Abstract
Mango is an extremely perishable fruit with a short postharvest time, and a considerable proportion of harvested mangoes become spoiled due to the postharvest decay in mango-producing areas of the world. The current study was designed to evaluate the effects of chitosan on the storage life of mango. Mango samples were coated with 750, 1000, and 1500 ppm chitosan solution, before storing them in the open or zip-bags under ambient and refrigeration conditions for different storage periods. Changes in different physical and chemical parameters were recorded to evaluate the treatments’ effectiveness in extending fruit shelf-life and sustaining postharvest quality of mangoes. The results showed that chitosan coating was able to reduce weight loss up to 65% in comparison to the uncoated control. Total mold and bacterial counts were also significantly lower in postharvest mangos when they were coated with chitosan compared to the uncoated samples. In addition, different fruit quality attributes, such as vitamin C content, titratable acidity, sugar content, ash, and protein content were also retained to a considerable extent by the chitosan coatings. Chitosan at refrigeration temperature (4 °C) with zip-bag packaging had a greater positive effect on fruit shelf-life, weight maintenance, and quality attributes than ambient temperature. Among the different coating concentrations, 1000 ppm chitosan solutions could provide better performance to extend the shelf-life of mango fruit while maintaining quality attributes. Altogether, our findings suggest that chitosan coating effectively prolongs the storage life of mango fruit and maintains fruit quality during storage, and offers promising potential for successful commercialization of this edible coating for mango growers and the industry.
1. Introduction
Mango (Mangifera indica L.) is considered as the king of oriental fruits, and has been widely cultivated in Bangladesh for 4000 years [1]. In 2020, Bangladesh ranked 10th by producing more than 1.4 million tons of mango among the mango-producing countries [2,3]. Mango is preferred by consumers because of its excellent tropical flavor and aroma, high antioxidant and anti-carcinogenic properties, and because it is a rich source of nutrients and several rare bioactive compounds [4]. However, the mango market is adversely affected because it is prone to numerous postharvest diseases and is highly perishable in nature. Moreover, being a climacteric fruit, the ripening process in mango fruit occurs very rapidly, which makes them soft and, hence, susceptible to attack by various pathogens [5]. These associated problems further limit their storage, handling, and transport potential over long distances for marketing and consumption, which ultimately affects the global mango trade. It has been estimated that about 25–45% of off-flavored, broken textured, and microbiologically spoiled mango goes to waste due to postharvest decay [6,7,8]. Therefore, to reduce the associated postharvest loss and to extend the postharvest shelf-life, different approaches, such as plant growth regulators, low temperatures, modified atmosphere packaging, irradiation, and coatings have been applied.
Similarly, application of chemical fungicides has long been used as a prevailing and effective technique to extend the shelf-life of harvested mangoes by reducing decay [9,10]. However, due to the negative environmental impact, formation of chemical residue, development of pathogen population resistance, and improvements in global standards of living and consumption, the application of chemical fungicides is no longer preferred to prevent postharvest decay in harvested mango [11]. In this context, there is an urgent need to explore alternative pollution-free postharvest decay control strategies for facilitating the development of the mango industry in respect to maintaining overall fruit quality and improving preservation efficiency. Edible biocompatible polymer coatings are a very promising technology which play an important role because they are considered as an efficient, safe means of preservation, leave no residue, are environmentally sound and not susceptible to drug resistance, and can successfully avoid moisture and aroma loss as well as inhibit the oxygen penetration to the plant tissue or microbial growth [12].
Chitosan, a naturally occurring alkaline polysaccharide, is a biodegradable macromolecule, originated from the deacetylation of nontoxic and bio-functional chitin [13]. Chitosan is one of the well-studied biopolymers, and it has a wide range of application prospects in agricultural production due to its inherent antimicrobial activity, low cost, abundant availability, nontoxicity, good film-forming properties, and biocompatibility. The strong inherent antimicrobial and antifungal properties of chitosan make it effective against fruit decay through the improvement of shelf-life and by inhibiting microbial infestation. Exogenous chitosan treatment decreases the transpiration and loss of firmness, increases the antioxidant capacity, and improves the overall quality of the harvested fruit [14]. By modulating the physiological metabolism of fruits, chitosan treatments are able to preserve the freshness for a longer period of time [15]. Previously, chitosan-based coatings, alone or incorporated with active agents, were successfully used in different fruits, such as guava, green tomato, plum, strawberry, mango, and kiwi, [16,17,18,19] to extend their storage life. In many countries, packaging has been introduced in farms to minimize quality breakdown of postharvest fruits, and this is one of the most frequently adopted postharvest practices. Packaging helps to modify the atmosphere during storage and transportation of fruits and vegetables, which helps to maintain them in utilized volumes, protect them from hazards and allowing for easy handling. Zip-bags are one of the readily available packaging materials that can be used as an effective technique for sealing actively respiring mangoes and to protect them from pathogens and contaminants in order to improve shelf-life [20]. The main cause of postharvest degradation is the enhanced metabolism due to the natural senescence mechanism or stress, which is influenced the by temperature and humidity around the fruits or vegetables [21]. Therefore, it is necessary to modify storage temperature to inhibit metabolism related to quality and shelf-life degradation during different marketing levels.
However, to date, there is no report of using chitosan and zip-bags for the improvement of postharvest shelf-life of mango in Bangladesh to prevent the significant economic loss due to the rapid perishability of harvested mango. Moreover, until now, very little work has been carried out to study the effect of chitosan, low temperature, and zip-bag packaging regarding the biochemical responses of mango fruit. Keeping this mind, the current study was designed to investigate the potential effects of a package of practices including temperature, open or zip-bag packaging, and chitosan coatings, on the shelf-life extension of mango fruit as well as the quality attributes during postharvest storage to determine the interaction between chitosan and mango fruit apart from their protection against decay.
2. Materials and Methods
2.1. Plant Material
Mature (75–82 days old) mango fruits (Mangifera indica L. cv. Gopal vogh) were harvested from a local orchard located in Rajshahi district, Bangladesh. In this study, we selected Gopal vogh as an experimental mango variety because it is one of the widely cultivated early varieties in Bangladesh with a delicious fruity sweet taste. The attribute of a golden colored non-fibrous tender texture makes it popular among consumers, but at the same time the short shelf-life makes it difficult to store. The harvested fruit were transferred immediately to the Institute of Radiation and Polymer Technology (IRPT) laboratory, Bangladesh Atomic Energy Commission, Savar, Dhaka, Bangladesh. Uniform-sized fruits were used for this research, and selections were made based on the uniformity of ripeness, size, color, shape, and the absence of defects, injuries, and fungal infection.
2.2. Preparation of Chitosan Solution
Chitosan was extracted from prawn shell waste in the laboratory using the deacetylation process described by Rashid et al. [22]. The high molecular weight chitosan solution (molecular weight = 83 KD, viscosity less than 200 mPa-s, deacetylation degree 82.7%, 2% acetic acid in 55 °C) was obtained by applying a dose of 3.2 kGy per hour generated from a 120 k Curie radiation source [22]. Laboratory grade chemicals were used for the solution preparation. Among different doses of gamma irradiated chitosan solution, 40 kGy was selected based on initial observatory test on the weight loss, smell, color, and texture of mango samples.
2.3. Treatments and Sample Preparation
The experimental procedure and treatments are depicted in Figure 1. In a nutshell, the collected mangoes were washed with distilled water and dried at room temperature, followed by dipping into different irradiated chitosan solutions, e.g., 0 (control), 750, 1000, and 1500 ppm, for two minutes. After air drying at room temperature, control and chitosan-coated samples were stored in the open or in zip-bags at ambient (25–28 °C) and refrigeration (4 °C) temperature, respectively, with 70 to 85% of relative humidity during the entire storage period. The overripened and spoilage state of the samples stored at the ambient temperature were investigated daily, while the samples stored in the refrigeration temperature environment were investigated at five day intervals.
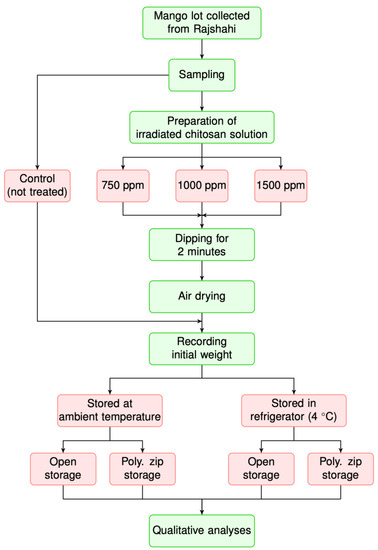
Figure 1.
Schematic flow of the experimental procedure. The red-filled boxes denote the experimental factors.
2.4. Weight Loss Determination
Weight loss of mangoes was determined at definite time intervals based on the initial weight. The percentage of weight loss was calculated using the following equation:
2.5. Total Bacteria Count (TBC) and Total Mold Count (TMC) Determination
Plate count agar (PCA) and potato dextrose agar (PDA) media were used to estimate the bacterial and fungal counts, respectively. For this, 10 mL of saline water was mixed with 1 g of fruit sample. Then, 10 µL of mixed sample was spread in PCA and PDA plates. The plates were incubated for 24 h at 37 °C and 25 °C, respectively. Finally, the colony forming units (cfu) were counted with three replications.
2.6. Chemical Analyses
After three weeks of storage, chitosan-treated samples were analyzed to determine moisture content, ash content, acidity, and vitamin C, sugar, and protein contents by standard methods. Due to the high spoilage rate observed in untreated mango fruit, these samples were subjected for chemical analysis after one week. Three replications were considered for the whole experimentation.
2.6.1. Determination of Vitamin C (Vit-C) Content
The concentration of vitamin C in fruit samples was determined using the titrimetric method [23]. 2,6-dichlorophenol indophenol dye was used to determine the end point of titration. The following formula was used for vitamin C content determination:
2.6.2. Determination of Ash Content
The total ash content was determined using the AOAC [24] method. Briefly, 2.0 g of mango pulp was measured and transferred into a clean and dry crucible. Then, the crucible was kept in a muffle furnace at 550 °C for 6 h and cooled at a desiccator and weighed.
The ash content was calculated as follows:
2.6.3. Determination of Total Protein Content
The protein content of mango pulp was determined using the AOAC [25] method with some modifications.
The following formulas were used for the calculation of N and protein content:
where the normality of H2SO4 = 0.2, and mL equivalent of N = 1.4. The following equation was employed to calculate protein content:
where protein factor = 5.5
2.6.4. Determination of Titratable Acidity
Titratable acidity was determined by dissolving approximately 5 g of homogenized mango pulp in 40 mL of distilled water and filtering the solution through cotton wool. Then, the filtrate (5 mL) was titrated against 0.1 N NaOH using 1% phenolphthalein solution as an indicator [26]. The appearance of a pink color signaled the endpoint of the titration reaction (pH 8.1). The results were expressed as the percentage of citric acid (%) per 100 mL of juice [17,18].
2.6.5. Determination of Sugar Content
The total soluble sugars of mango pulp were determined using the methods described by DuBois et al. [27]. In brief, approximately 0.5 g filtered mango pulp sample were mixed well with 10 mL of 80% ethanol following centrifugation for 20 min at 2000× g. Then, the extraction solution was prepared by adding 1 mL of supernatant in the prepared 1 mL (5%) phenol solution. Then, 5 mL of 95.5% H2SO4 was added to the sample and we allowed the test tubes to stand for 10 min before vortexing for 30 sec. Following this, the test tubes were placed in a water bath at room temperature for 20 min for color development. Finally, the absorbance was recorded at 490 nm using a spectrophotometer (UV 1800 Shaanxi, China), and the results were expressed in µg/g of fresh weight of mango pulp sample.
2.7. Statistical Analysis
The experiment was performed in a completely randomized design (CRD) with three replications with a two-factorial interaction of storage and chitosan treatments. The first factor corresponded to the four levels of storage conditions i.e., ambient temperature open storage, ambient temperature zip-bag storage, refrigeration temperature open storage, and refrigeration temperature open storage, while the second factor corresponded to different concentrations of chitosan solution, i.e., 0 (control), 750 ppm, 1000 ppm, and 1500 ppm. Two fruits were sampled from each replication for each analysis. The data analyses were carried out using the statistical package ‘R’ [28]. All data were subjected to two-way ANOVA. The averages of treatment means were compared by two-way ANOVA followed by Tukey’s post hoc test (p = 0.05).
3. Results
3.1. Weight Loss and Shelf-Life
The effects of chitosan coatings, storage temperature (ambient and refrigeration), and conditions (open or in a zip-bag) were significant on fruit weight loss. Chitosan-coated and uncoated (control) Gopal vogh mangoes kept in the open or in a zip-bag were observed daily for 10- and 11-day storage periods (DSP) at ambient temperature, respectively (Figure 2a,c). However, weight changes in refrigeration temperature-stored mangoes were observed at 5-day intervals for 40 and 45 DSP by storing them in the open or in a zip-bag, respectively (Figure 2b,d). It is evident from the results that chitosan coatings significantly reduced the weight loss of mango fruit during storage compared to the controls (Figure 2a–d). Under ambient temperature storage, control fruit presented higher weight losses over time, reaching 25.6% and 3.4% for the open or zip-bag, respectively, at 6 DSP. However, chitosan-treated fruits showed lower values for weight loss, ranging from 12.2 to 13.2% and 1.2 to 1.4% for the open or zip-bag samples, respectively, at the same storage period; the estimated reduced weight loss is about 44–50% for the open and 51–65% for the zip-bags compared to the control mangoes. However, refrigeration storage temperature significantly increased the storage duration of coated and uncoated mangoes by reducing the weight loss. At 25 DSP, weight loss of the uncoated fruits in the open or in zip-bags were recorded as 28.5 and 1.1%, respectively, while the weight loss of the chitosan-coated samples varied from 14.1 to 15.5% and 0.50 to 0.75% for the open or zip-bagged samples, respectively (Figure 2b,d).
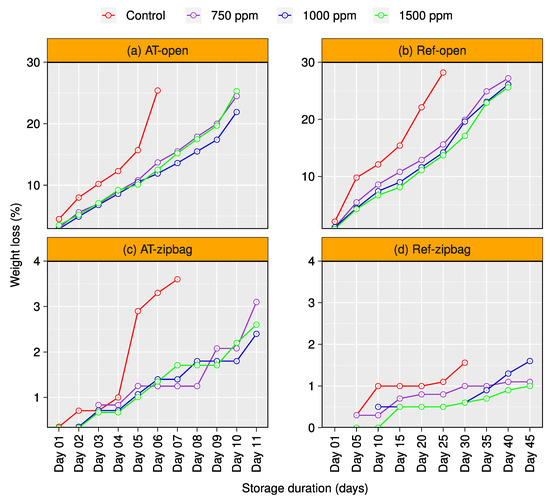
Figure 2.
Evaluation of weight loss (%) of chitosan-treated and untreated Gopal vogh mango stored in the open or in zip-bags at ambient temperature and refrigeration (Ref) temperature (a–d).
Based on our results, zip-bag packaged mangoes were found to be more capable of retaining weight than open-stored fruits during the storage period at all conditions. Chitosan coatings significantly reduced the weight loss irrespective of storage environments, which led to an increase in the storage life. Among the different chitosan treatments, mangoes coated with 1000 ppm chitosan maintained weight efficiently compared to control and other chitosan treatments throughout the storage periods in all storage environments, with a few exceptions (Figure 2a–d).
3.2. Microbiological Changes
3.2.1. Total Bacteria Count (TBC)
Chitosan-coated and control mango fruits stored in the open or zip-bagged were evaluated to estimate the microbial changes (TBC and TMC) after 1 week of ambient and 3 weeks of refrigeration temperature storage (Figure 3 and Figure 4). The amount of TBC was significantly (p ≤ 0.05) reduced for both the open and zip-bag-stored chitosan-treated mangoes compared to the controls for both storage (ambient and refrigeration) environments (Figure 3a,c). The occurrence of TBC was found to be higher in ambient storage than in refrigeration. Fruits treated with chitosan contained about 52–73% reduced TBC compared to the untreated fruits, while 1000 ppm chitosan treatment provided the highest (73%) reduction in TBC (Figure 3b).
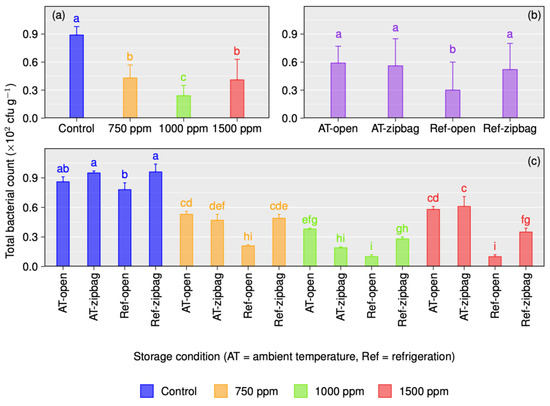
Figure 3.
Total bacteria count (TBC) of different concentration of chitosan-treated and untreated Gopal vogh mango stored in the open or in zip-bags at ambient temperature and refrigeration temperature (a–c). Means with the same letters are not statistically different from each other as per Tukey’s post hoc test (p = 0.05).
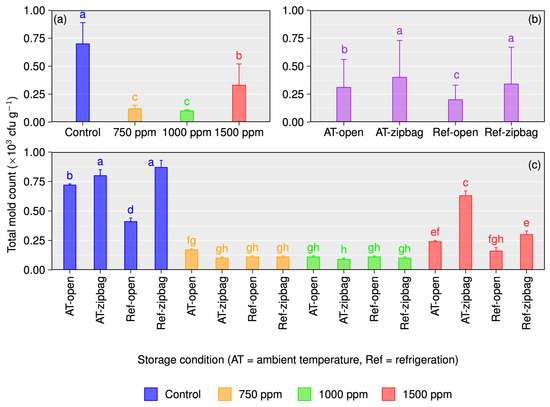
Figure 4.
Total mold count (TBC) for different concentrations of chitosan-treated and untreated Gopal vogh mango stored in the open or zip-bagged at ambient temperature and refrigeration temperature (a–c). Means with the same letters are not statistically different from each other as per Tukey’s post hoc test (p = 0.05).
In terms of storage environment and chitosan treatment interaction, the amount of TBC on chitosan-treated mangoes stored in the open ranged from 0.38 × 102 to 0.58 × 102 cfu/g, which was comparatively higher than the zip-bagged samples, varying from 0.19 × 102 to 0.61 × 102 cfu/g TBC under the ambient storage environment. However, contrasting results were recorded for refrigeration storage and control samples, where TBC was higher in the zip-bag sample than for the open-stored fruits (Figure 3c). Among the different chitosan concentrations and storage environments, the significantly lowest (0.10 × 102 cfu/g) amounts of TBC were recorded for mangoes coated with 1000 and 15,000 ppm chitosan solution for both storage environments (Figure 3c).
3.2.2. Total Mold Count (TMC)
As with TBC, chitosan-treated mangoes had a significantly lower total mold count (TMC) than control samples under all storage circumstances (Figure 4a–c).
In the case of ambient temperature storage, the occurrence of TMC was 0.72 × 103 cfu/g (open) and 0.80 × 103 cfu/g (zip-bagged) for control samples, which was significantly higher than for the different concentrated chitosan-treated mangoes, ranging from 0.11 × 103 to 0.24 × 103 and 0.09 × 103 to 0.63 × 103 for open or zip-bagged storage, respectively (Figure 4c). Similar trends were also observed for storing mangoes at refrigeration temperature either in the open or zip-bagged. However, our results revealed that fruits treated with 1500 ppm chitosan recorded increased amounts of TMC compared to 750 and 1000 ppm chitosan treatments under all storage conditions. Among the different chitosan treatments, 1000 ppm was the most efficient in controlling the TMC for all storage environment fruits.
3.3. Nutritional Changes
Chitosan-coated and uncoated fruits were subjected to biochemical analyses to determine the nutritional changes in the ambient and refrigeration storage fruits. Two-way ANOVA results showed the main effects of factors (storage conditions, chitosan treatments) and interaction effects of storage conditions and chitosan treatments were significant on vitamin C, titratable acidity, sugars, ash, and protein (p < 0.05) (Table 1).

Table 1.
Vitamin C, titratable acidity, sugar, ash, and protein content of chitosan-treated and untreated Gopal vogh mango stored in the open or in zip-bags at ambient temperature or refrigeration temperature.
3.3.1. Vitamin C (Vit-C) Content
After the respective storage periods, the lowest amount of ascorbic acid (vitamin C) was observed for control mangoes, whereas an increase in ascorbic acid (Vit-C) content was observed for chitosan-treated mangoes. The highest (4.40%) amount of Vit-C was observed for refrigerated fruits stored in zip-bags, while the lowest (2.0%) Vit-C was recorded for ambient open-stored fruits. Among the chitosan treatments, mangoes coated with 750 ppm chitosan performed well in controlling Vit-C degradation in both storage environments. In terms of storage environment and states, refrigerated and zip-bagged coated mangoes were more efficient in maintaining Vit-C degradation than the ambient temperature and zip-bagged mangoes (Table 1). Packaging mangoes in zip-bags had a highly significant effect in maintaining higher Vit-C, thus, prolonging fruit senescence. For example, chitosan-coated mangoes stored in zip-bags displayed about 8 to 37% and 2 to 15% more Vit-C content than those stored in the open under ambient and refrigeration storage, respectively (Table 1).
3.3.2. Titratable Acidity (TA)
Titratable acidity is responsible for the sourness of fruit. The TA values in chitosan-coated fruits were found to be higher than the untreated (control) mangoes at all storage environments and states. Results showed that chitosan-treated mangoes stored in refrigerated environments showed higher amounts of TA compared to the ambient environment storage (Table 1). The ambient temperature zip-bagged fruits displayed 75 to 85% more TA than open-stored fruits. However, contrasting results were recorded for refrigerated zip-bag-stored fruits, which displayed 18 to 36% reduced TA compared to open-stored fruits. The highest amount of acidity was observed for 1000 ppm chitosan-coated mangoes, whereas the lowest value was observed for 1500 ppm under all storage environments.
3.3.3. Sugar Content
The amount of sugar content in stored mangoes decreased with increasing chitosan concentration in most of the cases (Table 1). Sugar content was high in untreated mangoes compared to the chitosan-coated fruits in all storage environments. The chitosan-treated mangoes delayed about 8 to 14% (open) and 0.2 to 6% (zip-bagged) of sugar accumulation for the ambient temperature storage, while the values were 25 to 30% (open) and 3 to 17% (zip-bagged) for refrigerated mangoes (Table 1). Mangoes treated with 1000 ppm chitosan solutions showed the lowest sugar content in all storage environments and states except for the refrigeration with open storage environments. Overall, mangoes stored under refrigeration showed lower amounts of sugar content than ambient temperature storage.
3.3.4. Ash Content
Chitosan-treated mangoes showed relatively high ash content compared to control mangoes (Table 1). At ambient temperature storage, untreated mangoes showed a similar amount of ash content to chitosan-coated mangoes. However, an increasing amount of ash content was recorded for chitosan-treated mangoes stored under refrigeration. Under refrigeration storage, chitosan-treated mangoes showed a 7 to 14% and 1 to 79% increase in ash content for open or zip-bag stored mangoes, respectively, compared with the untreated mangoes (Table 1).
3.3.5. Protein Content
The protein content of the chitosan-coated and control samples were presented in Table 1. Protein content was subsequently lower in untreated (control) mangoes in all storage environments and conditions than in the chitosan-coated mangoes. Under ambient temperature, chitosan-coated mangoes displayed 7 to 14% and 1 to 79% more protein compared to the control samples. Similar trends were also noted for refrigeration storage environments. In all storage environments, zip-bag packaged mangoes were found to be more effective in maintaining protein content than open conditions during the storage period. Chitosan coatings on mangoes at 1000 ppm preserved the most protein compared to the other chitosan treatments and untreated mangoes for all storage environments and conditions (Table 1). Based on the results, it is quite evident that mangoes stored in zip-bags preserved relatively more protein than the open condition under all storage environments.
4. Discussion
Mango is an economically important, excellent source of nutritious fruit, and is a significant source of income for many farmers in tropical areas, especially in Bangladesh. Short shelf-life and a lack of proper postharvest management has limited mango exports to distant markets and leads to a significant monetary loss due to the rapid postharvest decay [29]. Chitosan is a natural edible coating and has been used in an attempt to preserve and to increase the postharvest life of different fruits because of its film-forming, biochemical, inherent antifungal, and eliciting properties [30]. Zip-bags are the most used polymers for food, due to their low cost, high availability, and because they work as a barrier between fruits and the external environment. Therefore, in our current study we investigate the potential of chitosan coating, zip-bags, and low temperature to improve the postharvest shelf-life of harvested mature mangoes. The present study results clearly demonstrated that chitosan might be a viable alternative in extending the postharvest shelf-life of mango and that it functions as a controlling agent of the nutrient levels under ambient temperature and refrigeration environment in both open and zip-bag storage conditions.
Mango fruit ripening is characterized by textural softening, changes in their green color to yellow, sugar content, and acidity [18]. Our results showed that chitosan, low temperature, and zip-bag packaging can effectively delay ripening of the mango fruit by reducing the fresh weight loss of fruit. Loss of water from the fruit during postharvest is directly associated with the reduction in weight and fruit shriveling [31,32]. In our study, we found that chitosan-treated mangoes reduced up to 65% of the weight loss of postharvest mangoes under ambient temperature and refrigeration environments. Mango fruits coated with 1000 ppm chitosan were found to be more efficient in maintaining fruit weight during the storage period. Our results corroborated with other researchers’ findings that the use of chitosan-based formulations, alone or incorporated with active agents, reduced postharvest weight loss and provide better results in extending the shelf-life of mango [17,18,33]. These positive effects may be due to fact that chitosan coating acts as a physical barrier for water loss by reducing the respiration and transpiration process through the fruit surfaces, and thereby delayed the fruit ripening and prolonged the shelf-life of harvested mangoes [30]. However, compared to the refrigerated storage, a steep reduction in weight loss was noted in ambient temperature, which is consistent with the findings of Kumar et al. [34]. Moreover, mangoes stored in the open-air recorded a greater reduction in weight loss than zip-bagged mangoes irrespective of the storage environments studied. Similarly, Tefera et al. [35] found greater weight retention for polythene bag-packaged mangoes during the storage period. Low temperature provides the advantage of extending the storage life by maintaining constant relative humidity, lowering the cell metabolism rate, and by delaying the senescence and ripening of fruits. Packaging plays a role to change the surrounding atmosphere by reducing O2 and increasing CO2 and lowers the transpiration rate relative to fresh fruits, which in turn limits wilting, shriveling, color changes, senescence, and retains the firmness of fresh fruit. An increasing level of CO2 in the surrounding atmosphere reduces the activities of pectin esterase, which reduces pectin depolymerization and helps to retain firmness in fruits [36]. Moreover, packaging increases the water vapor barrier inside the zip-bag and reduces the water loss from fruit cells [37].
The postharvest quality of mangoes is influenced by bacteria and mold [38,39]. Harvested fruits coated with chitosan formulations were known to inhibit the incidences of postharvest diseases. Our current study results clearly indicated that chitosan coating was effective in reducing the microbial (TBC and TMC) incidence in mango fruits. The reduction in the growth of microbial populations treated with chitosan is in agreement with previous studies observed by Ngo et al. [40] in mango and Fortunati et al. [41] in kiwifruit. Chitosan prevents the microbial growth through the antimicrobial activity mechanisms that involve interaction with microorganism’s cell wall, cell membrane, and cytoplasmic constituents via electrostatic interactions [30,33]. Consequently, inhibition of microbial population growth during the postharvest storage of mangoes prevents them from rapid decay and, thus, increases their shelf-life. Moreover, temperatures below 5 °C can effectively inhibit the growth rate and degree of pathogenicity of most of the postharvest pathogens [42,43]. Similar results were also observed in this study. The resonant combination of chitosan coating and refrigeration was highly effective in delaying the microbial incidence.
Chitosan coating and storage environment and state also affect the loss of nutrient content, chemical characteristics, and quality attributes of the postharvest mangoes. Chitosan treatment maintains the fruit quality by retaining the amount of vitamin C (ascorbic acid), titratable acidity, and ash and protein content while reducing the sugar content during postharvest storage in all conditions. Vitamin C is one of the most well-known antioxidant compounds, and it protects postharvest storage fruits from the damaging effects of reactive oxygen species [17]. The results of our study indicate that the content of Vit-C was significantly higher in chitosan-coated mangoes compared to the untreated mangoes during storage. The refrigerated (4 °C) mango fruits displayed more Vit-C than ambient temperature-stored fruits which is in line with Tefera et al. [35]. Therefore, mangoes with chitosan coatings and low temperature show delayed fruit ripening, since coating reduces ascorbic acid degradation and helps to improve shelf-life. Additionally, titratable acidity and sugar contents are important sensory attributes used as maturity- and ripening-related indices in fruit quality measurements. Citric and malic acid are the two most abundant organic acids in mango responsible for fruit acidity [18]. As the fruit storage period increases, fruit acidity declines, and a rapid decrease in titratable acidity indicates senescence [17]. An increased amount of titratable acidity was observed in chitosan-coated mangoes compared to the uncoated mangoes at all storage temperatures in the current study. Moreover, chitosan coating significantly delayed sugar accumulation and starch degradation, retarding the ripening process. However, mangoes stored at ambient temperature showed accelerated fruit ripening due to the higher accumulation of sugars in mango fruits than refrigeration temperature. Moreover, zip-bag packaged fruits showed reduced sugar content and improved shelf-life compared to open-stored mangoes. Changes in the levels of titratable acidity and sugar contents were significantly reduced by chitosan coatings and temperature, extending mango fruits’ shelf-life, corroborating the findings reported by other researchers [17,18,33,44,45]. In our recent findings, maintenance of Vit-C content and acidity and reduced sugar accumulation effects seem to be due the fact that chitosan coating forms a thin layer on the fruit’s surface and creates a physical barrier to delay the physiological and biochemical process, such as slower conversion of starch into sugars, by modifying the gas exchange within the atmosphere, which directly decreases the respiration rate and fruit ripening [17,18,44,46,47]. Again, zip-bag packaging also improves the shelf-life and quality by slowing down the degradation of titratable acidity due to the reduction in their consumption as respiratory substrates [48]. Zip-bag packaged fruits stored in low temperatures also tend to show a decrease in ethylene concentration and fruit metabolism [49], which explains the occurrence of the higher value of Vit-C and lower value of reducing sugar by deferring the respiration and ripening of the packaged fruits. Moreover, it was reported that storing fruit at high temperatures amplifies enzymatic catalysis that promotes biochemical breakdown of compounds [49], which ultimately leads to quality decay. Furthermore, ash and protein contents were recorded as being comparatively higher in chitosan-coated mangoes than in untreated mangoes, which clearly indicates the usefulness of edible coatings in preserving the nutritional qualities of post-harvested mangoes during storage periods and extending the shelf-life of mango.
5. Conclusions
Our findings indicate that chitosan coating has great potential and could be beneficial in extending shelf-life and preserving the quality of postharvest storage mango fruits (cv. Gopal vogh). Chitosan coating successfully inhibited decay, controlled weight loss, and decreased postharvest diseases by reducing TBC and TMC in mango fruit during storage. Moreover, chitosan treatments also conferred benefits by retaining quality attributes, such as vitamin C, titratable acidity, and the sugars, ash, and protein content of the mango fruit during storage. Our results demonstrated that coating with 1000 ppm chitosan solution and zip-bag packaging in low temperatures may provide better performance to extend the shelf-life of mango fruit while maintaining quality attributes. Therefore, chitosan formulation seems to be a promising and sustainable solution to maintain postharvest quality and to extend the shelf-life of mangoes. However, a comprehensive study is needed for successful commercialization of the edible coating for the mango fruit growers and industry in Bangladesh, which would ultimately reduce the associated monetary loss due to the postharvest damages to mango. Our future efforts would be directed to study different parameters, such as enzymatic studies, antioxidant activity, membrane damage, etc., associated with increasing the storage life of chitosan-coated mangoes.
Author Contributions
Conceptualization, N.P., A.R. and M.A.K. (Md. Abdul Kader); methodology, N.P., M.A.K. ( Md. Abdul Kader) and M.A.S.; software, M.H.R.; validation, M.E.M., F.M.J.U. and M.H.K.; formal analysis, M.H.R. and J.R.; investigation, M.E.M. and M.A.K. (Md. Abdul Kader); resources, M.E.M. and M.A.K. (Mubarak A. Khan); data curation, A.R. and M.H.R.; writing—original draft preparation, N.P., J.R., A.R. and M.H.R.; writing—review and editing, M.H.R., A.R., J.R., M.A.S., N.C.P., M.A.M. and S.I.; visualization, J.R., A.R., M.A.S. and M.H.R.; supervision, M.A.K. (Mubarak A. Khan) and M.E.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Acknowledgments
Thank you to the Institute of Radiation and Polymer Technology (IRPT) laboratory, Bangladesh Atomic Energy Commission, Savar, Dhaka, for providing logistical support for the research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sarker, S.R.; Islam, M.R.; Hossain, I. Prevalence and eco-friendly management of some important nursery diseases of mango in Bangladesh. J. Agric. Sci. 2015, 8, 205. [Google Scholar] [CrossRef][Green Version]
- FAOSTAT. Food and Agriculture Organization of The United Nations. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 1 April 2022).
- Tridge. Fresh Mango. Available online: https://www.tridge.com/intelligences/mango/production (accessed on 1 April 2022).
- Afifa, K.; Kamruzzaman, M.; Mahfuza, I.; Afzal, H.; Arzina, H.; Roksana, H. A comparison with antioxidant and functional properties among five mango (Mangifera indica L.) varieties in Bangladesh. Int. Food Res. J. 2014, 21, 1501. [Google Scholar]
- Giovannoni, J.; Nguyen, C.; Ampofo, B.; Zhong, S.; Fei, Z. The epigenome and transcriptional dynamics of fruit ripening. Annu. Rev. Plant Biol. 2017, 68, 61–84. [Google Scholar] [CrossRef] [PubMed]
- Alam, S.M.K.; Rahman, M.A.; Reza, M.H.; Amin, M.N.; Hussen, M.A.M. Postharvest loss assessment of mango at different stages of supply chain through traditional and improved handling practices. Adv. Plants Agric. Res. 2019, 9, 384–388. [Google Scholar]
- Begum, M.; Marium, B.; Farid, M.S.; Hasan, M. Post-harvest loss assessment and marketing practices of fruits: An empirical study of Maulvibazar District in Bangladesh. J. Econ. Manag. Trade 2022, 28, 15–27. [Google Scholar] [CrossRef]
- Rahman, M.A.; Saha, M.G.; Nasrin, T.A.A.; Islam, M.N.; Uddin, M.S.; Arfin, M.S. Postharvest loss assessment of mango in the existing value chain of Bangladesh. J. Bangladesh Hortic. 2017, 3, 12–22. [Google Scholar]
- Diskin, S.; Sharir, T.; Feygenberg, O.; Maurer, D.; Alkan, N. Fludioxonil—A potential alternative for postharvest disease control in mango fruit. Crop. Prot. 2019, 124, 104855. [Google Scholar] [CrossRef]
- Swart, S.H.; Serfontein, J.J.; Swart, G.; Labuschagne, C. Chemical control of post-harvest diseases of mango: The effect of fludioxonil and prochloraz on soft brown rot, stem-end rot and anthracnose. Acta Hortic. 2009, 820, 503–510. [Google Scholar] [CrossRef]
- Sellitto, V.M.; Zara, S.; Fracchetti, F.; Capozzi, V.; Nardi, T. Microbial biocontrol as an alternative to synthetic fungicides: Boundaries between pre- and postharvest applications on vegetables and fruits. Fermentation 2021, 7, 60. [Google Scholar] [CrossRef]
- Jianglian, D. Application of chitosan based coating in fruit and vegetable preservation: A review. J. Food Process. Technol. 2013, 4, 1–4. [Google Scholar] [CrossRef]
- Zhao, J.; Pan, L.; Zhou, M.; Yang, Z.; Meng, Y.; Zhang, X. Comparative physiological and transcriptomic analyses reveal mechanisms of improved osmotic stress tolerance in annual ryegrass by exogenous chitosan. Genes 2019, 10, 853. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, Z.; Tang, W.; Zhang, Q.; Lu, B.; Li, Q.; Zhang, G. Impact of chitosan, sucrose, glucose, and fructose on the postharvest decay, quality, enzyme activity, and defense-related gene expression of strawberries. Horticulturae 2021, 7, 518. [Google Scholar] [CrossRef]
- Badawy, M.E.I.; Rabea, E.I.; El-Nouby, M.A.M.; Ismail, R.I.A.; Taktak, N.E.M. Strawberry shelf life, composition, and enzymes activity in response to edible chitosan coatings. Int. J. Fruit Sci. 2016, 17, 117–136. [Google Scholar] [CrossRef]
- Drevinskas, T.; Naujokaitytė, G.; Maruška, A.; Kaya, M.; Sargin, I.; Daubaras, R.; Česonienė, L. Effect of molecular weight of chitosan on the shelf life and other quality parameters of three different cultivars of Actinidia kolomikta (kiwifruit). Carbohydr. Polym. 2017, 173, 269–275. [Google Scholar] [CrossRef]
- Shah, S.; Hashmi, M.S. Chitosan–aloe vera gel coating delays postharvest decay of mango fruit. Hortic. Environ. Biotechnol. 2020, 61, 279–289. [Google Scholar] [CrossRef]
- Silva, G.M.C.; Silva, W.B.; Medeiros, D.B.; Salvador, A.R.; Cordeiro, M.H.M.; da Silva, N.M.; Santana, D.B.; Mizobutsi, G.P. The chitosan affects severely the carbon metabolism in mango (Mangifera indica L. cv. Palmer) fruit during storage. Food Chem. 2017, 237, 372–378. [Google Scholar] [CrossRef]
- Parvin, N.; Kader, M.A.; Huque, R.; Molla, M.E.; Khan, M.A. Extension of shelf-life of tomato using irradiated chitosan and its physical and biochemical characteristics. Int. Lett. Nat. Sci. 2018, 67, 16–23. [Google Scholar] [CrossRef]
- Hailu, M.; Seyoum Workneh, T.; Belew, D. Effect of packaging materials on shelf life and quality of banana cultivars (Musa spp.). J. Food Sci. Technol. 2014, 51, 2947–2963. [Google Scholar] [CrossRef]
- Wills, R.H.H.; Lee, T.H.; Graham, D.; McGlasson, W.B.; Hall, E.G. Postharvest. An Introduction to the Physiology and Handling of Fruit and Vegetables, 3rd ed.; Springer: New York, NY, USA, 1989. [Google Scholar]
- Rashid, T.U.; Rahman, M.M.; Kabir, S.; Shamsuddin, S.M.; Khan, M.A. A new approach for the preparation of chitosan from γ-irradiation of prawn shell: Effects of radiation on the characteristics of chitosan. Polym. Int. 2012, 61, 1302–1308. [Google Scholar] [CrossRef]
- Harris, L.J.; Ray, S.N. Determination of plasma Ascorbic acid by 2, 6-dichorphenol indophenols titration. Lancet 1935, 1, 462. [Google Scholar] [CrossRef]
- AOAC. Association of Official Analytical Chemists, 12th ed.; Method 14.006; The Association of Official Analytical Chemists: Gaithersburg, MD, USA, 1975. [Google Scholar]
- AOAC. Association of Official Analytical Chemists, 17th ed.; Method 7.056; The Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2002. [Google Scholar]
- Ranganna, S. Handbook of Analysis and Quality Control for Fruit and Vegetable Products, 2nd ed.; Tata McGraw-Hill Education: New York, NY, USA, 1986. [Google Scholar]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 12 August 2022).
- Ntsoane, M.L.; Zude-Sasse, M.; Mahajan, P.; Sivakumar, D. Quality assesment and postharvest technology of mango: A review of its current status and future perspectives. Sci. Hortic. 2019, 249, 77–85. [Google Scholar] [CrossRef]
- Kumarihami, H.M.P.C.; Kim, Y.-H.; Kwack, Y.-B.; Kim, J.; Kim, J.G. Application of chitosan as edible coating to enhance storability and fruit quality of kiwifruit: A review. Sci. Hortic. 2022, 292, 110647. [Google Scholar] [CrossRef]
- Sogvar, O.B.; Saba, M.K.; Emamifar, A. Aloe vera and ascorbic acid coatings maintain postharvest quality and reduce microbial load of strawberry fruit. Postharvest Biol. Technol. 2016, 114, 29–35. [Google Scholar] [CrossRef]
- Bambalele, N.L.; Mditshwa, A.; Magwaza, L.S.; Tesfay, S.Z. Recent advances on postharvest technologies of mango fruit: A review. Int. J. Fruit Sci. 2021, 21, 565–586. [Google Scholar] [CrossRef]
- Prashanth, K.V.H.; Baskaran, R.; DhanyaSri, E.B. Rajashekaramurthy, Bioactive chitosan based coatings: Functional applications in shelf life extension of Alphonso mango—A sweet story. Pure Appl. Chem. 2016, 88, 853–863. [Google Scholar] [CrossRef]
- Kumar, N.; Pratibha, N.; Petkoska, A.T.; Al-Hilifi, S.A.; Fawole, O.A. Effect of Chitosan–Pullulan Composite Edible Coating Functionalized with Pomegranate Peel Extract on the Shelf Life of Mango (Mangifera indica). Coatings 2021, 11, 764. [Google Scholar] [CrossRef]
- Tefera, A.; Seyoum, T.; Woldetsadik, K. Effect of Disinfection, Packaging, and Storage Environment on the Shelf Life of Mango. Biosyst. Eng. 2007, 96, 201–212. [Google Scholar] [CrossRef]
- Kahramanoğlu, İ.; Usanmaz, S. Improving Postharvest Storage Quality of Cucumber Fruit by Modified Atmosphere Packaging and Biomaterials. HortScience 2019, 54, 2005–2014. [Google Scholar] [CrossRef]
- Chiabrando, V.; Garavaglia, L.; Giacalone, G. The Postharvest Quality of Fresh Sweet Cherries and Strawberries with an Active Packaging System. Foods 2019, 8, 335. [Google Scholar] [CrossRef]
- Jha, S.N.; Jaiswal, P.; Narsaiah, K.; Bhardwaj, R.; Sharma, R.; Kumar, R.; Basediya, A.L. Post-harvest micro-flora on major cultivars of Indian mangoes. Sci. Hortic. 2010, 125, 617–621. [Google Scholar] [CrossRef]
- Govender, V.; Korsten, L.; Sivakumar, D. Semi-commercial evaluation of Bacillus licheniformis to control mango postharvest diseases in South Africa. Postharvest Biol. Technol. 2005, 38, 57–65. [Google Scholar] [CrossRef]
- Ngo, T.M.P.; Nguyen, T.H.; Dang, T.M.Q.; Do, T.V.T.; Reungsang, A.; Chaiwong, N.; Rachtanapun, P. Effect of Pectin/Nanochitosan-Based Coatings and Storage Temperature on Shelf-Life Extension of “Elephant” Mango (Mangifera indica L.) Fruit. Polymers 2021, 13, 3430. [Google Scholar] [CrossRef]
- Fortunati, E.; Giovanale, G.; Luzi, F.; Mazzaglia, A.; Kenny, J.; Torre, L.; Balestra, G. Effective postharvest preservation of kiwifruit and romaine lettuce with a chitosan hydrochloride coating. Coatings 2017, 7, 196. [Google Scholar] [CrossRef]
- Erkmen, O.; Faruk Bozoglu, F.T. Food Microbiology: Principles into Practice; Wiley: Hoboken, NJ, USA, 2016. [Google Scholar]
- Lers, A. 27-Potential application of biotechnology to maintain fresh produce postharvest quality and reduce losses during storage. In Plant Biotechnology and Agriculture; Altman, A., Hasegawa, P.M., Eds.; Academic Press: San Diego, CA, USA, 2012; pp. 425–441. [Google Scholar]
- Hesami, A.; Kavoosi, S.; Khademi, R.; Sarikhani, S. Effect of chitosan coating and storage temperature on shelf-life and fruit quality of Ziziphus mauritiana. Int. J. Fruit Sci. 2021, 21, 509–518. [Google Scholar] [CrossRef]
- Suseno, N.; Savitri, E.; Sapei, L.; Padmawijaya, K.S. Improving shelf-life of cavendish banana using chitosan edible coating. Procedia Chem. 2014, 9, 113–120. [Google Scholar] [CrossRef]
- Basumatary, I.B.; Mukherjee, A.; Katiyar, V.; Kumar, S.; Dutta, J. Chitosan-based antimicrobial coating for improving postharvest shelf life of pineapple. Coatings 2021, 11, 1366. [Google Scholar] [CrossRef]
- Sritananan, S.; Uthairatanakij, A.; Jitareerat, P.; Photchanachai, S.; Vongcheeree, S. Effects of Irradiation and Chitosan Coating on Physiological Changes of Mangosteen Fruit Stored at Room Temperature; International Symposium on New Frontiers of Food and Non-Food Products; KMUTT: Bangkok, Thailand, 2005; pp. 22–23. [Google Scholar]
- Vilvert, J.C.; de Freitas, S.T.; Ferreira, M.A.R.; Leite, R.H.d.L.; dos Santos, F.K.G.; Costa, C.d.S.R.; Aroucha, E.M.M. Chitosan and graphene oxide-based biodegradable bags: An eco-friendly and effective packaging alternative to maintain postharvest quality of ‘Palmer’ mango. Lwt 2022, 154, 112741. [Google Scholar] [CrossRef]
- Azene, M.; Workneh, T.S.; Woldetsadik, K. Effect of packaging materials and storage environment on postharvest quality of papaya fruit. J. Food Sci. Technol. 2014, 51, 1041–1055. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).