1. Introduction
Recent historical events, such as the COVID-19 pandemic and new geo-political arrangements, have demonstrated the need for more resilient food systems capable of ensuring food self-sufficiency, especially in response to sudden changes [
1]. This need for resilience of the food supply chain particularly relates to the urban context, where half of the world’s population is currently living [
2]. Urban agriculture (UA) (e.g., home gardening, vertical farming, rooftop agriculture) has been identified as a viable solution to promote local production, ensure food security, reduce food waste and create more sustainable food systems [
3].
In its most advanced form, UA is applied in or on urban structures as a building-integrated agriculture (BIA) system [
4]. Rooftop farming is an example of BIA, applicable both in unprotected (open-air farms) and protected conditions (rooftop greenhouses) [
5]. The latter case represents a sustainable method of building and cultivation integration, linking the metabolisms of the two systems and symbiotic exchange of CO
2, heat, and water, favoring the optimization of cultivation inputs, energy saving, and emission reduction [
6]. In particular, an integrated rooftop greenhouse (i-RTG) was shown to be able to recycle about 342 kWh m
−2 yr
−1 from the main building, which, compared with a traditional fossil-fuel-heated greenhouse, can result in a CO
2 retention of about 114 kg CO
2 (eq) m
−2 yr
−1, corresponding to about 20 EUR m
−2 yr
−1 in economic savings [
7]. However, despite the potential environmental and economic benefits, production in an urban i-RTG may present some limitations due to the shading of surrounding buildings, as well as the bulky structural items, and the loss of transmissivity of fireproof covering materials (e.g., polycarbonate). In fact, given the integration in a city context, the structure must comply with the municipality’s structural and fire safety codes, with consequent constraints affecting the greenhouse light environment [
8,
9].
Supplementary LED light is receiving wide application in various greenhouse production contexts characterized by reduced solar radiation, such as the cultivation of high-density crops, at high latitudes or during darker seasons [
10,
11,
12]. In the Mediterranean region, supplementary lighting is still limited in use and applied mainly between October and March [
13]. However, a more extensive application might be of interest also for the Southern European sector, especially considering the low transmissivity of the chosen plastic roofing materials (60% of PAR reduction) or the need to whitewash greenhouses to protect plants from excessive heat during the summer period [
13]. These factors, together with increasing competition from Nordic countries, make it necessary for a technological upgrade in the Mediterranean greenhouse sector [
14]. Although research has already begun to investigate the effects of supplementary LED light, even in Southern Europe [
15,
16,
17], nothing has yet been demonstrated regarding its application in a low-solar-irradiance BIA context, whether in warm or cold climates. The present research aims to identify the potential benefits and limitations of supplementary LED lighting in the context of agriculture applied to buildings, with a specific focus on the Mediterranean.
2. Materials and Methods
2.1. Plants Growing Conditions
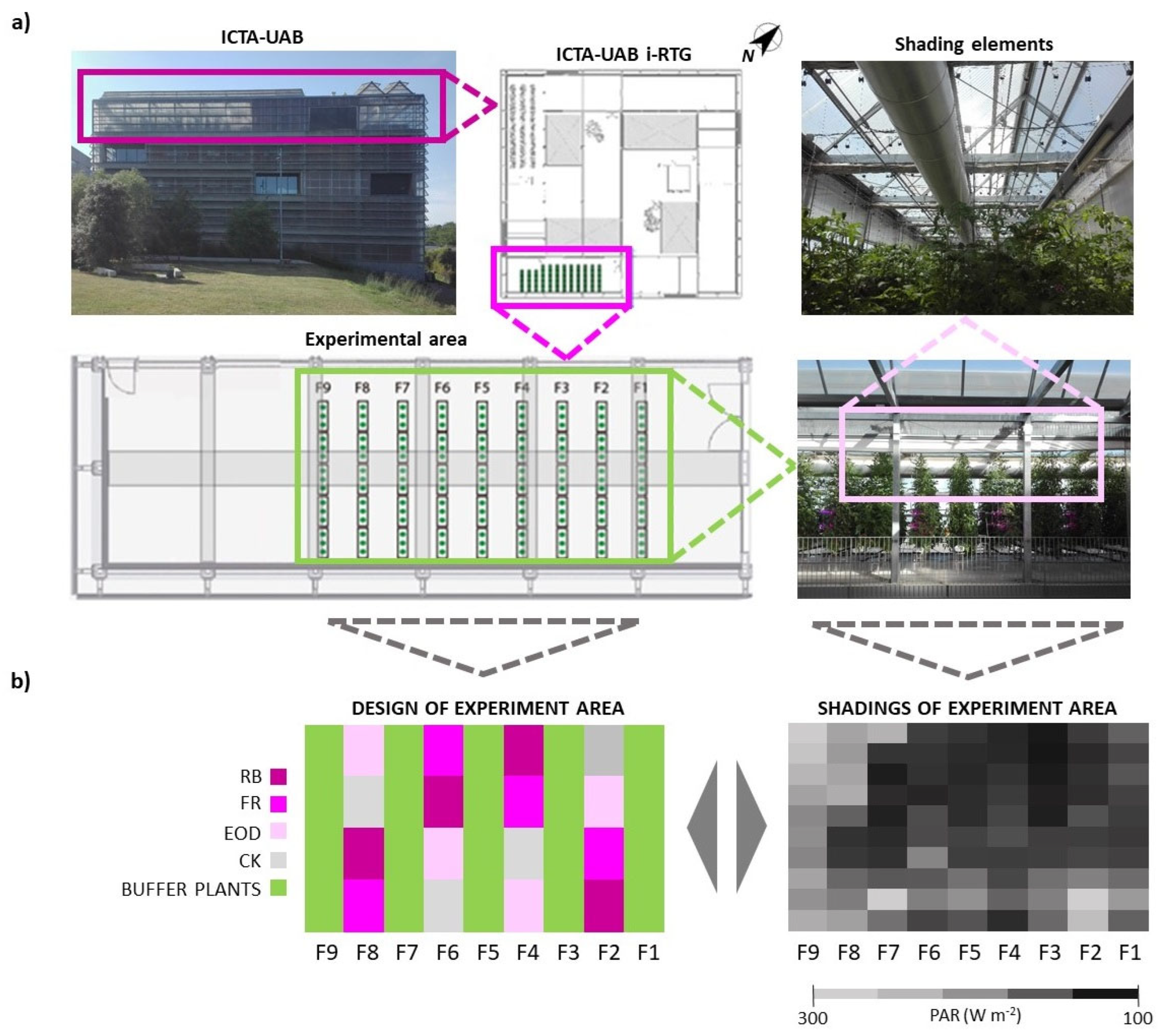
The experiment was performed in the i-RTG at the Institute of Environmental Science and Technology (ICTA-UAB) of the Universitat Autònoma de Barcelona (Catalunya, Spain) (41°49′78″ N, 2°10′89″ E) (
Figure 1a). The i-RTG structure consisted of reinforced steel pillars and polycarbonate cladding to satisfy the Spanish Technical Code of Edification and fire safety laws [
9].
Tomato plants (
Solanum lycopersicum L. cv. ‘Siranzo’, Rijk Zwaan
®) were cultivated in the south-east oriented corner of the building using a high-wire hydroponic system. Perlite bags (40 L) were used to support plants. Plant distance within rows was 30 cm, while the distance between rows was 80 cm, for a planting density of 3.1 plants m
−2. In total, 9 rows (5 m in length each) of plants were cultivated, out of which 5 rows were used as buffers to avoid light treatment pollution among blocks (
Figure 1b). The plants were sown in peat in late January and transplanted into perlite bags in mid-March. The experiment was terminated at the end of July.
Plants were fertigated with a closed-loop drip irrigation system using the rainwater collected in an underground tank and transported to the top floor by a pump. Irrigation shifts and fertilizer amounts were adjusted during the experiment according to the phenological stage and climatic conditions, maintaining an average pH of 7 and an EC of 1.7 dS m
−1. Nutrient solution (
Table 1), drainage, and rainwater were checked daily to maintain stable nutrition, pH and EC. Temperature (25 ± 4 °C), relative humidity (61 ± 14%), and outdoor (294 ± 344 W m
−2) and indoor (110 ± 136 W m
−2) radiation were constantly monitored using computer-monitored sensors. Passive ventilation was automatically adjusted according to environmental conditions by opening top and lateral windows. The residual heat coming from the lower floors of the building was used to warm-up the greenhouse during cool days.
2.2. Light Treatment and Experimental Design
Each light treatment was provided by a couple of LED inter-lighting lamps (Philips GreenPower LED, Philips®, Amsterdam, The Netherlands) located at 50 cm and 80 cm of height, and at 30 cm of distance from the stem. A control using natural light only (CK), plus three different supplemental LED lighting regimes, were evaluated. Lighting treatments consisted of:
- (1)
Red (660 nm) and blue (465 nm) light with R:B ratio of 3, a total photosynthetic photon flux density (PPFD) of 170 µmol m−2 s−1 (85 µmol m−2 s−1 per each lamp, measured at 30 cm from the plant) and a photoperiod of 16 h d−1 (8 a.m.–12 a.m.) (namely RB);
- (2)
RB treatment with an addition of 40 µmol m−2 s−1 of far-red light (730 nm) during the whole photoperiod (namely FR);
- (3)
RB treatment with an addition of 40 µmol m−2 s−1 of far-red, added only for 30 min right after the end of photoperiod (end-of-day) (namely EOD).
Lighting treatments started in mid-April until the end of the trial. A Latin square design was used to reduce systematic error determined by the shading of air conducts and structural elements on plants (
Figure 1b). The experiment was divided into 4 replicates (n = 4) containing 3 plants per treatment (12 plants per treatment). Lines of buffer plants were used to screen the radiation coming from parallel rows, and 1 or 2 buffer plants were used between two adjacent treatments to avoid light interactions on the row (
Figure 1b).
2.3. Plant Vegetative, Physiological and Biochemical Measurements
Stem and collar diameter were measured every two weeks from the beginning of lighting treatment until the end of May, at 1 cm under the fruit truss and 1 cm from the perlite bag, respectively. Internodes length was measured weekly as the distance between two consecutive fruit trusses. Apical growth and the number of clusters were measured every week until plant topping occurred in the last week of June. Final fresh weight of the entire plant biomass (e.g., leaves and stems) was measured with a digital scale at the end of the experiment.
Leaf area was evaluated on the last week of May using Easy Leaf Area software (Department of Plant Sciences, University of California, Davis, CA, USA) [
18] on the first leaf above the third fruit truss of each plant. The same leaves were measured as fresh and after drying at 60 °C per 4 days. Weight measurements were used to evaluate leaf dry matter content (LDMC), as the ratio between leaf dry mass and leaf fresh mass (mg g
−1), and specific leaf area (SLA), as the ratio between leaf area and leaf dry mass (m
2 kg
−1) [
19].
Chlorophyll content of leaves was evaluated in the first week of June, considering three points of the first leaf right under the third fruit truss of each plant. A Chlorophyll Content Meter CCM-200 (Opti-Sciences
®, Hudson, NY, USA) was used to non-destructively estimate the content based on the ratio of light transmittance at 653 nm and at 931 nm [
20].
Analysis of micro- and macro-elements (B, Mn, Fe, Cu, Zn, Na, Mg, K, P, S, and Ca) of plant biomass was carried out through a digestion process with HNO
3 and analyzed using ICP-OES optical spectrometry (Optima 4300DV, Perkin-Elmer, Waltham, MA, USA) as reported in Arcas-Pilz et al. [
21]. The analysis was conducted on leaf samples collected weekly, and plant stems collected at the end of the experiment. Leaves and stems were analyzed separately.
2.4. Fruit Development and Yield
Fruit development was monitored on the proximal fruit of the first produced cluster, representative of spring development (Follow-up 1), and the fourth cluster, representative of summer development (Follow-up 2), by measuring the equatorial and polar diameter. Evaluations were taken two times per week during the first three weeks and once a week during the last three weeks, starting from 12 days after anthesis until stabilization of fruit growth (around the turning phase). A digital Vernier caliper was used to measure the polar and equatorial dimensions (cm), estimating the volume of the fruit as the volume of an ellipsoid of rotation V = (4/3) πab
2, where a is one half of the polar radius and b is one half of the equatorial radius [
22]. Ripening was evaluated on the same fruit two weeks before harvesting using a DA-Meter (SINTELEIA
®, Bologna, Italy), which non-destructively evaluated the chlorophyll degradation and correlated it with a ripening index.
From the beginning of June until the end of the trial, fruits were harvested once per week (in total 6 clusters per plant). Fresh weight of the total clusters of each treatment was measured with a digital scale. The number of fruits per cluster was counted for each plant. At harvesting, fruits were divided, counted, and weighed as consumable and non-consumable, in which consumable was considered a mature red or dark orange tomato, and not consumable was considered a fruit backward in growth and ripening, mostly green and with small dimensions (<3 cm). This definition was not established on the basis of commercial parameters but rather on the assessment of the edibility of tomatoes to be used for local and direct consumption by the office workers. The average fresh weight of an individual fruit per treatment was estimated by dividing the total fresh weight by the total number of fruits. The total fresh mass of fruit produced by the plant was estimated by multiplying the number of fruits of each plant by the average fresh weight of an individual fruit for each treatment.
2.5. Fruit Quality and Biochemical Analysis
2.5.1. Fruit Qualitative Evaluation
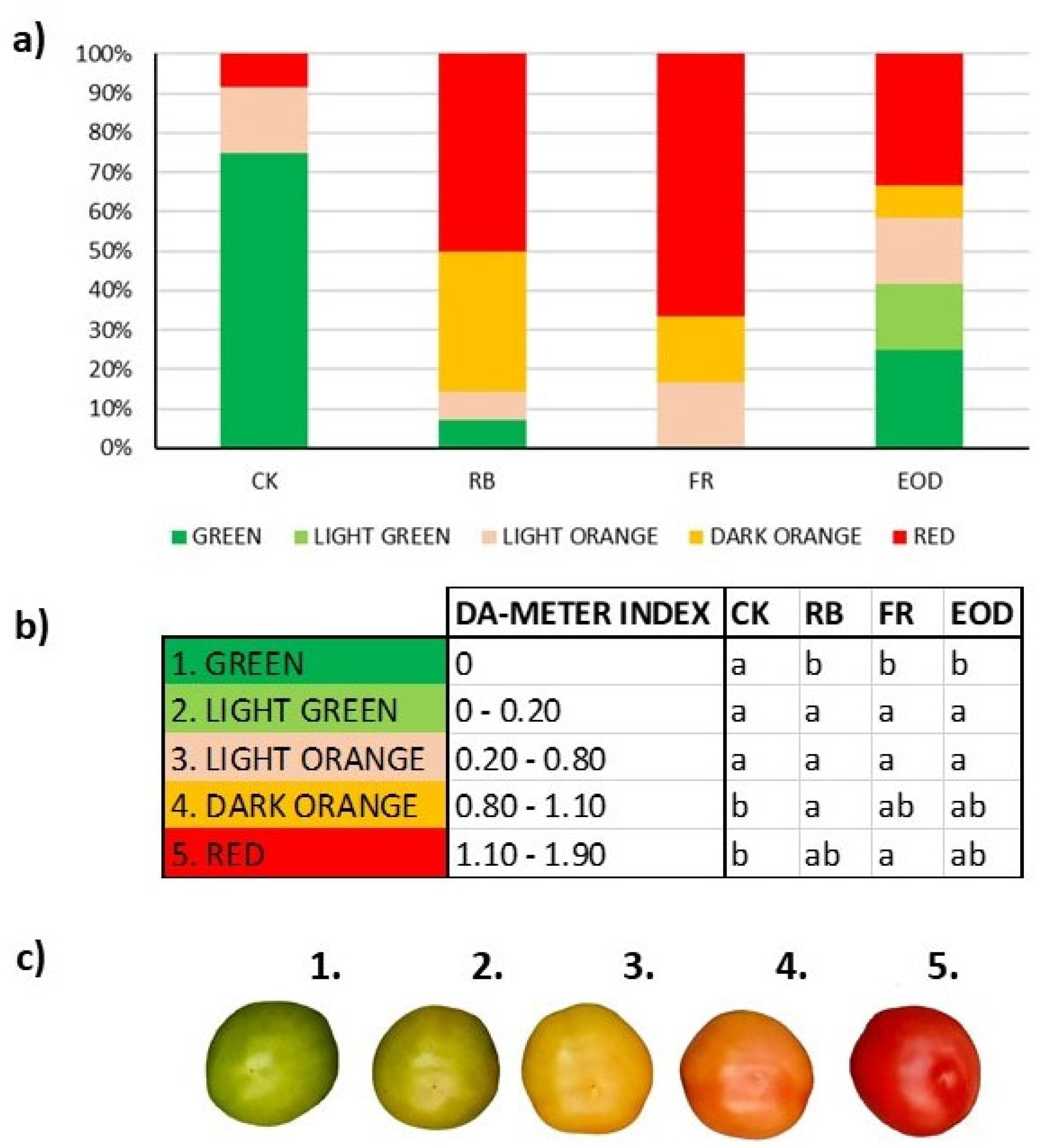
Fruit qualitative evaluations were performed two times on mature tomatoes (spring development, Follow-up 1, and summer development, Follow-up 2), and one time on immature tomatoes at the turning stage (summer development, Follow-up 2). Mature tomatoes were selected considering a DA-Meter range index between 1.30 and 1.50, whereas immature tomatoes were selected considering a range index between 0.20 and 0.40.
Fruit hardness was evaluated using a fruit hardness tester (Turoni®, Forlì, Italy) on four opposite sides of the equatorial diameter of the fruit. The instrument non-destructively measured the elasticity of fruit exocarp, expressing it as an index ranging from 0 to 100. Soluble solid content and acidity were measured on fruit juice using a pocket Brix and acidity meter (PAL-BX|ACID3, Atago®, Tokyo, Japan). Fruit dry matter content (FDMC) was measured as the ratio between fruit fresh weight and dried fruit.
2.5.2. Lycopene and β-Carotene Content
Lycopene and β-carotene content were evaluated on tomato samples frozen at −20 °C, using the methodology described by Anthon and Barrett [
23], with slight modifications. Briefly, an extraction solution was prepared by mixing hexane, acetone, and ethanol in a
v:
v proportion of 2:1:1 and 0.5 g L
−1 of butylated hydroxytoluene (BHT). Thereafter, 0.5 g of frozen sample including exocarp and mesocarp, trying to avoid the green parts (e.g., petiole), were pestled and mixed with 10 mL of extraction solution. The material was left in darkness for 30 min and then centrifuged at 5000 rpm for 5 min. Finally, 1 mL of supernatant was read at 503 nm for lycopene and 444 nm for β-carotene with a spectrophotometer (8453 UV-Visible Spectrophotometer, HP
®, Palo Alto, CA, USA).
The lycopene content was calculated using the following formula:
where X is the amount of hexane (mL), Y the weight of the fruit tissue (g), A503 is the absorbance at 503 nm, and 3.12 is the extinction coefficient. β-Carotene was calculated with the following equation:
where A444 is the absorbance at 444 nm, A503 is the absorbance at 503 nm, 0.55 is the ratio of the final hexane layer volume to the volume of mixed solvents added for hexane:acetone:ethanol (2:1:1), V is the volume of mixed solvents added, W is the fresh weight of the sample, and 537 (g mole
−1) is the molecular weight of β-carotene.
2.5.3. Total Polyphenols and Total Antioxidant Capacity
Total polyphenols and total antioxidant capacity were evaluated on tomato samples frozen at −20 °C. The extraction was performed by placing 4 g of samples in tubes and adding 8 mL of extraction mixture (60% methanol, 30% H2O, 10% acetone). The process was carried out by centrifugation at 5000 rpm for 10 min at 4 °C. Supernatant was collected and used for antioxidant and total phenol analysis.
Total antioxidant capacity was analyzed using the FRAP (Ferric Reducing Antioxidant Power) method, developed following the method of Benzie and Strain [
24], with slight modifications. Briefly, a reaction mixture containing acetate buffer (pH 3.6), 300 mM of 2,4,6-Tris(2-pyridyl)-s-triazine (TPTZ) solution (in 40 mM HCl) and 20 mM FeCl
3 was prepared in a
v:
v:
v proportion of 10:1:1 and incubated at 37 °C for 2 h in darkness. Then, 1.2 mL of reaction mixture was added to 20 µL of supernatant and incubated for 1 h at room temperature in darkness. Calibration standards were prepared by dissolving 28 mg of FeSO
4 in 10 mL of H
2O (10 mM) and subsequently diluting 1 mL of the solution with 1 mL of H
2O (5 mM). Six Eppendorf tubes containing 40 µL of H
2O were prepared and 20 µL of 5 mM solution were added to the first Eppendorf (2.5 mM). The operation was repeated moving 20 µL of solution, from one tube to another 6 times, in order to halve the concentration at each dilution. A blank with 40 µL of H
2O was also prepared. Samples and standards were read at 593 nm with a spectrophotometer (8453 UV-Visible Spectrophotometer, HP
®, Palo Alto, CA, USA).
Total polyphenols were determined using the methodology described by Waterhouse [
25], with slight modifications. Briefly, 50 µL of sample extract were added to 800 µL of extraction mixture (H
2O and Folin–Ciocalteu reagent in a
v:
v proportion of 15:1). Calibration standards were prepared by dissolving 80 mg of gallic acid in 10 mL of H
2O (8 mg mL
−1) and subsequently diluting 0.5 mL of the solution with 4.5 mL of H
2O (800 µg mL
−1). Six Eppendorf tubes containing 50 µL of H
2O were prepared and 50 µL of 800 µg mL
−1 solution were added to the first (400 µg mL
−1). The operation was repeated moving 50 µL of solution from one tube to another for 6 times, in order to halve the concentration at each dilution. A blank with 50 µL of H
2O was also prepared. After an incubation of 5 min, samples and standards were added with 150 µL of 20% Na
2CO
3, incubated for 1 h at room temperature and then read at 765 nm with a spectrophotometer (8453 UV-Visible Spectrophotometer, HP
®, Palo Alto, CA, USA).
2.6. Chilling Injury Analysis
In mid-June, both mature (DA-Meter Index 1.30–1.50) and immature tomatoes (DA-Meter Index 0.20–0.40) were harvested and stored for one week at 4 °C. After cold storage, fruits were moved into a dark room at 20 °C, 60% RH and 600–660 ppm of CO2 for 2 weeks. Measurements took place right after 4 °C chilling (T1) and 10 days after 20 °C storage (T2).
Non-destructive analyses, including fresh weight loss, hardness, and ripening, were performed on the same tomatoes at T1 and T2 as described above. Fruit fresh weight was also evaluated before chilling. Destructive analyses, including soluble solids, lycopene content, β-carotene content, antioxidant activity, and total phenol content, were performed at the end (T2) of chilling injury evaluation as described above.
Chilling injury index was visually attributed at T2 according to Vega-Garcìa et al. [
26] and Affandi et al. [
27]. In particular, the criteria to evaluate the symptoms of chilling damage consisted of: uneven ripening and color development (U), pitting (P), and decay (D). A five-point scale was used to attribute the severity of the symptoms based on the percentage of affected fruit surface: 0 = no injury, 1 ≤ 10%, 2 = 11% to 25%, 3 = 26% to 40%, and 4 ≥ 40%.
2.7. Energy Cost Assessment
The energy cost assessment was calculated based on the actual consumption of a lamp with standard RB treatment (0.044 kW) applied for 16 h per day (0.704 kWh d
−1). Costs were estimated per plant per day, assuming that treatments were carried out with double lamps and that each pair of lamps covered about four plants. The price of electricity was acquired from EUROSTAT [
28] dataset considering household electricity prices for Italy (0.176 € kWh
−1) and Spain (0.188 € kWh
−1) in 2021, the two main producers of tomato in the Mediterranean area.
2.8. Statistical Analysis
Statistical analysis was performed by applying one-way ANOVA and Tukey’s test to compare means. Data were analyzed by using SPSS software. The Marascuilo procedure was used to compare multiple proportions in case of maturation degree and chilling injury index evaluation. Statistics considered a 5% significance level (p ≤ 0.05).
3. Results
The influence of LED light on plants vegetative parameters was not significant. In particular, stem and collar diameter measurements showed no significant differences between light treatments and unlighted control at each time point (data not shown). Concurrently, an absence of differences was observed in the average internode length, although the third internode of CK plants showed a significant increase in length compared with RB treatment, featuring mean lengths of 31 ± 2 and 27 ± 4 cm, respectively. This difference disappeared in the following internodes. However, the average elongation of the plant apex showed a significantly greater length in the unilluminated control than in the RB treatment, with mean lengths of 29 ± 3 and 25 ± 3 cm, respectively. The final fresh weight of the entire plant, leaves and stems, did not show any difference (data not shown). In the same way, leaf area, LDMC, SLA, and chlorophyll content did not present statistically significant changes among treatments. Lighting regimes did not affect leaves’ micro- and macro-elements content (
Table 2). At the same time, stems presented significantly different accumulations of Fe, Cu, Mg, K, and P elements depending on light treatment. In particular, Cu and P were shown to have a greater accumulation in CK plants than in LED-treated plants, as reported in
Table 2.
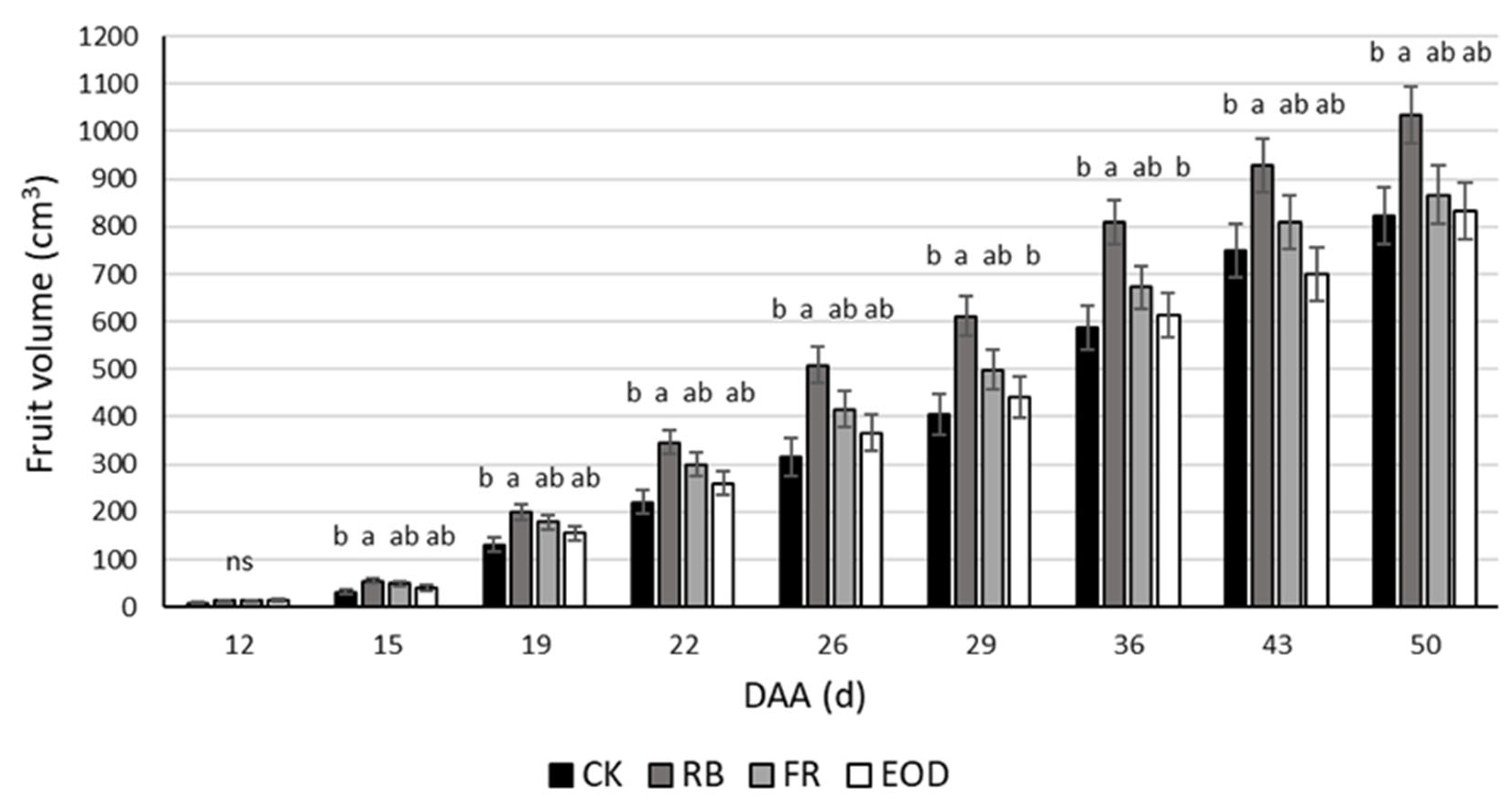
The increase in volume of proximal fruits showed different trends depending on the season. In particular, fruits of Follow-up 1, representative of the spring season, were not affected by the lighting regimes (data not shown). In contrast, fruits of Follow-up 2, representative of the summer period, showed clear differences in growth, particularly comparing CK and RB treatments (
Figure 2). Evaluation of ripening degree showed the same seasonal difference, demonstrating no difference for representative spring season fruit. In contrast, summer fruit seemed to present a faster ripening when exposed to LED light treatments, showing 35% more red fruit compared with CK plants (
Figure 3).
Control tomatoes were approximately 9.3% lighter than tomatoes grown under light treatments (approximately 93.8 g for CK and 103.5 g in LED-treated plants). Fruit number was also lower (−7.2%) in control plants than in LED-treated plants (38.4 fruits plant−1 in CK and 41.4 fruits plant−1 in LED-treated plants). The statistical evaluation shows a significantly higher yield of LED-treated plants (RB, FR, and EOD) as compared with those grown with natural light only (CK). In particular, plants grown without supplemental LED light showed around 17% lower yield as compared with those grown with LED light (3.6 kg plant−1 in CK and 4.4 in LED-treated plants). Regarding yields for consumable and non-consumable tomatoes of each treatment, plants treated with LED lights produced a lower number of consumable red tomatoes (−9.3%) than control plants, although these fruits were larger in size (+10.5%). On the contrary, non-consumable green tomatoes of LED treatments were higher in number (+8.8%) than those in control, still presenting a larger average size (+13.7%).
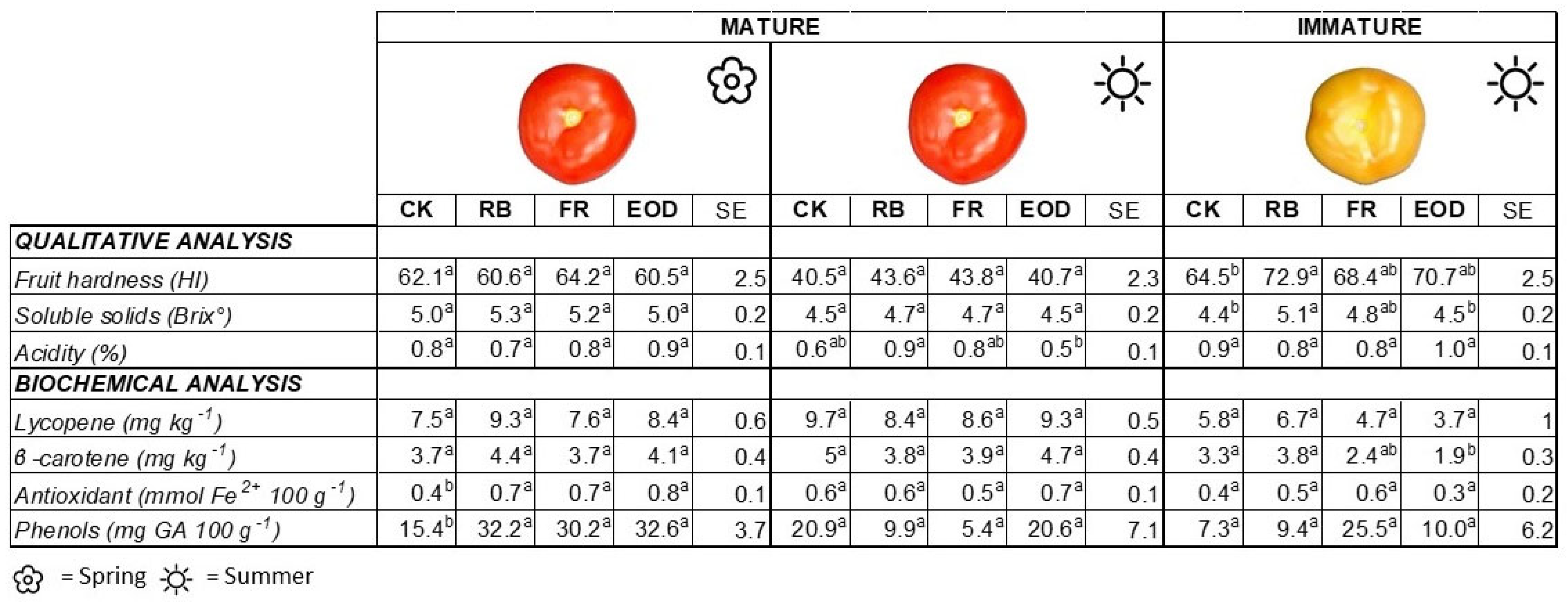
Qualitative and biochemical parameters showed different trends depending on the period in which the fruits developed (e.g., spring or summer) and the ripening stage at harvest (e.g., mature or immature) (
Figure 4). In particular, observing representative fruits from the spring period, no significant differences were observed concerning the evaluation of qualitative parameters. However, a significantly lower content of antioxidants and phenols was observed in control fruits’ phenol content and antioxidant capacity compared with those grown with LED light treatments (
Figure 4). In the case of summer fruits harvested at the mature stage, qualitative analyses showed lower acidity in fruits from the EOD treatment than the RB treatment (a reduction by 44.4% in EOD compared with RB), but no difference at the biochemical level. Finally, the evaluation of immature fruits, carried out only for the summer period, showed significantly higher hardness in fruits obtained from the RB treatment as compared with those produced by CK plants (11.5% less in CK compared with RB), and significantly higher soluble solid content in the RB than CK and EOD (13.7% less in CK and EOD compared with RB). For the biochemical analysis, the immature fruits from the summer period had a statistically significantly lower β-carotene content than RB and CK treatments (
Figure 4). FDMC of selected fruits did not show any significant difference among treatments (data not shown).
Cold storage showed different results on tomatoes depending on the ripeness degree. In particular, immature tomatoes showed no significant differences over time, except for polyphenol content. Indeed, polyphenols were found to be statistically lower in EOD and FR treatments compared with RB at T2, whereas no differences were observed among LED treatments and control (31.8 ± 8 mg GA 100 g
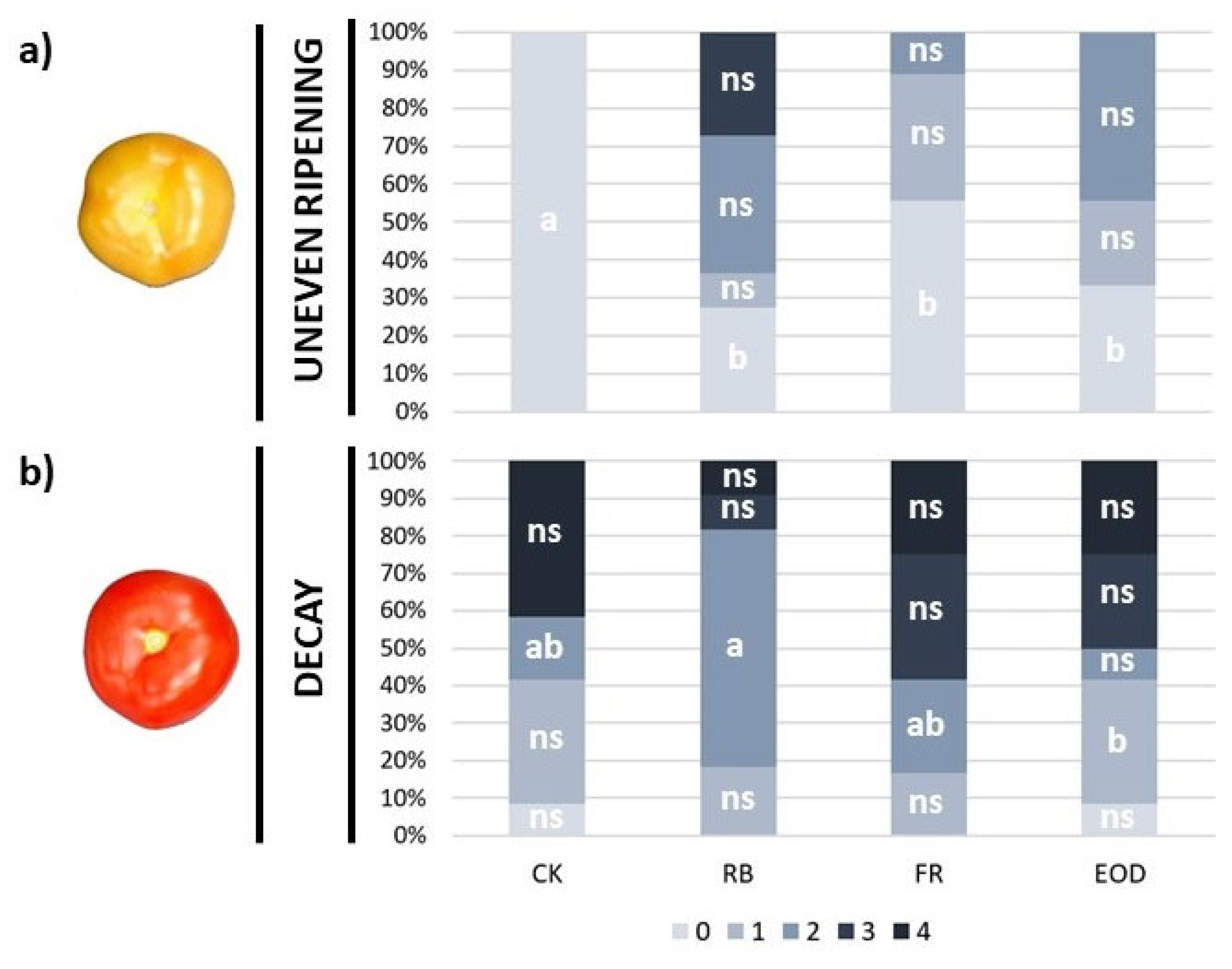
−1 in CK, 39.2 ± 6 in RB, 26.1 ± 4 in FR and 19.9 ± 7 in EOD). Immature fruits also showed more irregular ripening with the application of additional LED treatments, whereas no uneven ripening was observed in CK fruit (
Figure 5a). No significant statistical changes were observed in the case of pitting or decay evaluation for immature tomatoes (data not shown). On the other hand, ripe fruits showed significantly higher weight loss in CK compared with FR and EOD treatments immediately after 4 °C chilling (T1) (1.6 ± 0.1% in CK, 1.1 ± 0.4 in RB, 0.7 ± 0.3 in FR, and 0.6 ± 0.4 in EOD). However, differences among treatments disappeared after 10 days (T2). Differences in mature tomatoes were also observed in the case of β-carotene content, where RB treatment showed a significantly higher concentration than EOD treatment at T2 (4.9 ± 0.7 mg kg
−1 in CK, 5.9 ± 0.9 in RB, 4.6 ± 0.7 in FR, and 4.1 ± 0.9 in EOD). Regarding chilling injury assessment, ripe tomatoes treated with RB light showed a significantly higher number of fruits in the intermediate class of decay compared with the other treatments 10 days after chilling (T2) (
Figure 5b). However, although not significant, CK presented 80% more fruits in the highest decay class compared with fruits treated with RB light. No significant changes were observed in the case of pitting and uneven ripening evaluation for ripened tomatoes (data not shown).
Evaluation of energy consumption per plant showed an average value of about 0.022 kW, which multiplied by 16 h of treatment results in a daily consumption of about 0.352 kWh per plant. Considering this consumption and the household electricity costs reported above [
28], the daily cost to obtain a yield increase of 17% during the spring–summer period is about 0.06 EUR plant
−1 d
−1 for Italy and 0.07 EUR plant
−1 d
−1 for Spain, resulting in an increased cost by 1.27 EUR kg
−1 in Italy and 1.35 EUR kg
−1 in Spain.
4. Discussion
The amount of light radiation intercepted by plants depends on both the leaf area index (LAI, total leaf area per unit ground area) and a light extinction coefficient (k) influenced by morphological factors [
29]. The capacity to modify plant architecture and consequently increase light interception is fundamental for improving photosynthetic rate. Specific wavelengths are known to influence and modify some vegetative and morphological traits of tomato plants. In particular, far-red radiation has been shown to have effects on the elongation of tomato internodes, leaf morphology, and inclination [
12,
30,
31], being related to the so-called shade avoidance syndrome [
32]. This phenomenon is determined by a low R:FR perception by plant phytochromes, triggering different responses that also involve leaf development, apical dominance, internode extension, chloroplast development, and assimilate partitioning, among others [
33]. In natural conditions, without the artificial addition of far-red light, this phenomenon can be triggered by the far-red reflected by the leaves of the surrounding canopy as a signal of competition [
34]. Furthermore, a natural low R:FR ratio can also occur at the end of the day [
35], when the sun is low and longer wavelengths can travel further in the atmosphere.
In this report, the effects of different LED supplemental lighting spectral conditions, with or without far-red, have been evaluated on greenhouse tomato growth. Most vegetative and physiological traits were not affected by lighting regimes, contrary to what was reported by other researches, where some parameters, such as plant height or leaf area, were increased by far-red addition as compared with red and blue treatment alone [
36]. The average apical elongation was the only parameter showing statistically significant differences, resulting in CK values higher than RB, but not than FR and EOD, as a possible consequence of the elongation effect given by far-red presence. On the other hand, the absence of a statistical difference among FR and EOD compared with RB may be attributed to the presence of the blue wavelength beside far-red light, known to have a possible dwarfing effect on plants, therefore lowering LED-treated plants’ height [
37]. Concerning mineral element evaluation, previous studies suggested that light treatments might impact their accumulation in some plant organs [
38,
39]. In particular, Samuolienė et al. [
40] observed a higher accumulation of elements in tomato leaves grown with supplemental green LED light. In the present research, however, no differences were observed in leaves, although the different lighting treatments seem to have played different effects on the accumulation of specific elements in plant stems (
Table 2).
Supplemental LED light can increase the photosynthetic rate of tomato plants and consequently influence fruit size and weight [
41]. However, the effect of light on fruit growth can be affected by its position on the cluster, given the greater sink strength that a proximal fruit can have compared with a distal fruit. The sink strength of proximal fruit is related to higher cell division due to competitive assimilating processes triggered in the first phase of fruit development after pollination [
42]. In the following stages, the fruit accumulates most of its dry matter and undergoes a process of cell enlargement until it reaches the final stage of ripening, where it stops accumulating carbohydrates [
43]. In high-wire systems, where tomato plants are periodically lowered, fruits in the cell division stage are at the top of the plant, away from the LED lamps, whereas during the cell enlargement stage, fruits are at the bottom of the plant near the lamps. It might be possible to think that the lamps can only affect the second development stage, being in direct contact with the fruits. However, when comparing the volume growth over time of representative tomato fruits from spring (Follow-up 1) and summer (Follow-up 2) phases, different conclusions may be drawn. Whereas earlier in the season no significant differences were observed between treatments, later, RB treatment showed a significantly greater dimension of fruits than CK, already after the second measurement (T2) (
Figure 2). Whereas the light treatment for spring fruits was started after anthesis, on already formed fruit, in summer fruits, the treatment was already in progress since the early flowering. From these observations, it can be deduced that, despite being distant from the lamps, summer fruits have been affected by LED light, accumulating a major quantity of assimilates since the cell division stage. A similar response was also reported by Paponov et al. [
11], although differences were mainly observed in fruits at the intermediate position in the cluster.
The presence of far-red might influence the enhancement of tomatoes fresh weight [
31,
44]. However, as formerly observed in other studies performed in the Mediterranean area [
17], the presence of additional far-red did not seem to have specifically affected plant yield. However, a general increase in average fruit weight and fruit number was observed in all LED treatments compared with the control. Total yield had a percentage increase (17%) very similar to those observed in other studies comparing the use of supplemental LED light with natural light alone [
15,
16,
41,
45,
46]. By dividing the number of consumable fruits from non-consumable fruits, it was observed that plants under LED treatment produced fewer but larger red tomatoes, and more and bigger green tomatoes than the control. This observation seems to confirm the previous results by Paponov et al. [
11] on the ability of LED light to increase the number of cells and thus the sink strength in the early developmental stage of fruit at a different height on the truss. During the ripening stage, although fewer in number than the control, proximal fruits of LED-treated plants were found to have an earlier ripening (
Figure 3) as already observed by some authors [
15,
47]. The higher ripening rate in proximal fruits of LED-treated plants could be attributed to a greater competition and consequent accumulation of some biochemical compounds such as phytoene or melatonin, responsible for ripening processes [
47,
48].
Additional LED light is known to positively affect several quality attributes of tomatoes, although some inconsistencies among published research have been shown, probably determined by different environmental conditions and genotypes [
39,
49]. On ripe fruits, a significant increase in antioxidant capacity and phenol content in the LED-treated fruits was observed in spring, but no significant differences between lighting treatments was detected in the summer (
Figure 4). The effect of LED light on qualitative attributes may also change according to the fruit ripening stage, as observed in immature tomatoes in relation to sugar content and fruit hardness. Accordingly, Fanwoua et al. [
44] already observed that the effects of LED light on pericarp sugar concentrations could change based on fruit age, as a consequence of different fruit water content that would affect the sugar dilution.
To the best of our knowledge, limited research has investigated the effects of supplemental LED light applied at the cultivation stage on chilling injury and the post-harvest quality of tomatoes. In particular, Affandi et al. [
27] observed that high levels of supplemental far-red light might improve cold tolerance in both mature green and red tomatoes, reducing pitting, decay, and weight loss. In our research, a significant effect on cold tolerance was not observed. However, far-red light, applied both throughout the day and at the end-of-the-day, would seem to confirm an ability to delay weight loss, especially during the chilling phase (T1). In contrast to a former report by Affandi et al. [
27], mature green tomatoes treated with far-red light did not seem to have a faster turning to red compared with RB treatment, whereas the best performance was observed in the CK case.
Based on the results of our research, the application of additional LED light seems not economically feasible. In fact, considering the productive capacity of our experiment, the energy cost per kg is 1.27 EUR kg
−1 in Italy and 1.35 EUR kg
−1 in Spain, whereas selling prices reported by EUROSTAT [
28] in 2021 are 1.15 EUR kg
−1 and 0.74 EUR kg
−1 for Italy and Spain, respectively. Other authors have already observed scarce economic feasibility, estimating energy consumption of about 28.8 kWh to increase tomato yield by 1 kg [
50]. However, these observations need to be put into context. In particular, the country’s socio-economic condition, geo-political situation, access to renewable energy, as well as the cultivation latitude, and greenhouse light accessibility and transmissivity, may all influence the feasibility of supplementary LED lighting application to increase tomato yield. Besides that, greenhouse design, management of cultivation period, and lighting strategy are other important aspects to adapt to maximize the economic benefit. For instance, it has been observed that in a Northern European context, a year-round production with high technology investment (e.g., supplemental lighting, heat pump) can provide the most elevated economic return [
51]. This contextual approach could also be applied in the Mediterranean area, modifying some parameters according to the different climatic and socio-economic conditions. To give an example, our study looked only at the spring–summer time span, although the application during the cold-season could have resulted in more cost-effective results. Furthermore, our research referred to household on-grid electricity costs, applied to an urban BIA context intended for direct consumption by building users. Integration of alternative energy sources such as solar panels may drastically reduce the electricity cost, leading to a net profit increase both at domestic and commercial levels. Integrating the research with different lighting strategies (e.g., pulsed light, cold-season lighting, photoperiod reduction) should be considered better to estimate the economic benefits for the Mediterranean area.