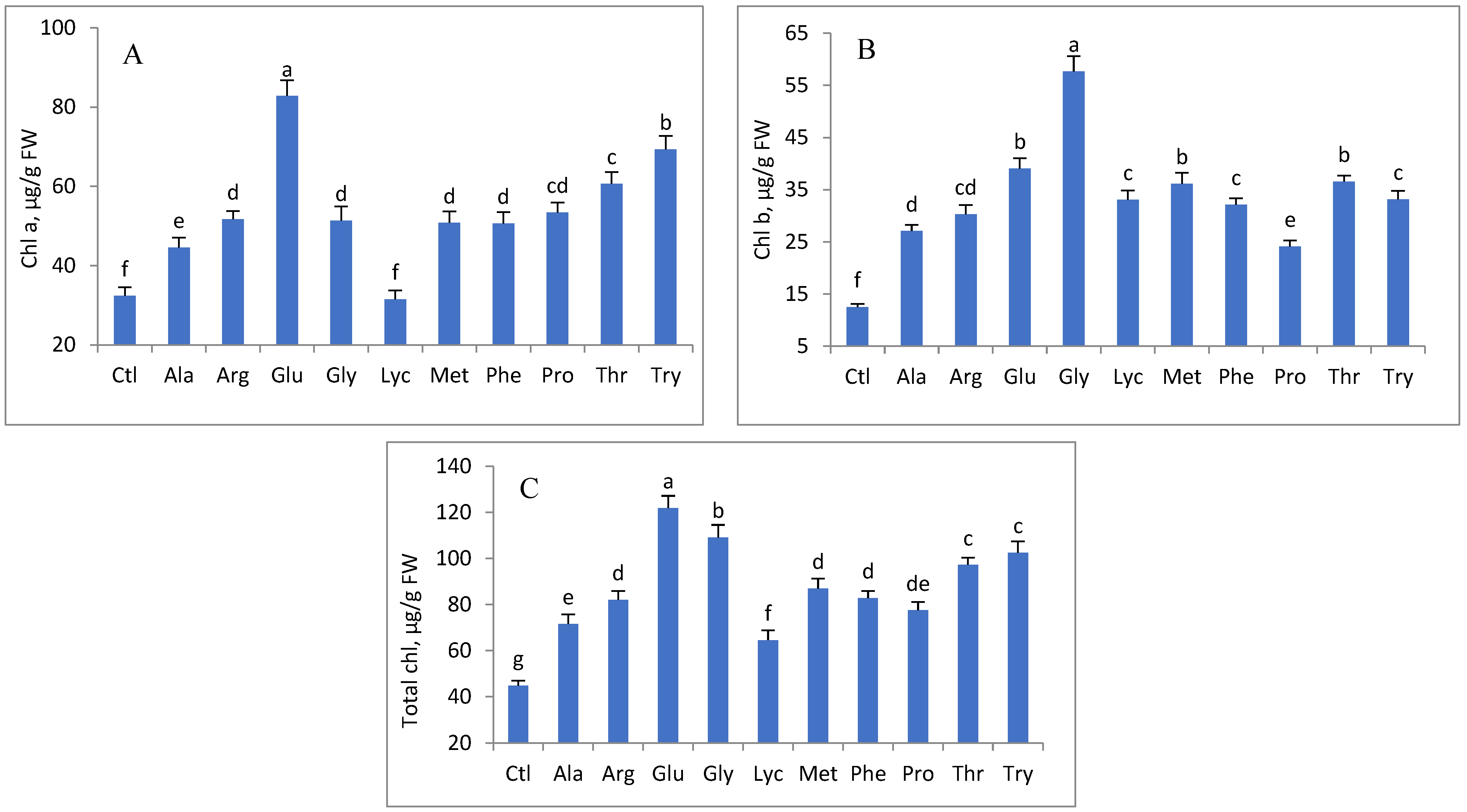
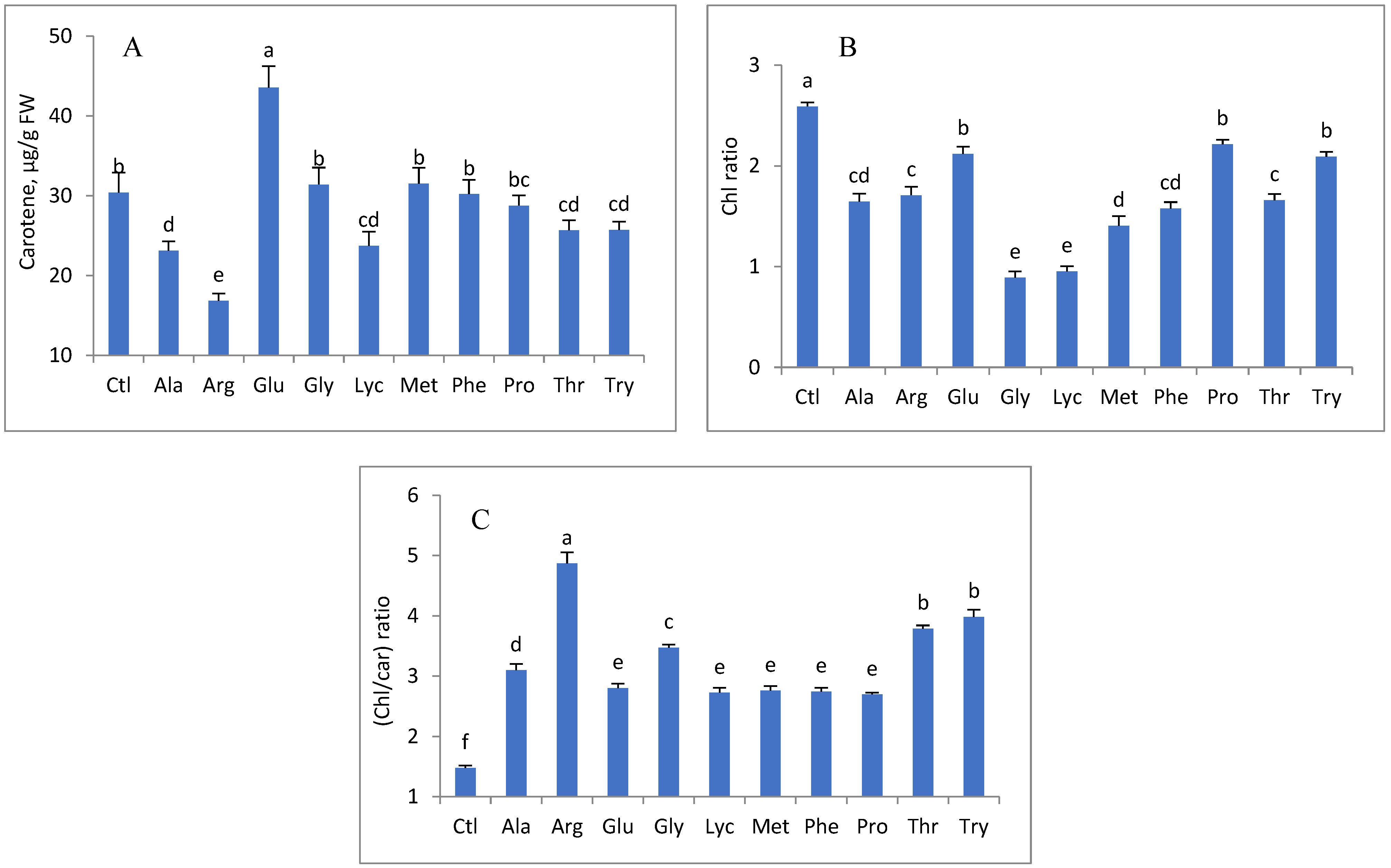
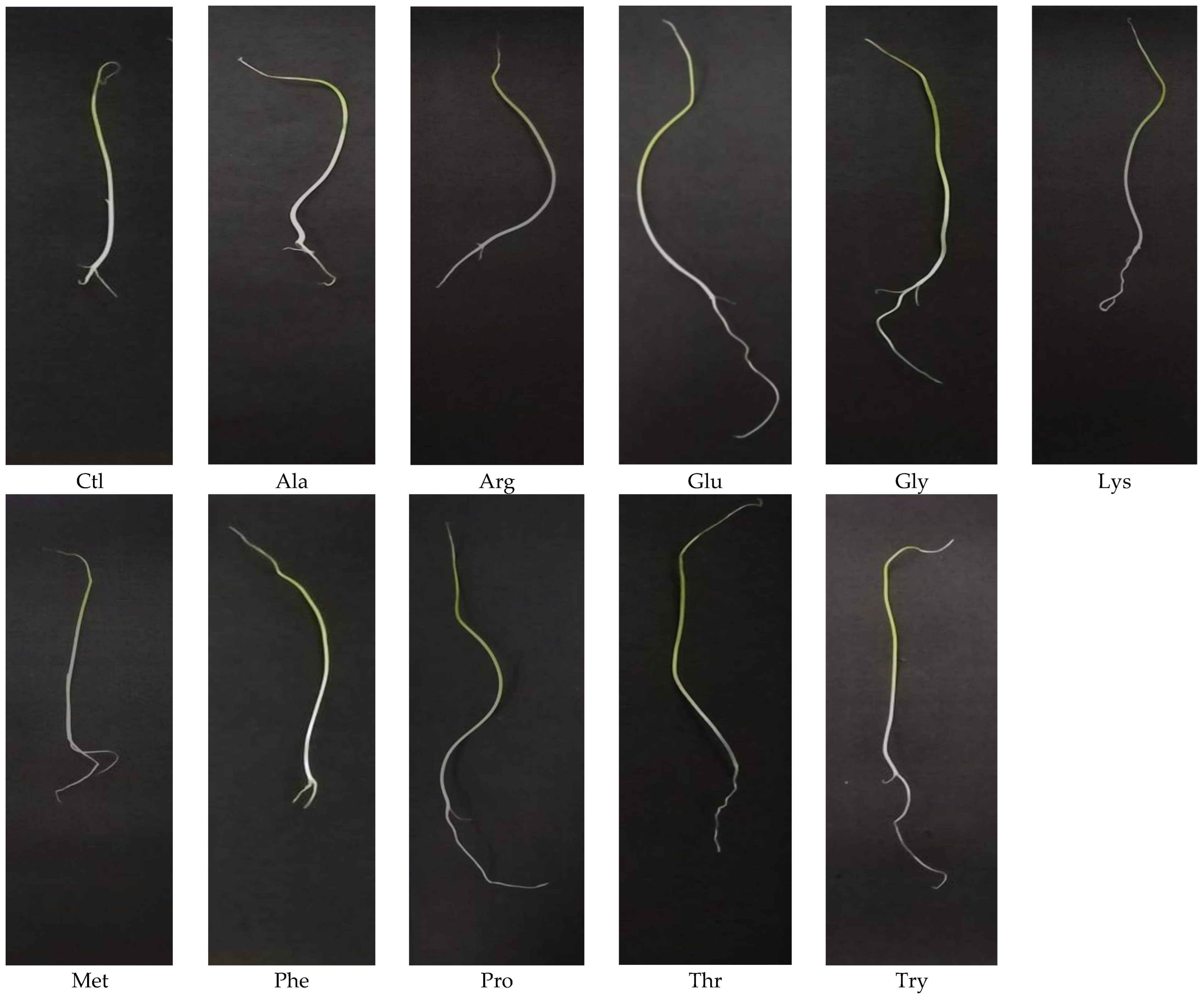
3. Results
Principal Component Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Abdelkader, M.M.; Gaplaev, M.S.; Terekbaev, A.A.; Puchkov, M.Y. The Influence of Biostimulants on Tomato Plants Cultivated under Hydroponic Systems. J. Hortic. Res. 2021, 29, 107–116. [Google Scholar] [CrossRef]
- Righini, H.; Roberti, R.; Cetrullo, S.; Flamigni, F.; Quintana, A.M.; Francioso, O.; Panichi, V.; Cianchetta, S.; Galletti, S. Jania adhaerens Primes Tomato Seed against Soil-Borne Pathogens. Horticulturae 2022, 8, 746. [Google Scholar] [CrossRef]
- Jardin, P.D. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- El-Nakhel, C.; Cozzolino, E.; Ottaiano, L.; Petropoulos, S.A.; Nocerino, S.; Pelosi, M.E.; Rouphael, Y.; Mori, M.; Di Mola, I. Effect of Biostimulant Application on Plant Growth, Chlorophylls and Hydrophilic Antioxidant Activity of Spinach (Spinacia oleracea L.) Grown under Saline Stress. Horticulturae 2022, 8, 971. [Google Scholar] [CrossRef]
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in Plant Science: A Global Perspective. Front. Plant Sci. 2017, 7, 2049. [Google Scholar] [CrossRef] [PubMed]
- Alcazar, R.; Altabella, T.; Marco, F.; Bortolotti, C.; Reymond, M.; Koncz, C.; Carrasco, P.; Tiburcio, A.F. Polyamines: Molecules with regulatory functions in plant abiotic stress tolerance. Planta 2010, 231, 1237–1249. [Google Scholar] [CrossRef]
- Lönnerdal, B. Dietary Factors Influencing Zinc Absorption. J. Nutr. 2000, 130, 1378S–1383S. [Google Scholar] [CrossRef]
- Atilio, J.B.; Causin, H.F. The central role of amino acids on nitrogen utilization and plant growth. J. Plant Physiol. 1996, 149, 358–362. [Google Scholar] [CrossRef]
- Amin, A.; Gharib, F.A.; El-Awadi, M.; Rashad, E.-S.M. Physiological response of onion plants to foliar application of putrescine and glutamine. Sci. Hortic. 2011, 129, 353–360. [Google Scholar] [CrossRef]
- Meister, A. Selective Modification of Glutathione Metabolism. Science 1983, 220, 472–477. [Google Scholar] [CrossRef]
- Das, C.; Sengupta, T.; Chattopadhyay, S.; Setua, M.; Das, N.K.; Saratchandra, B. Involvement of kinetin and spermidine in controlling salinity stress in mulberry (Morus alba L. cv. S1). Acta Physiol. Plant 2002, 24, 53–57. [Google Scholar] [CrossRef]
- Zulfiqar, F. Effect of seed priming on horticultural crops. Sci. Hortic. 2021, 286, 110197. [Google Scholar] [CrossRef]
- Karim, M.N. Stimulatory effect of seed priming as pretreatment factors on germination and yield performance of yard long bean (Vigna unguiculata). Horticulturae 2020, 6, 104. [Google Scholar] [CrossRef]
- Sorrentino, M.; De Diego, N.; Ugena, L.; Spíchal, L.; Lucini, L.; Miras-Moreno, B.; Zhang, L.; Rouphael, Y.; Colla, G.; Panzarová, K. Seed Priming With Protein Hydrolysates Improves Arabidopsis Growth and Stress Tolerance to Abiotic Stresses. Front. Plant Sci. 2021, 12, 626301. [Google Scholar] [CrossRef] [PubMed]
- Al-Amri, S.M. Improved growth, productivity and quality of tomato (Solanum lycopersicum L.) plants through application of shikimic acid. Saudi J. Biol. Sci. 2013, 20, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Yan, M. Seed priming stimulate germination and early seedling growth of Chinese cabbage under drought stress. S. Afr. J. Bot. 2015, 99, 88–92. [Google Scholar] [CrossRef]
- Piri, R.; Moradi, A.; Balouchi, H.; Salehi, A. Improvement of cumin (Cuminum cyminum) seed performance under drought stress by seed coating and biopriming. Sci. Hortic. 2019, 257, 108667. [Google Scholar] [CrossRef]
- Ashraf, R.; Sultana, B.; Riaz, S.; Mushtaq, M.; Iqbal, M.; Nazir, A.; Atif, M.; Zafar, Z. Fortification of phenolics, antioxidant activities and biochemical attributes of radish root by plant leaf extract seed priming. Biocatal. Agric. Biotechnol. 2018, 16, 115–120. [Google Scholar] [CrossRef]
- Nessim, A.; Kasim, W. Physiological Impact of Seed Priming with CaCl2 or Carrot Root Extract on Lupinus termis Plants Fully Grown under Salinity Stress. Egypt. J. Bot. 2019, 59, 763–777. [Google Scholar] [CrossRef]
- Valivand, M.; Amooaghaie, R.; Ahadi, A. Seed priming with H2S and Ca2+ trigger signal memory that induces cross-adaptation against nickel stress in zucchini seedlings. Plant Physiol. Biochem. 2019, 143, 286–298. [Google Scholar] [CrossRef]
- Paul, S.; Dey, S.; Kundu, R. Seed priming: An emerging tool towards sustainable agriculture. Plant Growth Regul. 2021, 97, 215–234. [Google Scholar] [CrossRef]
- Abdelkader, M.; Zargar, M.; Murtazova, K.M.-S.; Nakhaev, M.R. Life Cycle Assessment of the Cultivation Processes for the Main Vegetable Crops in Southern Egypt. Agronomy 2022, 12, 1527. [Google Scholar] [CrossRef]
- Jisha, K.C.; Vijayakumari, K.; Puthur, J.T. Seed priming for abiotic stress tolerance: An overview. Acta Physiol. Plant 2012, 35, 1381–1396. [Google Scholar] [CrossRef]
- Paparella, S.; Araujo, S.S.; Rossi, G.; Wijayasinghe, M.; Carbonera, D.; Balestrazzi, A. Seed priming: State of the art and new perspectives. Plant Cell Rep. 2015, 34, 1281–1293. [Google Scholar] [CrossRef] [PubMed]
- Bryksová, M.; Hybenová, A.; Hernándiz, A.E.; Novák, O.; Pěnčík, A.; Spíchal, L.; De Diego, N.; Doležal, K. Hormopriming to Mitigate Abiotic Stress Effects: A Case Study of N9-Substituted Cytokinin Derivatives With a Fluorinated Carbohydrate Moiety. Front. Plant Sci. 2020, 11, 599228. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Reche, J.; Vallejo-Marín, M.; Quilliam, R.S. Quantifying the potential of ‘on-farm’seed priming to increase crop performance in developing countries. A meta-analysis. Agron. Sustain. Dev. 2018, 38, 64. [Google Scholar] [CrossRef]
- Gallardo, K.; Job, C.; Groot, S.P.; Puype, M.; Demol, H.; Vandekerckhove, J.; Job, D. Proteomic Analysis of Arabidopsis Seed Germination and Priming. Plant Physiol. 2001, 126, 835–848. [Google Scholar] [CrossRef]
- Weitbrecht, K.; Müller, K.; Leubner-Metzger, G. First off the mark: Early seed germination. J. Exp. Bot. 2011, 62, 3289–3309. [Google Scholar] [CrossRef]
- Steinbrecher, T.; Leubner-Metzger, G. The biomechanics of seed germination. J. Exp. Bot. 2016, 68, 765–783. [Google Scholar] [CrossRef]
- Chen, K.; Arora, R. Priming memory invokes seed stress-tolerance. Environ. Exp. Bot. 2013, 94, 33–45. [Google Scholar] [CrossRef]
- Teixeira, W.F.; Fagan, E.B.; Soares, L.H.; Umburanas, R.C.; Reichardt, K.; Neto, D.D. Foliar and Seed Application of Amino Acids Affects the Antioxidant Metabolism of the Soybean Crop. Front. Plant Sci. 2017, 8, 327. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Yu, H.; Li, Q.; Gao, Y.; Sallam, B.N.; Wang, H.; Liu, P.; Jiang, W. Exogenous Application of Amino Acids Improves the Growth and Yield of Lettuce by Enhancing Photosynthetic Assimilation and Nutrient Availability. Agronomy 2019, 9, 266. [Google Scholar] [CrossRef]
- Selvi, D.T.; Saraswathy, S. Seed viability, seed deterioration and seed quality improvements in stored onion seeds: A review. J. Hortic. Sci. Biotechnol. 2017, 93, 1–7. [Google Scholar] [CrossRef]
- El-Damarany, A.M.; El-Shaikh, K.A.A.; Obiadalla-Ali, H.A.; Abdel-Kader, M.M. Effect of Mother Bulb Size and Planting Space on Seed Production of Onion (Allium cepa, L.) Cultivar Giza 6 Mohassan. J. Agril. Vet. Sci. 2015, 8, 187–200. [Google Scholar] [CrossRef]
- Khokhar, K. Part 1 Chapter 2 Onion: Seed Viability and Germination. In Onion—An Ancient Crop and Modern Practices; Noor Publishing: Pakistan, 2019. [Google Scholar]
- El-Damarany, A.M.; El-Shaikh, K.A.A.; Obiadalla-Ali, H.A.; Abdel-Kader, M.M. Effect of Nitrogen and Potassium Fertilization on Seed Production of Onion (Allium cepa L.) Improved Giza 6 Cultivar. Am.-Euras. J. Agric. Environ. Sci. 2016, 16, 1296–1303. [Google Scholar]
- Tan, J.W.; Kester, S.T.; Su, K.; Hildebrand, D.F.; Geneve, R.L. Seed Priming and Pericarp Removal Improve Germination in Low-Germinating Seed Lots of Industrial Hemp. Crops 2022, 2, 407–414. [Google Scholar] [CrossRef]
- Malik, C.P. Seed deterioration: A review. Int. J. Life Sci. Biotechnol. Pharma Res. 2013, 2, 374–385. [Google Scholar]
- Khan, W.; Rayirath, U.P.; Subramanian, S.; Jithesh, M.N.; Rayorath, P.; Hodges, D.M.; Critchley, A.T.; Craigie, J.S.; Norrie, J.; Prithiviraj, B. Seaweed Extracts as Biostimulants of Plant Growth and Development. J. Plant Growth Regul. 2009, 28, 386–399. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y.; Canaguier, R.; Svecova, E.; Cardarelli, M. Biostimulant action of a plant-derived protein hydrolysate produced through enzymatic hydrolysis. Front. Plant Sci. 2014, 5, 448. [Google Scholar] [CrossRef]
- Abdel-Mawgoud, A.M.R.; El-Bassiouny, A.M.; Ghoname, A.; Abou-Hussein, S.D. Foliar application of amino acids and micronutrients enhance performance of green bean crop under newly reclaimed land conditions. Aust. J. Basic Appl. Sci. 2011, 5, 51–55. [Google Scholar]
- Koukounaras, A.; Tsouvaltzis, P.; Siomos, A.S. Effect of root and foliar application of amino acids on the growth and yield of greenhouse tomato in different fertilization levels. J. Food Agric. Environ. 2013, 11, 644–648. [Google Scholar]
- Bujalski, W.; Nienow, A. Large-scale osmotic priming of onion seeds: A comparison of different strategies for oxygenation. Sci. Hortic. 1991, 46, 13–24. [Google Scholar] [CrossRef]
- Shooshtari, F.Z.; Souri, M.K.; Hasandokht, M.R.; Jari, S.K. Glycine mitigates fertilizer requirements of agricultural crops: Case study with cucumber as a high fertilizer demanding crop. Chem. Biol. Technol. Agric. 2020, 7, 19. [Google Scholar] [CrossRef]
- Ambreen, S.; Athar, H.-U.; Khan, A.; Zafar, Z.U.; Ayyaz, A.; Kalaji, H.M. Seed priming with proline improved photosystem II efficiency and growth of wheat (Triticum aestivum L.). BMC Plant Biol. 2021, 21, 502. [Google Scholar] [CrossRef]
- Alfosea-Simón, M.; Zavala-Gonzalez, E.A.; Camara-Zapata, J.M.; Martínez-Nicolás, J.J.; Simón, I.; Simón-Grao, S.; García-Sánchez, F. Effect of foliar application of amino acids on the salinity tolerance of tomato plants cultivated under hydroponic system. Sci. Hortic. 2020, 272, 109509. [Google Scholar] [CrossRef]
- Noroozlo, Y.A.; Souri, M.K.; Delshad, M. Stimulation Effects of Foliar Applied Glycine and Glutamine Amino Acids on Lettuce Growth. Open Agric. 2019, 4, 164–172. [Google Scholar] [CrossRef]
- Abdelkader, M.; Geioushy, R.A.; Fouad, O.A.; Khaled, A.G.; Voronina, L. Investigation the activities of photosynthetic pigments, antioxidant enzymes and inducing genotoxicity of cucumber seedling exposed to copper oxides nanoparticles stress. Sci. Hortic. 2022, 305, 111364. [Google Scholar] [CrossRef]
- Kader, M.A. A comparison of seed germination calculation formulae and the associated interpretation of resulting data. J. Proc. R. Soc. New South Wales 2005, 138, 65–75. [Google Scholar]
- Herrera, R.M.H.; Santacruz-Ruvalcaba, F.; Ruiz-López, M.A.; Norrie, J.; Hernández-Carmona, G. Effect of liquid seaweed extracts on growth of tomato seedlings (Solanum lycopersicum L.). J. Appl. Phycol. 2013, 26, 619–628. [Google Scholar] [CrossRef]
- Wang, J.; Lu, W.; Tong, Y.; Yang, Q. Leaf Morphology, Photosynthetic Performance, Chlorophyll Fluorescence, Stomatal Development of Lettuce (Lactuca sativa L.) Exposed to Different Ratios of Red Light to Blue Light. Front. Plant Sci. 2016, 7, 250. [Google Scholar] [CrossRef]
- Wellburn, A.R.; Lichtenthaler, H. Formulae and Program to Determine Total Carotenoids and Chlorophylls A and B of Leaf Extracts in Different Solvents. In Advances in Photosynthesis Research; Springer: Berlin/Heidelberg, Germany, 1984; pp. 9–12. [Google Scholar]
- Trineeva, O.V.; Slivkin, A.I. Comparative characteristics of pigment composition from raw materials and oil extract of nettle leaves. Drug Dev. Regist. 2016, 1, 142–148. [Google Scholar]
- Abdelkader, M.M.; Elsayed, H.M.A. Biodiversity of Photosynthetic Pigments, Macronutrients Uptake and Fruit Quality of Tomato Genotypes. Russ. J. Plant Physiol. 2022, 69, 50. [Google Scholar] [CrossRef]
- Kan, C.-C.; Chung, T.-Y.; Juo, Y.-A.; Hsieh, M.-H. Glutamine rapidly induces the expression of key transcription factor genes involved in nitrogen and stress responses in rice roots. BMC Genom. 2015, 16, 731. [Google Scholar] [CrossRef]
- Wu, L.; Huo, W.; Yao, D.; Li, M. Effects of solid matrix priming (SMP) and salt stress on broccoli and cauliflower seed germination and early seedling growth. Sci. Hortic. 2019, 255, 161–168. [Google Scholar] [CrossRef]
- Thakur, M.; Tiwari, S.; Kataria, S.; Anand, A. Recent advances in seed priming strategies for enhancing planting value of vegetable seeds. Sci. Hortic. 2022, 305, 111355. [Google Scholar] [CrossRef]
- Gardarin, A.; Coste, F.; Wagner, M.-H.; Dürr, C. How do seed and seedling traits influence germination and emergence parameters in crop species? A comparative analysis. Seed Sci. Res. 2016, 26, 317–331. [Google Scholar] [CrossRef]
- Wojtyla, Ł.; Lechowska, K.; Kubala, S.; Garnczarska, M. Molecular processes induced in primed seeds—increasing the potential to stabilize crop yields under drought conditions. J. Plant Physiol. 2016, 203, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Sarojnee, D.Y.; Navindra, B.; Chandrabose, S. Effect of naturally occurring amino acid stimulants on the growth and yield of hot peppers. J. Anim. Plant Sci. 2009, 5, 414–424. [Google Scholar]
- Neeraja, G.; Reddy, I.P. Effect of Growth Promoters on Growth and Yield of Tomato cv. Marutham. J. Res. ANGRAU 2005, 33, 68–70. [Google Scholar]
- Fernández, V.; Eichert, T. Uptake of Hydrophilic Solutes Through Plant Leaves: Current State of Knowledge and Perspectives of Foliar Fertilization. Crit. Rev. Plant Sci. 2009, 28, 36–68. [Google Scholar]
- Gunes, A.; Post, W.N.K.; Kirkby, E.A.; Aktas, M. Influence of partial replacement of nitrate by amino acid nitrogen or urea in the nutrient medium on nitrate accumulation in NFT grown winter lettuce. J. Plant Nutr. 1994, 17, 1929–1938. [Google Scholar] [CrossRef]
- Gonzalez, C.; Zheng, Y.; Lovatt, C. Properly timed foliar fertilization can and should result in a yield benefit and net increase in grower income. Acta Hortic. 2010, 273–286. [Google Scholar] [CrossRef]
- Kamar, M.E.; Omar, A. Effect of nitrogen levels and spraying with aminal-forte (amino acids salvation) on yield of cucumber and potatoes. J. Agric. Sci. Mansoura Univ. 1987, 12, 900–907. [Google Scholar]
- El-Shabasi, M.S.; Mohamed, S.M.; Mahfouz, S.A. Effect of foliar spray with amino acids on growth, yield and chemical composition of garlic plants. In Proceedings of the Sixth Arabian Conference for Horticulture, Ismailia, Egypt, 20–22 March 2005. [Google Scholar]
- Shekari, G.; Javanmardi, J. Effects of foliar application pure amino acid and amino acid containing fertilizer on broccoli (Brassica oleracea L. var. italica) transplant. Adv. Crop Sci. Technol. 2017, 5, 280. [Google Scholar] [CrossRef]
- Basha, D.; El-Aila, H.I. Response of foliar spraying with amino acids and integrated use of nitrogen fertilizer on radish (Raphanus sativus L.) plant. Int. J. ChemTech Res. 2015, 8, 135–140. [Google Scholar]
- Kauffman, G.L.; Kneivel, D.P.; Watschke, T.L. Effects of a biostimulant on the heat tolerance associated with photosynthetic capacity, membrane thermostability, and polyphenol production of perennial ryegrass. Crop Sci. 2007, 47, 261–267. [Google Scholar] [CrossRef]
- Kolomazník, K.; Pecha, J.; Friebrová, V.; Janáčová, D.; Vašek, V. Diffusion of biostimulators into plant tissues. Heat Mass Transf. 2012, 48, 1505–1512. [Google Scholar] [CrossRef]
- Li, Y.; He, N.; Hou, J.; Xu, L.; Liu, C.; Zhang, J.; Wang, Q.; Zhang, X.; Wu, X. Factors Influencing Leaf Chlorophyll Content in Natural Forests at the Biome Scale. Front. Ecol. Evol. 2018, 6, 64. [Google Scholar] [CrossRef]
- Garcia, A.L.; Madrid, R.; Gimeno, V.; Rodriguez-Ortega, W.M.; Nicolas, N.; Garcia-Sanchez, F. The effects of amino acids fertilization incorporated to the nutrient solution on mineral composition and growth in tomato seedlings. Span. J. Agric. Res. 2011, 9, 852. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhou, J.; Li, R.; Wang, H.; Wang, J. Effect of exogenous amino acids on Cu uptake and translocation in maize seedlings. Plant Soil 2007, 292, 105–117. [Google Scholar] [CrossRef]
- Souri, M.K.; Hatamian, M. Aminochelates in plant nutrition: A review. J. Plant Nutr. 2019, 42, 67–78. [Google Scholar] [CrossRef]
- Brian, G.; Lea, P.J. Glutamate in plants: Metabolism, regulation, and signaling. J. Exp. Bot. 2007, 58, 2339–2358. [Google Scholar]
- Semida, W.M.; Abdelkhalik, A.; Rady, M.O.; Marey, R.A.; El-Mageed, T.A.A. Exogenously applied proline enhances growth and productivity of drought stressed onion by improving photosynthetic efficiency, water use efficiency and up-regulating osmoprotectants. Sci. Hortic. 2020, 272, 109580. [Google Scholar] [CrossRef]
- Wahid, A.; Jamil, A. Inducing salt tolerance in canola (Brassica napus L.) by exogenous application of glycinebetaine and proline: Response at the initial growth stages. Pak. J. Bot. 2009, 41, 1311–1319. [Google Scholar]
- Chiang, H.-H.; Dandekar, A.M. Regulation of proline accumulation in Arabidopsis thaliana (L.) Heynh during development and in response to desiccation. Plant Cell Environ. 1995, 18, 1280–1290. [Google Scholar] [CrossRef]
- Szabados, L.; Savouré, A. Proline: A multifunctional amino acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Becker, D.F. Connecting proline metabolism and signaling pathways in plant senescence. Front. Plant Sci. 2015, 6, 552. [Google Scholar] [CrossRef]
- Altuntaş, C.; Demiralay, M.; Muslu, A.S.; Terzi, R. Proline-stimulated signaling primarily targets the chlorophyll degradation pathway and photosynthesis associated processes to cope with short-term water deficit in maize. Photosynth. Res. 2020, 144, 35–48. [Google Scholar] [CrossRef]
- Verslues, P.E.; Sharma, S. Proline Metabolism and Its Implications for Plant-Environment Interaction. Arab. Book/Am. Soc. Plant Biol. 2010, 8, e0140. [Google Scholar] [CrossRef]
- Signorelli, S.; Dans, P.D.; Coitiño, E.L.; Borsani, O.; Monza, J. Connecting Proline and γ-Aminobutyric Acid in Stressed Plants through Non-Enzymatic Reactions. PLoS ONE 2015, 10, e0115349. [Google Scholar] [CrossRef]
- Korkmaz, A.; Gerekli, A.; Yakupoğlu, G.; Karaca, A.; Köklü, Ş. Seed treatment with tryptophan improves germination and emergence of pepper under salinity stress. In Proceedings of the XXX International Horticultural Congress IHC2018: II International Symposium on Soilless Culture and VIII International 1273, Istanbul, Turkey, 12–16 August 2018; pp. 441–448. [Google Scholar]
- Abbas, S.H.; Muhammad, S.; Saleem, M.; Mahmood, T.; Aziz, I.; Qamar, M.; Majeed, A.; Arif, M. Effect of L-tryptophan on plant weight and pod weight in chickpea under rainfed conditions. Sci. Tech. Dev. 2013, 32, 277–280. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelkader, M.; Voronina, L.; Puchkov, M.; Shcherbakova, N.; Pakina, E.; Zargar, M.; Lyashko, M. Seed Priming with Exogenous Amino Acids Improves Germination Rates and Enhances Photosynthetic Pigments of Onion Seedlings (Allium cepa L.). Horticulturae 2023, 9, 80. https://doi.org/10.3390/horticulturae9010080
Abdelkader M, Voronina L, Puchkov M, Shcherbakova N, Pakina E, Zargar M, Lyashko M. Seed Priming with Exogenous Amino Acids Improves Germination Rates and Enhances Photosynthetic Pigments of Onion Seedlings (Allium cepa L.). Horticulturae. 2023; 9(1):80. https://doi.org/10.3390/horticulturae9010080
Chicago/Turabian StyleAbdelkader, Mostafa, Luidmila Voronina, Mikhail Puchkov, Natalya Shcherbakova, Elena Pakina, Meisam Zargar, and Marina Lyashko. 2023. "Seed Priming with Exogenous Amino Acids Improves Germination Rates and Enhances Photosynthetic Pigments of Onion Seedlings (Allium cepa L.)" Horticulturae 9, no. 1: 80. https://doi.org/10.3390/horticulturae9010080
APA StyleAbdelkader, M., Voronina, L., Puchkov, M., Shcherbakova, N., Pakina, E., Zargar, M., & Lyashko, M. (2023). Seed Priming with Exogenous Amino Acids Improves Germination Rates and Enhances Photosynthetic Pigments of Onion Seedlings (Allium cepa L.). Horticulturae, 9(1), 80. https://doi.org/10.3390/horticulturae9010080







