In-Orchard Sizing of Mango Fruit: 2. Forward Estimation of Size at Harvest
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Harvest Maturity Estimation
2.3. Fruit Measurements
2.4. Statistics
3. Results and Discussion
3.1. Estimation of Fruit Mass from Linear Dimensions
3.2. Sampling
3.3. Prediction of Fruit Mass at Harvest
3.4. Prediction of Tray Size Distribution at Harvest
3.5. Recommendations for Future Work
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anderson, N.; Walsh, K.; Wulfsohn, D. Technologies for forecasting tree fruit load and harvest timing from ground, sky and time. Agronomy 2021, 11, 1409. [Google Scholar] [CrossRef]
- Anderson, N.; Walsh, K.; Koirala, A.; Wang, Z.; Amaral, M.H.; Dickinson, G. Estimation of fruit load in Australian mango orchards using machine vision. Agronomy 2021, 11, 1711. [Google Scholar] [CrossRef]
- Spreer, W.; Müller, J. Estimating the mass of mango fruit (Mangifera indica, cv. Chok Anan) from its geometric dimensions by optical measurement. Comput. Electron. Agric. 2011, 75, 125–131. [Google Scholar] [CrossRef]
- Wang, Z.; Walsh, K.B.; Verma, B. On-tree mango fruit size estimation using RGB-D images. Sensors 2017, 17, 2738. [Google Scholar] [CrossRef] [PubMed]
- Anderson, N.; Subedi, P.; Walsh, K. Manipulation of mango fruit dry matter content to improve eating quality. Sci. Hortic. 2017, 226, 316–321. [Google Scholar] [CrossRef]
- Utai, K.; Nagle, M.; Hämmerle, S.; Spreer, W.; Mahayothee, B.; Müller, J. Mass estimation of mango fruits (Mangifera indica L.; cv. ‘Nam Dokmai’) by linking image processing and artificial neural network. Eng. Agric. Environ. Food 2019, 12, 103–110. [Google Scholar] [CrossRef]
- Wang, Z.; Koirala, A.; Walsh, K.; Anderson, N.; Verma, B. In field fruit sizing using a smart phone application. Sensors 2018, 18, 3331. [Google Scholar] [CrossRef] [PubMed]
- Neupane, C.; Koirala, A.; Wang, Z.; Walsh, K. Evaluation of depth cameras for use in fruit localization and sizing: Finding a successor to Kinect v2. Agronomy 2021, 11, 1780. [Google Scholar] [CrossRef]
- Carella, A.; Gianguzzi, G.; Scalisi, A.; Farina, V.; Inglese, P.; Bianco, R. Fruit growth stage transitions in two mango cultivars grown in a Mediterranean environment. Plants 2021, 10, 1332. [Google Scholar] [CrossRef] [PubMed]
- Costa, G.; Noferini, M.; Bucchi, F.; Corelli-Grappadelli, L. Methods for early forecasting apple size at harvest. Acta Hortic. 2004, 636, 651–659. [Google Scholar] [CrossRef]
- Khurshid, T.; Braysher, B. Early fruit size prediction model using cubic smoothing splines for ‘Washington Navel’ (Citrus sinensis L. Osbeck) oranges in Australia. Int. J. Fruit Sci. 2009, 9, 394–408. [Google Scholar] [CrossRef]
- Da Silva, D.F.; Salomão, L.C.; Pereira, L.D.; Valle, K.D.; Assunção, H.F.; Cruz, S.C. Development and maturation of mango fruits CV. ‘ubá’ in Visconde do rio branco, Minas Gerais State, Brazil. Rev. Ceres 2018, 65, 507–516. [Google Scholar] [CrossRef]
- Souza, J.; Leonel, S.; Modesto, J.; Ferraz, R.; Gonçalves, B. Phenological cycles, thermal time and growth curves of mango fruit cultivars in subtropical conditions. Br. J. Appl. Sci. Technol. 2015, 9, 100–107. [Google Scholar] [CrossRef]
- Anila, R.; Radha, T. Studies on fruit drop in mango varieties. Coll. Hortic. J. Trop. Agric. 2003, 41, 30–32. [Google Scholar]
- Ometto, J. Bioclimatologia vegetal [Plant Bioclimatology]. Agron. Ceres 1981, 1, 129–155. [Google Scholar]
- Moore, C. Developing a Crop Forecasting System for the Australian Mango Industry. Available online: https://www.horticulture.com.au/globalassets/hort-innovation/historic-reports/developing-a-crop-forecasting-system-for-the-australian-mango-industry-mg05004.pdf (accessed on 3 March 2022).
- Amaral, M.H.P. Benchmarking New Methods for Estimation of Quantity and Harvest Timing of the Mango Crop. Master’s Thesis, Central Queensland University, Rockhampton, Australia, 2022. [Google Scholar]
- Walsh, K.B. In-field estimation of fruit quality and quantity. Agronomy 2022, 12, 1074. [Google Scholar] [CrossRef]
- Scalisi, A.; O’Connell, G.M.; Stefanelli, D.; Lo Biancco, R. Fruit and leaf sensing for continuous detection of Nectarine Water Status. Front. Plant Sci. 2019, 10, 805. [Google Scholar] [CrossRef] [PubMed]
- Walsh, K.B.; Long, R.L.; Middleton, S.G. Use of near infra-red spectroscopy in evaluation of source-sink manipulation to increase the soluble sugar content of stonefruit. J. Hortic. Sci. Biotechnol. 2007, 82, 316–322. [Google Scholar] [CrossRef]
- Kernot, I.; Meurant, N. Mango Information Kit; Agrilink, Dept. of Primary Industries: Nambour, Queensland, 1999. Available online: http://era.daf.qld.gov.au/id/eprint/1647/1/0tit-mango.pdf (accessed on 12 November 2021).
- Neupane, C.; Koirala, A.; Walsh, K.B. In-orchard sizing of Mango Fruit: 1. comparison of machine vision-based methods for on-the-go estimation. Horticulturae 2022, 8, 1223. [Google Scholar] [CrossRef]
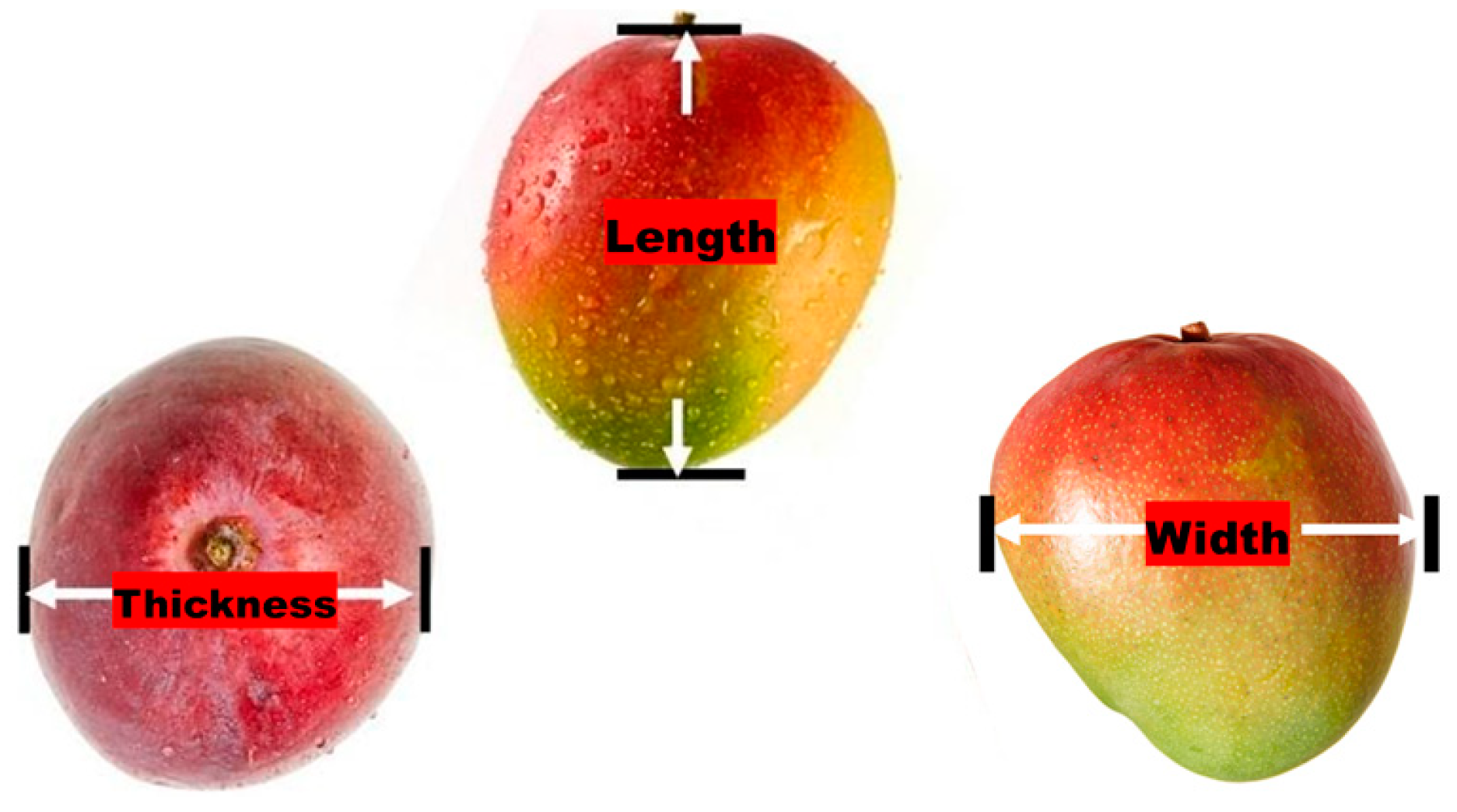
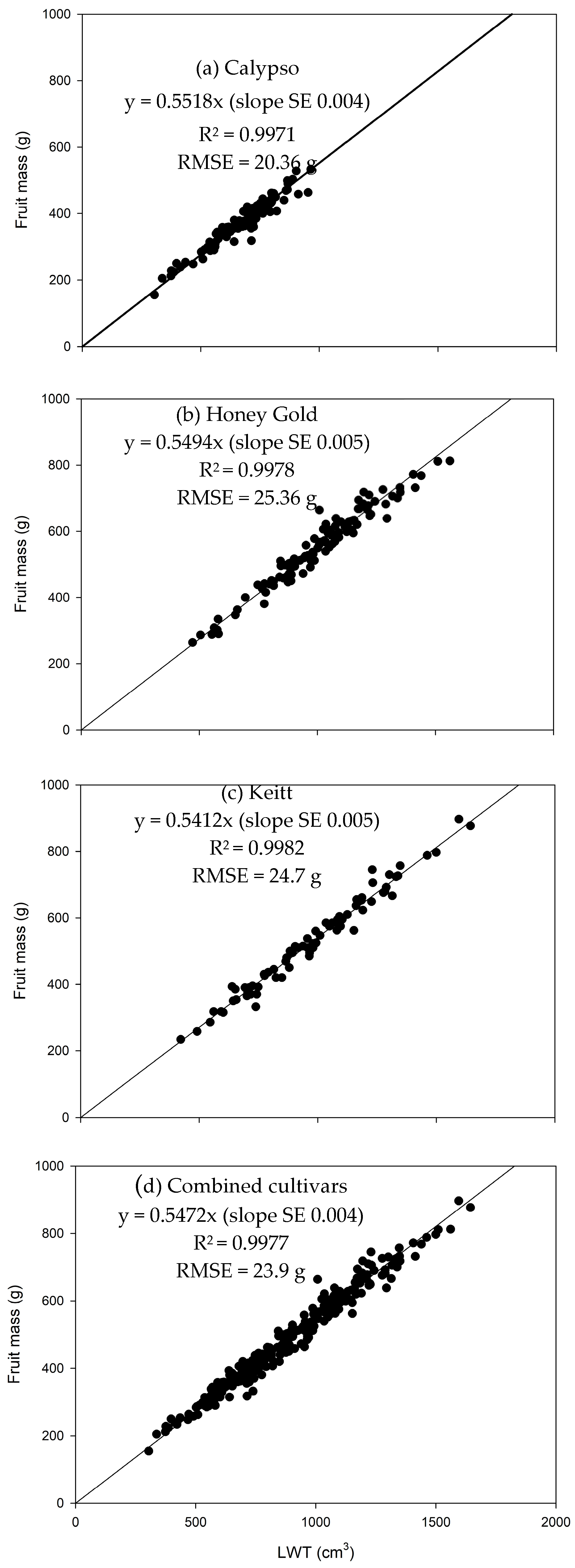
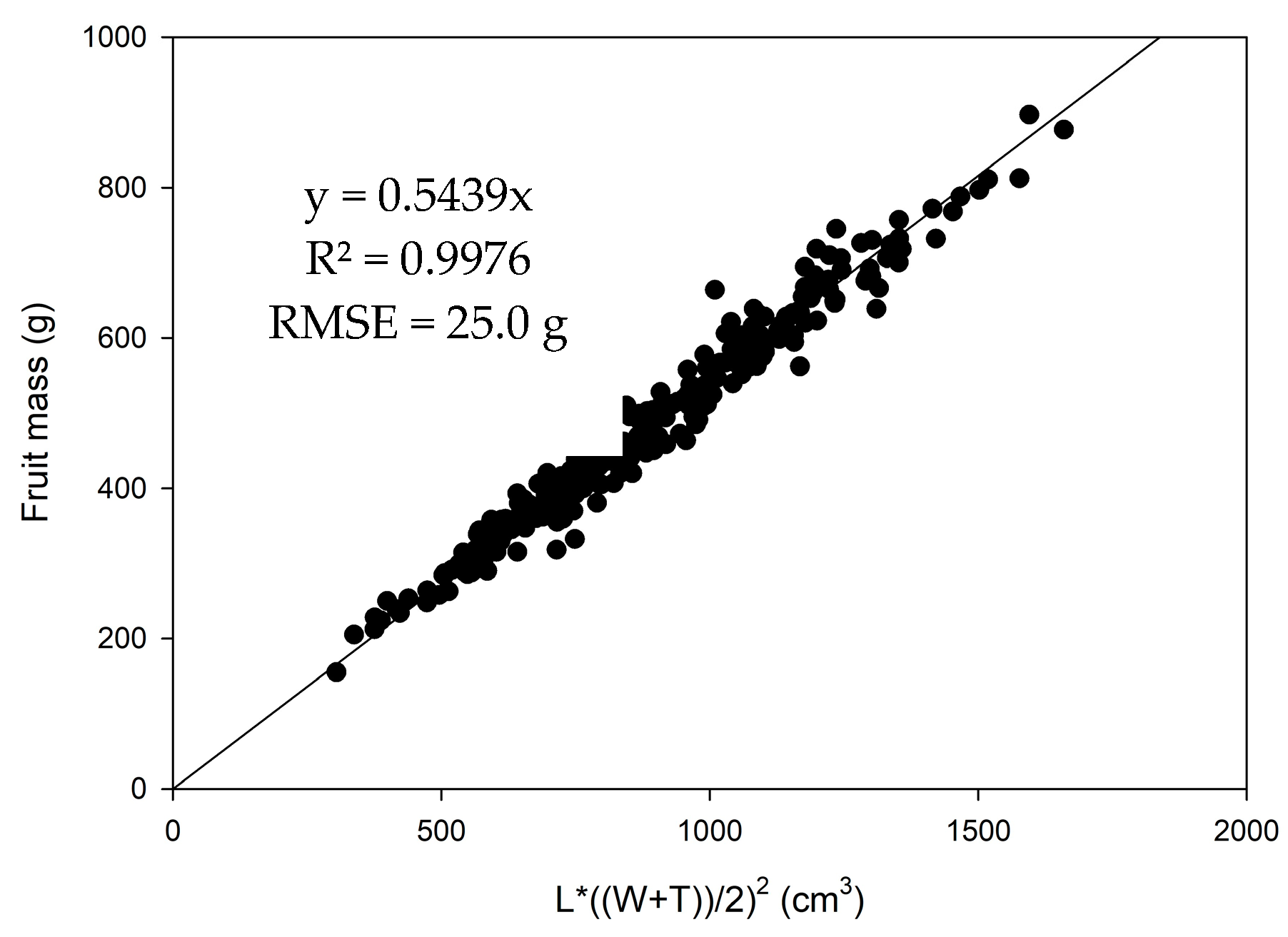
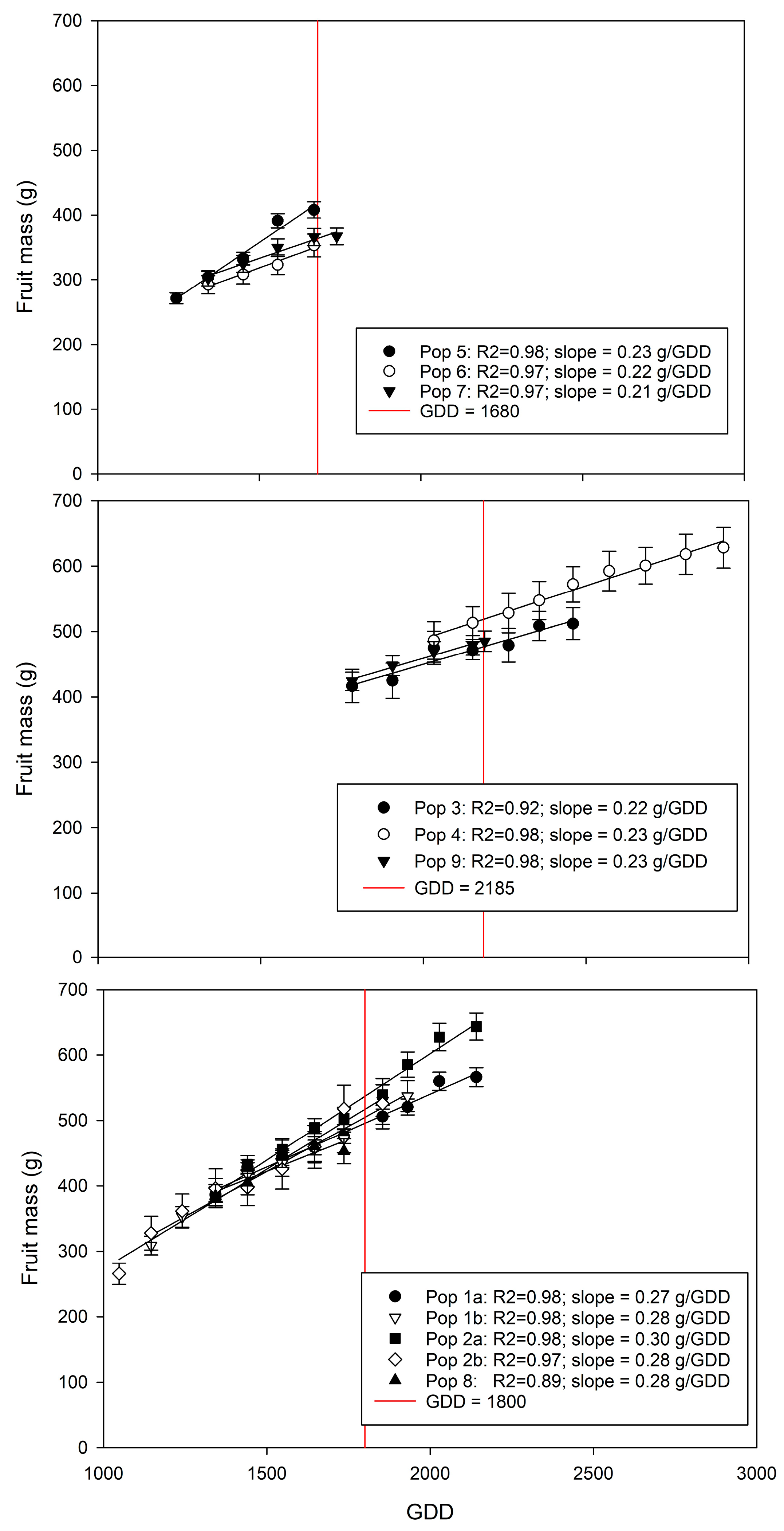
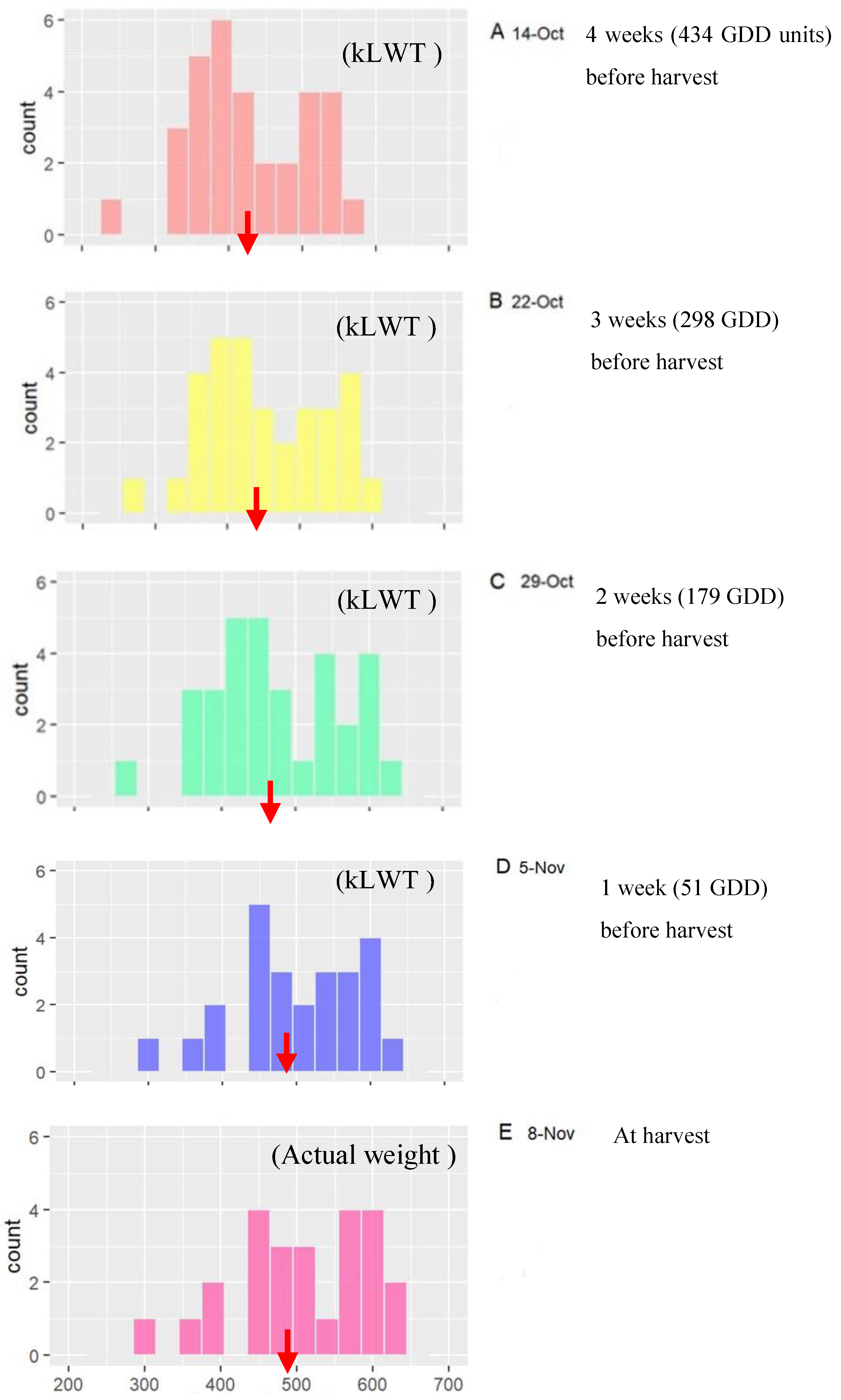
| Farm | Population | Cultivar | Season | Fruit Sample Size (n) | Monitored Period (weeks) | Day-Month of Initial Assessment (GDD) | Day-Month of Final Assessment (GDD) |
|---|---|---|---|---|---|---|---|
| A | 1a | Honey Gold | 2020/21 | 25 | 7 | 19-11 (1250) | 14-01 (1886) |
| A | 1b | Honey Gold | 2020/21 | 25 | 7 | 19-11 (1328) | 14-01 (1965) |
| A | 2a | Honey Gold | 2020/21 | 25 | 9 | 19-11 (1250) | 14-01 (1886) |
| A | 2b | Honey Gold | 2020/21 | 15 | 9 | 19-11 (1318) | 14-01 (2141) |
| A | 3 | Keitt | 2020/21 | 26 | 7 | 22-12 (1796) | 02-02 (2456) |
| A | 4 | Keitt | 2020/21 | 17 | 9 | 14-01 (2053) | 11-03 (2922) |
| B | 5 | Calypso | 2021/22 | 44 | 5 | 02-09 (1243) | 30-09 (1669) |
| B | 6 | Calypso | 2021/22 | 27 | 4 | 02-09 (1410) | 23-09 (1739) |
| C | 7 | Calypso | 2021/22 | 29 | 4 | 03-11 (1369) | 29-11 (1739) |
| C | 8 | Honey Gold | 2021/22 | 20 | 4 | 03-11 (1369) | 29-11(1739) |
| D | 9 | Keitt | 2021/22 | 32 | 5 | 14-10 (1755) | 8-11 (2188) |
| Population | GDD at Measurement Start (Weeks before Harvest) | Period (Weeks before Harvest) | Slope (g/week) | Predicted Mass at Harvest Maturity | Actual Mass (LWT) at Harvest Maturity | Percentage Error (%) |
|---|---|---|---|---|---|---|
| 1a | 505.1 (5) | 5 and 4 | 42.6 | 599 | 506 | 18 |
| 1a | 421.2 (4) | 4 and 3 | 12.5 | 479 | 506 | −5 |
| 1b | 523.6 (5) | 5 and 4 | 41.5 | 560 | 487 | 15 |
| 1b | 418.5 (4) | 4 and 3 | 23.2 | 487 | 487 | 0 |
| 2a | 505.1 (5) | 5 and 4 | 52.2 | 686 | 590 | 16 |
| 2a | 421.2 (4) | 4 and 3 | 24.3 | 574 | 590 | −3 |
| 2b | 523.6 (5) | 5 and 4 | 1.2 | 444 | 526 | −15 |
| 2b | 431.0 (4) | 4 and 3 | 30.2 | 560 | 526 | 6 |
| 8 | 371.3 (4) | 4 and 3 | 20.1 | 465 | 453 | 3 |
| 5 | 443.1 (4) | 4 and 3 | 32.7 | 402 | 408 | −2 |
| 6 | 434.0 (4) | 4 and 3 * | 16.0 | 339 | 353 | −4 |
| 7 | 371.3 (4) | 4 and 3 | 22.6 | 393 | 367 | 7 |
| 3 | 469.5 (4) | 4 and 3 | 8.1 | 449 | 479 | −6 |
| 4 | 427.8 (4) | 4 and 3 | 26.9 | 594 | 572 | 4 |
| 9 | 433.9 (4) | 4 and 3 | 25.1 | 523 | 485 | 8 |
| Mean ± SD | 514.3 ± 9.2 | 5 and 4 | 34.4 ± 22.0 | 16.3 ± 1.3 * | ||
| Mean ± SD | 422.1 ± 27.4 | 4 and 3 | 19.6 ± 7.1 | 4.5 ± 2.4 * |
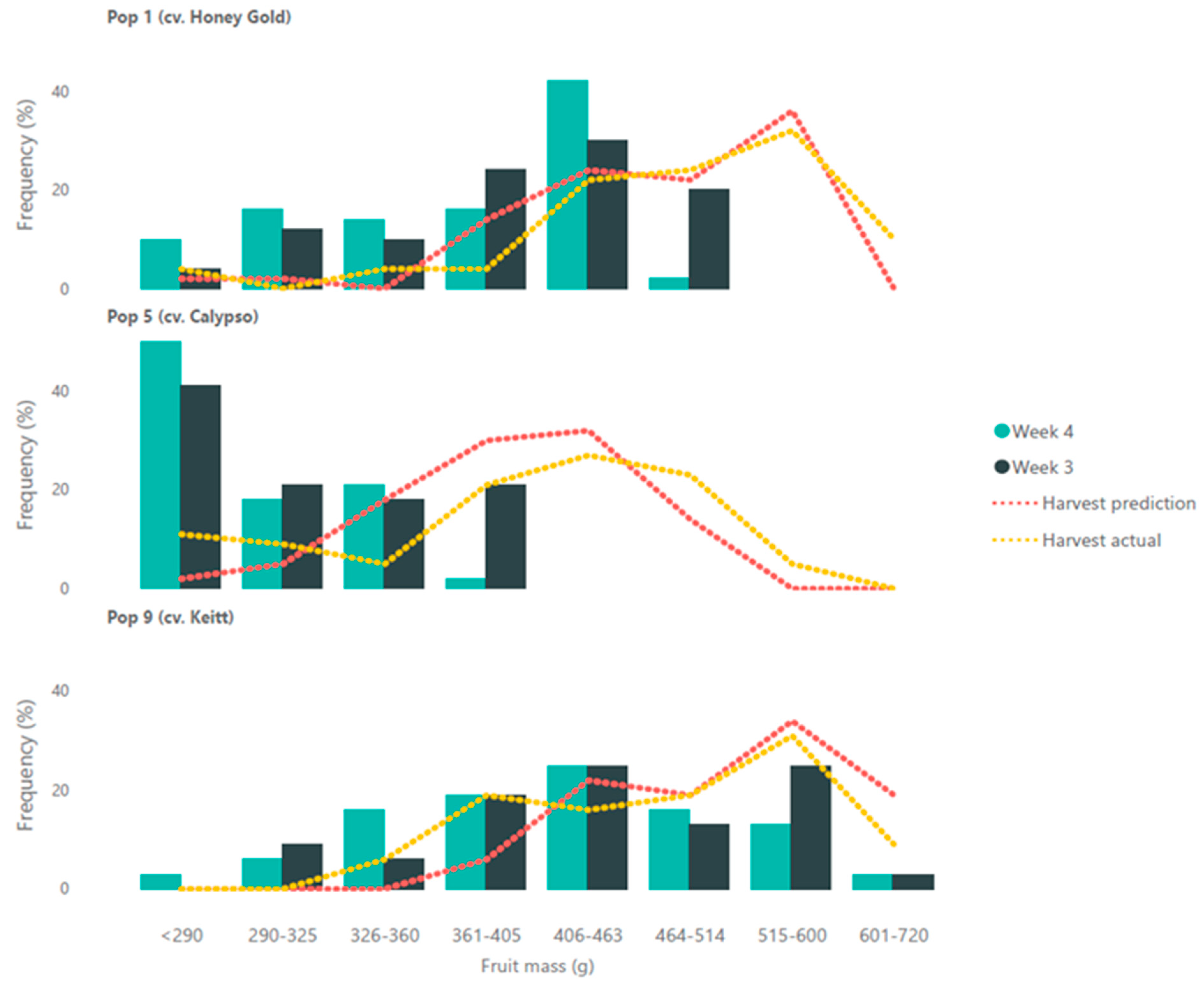
| Population | 1 | 1 | 1 | 1 | 5 | 5 | 5 | 5 | 9 | 9 | 9 | 9 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fruit mass | (i) week 4 | (ii) week 3 | (iii) harvest prediction | (iv) harvest actual | (i) week 4 | (ii) week 3 | (iii) harvest prediction | (iv) harvest actual | (i) week 4 | (ii) week 3 | (iii) harvest prediction | (iv) harvest actual |
| (g) | (%) | (%) | (%) | (%) | (%) | (%) | (%) | (%) | (%) | (%) | (%) | (%) |
| <290 | 10 | 4 | 4 | 4 | 59 | 39 | 5 | 11 | 6 | 0 | ||
| 290–325 | 16 | 12 | 18 | 23 | 9 | 9 | 3 | 9 | ||||
| 325–360 | 10 | 10 | 4 | 4 | 20 | 18 | 11 | 5 | 16 | 6 | 6 | |
| 361–405 | 18 | 24 | 18 | 4 | 2 | 18 | 34 | 20 | 16 | 16 | 9 | 19 |
| 405–463 | 40 | 24 | 24 | 22 | 32 | 27 | 25 | 28 | 16 | 16 | ||
| 464–514 | 6 | 26 | 28 | 24 | 9 | 23 | 16 | 13 | 22 | 19 | ||
| 515–600 | 22 | 32 | 5 | 16 | 25 | 31 | 31 | |||||
| 601–720 | 10 | 3 | 3 | 19 | 9 | |||||||
| >720 | ||||||||||||
| Comparison to distribution based on kLWT estimated mass (as presented in Figure 6), by column. | ||||||||||||
| X2 * | 2.8 | 1.4 | 11.3 | 0.0 | 0.6 | 5.5 | 2.8 | 0.3 | 1.9 | |||
| p value * | 0.723 | 0.910 | 0.080 | 1.000 | 0.880 | 0.360 | 0.900 | 1.000 | 0.749 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amaral, M.H.; Walsh, K.B. In-Orchard Sizing of Mango Fruit: 2. Forward Estimation of Size at Harvest. Horticulturae 2023, 9, 54. https://doi.org/10.3390/horticulturae9010054
Amaral MH, Walsh KB. In-Orchard Sizing of Mango Fruit: 2. Forward Estimation of Size at Harvest. Horticulturae. 2023; 9(1):54. https://doi.org/10.3390/horticulturae9010054
Chicago/Turabian StyleAmaral, Marcelo H., and Kerry B. Walsh. 2023. "In-Orchard Sizing of Mango Fruit: 2. Forward Estimation of Size at Harvest" Horticulturae 9, no. 1: 54. https://doi.org/10.3390/horticulturae9010054
APA StyleAmaral, M. H., & Walsh, K. B. (2023). In-Orchard Sizing of Mango Fruit: 2. Forward Estimation of Size at Harvest. Horticulturae, 9(1), 54. https://doi.org/10.3390/horticulturae9010054






