Modification of Light Characteristics Affect the Phytochemical Profile of Peppers
Abstract
1. Introduction
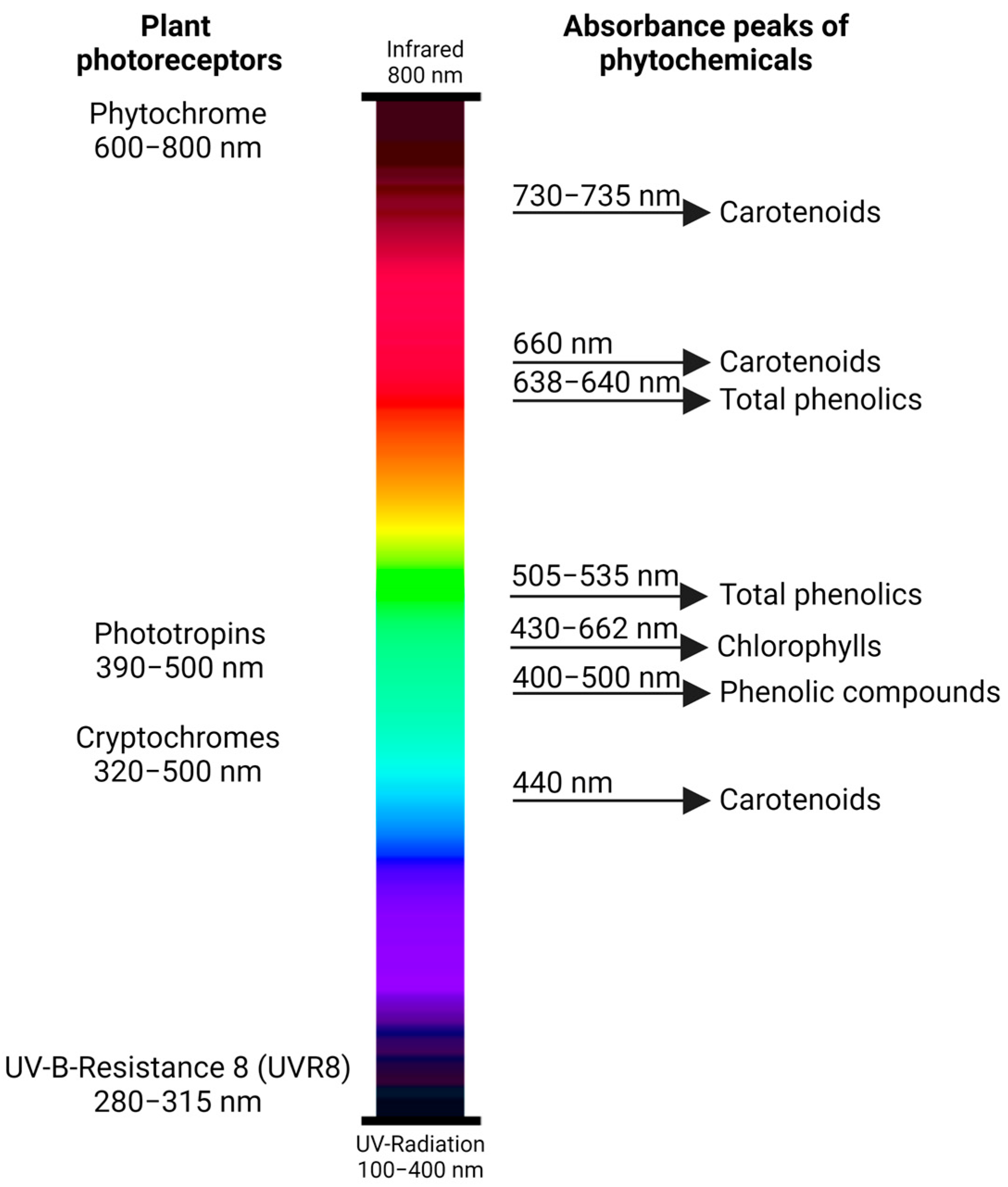
2. Light Interactions with Capsicum Plants
3. Effects of Light Characteristics on the Phytochemicals of Capsicum Fruits
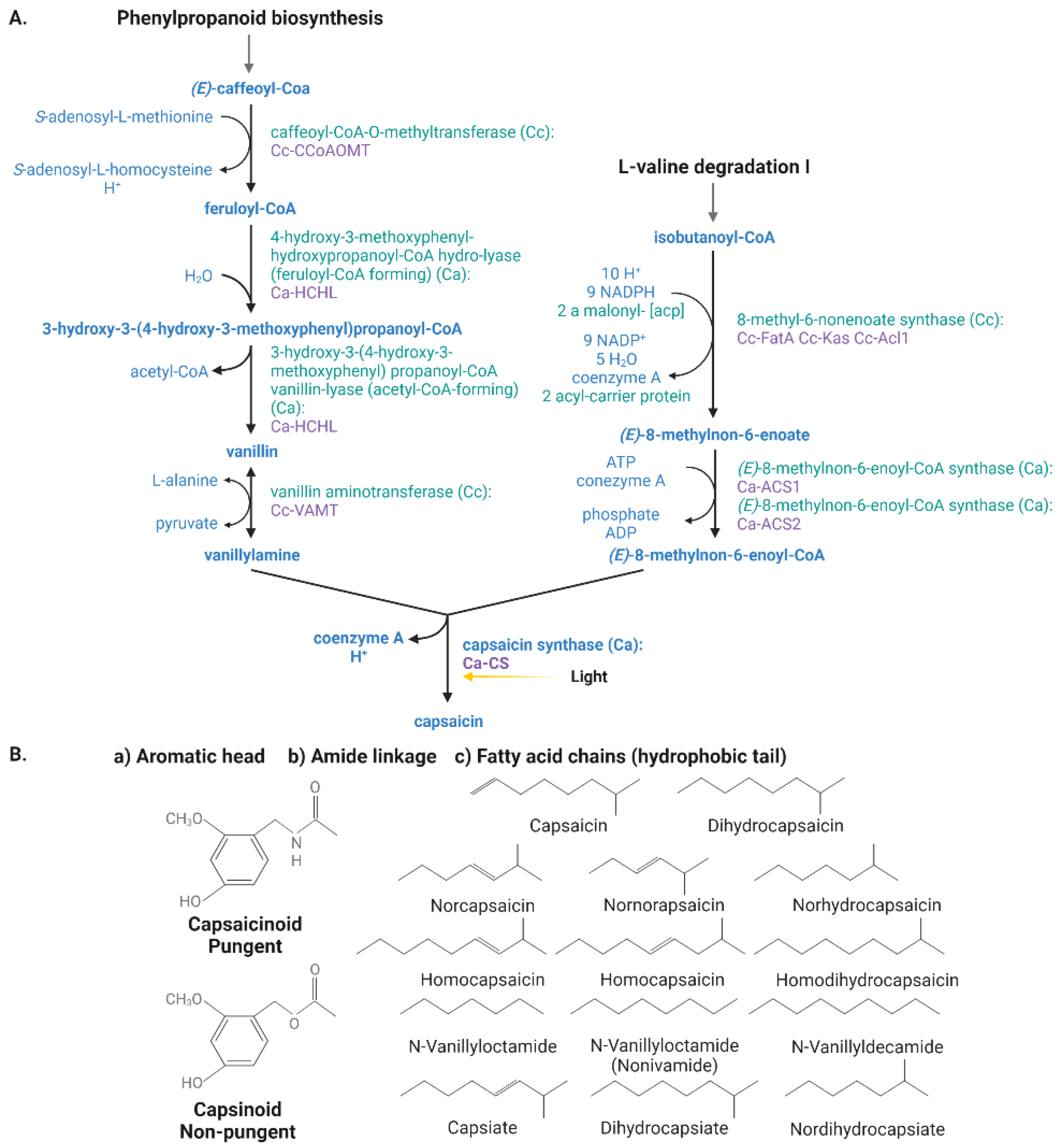
3.1. Capsaicinoids
3.1.1. Biosynthesis of Capsaicinoids
3.1.2. Effects of Light on Capsaicinoids
| Capsicum spp. | Light Treatment | Effects on Capsaicinoids Compared to Control | Biosynthetic Effect |
|---|---|---|---|
| C. chinense Jacq. Seven hot hybrid peppers | Light intensities (1200, 1313, 713, 1112, 774, and 783 μmol·m−2 ·s−1) in different locations with shading net with 50% shade | Reduced light intensity (713–783 µmol·m−2·s−1) and higher relative humidity increased capsaicinoid production in cultivars | Not reported [64] |
| C. chinense Jacq. ‘Bhut Jolokia’ ‘Akanee Pirote’ ‘Habanero’ | Shading nets with 50%, and 70% shade, and unshaded as control | ‘Bhut Jolokia’ showed the highest capsaicinoid yield under 70% shading, ‘Akanee Pirote’ under 50% shading, and habanero peppers showed the lowest capsaicinoid content under shading treatments | Levels of phenylalanine ammonia-lyase (PAL) increased under low light intensities [63] |
| C. annuum ‘Star flame’ ‘Fire flame’ | Colored shading nets: white, red, and green with 40% shade, and unshaded as control | Capsaicinoid content increased in color-shading treatments, specifically in green treatment in both cultivars | A high average temperature of 22–28 °C may have promoted capsaicinoid biosynthesis [65] |
| C. annuum ‘Super hot’ | Greenhouse conditions with LED lighting treatments: blue, red, and a mixture of blue and red light, and 12 h of sunlight as control | Blue LEDs significantly increased nordihydrocapsaicin, capsaicin, dihydrocapsaicin, homocapsaicin, and homodihydrocapsaicin contents by 57, 43, 56, 28, and 54%, respectively | Capsaicin and dihydrocapsaicin accumulation helped in oxidative stress defense. Valine and phenylalanine increased in blue LED lights contributing to a higher content of capsaicinoids [68] |
| C. annuum ‘Cheonyang’ | LED lighting treatments: red, blue, and red plus blue, and fluorescent lamps as control | Blue LEDs increased capsaicinoid contents, red LEDs reduce two times the capsaicinoid content compared to fluorescent light | Not reported [36] |
| C. annuum ‘Shishito pepper’ | Continuous fluorescent illumination (150–350 µmol·m−2·s−1) at constant temperature (28 °C), and greenhouse conditions as control | Fewer seeds and higher concentration of capsaicin in fruits under continuous fluorescent illumination | There is a negative correlation between seed formation and capsaicin biosynthesis [67] |
| C. annuum Serrano ‘Tampiqueño 74′ Sweet pepper ‘California wonder’ | Artificial light in postharvest (50 µmol·m−2·s−2) and dark conditions as control | Light factors increased capsaicin content in ‘Tampiqueño 74′ | CaMYB31-expression analysis from placental tissue of pungent and non-pungent fruits showed a positive correlation with the structural genes Ca4H, Comt, KAS, pAMT, and AT3 expression, and with the content of capsaicin and dihydrocapsaicin during fruit development [60] |
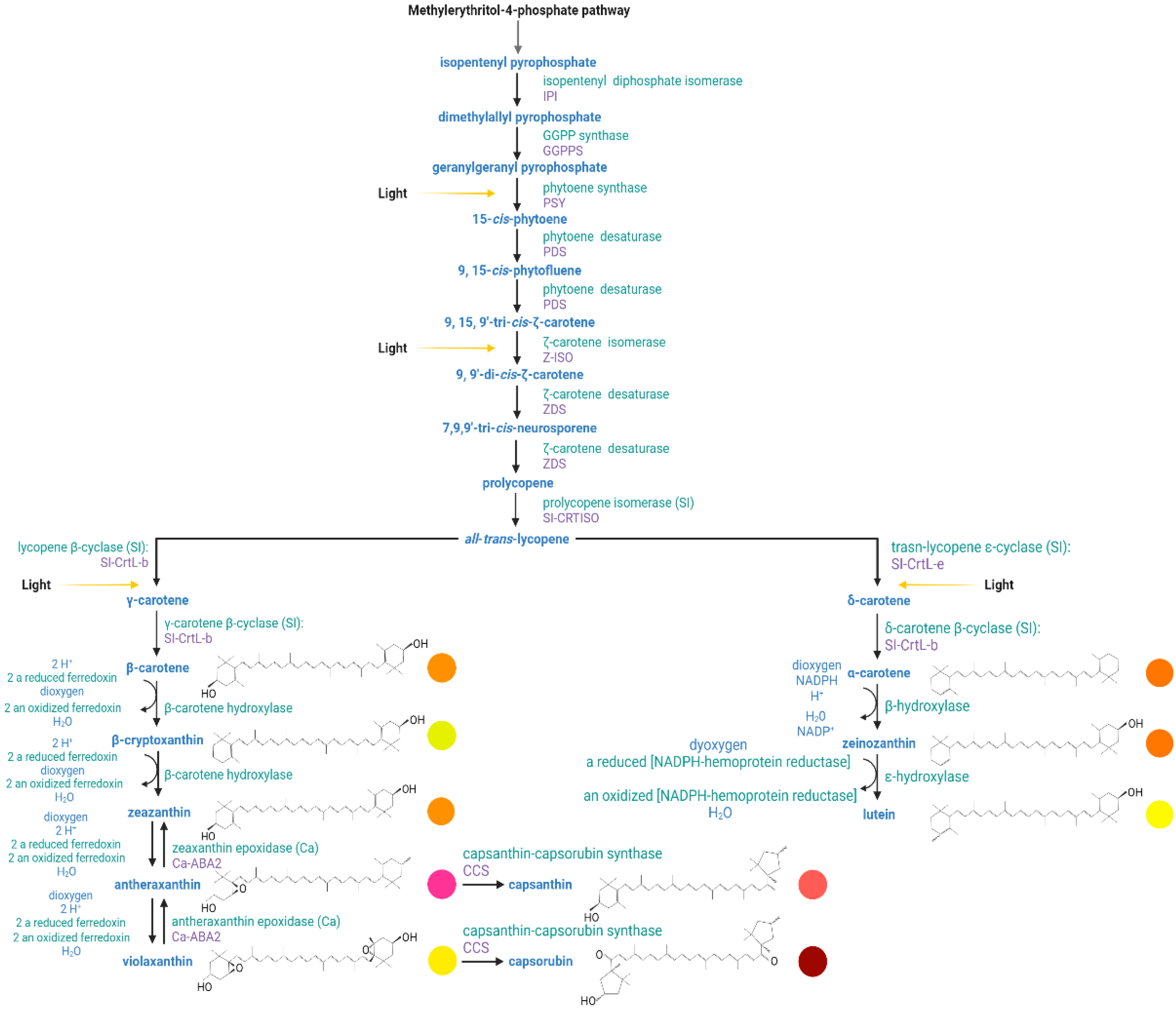
3.2. Carotenoids
3.2.1. Biosynthesis of Carotenoids
3.2.2. Effects of Light on Carotenoids
| Capsicum spp. | Light Treatment | Effects on Carotenoids Compared to Control | Biosynthetic Effect |
|---|---|---|---|
| C. annuum Sweet pepper | Colored shading net: white with 40% shade and controlled-temperature plastic tunnel environment | Controlled temperature plastic tunnel enhanced the accumulation of carotenoid components | Capsanthin biosynthesis was not affected by treatments in most of the cultivars; peppers showed a homogeneous behavior in β-cryptoxanthin biosynthesis, which was not significantly affected in most cultivars in any of the treatments. Shading effect influences a change in the active form of phytochrome, facilitating the degradation of phytochrome interacting factor (PIF1a) and activating PSY1 expression and carotenoid biosynthesis [8] |
| C. annuum Sweet pepper ‘Cameleon’ | Plastic tunnel plus colored shading nets: red, black, pearl, and blue shading nets with 40% shade, and open field as control | Black nets increased the carotenoid contents of β-carotene and lycopene | Not reported [11] |
| C. annuum Sweet pepper ‘Karpex’ | Colored shading nets: red, yellow, red, green, and white with 40% shade and unshaded as control | The unshaded control produced more than 50% less carotenoid than that under the white net. Peppers under the yellow and red nets produced the lowest content of carotenoids | Exposure to high temperature and radiation can lead to inhibition of carotenoid biosynthesis [91] |
| C. annuum Sweet pepper ‘Kapia’ | Colored shading nets: white, green, yellow, red, and unshaded as control | White shade net resulted in significantly higher carotenoid content compared to the green and the yellow nets | Not reported [92] |
| C. annuum ‘Fogo’ ‘NuMex’ ‘Sunset’ ‘Orange Grande’ | Shaded greenhouse with 40–50% shade, greenhouse conditions, and open field as control | Carotenoid concentrations decreased in fruits grown under increased light levels and increased in treatments with lower light intensity level | Not reported [90] |
| C. annuum Red and yellow sweet pepper | LED lighting treatments: natural light with red and blue LED, red and blue LED with far-red light, and natural light as control | In both colored fruits, carotenoid content was higher in LED treatments | Far-red light can act as a signal for starting plastid accumulation. Carotenoids changed by adding far-red light to the red and blue lighting [95] |
| C. annuum Red sweet pepper ‘Angus’ | UV lighting: UV-C, UV-B, UV-B+C, and no UV treatment as control | UV treatments induced carotenoid accumulation; after 14 days at 7 °C, UV-B and UV-C increased by 59% the total carotenoid content, and UVB + C by 94% | The active form of UVR8, a UV photoreceptor specific for UV-C and UV-B wavelengths, directly interacts with COP1 and regulates the expression of the HY5 gene, which promotes the production of carotenoids [94] |
| C. chinense Habanero pepper | Irradiation treatments: blue lamps (0, 1.5, and 3 min), and UV-C light (0, 0.5, and 1 min) at 4–5 °C | Both lights stimulated bioactive compounds. Carotenoid content increased only in the first days of storage | Blue and UV-C light may stimulate the synthesis of chlorophylls and total carotenoids [96] |
| C. annuum Sweet peppers | LED lighting treatments in postharvest: yellow light at a wavelength of 590 nm and dark conditions as control | LED light slightly accelerated the ripening of fruits and increased the content of β-carotene, α-tocopherol, γ-tocopherol, chlorophyll, and lutein. Fruits showed higher antioxidant potential | Not reported [97] |
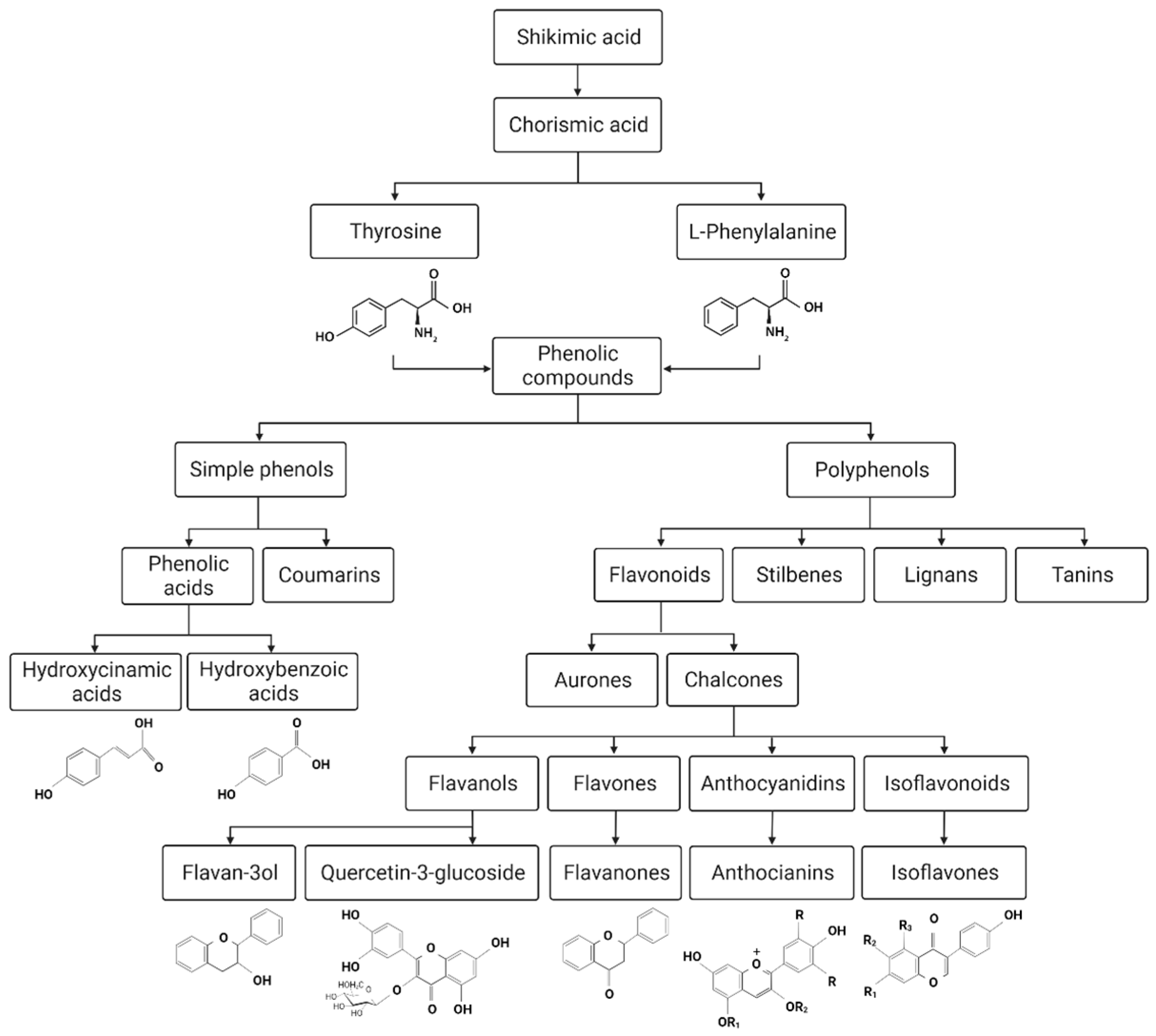
3.3. Phenolic Compounds
3.3.1. Biosynthesis of Phenolic Compounds
3.3.2. Effects of Light on Phenolic Compounds
| Capsicum spp. | Light Treatment | Effects on Phenolic Compounds Compared to Control | Biosynthetic Effect |
|---|---|---|---|
| C. annuum Sweet peppers c.v. ‘California Wonder’ | Polytrench greenhouse, shaded greenhouse (Polytrench + red shade net), and open field as control | The total contents of phenols and flavonoids were reduced by 35.2 and 14.6%, respectively, in the greenhouse treatment. | Not reported [106] |
| C. annuum Green sweet peppers | Colored shading nets: pearl, red, and yellow with 40% shade, and black net with 25% shade as control | Fruits produced under the pearl nets showed higher ascorbic acid content, and antioxidant scavenging activity after postharvest storage | Red–far-red photon ratio under the pearl net could have improved the ascorbic acid content and the antioxidant scavenging activity in green peppers [22] |
| C. annuum Sweet peppers | Colored shading nets: black, red, silver, white with 30% to 46% shade, and unshaded as control | Total phenols and flavonoids were among the highest in the unshaded treatment and under the white net, and the lowest content under the black net | Not reported [87] |
| C. annuum Sweet peppers, eleven cultivars | Colored shading net: white with 40% shade and controlled temperature plastic tunnel | White shade nets increased the accumulation of phenolic compounds and antioxidant activity in most of the studied cultivars | Not reported [8] |
| C. annuum c.v. ‘Takanotsume’ | LED lighting treatments: red (660 nm) and blue (470 nm) light at an intensity of 50 μmol·m−2·s−1 | The total phenolic, vitamin C content, and antioxidant capacity were higher in the blue LED-treated fruits | The blue LED was more effective in increasing the expression of the phytoene synthase (Psy) gene [78] |
| C. annuum Red sweet peppers | HPS and LED lighting in a glass greenhouse | LEDs at 622 nm enhanced phenolic compounds. HPS lighting supplemented with different LEDs was not efficient. | Not reported [111] |
| C. annuum Purple bell pepper | LED lighting treatments: white-red, and blue light | High blue-light fractions increased anthocyanin levels; white-red light is not efficient in the accumulation of anthocyanins | Increasing anthocyanin levels, via enhancing anthocyanin biosynthesis, was supported by kinetic modeling and higher expression levels of the anthocyanin biosynthetic genes CaMYB, CaCHS, CaDFR, CaANS and CaUFGT [85] |
| C. annuum Yellow, green, and red sweet peppers | LED lighting treatments: red, blue, and white light, and darkness as control | Red LED light for 8 h per day during storage at 7 °C was beneficial to retain bioactive compounds such as phenols and flavonoids | PAL activity in the yellow and green peppers exposed to red LED light increased and was correlated with the number of bioactive compounds [24] |
4. Summary
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Wahyuni, Y.; Ballester, A.R.; Tikunov, Y.; de Vos, R.C.H.; Pelgrom, K.T.B.; Maharijaya, A.; Sudarmonowati, E.; Bino, R.J.; Bovy, A.G. Metabolomics and molecular marker analysis to explore pepper (Capsicum sp.) biodiversity. Metabolomics 2013, 9, 130–144. [Google Scholar] [CrossRef]
- De Sá Mendes, N.; de Andrade Gonçalves, É.C.B. The role of bioactive components found in peppers. Trends Food Sci. Technol. 2020, 99, 229–243. [Google Scholar] [CrossRef]
- Cisternas-Jamet, J.; Salvatierra-Martínez, R.; Vega-Gálvez, A.; Stoll, A.; Uribe, E.; Goñi, M.G. Biochemical composition as a function of fruit maturity stage of bell pepper (Capsicum annum) inoculated with Bacillus amyloliquefaciens. Sci. Hort. 2020, 263, 109107. [Google Scholar] [CrossRef]
- Antonio, A.S.; Wiedemann, L.S.M.; Veiga Junior, V.F. The genus: Capsicum: A phytochemical review of bioactive secondary metabolites. RSC Adv. 2018, 8, 25767–25784. [Google Scholar] [CrossRef]
- Wahyuni, Y.; Ballester, A.R.; Sudarmonowati, E.; Bino, R.J.; Bovy, A.G. Metabolite biodiversity in pepper (Capsicum) fruits of thirty-two diverse accessions: Variation in health-related compounds and implications for breeding. Phytochemistry 2011, 72, 1358–1370. [Google Scholar] [CrossRef]
- Namdeo, A.G. Plant cell elicitation for production of secondary metabolites: A Review. Pharmacogn. Rev. 2007, 1, 60–79. [Google Scholar]
- Berry, H.M.; Rickett, D.V.; Baxter, C.J.; Enfissi, E.M.A.; Fraser, P.D. Carotenoid biosynthesis and sequestration in red chilli pepper fruit and its impact on colour intensity traits. J. Exp. Bot. 2019, 70, 2637–2650. [Google Scholar] [CrossRef]
- Lekala, C.S.; Madani, K.S.H.; Phan, A.D.T.; Maboko, M.M.; Fotouo, H.; Soundy, P.; Sultanbawa, Y.; Sivakumar, D. Cultivar-specific responses in red sweet peppers grown under shade nets and controlled-temperature plastic tunnel environment on antioxidant constituents at harvest. Food Chem. 2019, 275, 85–94. [Google Scholar] [CrossRef]
- Pola, W.; Sugaya, S.; Photchanachai, S. Influence of postharvest temperatures on carotenoid biosynthesis and phytochemicals in mature green chili (Capsicum annuum L.). Antioxidants 2020, 9, 203. [Google Scholar] [CrossRef]
- Ncise, W.; Daniels, C.W.; Nchu, F. Effects of light intensities and varying watering intervals on growth, tissue nutrient content and antifungal activity of hydroponic cultivated Tulbaghia violacea L. under greenhouse conditions. Heliyon 2020, 6, e03906. [Google Scholar] [CrossRef]
- Ilić, Z.S.; Milenković, L.; Šunić, L.; Barać, S.; Mastilović, J.; Kevrešan, Ž.; Fallik, E. Effect of shading by coloured nets on yield and fruit quality of sweet pepper. Zemdirbyste 2017, 104, 53–62. [Google Scholar] [CrossRef]
- Valiente-Banuet, J.I.; Gutiérrez-Ochoa, A. Effect of irrigation frequency and shade levels on vegetative growth, yield, and fruit quality of piquin pepper (Capsicum annuum L. var. glabriusculum). HortScience 2016, 51, 573–579. [Google Scholar] [CrossRef]
- Selahle, K.M.; Sivakumar, D.; Jifon, J.; Soundy, P. Postharvest responses of red and yellow sweet peppers grown under photo-selective nets. Food Chem. 2015, 173, 951–956. [Google Scholar] [CrossRef] [PubMed]
- Russo, V.M.; Howard, L.R. Carotenoids in pungent and non-pungent peppers at various developmental stages grown in the field and glasshouse. J. Sci. Food. Agr. 2002, 82, 615–624. [Google Scholar] [CrossRef]
- Bian, Z.H.; Yang, Q.C.; Liu, W.K. Effects of light quality on the accumulation of phytochemicals in vegetables produced in controlled environments: A review. J. Sci. Food Agric. 2015, 95, 869–877. [Google Scholar] [CrossRef]
- Shafiq, I.; Hussain, S.; Raza, M.A.; Iqbal, N.; Asghar, M.A.; Raza, A.; Fan, Y.-F.; Mumtaz, M.; Shoaib, M.; Ansar, M.; et al. Crop photosynthetic response to light quality and light intensity. J. Integr. Agric. 2021, 19, 2–21. [Google Scholar] [CrossRef]
- Binotti, E.D.; Costa, E.; Binotti, F.F.D.S.; Batista, T.B. Chemical agents and shading levels for the production of pepper seedlings. Eng. Agric. 2018, 38, 450–456. [Google Scholar] [CrossRef]
- Casierra-Posada, F.; Matallana-Díaz, Y.A.; Zapata-Casierra, E. Growth of bell pepper plants (Capsicum annuum) affected by coloured covers. Gesunde Pflanzen. 2014, 66, 149–155. [Google Scholar] [CrossRef]
- Holopainen, J.K.; Kivimäenpää, M.; Julkunen-Tiitto, R. New light for phytochemicals. Trends Biotechnol. 2018, 36, 7–10. [Google Scholar] [CrossRef]
- Jiao, Y.; Lau, O.S.; Deng, X.W. Light-regulated transcriptional networks in higher plants. Nat. Rev. Genet. 2007, 8, 217–230. [Google Scholar] [CrossRef]
- Jovicich, E.; VanSickle, J.J.; Cantliffe, D.J.; Stoffella, P.J. Greenhouse-grown colored peppers: A profitable alternative for vegetable production in Florida? Hort. Technol. 2005, 15, 355–369. [Google Scholar] [CrossRef]
- Mashabela, M.N.; Selahle, K.M.; Soundy, P.; Crosby, K.M.; Sivakumar, D. Bioactive compounds and fruit quality of green sweet pepper grown under different colored shade netting during postharvest storage. J. Food Sci. 2015, 80, H2612–H2618. [Google Scholar] [CrossRef]
- Zermeño-González, A.; Claveria-Cigarrero, G.L.; Melendres-Alvarez, A.I.; Ramírez-Rodriguez, H.; Munguía-López, J.P.; Campos-Magaña, S.G.; Cadena-Zapata, M. Colored plastic covers and its relationship with radiation, growth and yield of a sweet yellow pepper (Capsicum annuum L.) crop. Agrociencia 2019, 53, 709–723. [Google Scholar]
- Maroga, G.M.; Soundy, P.; Sivakumar, D. Different postharvest responses of fresh-cut sweet peppers related to quality and antioxidant and phenylalanine ammonia lyase activities during exposure to light-emitting diode treatments. Foods 2019, 8, 359. [Google Scholar] [CrossRef]
- Hashim, M.; Ahmad, B.; Drouet, S.; Hano, C.; Abbasi, B.H.; Anjum, S. Comparative effects of different light sources on the production of key secondary metabolites in plants in vitro cultures. Plants 2021, 10, 1521. [Google Scholar] [CrossRef]
- Dueck, T.; van Ieperen, W.; Taulavuori, K. Light perception, signalling and plant responses to spectral quality and photoperiod in natural and horticultural environments. Environ. Exp. Bot. 2016, 121, 1–150. [Google Scholar] [CrossRef]
- Ilić, Z.S.; Fallik, E. Light quality manipulation improves vegetable quality at harvest and postharvest: A review. Environ. Exp. Bot. 2017, 139, 79–90. [Google Scholar] [CrossRef]
- Ilić, Z.S.; Milenković, L.; Šunić, L.; Fallik, E. Effect of coloured shade-nets on plant leaf parameters and tomato fruit quality. J. Sci. Food Agr. 2015, 95, 2660–2667. [Google Scholar] [CrossRef]
- Lester, G.E. Environmental regulation of human health nutrients (ascorbic acid, β-carotene, and folic acid) in fruits and vegetables. HortScience 2006, 41, 59–64. [Google Scholar] [CrossRef]
- Shahak, Y. Photo-selective netting for improved performance of horticultural crops. A review of ornamental and vegetable studies carried out in Israel. Acta Hortic. 2008, 770, 161–168. [Google Scholar] [CrossRef]
- Castellano, S.; Candura, A.; Scarascia Mugnozza, G. Relationship between solidity ratio, colour and shading effect of agricultural nets. Acta Hortic. 2008, 801, 253–258. [Google Scholar] [CrossRef]
- Sivakumar, D.; Jifon, J.; Soundy, P. Spectral quality of photo-selective shade nettings improves antioxidants and overall quality in selected fresh produce after postharvest storage. Food Rev. Int. 2018, 34, 290–307. [Google Scholar] [CrossRef]
- Castellano, S.; Mugnozza, G.S.; Russo, G.; Briassoulis, D.; Mistriotis, A.; Hemming, S.; Waajienberg, D. Plastic nets in agriculture: A general review of types and applications. Appl. Eng. Agric. 2008, 24, 799–808. [Google Scholar] [CrossRef]
- Santana, J.Q.; Balbino, M.A.; Tavares, T.R.; Bezerra, R.S.; Farias, J.G.; Ferreira, R.C. Effect of photoselective screens in the development and productivity of red and yellow sweet pepper. Acta Hortic. 2012, 956, 493–500. [Google Scholar] [CrossRef]
- Stamps, R.H. Use of colored shade netting in horticulture. HortScience 2009, 44, 239–241. [Google Scholar] [CrossRef]
- Gangadhar, B.H.; Mishra, R.K.; Pandian, G.; Park, S.W. Comparative study of color, pungency, and biochemical composition in chili pepper (Capsicum annuum) under different light-emitting diode treatments. HortScience 2012, 47, 1729–1735. [Google Scholar] [CrossRef]
- Al Murad, M.; Razi, K.; Jeong, B.R.; Samy, P.M.A.; Muneer, S. Light emitting diodes (LEDs) as agricultural lighting: Impact and its potential on improving physiology, flowering, and secondary metabolites of crops. Sustainability 2021, 13, 1985. [Google Scholar] [CrossRef]
- Neugart, S.; Baldermann, S.; Hanschen, F.S.; Klopsch, R.; Wiesner-Reinhold, M.; Schreiner, M. The intrinsic quality of brassicaceous vegetables: How secondary plant metabolites are affected by genetic, environmental, and agronomic factors. Sci. Hortic. 2018, 233, 460–478. [Google Scholar] [CrossRef]
- Jung, W.S.; Chung, I.M.; Hwang, M.H.; Kim, S.H.; Yu, C.Y.; Ghimire, B.K. Application of light-emitting diodes for improving the nutritional quality and bioactive compound levels of some crops and medicinal plants. Molecules 2021, 26, 1477. [Google Scholar] [CrossRef]
- Vera-Guzmán, A.M.; Chávez-Servia, J.L.; Carrillo-Rodríguez, J.C.; López, M.G. Mexico evaluación fitoquímica en chile (Capsicum annuum L. and C. pubescens Ruiz & Pav.) silvestre y cultivado en Oaxaca, México. Chil. J. Agr. Res. 2011, 71, 578–585. [Google Scholar] [CrossRef]
- Gurung, T.; Techawongstien, S.; Suriharn, B.; Techawongstien, S. Impact of environments on the accumulation of capsaicinoids in Capsicum spp. HortScience 2011, 46, 1576–1581. [Google Scholar] [CrossRef]
- Batiha, G.E.S.; Alqahtani, A.; Ojo, O.A.; Shaheen, H.M.; Wasef, L.; Elzeiny, M.; Ismail, M.; Shalaby, M.; Murata, T.; Zaragoza-Bastida, A.; et al. Biological properties, bioactive constituents, and pharmacokinetics of some Capsicum spp. And capsaicinoids. Int. J. Mol. Sci. 2020, 21, 5179. [Google Scholar] [CrossRef] [PubMed]
- Forero, M.D.; Quijano, C.E.; Pino, J.A. Volatile compounds of chile pepper (Capsicum annuum L. var. Glabriusculum) at two ripening stages. Flavour. Frag. J. 2009, 24, 25–30. [Google Scholar] [CrossRef]
- Mares-Quiñones, M.D.; Valiente-Banuet, J.I. Horticultural aspects for the cultivated production of piquin peppers (Capsicum annuum L. var. Glabriusculum) a review. HortScience 2019, 54, 70–75. [Google Scholar] [CrossRef]
- Reddy, K.K.; Ravinder, T.; Prasad, R.B.N.; Kanjilal, S. Evaluation of the antioxidant activity of capsiate analogues in polar, nonpolar, and micellar media. J. Agric. Food Chem. 2011, 59, 564–569. [Google Scholar] [CrossRef]
- Sarwa, K.K.; Das, P.J.; Mazumder, B. A nanovesicle topical formulation of Bhut Jolokia (hottest Capsicum): A potential anti-arthritic medicine. Expert Opin. Drug Del. 2014, 11, 661–676. [Google Scholar] [CrossRef]
- Almeida, M.; Nadal, J.; Klein, T.; De Paula, J.; Budel, J.; Novatski, A.; Campessato, E.A.; Farrago, P.V. Innovative phytoformulation containing capsaicinoids: Microparticles development, analytical method validation, and anti-ulcer effect. Pharmacogn. Mag. 2018, 14, 290–296. [Google Scholar] [CrossRef]
- Kuzma, M.; Fodor, K.; Almási, A.; Mózsik, G.; Past, T.; Perjési, P. Toxicokinetic study of a gastroprotective dose of capsaicin by HPLC-FLD method. Molecules 2019, 24, 2848. [Google Scholar] [CrossRef]
- Friedman, J.R.; Nolan, N.A.; Brown, K.C.; Miles, S.L.; Akers, A.T.; Colclough, K.W.; Seidler, J.M.; Rimoldi, J.M.; Valentovic, M.A.; Dasgupta, P. Anticancer activity of natural and synthetic capsaicin analogs. J. Pharmacol. Exp. Ther. 2018, 364, 462–473. [Google Scholar] [CrossRef]
- Fattori, V.; Hohmann, M.S.N.; Rossaneis, A.C.; Pinho-Ribeiro, F.A.; Verri, W.A. Capsaicin: Current understanding of its mechanisms and therapy of pain and other pre-clinical and clinical uses. Molecules 2016, 21, 844. [Google Scholar] [CrossRef]
- Cheok, C.Y.; Sobhi, B.; Adzahan, N.M.; Bakar, J.; Rahman, R.A.; Ab Karim, M.S.; Ghazali, Z. Physicochemical properties and volatile profile of chili shrimp paste as affected by irradiation and heat. Food Chem. 2017, 216, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Sricharoen, P.; Lamaiphan, N.; Patthawaro, P.; Limchoowong, N.; Techawongstien, S.; Chanthai, S. Phytochemicals in Capsicum oleoresin from different varieties of hot chilli peppers with their antidiabetic and antioxidant activities due to some phenolic compounds. Ultrason. Sonochem. 2017, 38, 629–639. [Google Scholar] [CrossRef]
- Nuñez-Palenius, H.G.; Ochoa-Alejo, N. Effect of phenylalanine and phenylpropanoids on the accumulation of capsaicinoids and lignin in cell cultures of chili pepper (Capsicum annuum L.). In Vitro Cell Dev. Biol. Plant. 2005, 41, 801–805. [Google Scholar] [CrossRef]
- Naves, E.R.; de Ávila Silva, L.; Sulpice, R.; Araújo, W.L.; Nunes-Nesi, A.; Peres, L.E.; Zsögön, A. Capsaicinoids: Pungency beyond Capsicum. Trends Plant Sci. 2019, 24, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Aza-González, C.; Núñez-Palenius, H.G.; Ochoa-Alejo, N. Molecular biology of capsaicinoid biosynthesis in chili pepper (Capsicum spp.). Plant Cell Rep. 2011, 30, 695–706. [Google Scholar] [CrossRef]
- Stewart, C.; Mazourek, M.; Stellari, G.M.; O’Connell, M.; Jahn, M. Genetic control of pungency in C. chinense via the Pun1 locus. J. Exp. Bot. 2007, 58, 979–991. [Google Scholar] [CrossRef] [PubMed]
- Burgos-Valencia, E.; Echevarría-Machado, I.; Narváez-Zapata, J.A.; Martínez-Estévez, M. Gene expression related to the capsaicinoids biosynthesis in the Capsicum genus: Molecular and transcriptomic studies. Braz. J. Bot. 2020, 43, 201–212. [Google Scholar] [CrossRef]
- Caicedo-Lopez, L.H.; Guevara-Gonzalez, R.G.; Ramirez-Jimenez, A.K.; Feregrino-Pérez, A.A.; Contreras-Medina, L.M. Eustress application trough-controlled elicitation strategies as an effective agrobiotechnology tool for capsaicinoids increase: A review. Phytochem. Rev. 2022, 21, 1941–1968. [Google Scholar] [CrossRef]
- Díaz, J.; Pomar, F.; Bernal, Á.; Merino, F. Peroxidases and the metabolism of capsaicin in Capsicum annuum L. Phytochem. Rev. 2004, 3, 141–157. [Google Scholar] [CrossRef]
- Arce-Rodríguez, M.L.; Ochoa-Alejo, N. An R2R3-MYB transcription factor regulates capsaicinoid biosynthesis. Plant Physiol. 2017, 174, 1359–1370. [Google Scholar] [CrossRef]
- Kim, J.S.; Park, M.; Lee, D.J.; Kim, D. Characterization of putative capsaicin synthase promoter activity. Mol. Cells 2009, 28, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Pérez, J.C. Bell pepper (Capsicum annum L.) crop as affected by shade level: Fruit yield, quality, and postharvest attributes, and incidence of phytophthora blight (caused by Phytophthora capsici Leon.). HortScience 2014, 49, 891–900. [Google Scholar] [CrossRef]
- Jeeatid, N.; Techawongstien, S.; Suriharn, B.; Bosland, P.W.; Techawongstien, S. Light intensity affects capsaicinoid accumulation in hot pepper (Capsicum chinense Jacq.) cultivars. Hortic. Environ. Biotech. 2017, 58, 103–110. [Google Scholar] [CrossRef]
- Jeeatid, N.; Suriharn, B.; Techawongstien, S.; Chanthai, S.; Bosland, P.W.; Techawongstien, S. Evaluation of the effect of genotype-by-environment interaction on capsaicinoid production in hot pepper hybrids (Capsicum chinense Jacq.) under controlled environment. Sci. Hortic. 2018, 235, 334–339. [Google Scholar] [CrossRef]
- Nagy, Z.; Daood, H.; Neményi, A.; Ambrózy, Z.; Pék, Z.; Helyes, L. Impact of shading net color on phytochemical contents in two chili pepper hybrids cultivated under greenhouse conditions. Korean J. Hortic. Sci. 2017, 35, 418–430. [Google Scholar] [CrossRef]
- Phimchan, P.; Chanthai, S.; Bosland, P.W.; Techawongstien, S. Enzymatic changes in phenylalanine ammonia-lyase, cinnamic-4-hydroxylase, capsaicin synthase, and peroxidase activities in Capsicum under drought stress. J. Agric. Food Chem. 2014, 62, 7057–7062. [Google Scholar] [CrossRef]
- Murakami, K.; Ido, M.; Masuda, M. Fruit Pungency of “Shishito” pepper as affected by a dark interval in continuous fluorescent illumination with temperature alteration. J. Soc. High Tech. Agric. 2006, 18, 284–289. [Google Scholar] [CrossRef]
- Yap, E.S.P.; Uthairatanakij, A.; Laohakunjit, N.; Jitareerat, P.; Vaswani, A.; Magana, A.A.; Morre, J.; Maier, C.S. Plant growth and metabolic changes in ‘Super Hot’ chili fruit (Capsicum annuum) exposed to supplemental LED lights. Plant Sci. 2021, 305, 110826. [Google Scholar] [CrossRef]
- Quian-Ulloa, R.; Stange, C. Carotenoid biosynthesis and plastid development in plants: The role of light. Int. J. Mol. Sci. 2021, 22, 1184. [Google Scholar] [CrossRef]
- Botella-Pavía, P.; Rodríguez-Concepción, M. Carotenoid biotechnology in plants for nutritionally improved foods. Physiol. Plantarum. 2006, 126, 369–381. [Google Scholar] [CrossRef]
- Gómez-García, M.D.R.; Ochoa-Alejo, N. Biochemistry and molecular Biology of carotenoid biosynthesis in chili peppers (Capsicum spp.). Int. J. Mol. Sci. 2013, 14, 19025–19053. [Google Scholar] [CrossRef] [PubMed]
- Nisar, N.; Li, L.; Lu, S.; Khin, N.C.; Pogson, B.J. Carotenoid metabolism in plants. Mol. Plant. 2015, 8, 68–82. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Pérez, T.; Gómez-García, M.D.R.; Valverde, M.E.; Paredes-López, O. Capsicum annuum (hot pepper): An ancient Latin-American crop with outstanding bioactive compounds and nutraceutical potential. A review. Compr. Rev. Food Sci. F 2020, 19, 2972–2993. [Google Scholar] [CrossRef] [PubMed]
- Hassan, N.M.; Yusof, N.A.; Yahaya, A.F.; Rozali, N.N.M.; Othman, R. Carotenoids of Capsicum fruits: Pigment profile and health-promoting functional attributes. Antioxidants 2019, 8, 469. [Google Scholar] [CrossRef]
- Mercy, E.R.; David, U. Potential health benefits of conventional nutrients and phytochemicals of Capsicum peppers. Pharm. Pharmacol. Int. J. 2018, 6, 62–69. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, E.; Sánchez-Prieto, M.; Olmedilla-Alonso, B. Assessment of carotenoid concentrations in red peppers (Capsicum annuum) under domestic refrigeration for three weeks as determined by HPLC-DAD. Food Chem. 2020, 27, 100092. [Google Scholar] [CrossRef]
- Giuliano, G. Provitamin A biofortification of crop plants: A gold rush with many miners. Curr. Opin. Biotech. 2017, 44, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Pola, W.; Sugaya, S.; Photchanachai, S. Color development and phytochemical changes in mature green chili (Capsicum annuum L.) exposed to red and blue light-emitting diodes. J. Agric. Food Chem. 2020, 68, 59–66. [Google Scholar] [CrossRef]
- Villa-Rivera, M.G.; Ochoa-Alejo, N. Chili pepper carotenoids: Nutraceutical properties and mechanisms of action. Molecules 2020, 25, 5573. [Google Scholar] [CrossRef]
- Ghasemnezhad, M.; Sherafati, M.; Payvast, G.A. Variation in phenolic compounds, ascorbic acid and antioxidant activity of five coloured bell pepper (Capsicum annum) fruits at two different harvest times. J. Funct. Foods 2011, 3, 44–49. [Google Scholar] [CrossRef]
- Fraser, P.D.; Schuch, W.; Bramley, P.M. Phytoene synthase from tomato (Lycopersicon esculentum) chloroplasts-partial purification and biochemical properties. Planta 2020, 211, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Zhang, J.; Nageswaran, D.; Li, L. Carotenoid metabolism and regulation in horticultural crops. Hortic. Res. 2015, 2, 15036. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.J.; Kim, J.H.; Park, K.S.; Son, J.E.; Lee, J.M. Light-controlled fruit pigmentation and flavor volatiles in tomato and bellpepper. Antioxidants 2020, 9, 14. [Google Scholar] [CrossRef]
- Llorente, B.; D’Andrea, L.; Ruiz-Sola, M.A.; Botterweg, E.; Pulido, P.; Andilla, J.; Loza-Alvarez, P.; Rodriguez-Concepcion, M. Tomato fruit carotenoid biosynthesis is adjusted to actual ripening progression by a light-dependent mechanism. Plant J. 2016, 85, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Lv, J.; Zhang, Z.; Li, H.; Yang, B.; Chen, W.; Dai, X.; Li, X.; Yang, S.; Liu, L.; et al. Integrative transcriptome and proteome analysis identifies major metabolic pathways involved in pepper fruit development. J. Proteome Res. 2019, 18, 982–994. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Cao, Y.; Yu, H.; Wang, L.; Zhang, B. Genetic control and metabolite composition of fruit quality in Capsicum. Acta Hortic. Sin. 2019, 46, 1825–1841. [Google Scholar] [CrossRef]
- Díaz-Pérez, J.C.; St. John, K.; Kabir, M.Y.; Alvarado-Chávez, J.A.; Cutiño-Jiménez, A.M.; Bautista, J.; Gunawan, G.; Nambeesan, S.U. Bell Pepper (Capsicum annum L.) under Colored Shade Nets: Fruit Yield, Postharvest Transpiration, Color, and Chemical Composition. HortScience 2020, 55, 181–187. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, S.; He, X.; Li, Y.; Zhang, Y.; Chen, W. Response of total phenols, flavonoids, minerals, and amino acids of four edible fern species to four shading treatments. PeerJ. 2020, 21, e8354. [Google Scholar] [CrossRef]
- Howard, L.R.; Wildman, R.E.C. Antioxidant vitamin and phytochemical content of fresh and processed pepper fruit (Capsicum annuum). In Handbook of Nutraceuticals and Functional Foods, 2nd ed.; CRS Press, Ed.; Taylor and Francis: New York, NY, USA, 2016; pp. 165–191. [Google Scholar]
- Ambrózy, Z.; Daood, H.; Nagy, Z.; Darázsi Ledó, H.; Helyes, L. Effect of net shading technology and harvest times on yield and fruit quality of sweet pepper. Appl. Ecol. Environ. Res. 2016, 14, 99–109. [Google Scholar] [CrossRef]
- Ombódi, A.O.; Pék, Z.; Szuvandzsiev, P.; Lugasi, A.; Ledóné Darázsi, H.; Helyes, L. Effect of coloured shade nets on some nutritional characteristics of a kapia type pepper grown in plastic tunnel. Columella J. Agric. Environ. Sci. 2016, 25, 33. [Google Scholar] [CrossRef]
- Castillejo, N.; Martínez-Zamora, L.; Artés-Hernández, F. Postharvest UV radiation enhanced biosynthesis of flavonoids and carotenes in bell peppers. Postharvest Biol. Technol. 2022, 184, 111774. [Google Scholar] [CrossRef]
- Kim, D.; Son, J.E. Adding far-red to red, blue supplemental light-emitting diode interlighting improved sweet pepper yield but attenuated carotenoid content. Front. Plant Sci. 2022, 13, 938199. [Google Scholar] [CrossRef] [PubMed]
- ISO 21348; Definitions of Solar Irradiance Spectral Categories. ISO: Geneva, Switzerland, 2007.
- Pérez-Ambrocio, A.; Guerrero-Beltrán, J.A.; Aparicio-Fernández, X.; Ávila-Sosa, R.; Hernández-Carranza, P.; Cid-Pérez, S.; Ochoa-Velasco, C. Effect of blue and ultraviolet-C light irradiation on bioactive compounds and antioxidant capacity of habanero pepper (Capsicum chinense) during refrigeration storage. Postharvest Biol. Technol. 2018, 135, 19–26. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef] [PubMed]
- Kokalj, D.; Hribar, J.; Cigić, B.; Zlatić, E.; Demšar, L.; Sinkovič, L.; Šircelj, H.; Bizjak, G.; Vidrih, R. Influence of yellow light-emitting diodes at 590 nm on storage of apple, tomato and bell pepper fruit. Food Technol. Biotech. 2016, 54, 228. [Google Scholar] [CrossRef]
- Materska, M.; Perucka, I. Antioxidant activity of the main phenolic compounds isolated from hot pepper fruit (Capsicum annuum L.). J. Agric. Food Chem. 2005, 53, 1750–1756. [Google Scholar] [CrossRef]
- Murthy, H.N.; Lee, E.J.; Paek, K.Y. Production of secondary metabolites from cell and organ cultures: Strategies and approaches for biomass improvement and metabolite accumulation. Plant Cell Tiss. Org. 2014, 118, 1–16. [Google Scholar] [CrossRef]
- Meckelmann, S.W.; Riegel, D.W.; van Zonneveld, M.; Ríos, L.; Peña, K.; Mueller-Seitz, E.; Petz, M. Capsaicinoids, flavonoids, tocopherols, antioxidant capacity and color attributes in 23 native Peruvian chili peppers (Capsicum spp.) grown in three different locations. Eur. Food Res. Technol. 2014, 240, 273–283. [Google Scholar] [CrossRef]
- Moreno-Ramírez, Y.D.R.; Martínez-Ávila, G.C.G.; González-Hernández, V.A.; Castro-López, C.; Torres-Castillo, J.A. Free radical-scavenging capacities, phenolics and capsaicinoids in wild piquin chili (Capsicum annuum var. Glabriusculum). Molecules 2018, 23, 2655. [Google Scholar] [CrossRef]
- Lucci, P.; Saurina, J.; Núñez, O. Trends in LC-MS and LC-HRMS analysis and characterization of polyphenols in food. TrA Trend Anal. Chem. 2017, 88, 1–24. [Google Scholar] [CrossRef]
- Lemos, V.C.; Reimer, J.J.; Wormit, A. Color for life: Biosynthesis and distribution of phenolic compounds in pepper (Capsicum annuum). Agriculture 2019, 9, 81. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Sood, P.; Citovsky, V. The roles of plant phenolics in defense and communication during Agrobacterium and Rhizobium infection. Mol. Plant Pathol. 2010, 11, 705–719. [Google Scholar] [CrossRef] [PubMed]
- Barros, J.; Serrani-Yarce, J.C.; Chen, F.; Baxter, D.; Venables, B.J.; Dixon, R.A. Role of bifunctional ammonia-lyase in grass cell wall biosynthesis. Nat. Plants 2016, 2, 16050. [Google Scholar] [CrossRef] [PubMed]
- Lahbib, K.; Dabbou, S.; Bok, S.E.L.; Pandino, G.; Lombardo, S.; Gazzah, M.E.L. Variation of biochemical and antioxidant activity with respect to the part of Capsicum annuum fruit from Tunisian autochthonous cultivars. Ind. Crop Prod. 2017, 104, 164–170. [Google Scholar] [CrossRef]
- Angmo, P.; Dolma, T.; Phuntsog, N.; Chaurasia, O.P.; Stobdan, T. Effect of shading and high temperature amplitude on yield and phenolic contents of greenhouse capsicum (Capsicum annuum L.). J. Biol. Pharm. 2021, 4, 30–39. [Google Scholar] [CrossRef]
- Andersen, O.M.; Markham, K.R. Flavonoids. Chemistry, Biochemistry and Applications; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Pech, R.; Volná, A.; Hunt, L.; Bartas, M.; Červeň, J.; Pečinka, P.; Špunda, V.; Nezval, J. Regulation of Phenolic Compound Production by Light Varying in Spectral Quality and Total Irradiance. Int. J. Mol. Sci. 2022, 23, 6533. [Google Scholar] [CrossRef]
- Kavga, A.; Strati, I.F.; Sinanoglou, V.J.; Fotakis, C.; Sotiroudis, G.; Christodoulou, P.; Zoumpoulakis, P. Evaluating the experimental cultivation of peppers in low-energy-demand greenhouses. An interdisciplinary study. J. Sci. Food Agric. 2019, 99, 781–789. [Google Scholar] [CrossRef]
- Bae, J.H.; Park, Y.J.; Namiesnik, J.; Gülçin, I.; Kim, T.C.; Kim, H.-C.; Heo, B.-G.; Gorinstein, S.; Ku, Y.-G. Effects of artificial lighting on bioactivity of sweet red pepper (Capsicum annuum L.). Int. J. Food Sci. 2016, 51, 1378–1385. [Google Scholar] [CrossRef]
- Vicente, A.R.; Pineda, C.; Lemoine, L.; Civello, P.M.; Martinez, G.A.; Chaves, A.R. UV-C treatments reduce decay, retain quality and alleviate chilling injury in pepper. Postharvest Biol. Technol. 2005, 35, 69–78. [Google Scholar] [CrossRef]
- Ma, L.; Wang, Q.; Li, L.; Grierson, D.; Yuan, S.; Zheng, S.; Wang, Y.; Wang, B.; Bai, C.; Fu, A.; et al. UV-C irradiation delays the physiological changes of bell pepper fruit during storage. Postharvest Biol. Technol. 2021, 180, 111506. [Google Scholar] [CrossRef]
- Lemessa, A.; Popardowski, E.; Hebda, T.; Jakubowski, T. The Effect of UV-C Irradiation on the mechanical and physiological properties of potato tuber and different products. Appl. Sci. 2022, 12, 5907. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez-Viveros, Y.; Núñez-Palenius, H.G.; Fierros-Romero, G.; Valiente-Banuet, J.I. Modification of Light Characteristics Affect the Phytochemical Profile of Peppers. Horticulturae 2023, 9, 72. https://doi.org/10.3390/horticulturae9010072
Jiménez-Viveros Y, Núñez-Palenius HG, Fierros-Romero G, Valiente-Banuet JI. Modification of Light Characteristics Affect the Phytochemical Profile of Peppers. Horticulturae. 2023; 9(1):72. https://doi.org/10.3390/horticulturae9010072
Chicago/Turabian StyleJiménez-Viveros, Yamir, Héctor Gordon Núñez-Palenius, Grisel Fierros-Romero, and Juan Ignacio Valiente-Banuet. 2023. "Modification of Light Characteristics Affect the Phytochemical Profile of Peppers" Horticulturae 9, no. 1: 72. https://doi.org/10.3390/horticulturae9010072
APA StyleJiménez-Viveros, Y., Núñez-Palenius, H. G., Fierros-Romero, G., & Valiente-Banuet, J. I. (2023). Modification of Light Characteristics Affect the Phytochemical Profile of Peppers. Horticulturae, 9(1), 72. https://doi.org/10.3390/horticulturae9010072








