Growth, Phytochemicals, and Antioxidant Activity of Kale Grown under Different Nutrient-Solution Depths in Hydroponic
Abstract
1. Introduction
2. Materials and Methods
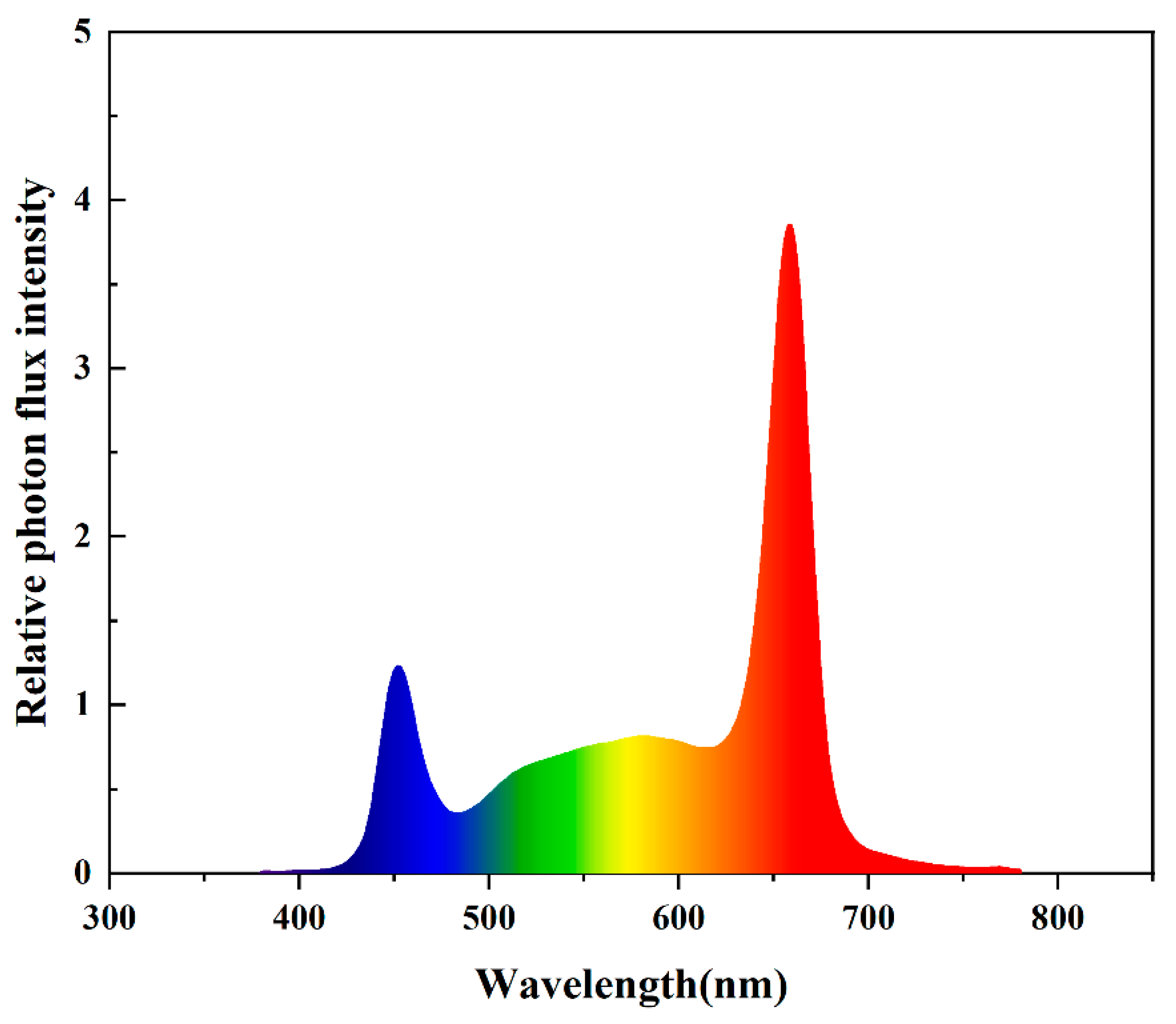
2.1. Plant Materials and Growth Condition
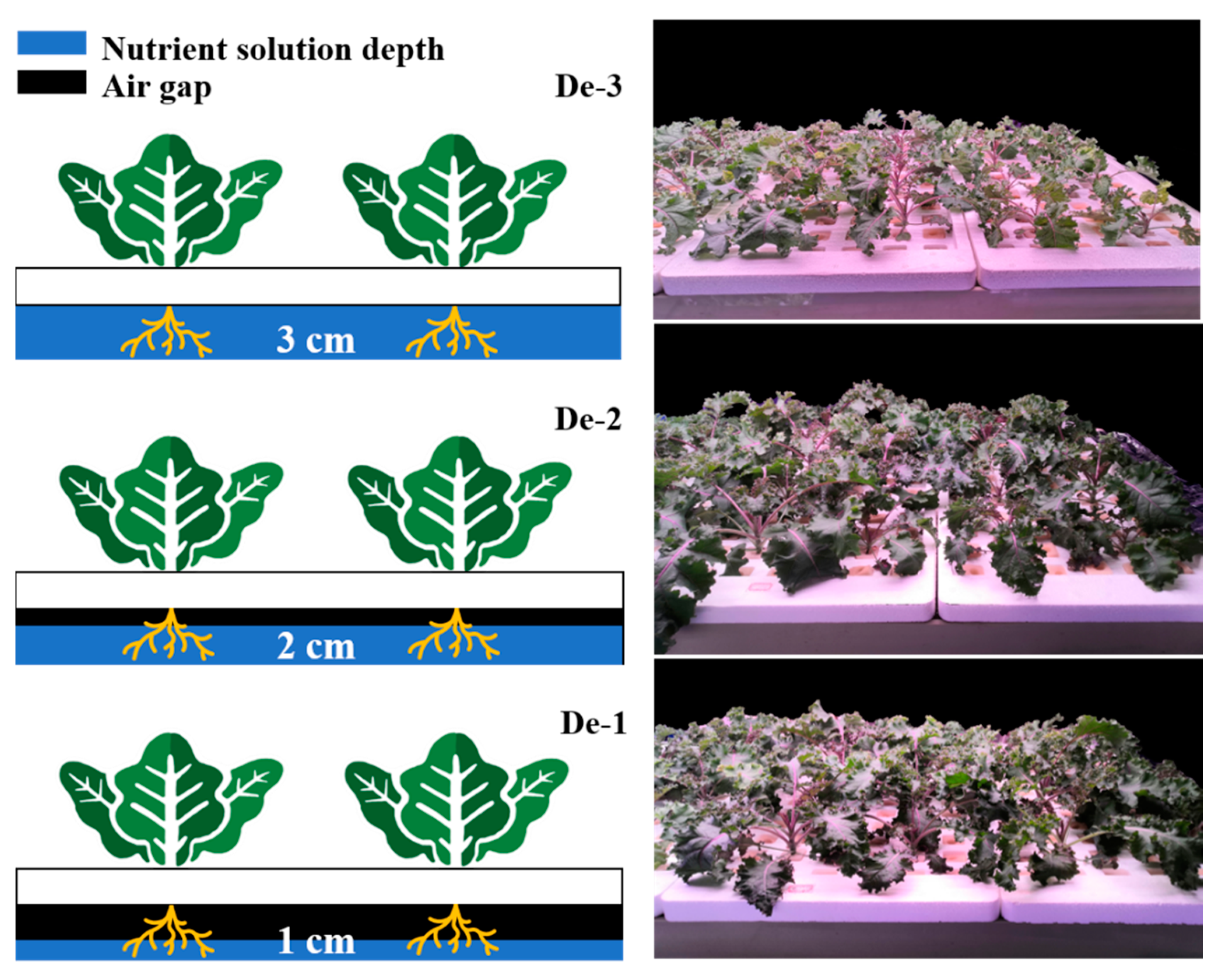
2.2. Experimental Designs
2.3. Agronomy Traits Measurements
2.4. Phytochemical Determinations
2.4.1. Chlorophyll (Chl) and Carotenoid Contents
2.4.2. Antioxidant Activity Measurements
2.4.3. Antioxidant Components Measurements
2.4.4. ROS, OXO, POD, H2O2, and Proline Assays
2.4.5. Proline Assays
2.4.6. Nutritional Compound Measurements
2.5. Glucosinolates Measurements
2.6. Minerals Measurements
2.7. Statistical Analysis
3. Results
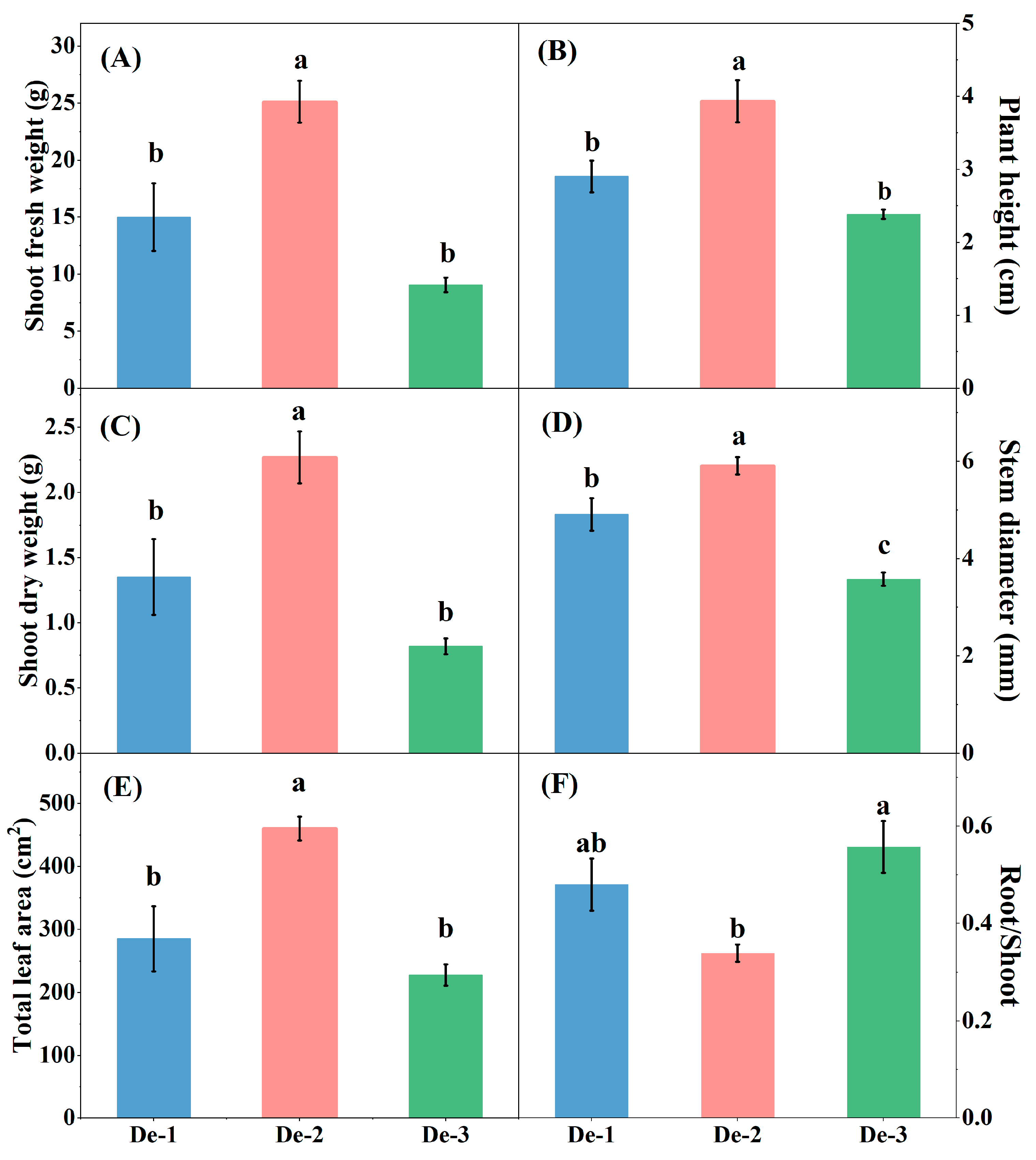
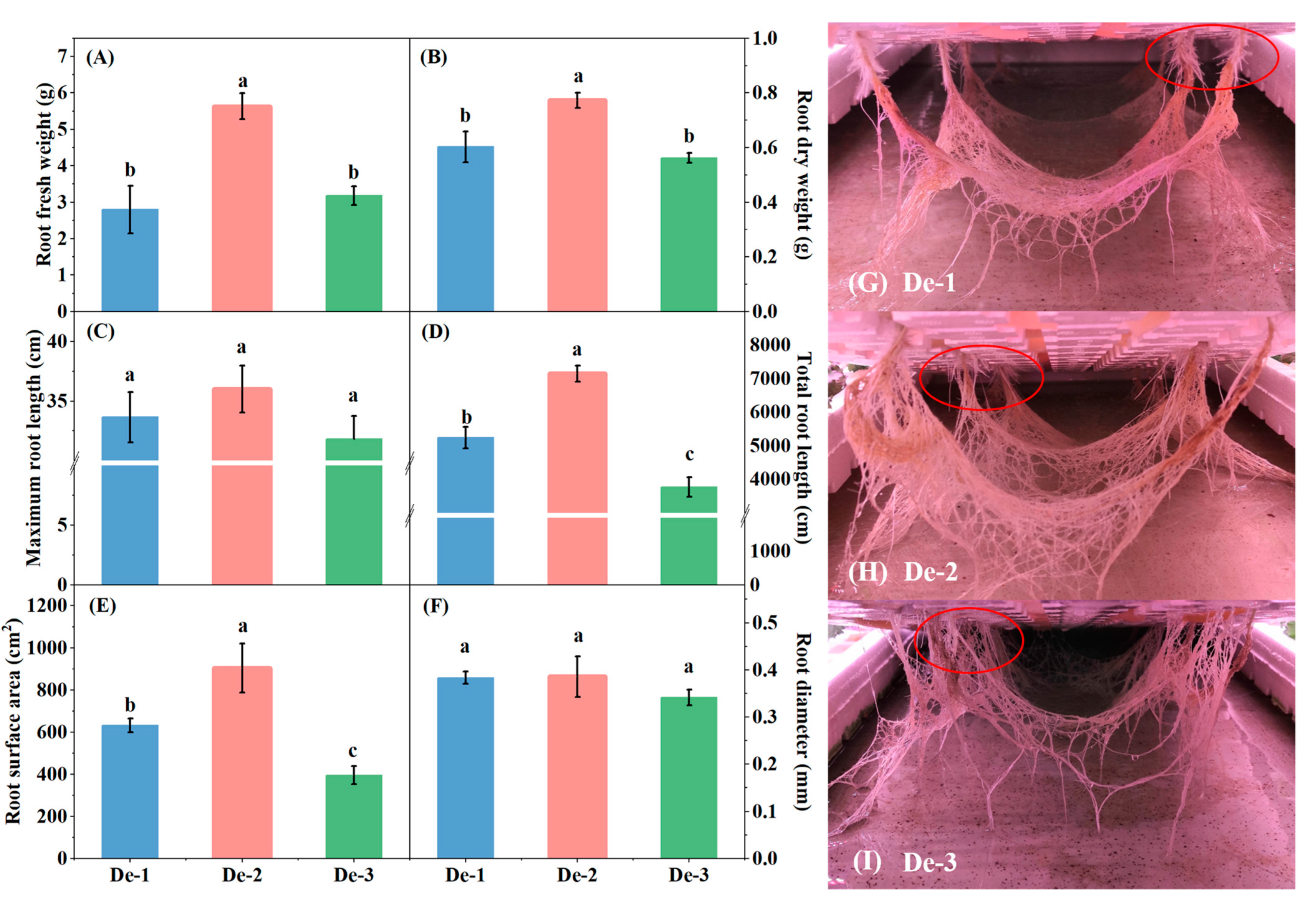
3.1. Effect of Nutrient-Solution Depth on Kale Plant Biomass and Root Morphological Properties
3.2. Effect of Nutrient-Solution Depth on the Photosynthetic Pigment Contents of Kale
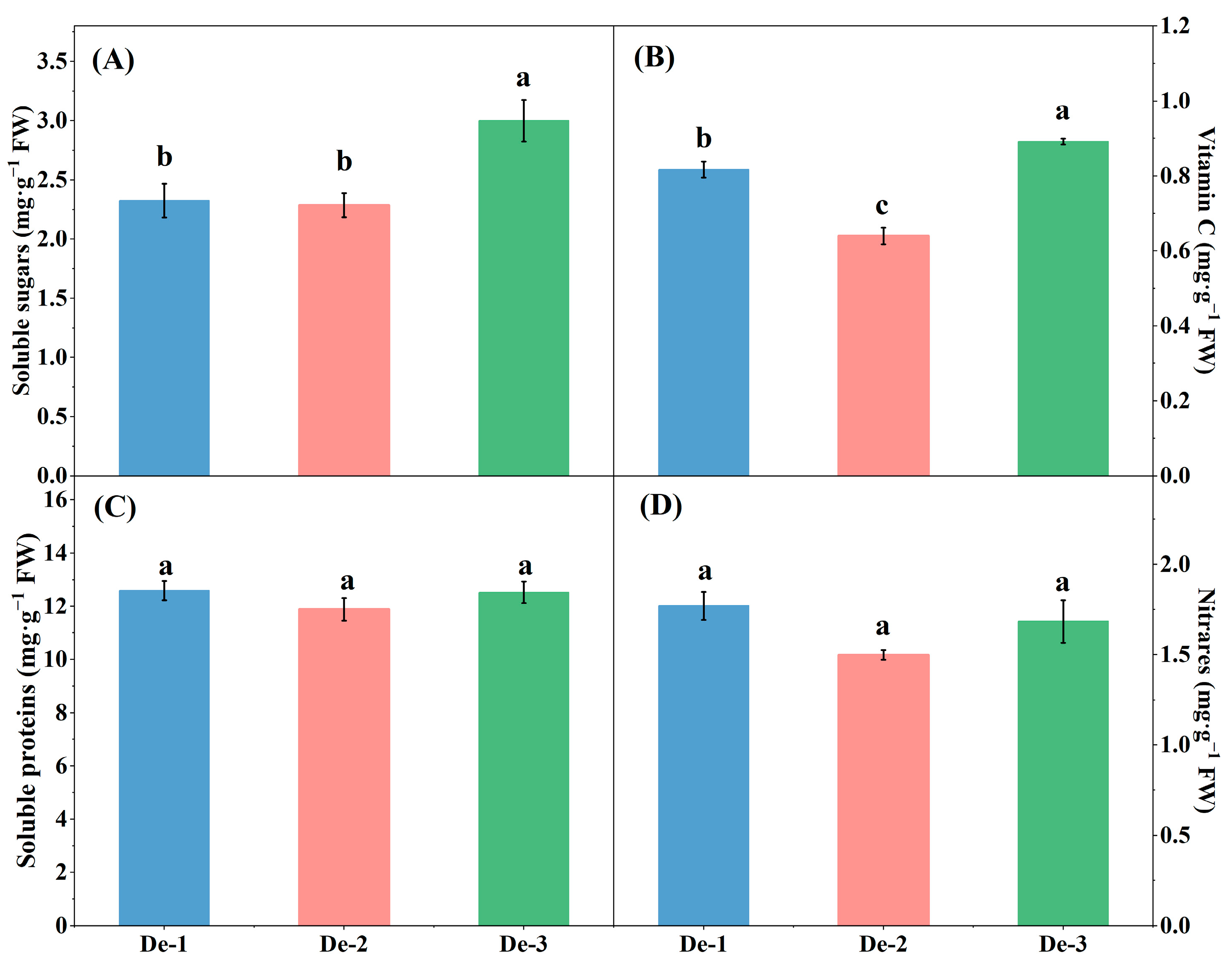
3.3. Effect of Nutrient-Solution Depth on Phytochemicals Contents of Kale
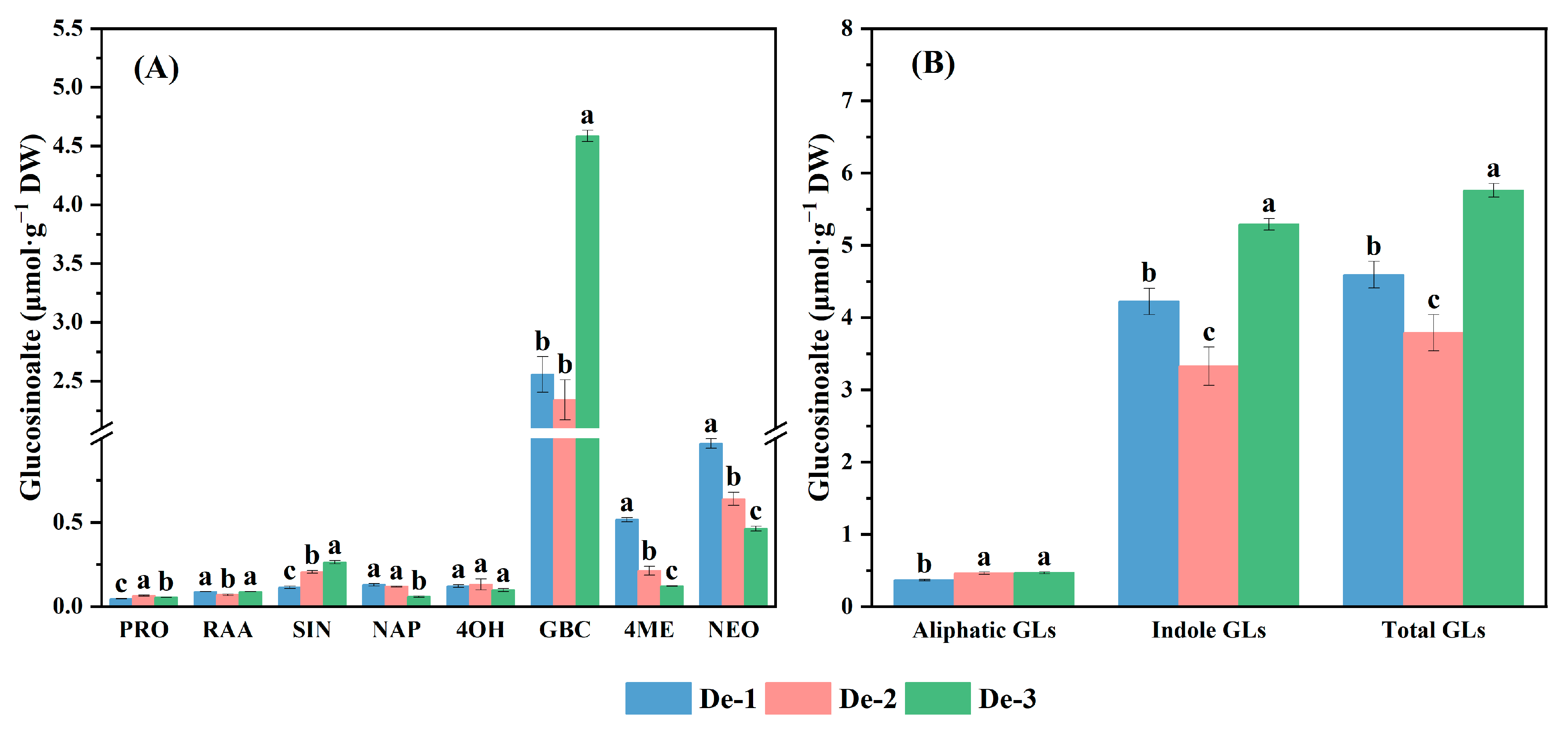
3.4. Effect of Nutrient-Solution Depths on Glucosinolate Composition and Content of Kale
3.5. Effect of Nutrient-Solution Depth on Mineral Elements Contents of Kale
3.6. Effect of Nutrient-Solution Depth on Antioxidant Enzyme Activities, Reactive Oxygen Species, Content of Hydrogen Peroxide, and Proline in Kale Root
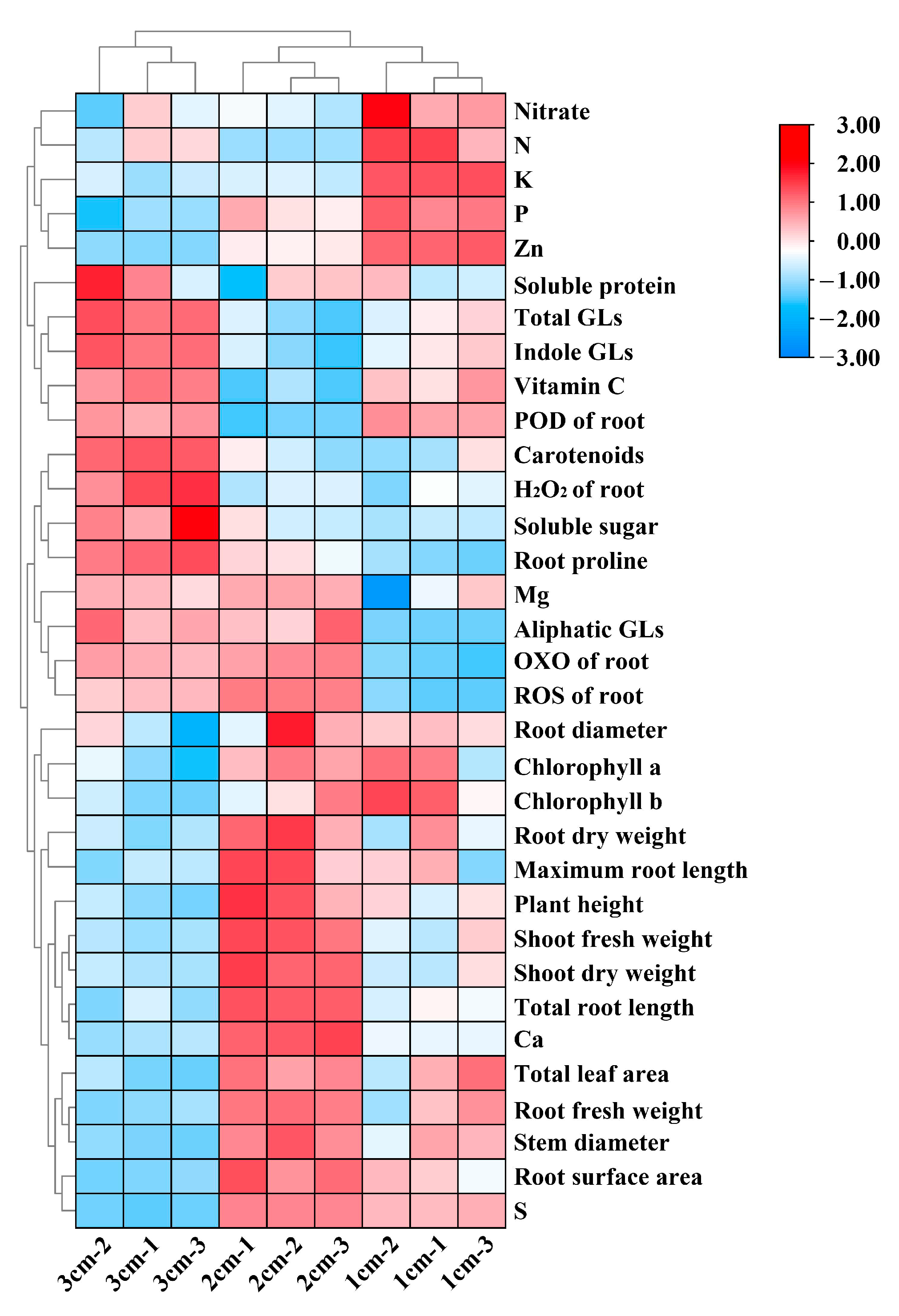
3.7. Heatmap Analysis of Growth and Nutritional Aspects of Kale under Different Nutrient-Solution Depths
3.8. Multivariate Principal Component Analysis
4. Discussion
4.1. Different Depths of Nutrient Solution Affected the Shoot Growth and Pigment in Kale
4.2. Different Depths of Nutrient Solution Affected the Roots Growth and Antioxidant Activity in Kale
4.3. Different Depths of Nutrient Solution Affected the Mineral Elements Contents in Kale
4.4. Different Depths of Nutrient Solution Affected the Kale Nutritive Quality
4.5. Different Depths of Nutrient Solution Affected the Glucosinolates Contents in Kale
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gu, D.; Andreev, K.; Dupre, M.E. Major trends in population growth around the world. China CDC Wkly. 2021, 3, 604. [Google Scholar] [CrossRef] [PubMed]
- Prosekov, A.Y.; Ivanova, S.A. Food security: The challenge of the present. Geoforum 2018, 91, 73–77. [Google Scholar] [CrossRef]
- Cifuentes-Torres, L.; Mendoza-Espinosa, L.G.; Correa-Reyes, G.; Daesslé, L.W. Hydroponics with wastewater: A review of trends and opportunities. Water Environ. J. 2020, 35, 166–180. [Google Scholar] [CrossRef]
- Sharma, N.; Acharya, S.; Kumar, K.; Singh, N.; Chaurasia, O.P. Hydroponics as an advanced technique for vegetable production: An overview. J. Soil Water Conserv. 2018, 17, 364–371. [Google Scholar] [CrossRef]
- Masaru, S.; Uenishi, M.; Miyamoto, K.; Suzuki, T. Effect of Root-Zone Temperature on the Growth and Fruit Quality of Hydroponically Grown Strawberry Plants. J. Agric. Sci. 2016, 8, 122–131. [Google Scholar] [CrossRef]
- Ikeura, H.; Takahashi, H.; Kobayashi, F.; Sato, M.; Tamaki, M. Effects of microbubble generation methods and dissolved oxygen concentrations on growth of Japanese mustard spinach in hydroponic culture. J. Hortic. Sci. Biotech. 2017, 93, 483–490. [Google Scholar] [CrossRef]
- Ren, X.; Lu, N.; Xu, W.; Zhuang, Y.; Takagaki, M. Optimization of the Yield, Total Phenolic Content, and Antioxidant Capacity of Basil by Controlling the Electrical Conductivity of the Nutrient Solution. Horticulturae 2022, 8, 216. [Google Scholar] [CrossRef]
- Chun, C.; Takakura, T. Rate of root respiration of lettuce under various dissolved oxygen concentrations in hydroponics. Environ. Control. Biol. 1994, 32, 125–135. [Google Scholar] [CrossRef]
- Ayi, Q.; Zeng, B.; Yang, K.; Lin, F.; Zhang, X.; van Bodegom, P.M.; Cornelissen, J.H.C. Similar Growth Performance but Contrasting Biomass Allocation of Root-Flooded Terrestrial Plant Alternanthera philoxeroides (Mart.) Griseb. in Response to Nutrient Versus Dissolved Oxygen Stress. Front. Plant Sci. 2019, 10, 111. [Google Scholar] [CrossRef]
- Tan, X.; Xu, H.; Khan, S.; Equiza, M.A.; Lee, S.H.; Vaziriyeganeh, M.; Zwiazek, J.J. Plant water transport and aquaporins in oxygen-deprived environments. J. Plant Physiol. 2018, 227, 20–30. [Google Scholar] [CrossRef]
- Morard, P.; Silvestre, J. Plant injury due to oxygen deficiency in the root environment of soilless culture: A review. Plant Soil 1996, 184, 243–254. [Google Scholar] [CrossRef]
- Ayi, Q.; Zeng, B.; Liu, J.; Li, S.; van Bodegom, P.M.; Cornelissen, J.H.C. Oxygen absorption by adventitious roots promotes the survival of completely submerged terrestrial plants. Ann. Bot. 2016, 118, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Suyantohadi, A.; Kyoren, T.; Hariadi, M.; Purnomo, M.H.; Morimoto, T. Effect of high consentrated dissolved oxygen on the plant growth in a deep hydroponic culture under a low temperature. IFAC Proc. Vol. 2010, 43, 251–255. [Google Scholar] [CrossRef]
- Song, W.; Zhang, S.; Huang, Z. Principle and efficiency of oxygen enrichment of dynamic solution surface in nutrient solution. Trans. Chin. Soc. Agric. Eng. 2003, 19, 194–198. (In Chinese) [Google Scholar]
- Voesenek, L.; Armstrong, W.; Bögemann, G.; Colmer, T.; McDonald, M. A lack of aerenchyma and high rates of radial oxygen loss from the root base contribute to the waterlogging intolerance of Brassica napus. Funct. Plant Biol. 1999, 26, 87–93. [Google Scholar] [CrossRef]
- Baiyin, B.; Tagawa, K.; Yamada, M.; Wang, X.; Yamada, S.; Yamamoto, S.; Ibaraki, Y. Study on Plant Growth and Nutrient Uptake under Different Aeration Intensity in Hydroponics with the Application of Particle Image Velocimetry. Agriculture 2021, 11, 1140. [Google Scholar] [CrossRef]
- Park, J.-S.; Kurata, K. Application of microbubbles to hydroponics solution promotes lettuce growth. HortTechnology 2009, 19, 212–215. [Google Scholar] [CrossRef]
- Jackson, M.B. Ethylene and responses of plants to soil waterlogging and submergence. Annu. Rev. Plant Physiol. 1985, 36, 145–174. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, D.; Adams, P. Improving aeration in hydroponics—Principle and practice. Acta Agric. Urae Zhejiangensis 1994, 6, 44–48. [Google Scholar]
- Son, J.E.; Kim, H.J.; Ahn, T.I. Hydroponic systems. In Plant Factory; Elsevier: Amsterdam, The Netherlands, 2020; pp. 273–283. [Google Scholar]
- Lu, N.; Shimamura, S. Protocols, issues and potential improvements of current cultivation systems. In Smart Plant Factory; Springer: Berlin/Heidelberg, Germany, 2018; pp. 31–49. [Google Scholar]
- Fu, L.; Jia, F.; Yang, Z.; Su, F.; Chen, H.; Yin, M.; Chen, J.; Hong, L.; Hu, D. Effects of hanging roots with different length on yield, quality and water dissolved oxygen of romaine lettuce. Southwest China J. Agric. Sci. 2015, 28, 1775–1779. [Google Scholar]
- da Silva, M.G.; Soares, T.M.; Gheyi, H.R.; Costa, I.P.; Vasconcelos, R.S. Growth, production and water consumption of coriander grown under different recirculation intervals and nutrient solution depths in hydroponic channels. Emir. J. Food Agr. 2020, 32, 281–294. [Google Scholar] [CrossRef]
- Baiyin, B.; Tagawa, K.; Yamada, M.; Wang, X.; Yamada, S.; Shao, Y.; An, P.; Yamamoto, S.; Ibaraki, Y. Effect of Nutrient Solution Flow Rate on Hydroponic Plant Growth and Root Morphology. Plants 2021, 10, 1840. [Google Scholar] [CrossRef] [PubMed]
- Khalid, W.; Sajid Arshad, M.; Imran, M.; Shabir Ahmad, R.; Imran, A.; Qaisrani, T.B.; Asghar, Z.; Husain, A.; Muhammad Anjum, F.; Ansar Rasul Suleria, H. Kale (Brassica oleracea var. sabellica) as miracle food with special reference to therapeutic and nutraceuticals perspective. Food Sci. Nutr. 2021, 10, 3175. [Google Scholar] [CrossRef]
- Park, Y.J.; Lee, H.M.; Shin, M.; Arasu, M.V.; Chung, D.Y.; Al-Dhabi, N.A.; Kim, S.J. Effect of different proportion of sulphur treatments on the contents of glucosinolate in kale (Brassica oleracea var. acephala) commonly consumed in Republic of Korea. Saudi J. Biol. Sci. 2018, 25, 349–353. [Google Scholar] [CrossRef]
- Mageney, V.; Neugart, S.; Albach, D.C. A Guide to the Variability of Flavonoids in Brassica oleracea. Molecules 2017, 22, 252. [Google Scholar] [CrossRef] [PubMed]
- Ashenafi, E.L.; Nyman, M.C.; Holley, J.M.; Mattson, N.S.; Rangarajan, A. Phenotypic plasticity and nutritional quality of three kale cultivars (Brassica oleracea L. var. acephala) under field, greenhouse, and growth chamber environments. Environ. Exp. Bot. 2022, 199, 104895. [Google Scholar] [CrossRef]
- Islam, S.; Reza, M.N.; Chowdhury, M.; Chung, S.-O.; Choi, I.-S. A review on effect of ambient environment factors and monitoring technology for plant factory. Precis. Agric. 2021, 3, 84. [Google Scholar]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Tadolini, B.; Juliano, C.; Piu, L.; Franconi, F.; Cabrini, L. Resveratrol inhibition of lipid peroxidation. Free Radical. Res. 2000, 33, 105–114. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. [14] Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Li, C.; Ge, Y.; Wan, D.; Hu, J.; Ying, C.; Wang, L. Optimization of extraction condition and quantification of total flavonoids in Elaeagni folium. Pharmacogn. J. 2011, 3, 8–12. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Kohyama, K.; Nishinari, K. Effect of soluble sugars on gelatinization and retrogradation of sweet potato starch. J. Agric. Food Chem. 1991, 39, 1406–1410. [Google Scholar] [CrossRef]
- Blakesley, R.W.; Boezi, J.A. A new staining technique for proteins in polyacrylamide gels using Coomassie Brilliant Blue G250. Anal. Biochem. 1977, 82, 580–582. [Google Scholar] [CrossRef]
- Chanwitheesuk, A.; Teerawutgulrag, A.; Rakariyatham, N. Screening of antioxidant activity and antioxidant compounds of some edible plants of Thailand. Food Chem. 2005, 92, 491–497. [Google Scholar] [CrossRef]
- Cataldo, D.A.; Maroon, M.; Schrader, L.E.; Youngs, V.L. Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Commun. Soil Sci. Plant Anal. 2008, 6, 71–80. [Google Scholar] [CrossRef]
- Grosser, K.; van Dam, N.M. A straightforward method for glucosinolate extraction and analysis with high-pressure liquid chromatography (HPLC). JoVE (J. Vis. Exp.) 2017, 121, e55425. [Google Scholar] [CrossRef]
- Waterland, N.L.; Moon, Y.; Tou, J.C.; Kim, M.J.; Pena-Yewtukhiw, E.M.; Park, S. Mineral content differs among microgreen, baby leaf, and adult stages in three cultivars of kale. HortScience 2017, 52, 566–571. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Shang, C.; Yan, Y.n.; Chen, L.; Xu, R.; Lin, Y.; Shao, G.; Zhong, F. Effects of rhizosphere oxygen environment on growth, physiology and quality of hydroponic lettuce. Acta Bot. Boreali-Occident. Sin. 2018, 38, 1895–1904. [Google Scholar]
- Kratky, B.A. Growing Lettuce in Non-Aerated, Non-Circulated Hydroponic Systems. J. Veg. Sci. 2005, 11, 35–42. [Google Scholar] [CrossRef]
- Trang, N.T.D.; Schierup, H.-H.; Brix, H. Leaf vegetables for use in integrated hydroponics and aquaculture systems: Effects of root flooding on growth, mineral composition and nutrient uptake. Afr. J. Biotechnol. 2010, 9, 4186–4196. [Google Scholar]
- Barickman, T.C.; Simpson, C.R.; Sams, C.E. Waterlogging Causes Early Modification in the Physiological Performance, Carotenoids, Chlorophylls, Proline, and Soluble Sugars of Cucumber Plants. Plants 2019, 8, 160. [Google Scholar] [CrossRef] [PubMed]
- Altaf, M.A.; Shahid, R.; Ren, M.-X.; Altaf, M.M.; Jahan, M.S.; Khan, L.U. Melatonin Mitigates Nickel Toxicity by Improving Nutrient Uptake Fluxes, Root Architecture System, Photosynthesis, and Antioxidant Potential in Tomato Seedling. J. Soil Sci. Plant Nut. 2021, 21, 1842–1855. [Google Scholar] [CrossRef]
- Li, Q.; Li, X.; Tang, B.; Gu, M. Growth Responses and Root Characteristics of Lettuce Grown in Aeroponics, Hydroponics, and Substrate Culture. Horticulturae 2018, 4, 35. [Google Scholar] [CrossRef]
- Nakano, Y. Response of tomato root systems to environmental stress under soilless culture. Jpn. Agric. Res. Q. JARQ 2007, 41, 7–15. [Google Scholar] [CrossRef]
- Tsukagoshi, H. Control of root growth and development by reactive oxygen species. Curr. Opin. Plant Biol. 2016, 29, 57–63. [Google Scholar] [CrossRef]
- Mittler, R. ROS Are Good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef]
- Tsukagoshi, H. Defective root growth triggered by oxidative stress is controlled through the expression of cell cycle-related genes. Plant Sci. 2012, 197, 30–39. [Google Scholar] [CrossRef]
- Tsukagoshi, H.; Busch, W.; Benfey, P.N. Transcriptional regulation of ROS controls transition from proliferation to differentiation in the root. Cell 2010, 143, 606–616. [Google Scholar] [CrossRef] [PubMed]
- Manzano, C.; Pallero-Baena, M.; Casimiro, I.; De Rybel, B.; Orman-Ligeza, B.; Van Isterdael, G.; Beeckman, T.; Draye, X.; Casero, P.; Del Pozo, J.C. The Emerging Role of Reactive Oxygen Species Signaling during Lateral Root Development. Plant Physiol. 2014, 165, 1105–1119. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, J.; Kuang, L.; Wang, N.; Zhang, G.; Jiang, L.; Wu, D. Effects of waterlogging stress on early seedling development and transcriptomic responses in Brassica napus. Mol. Breed 2020, 40, 1–14. [Google Scholar] [CrossRef]
- Li, N.; Sun, L.; Zhang, L.; Song, Y.; Hu, P.; Li, C.; Hao, F.S. AtrbohD and AtrbohF negatively regulate lateral root development by changing the localized accumulation of superoxide in primary roots of Arabidopsis. Planta 2015, 241, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Tan, J.; Lu, S.; Lin, C.; Hu, Y.; Guo, Z. Increased tolerance to oxidative stress in transgenic tobacco expressing a wheat oxalate oxidase gene via induction of antioxidant enzymes is mediated by H2O2. Physiol. Plant 2009, 136, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Voothuluru, P.; Makela, P.; Zhu, J.; Yamaguchi, M.; Cho, I.J.; Oliver, M.J.; Simmonds, J.; Sharp, R.E. Apoplastic Hydrogen Peroxide in the Growth Zone of the Maize Primary Root. Increased Levels Differentially Modulate Root Elongation Under Well-Watered and Water-Stressed Conditions. Front Plant Sci. 2020, 11, 392. [Google Scholar] [CrossRef] [PubMed]
- Dikilitas, M.; Simsek, E.; Roychoudhury, A. Role of proline and glycine betaine in overcoming abiotic stresses. Prot. Chem. Agents Amelior. Plant Abiotic Stress Biochem. Mol. Perspect. 2020, 59, 206–216. [Google Scholar]
- Baiyin, B.; Tagawa, K.; Yamada, M.; Wang, X.; Yamada, S.; Yamamoto, S.; Ibaraki, Y. Effect of Substrate Flow Rate on Nutrient Uptake and Use Efficiency in Hydroponically Grown Swiss Chard (Beta vulgaris L. ssp. cicla ‘Seiyou Shirokuki’). Agronomy 2021, 11, 2050. [Google Scholar] [CrossRef]
- Elzenga, J.T.M.; Veen, H.V. Waterlogging and plant nutrient uptake. In Waterlogging Signalling and Tolerance in Plants; Springer: Berlin/Heidelberg, Germany, 2010; pp. 23–35. [Google Scholar]
- Morard, P.; Lacoste, L.; Silvestre, J. Effect of Oxygen Deficiency on Mineral Nutrition of Excised Tomato Roots. J. Plant Nutr. 2004, 27, 613–626. [Google Scholar] [CrossRef]
- Yamauchi, T.; Tanaka, A.; Inahashi, H.; Nishizawa, N.K.; Tsutsumi, N.; Inukai, Y.; Nakazono, M. Fine control of aerenchyma and lateral root development through AUX/IAA-and ARF-dependent auxin signaling. Proc. Natl. Acad. Sci. USA 2019, 116, 20770–20775. [Google Scholar] [CrossRef]
- Arif, Y.; Singh, P.; Siddiqui, H.; Naaz, R.; Hayat, S. Transition metal homeostasis and its role in plant growth and development. In Microbial Biofertilizers and Micronutrient Availability; Springer: Berlin/Heidelberg, Germany, 2022; pp. 159–178. [Google Scholar]
- Qi, X.; Li, Q.; Shen, J.; Qian, C.; Xu, X.; Xu, Q.; Chen, X. Sugar enhances waterlogging-induced adventitious root formation in cucumber by promoting auxin transport and signalling. Plant Cell Environ. 2020, 43, 1545–1557. [Google Scholar] [CrossRef] [PubMed]
- Paciolla, C.; Fortunato, S.; Dipierro, N.; Paradiso, A.; De Leonardis, S.; Mastropasqua, L.; de Pinto, M.C. Vitamin C in Plants: From Functions to Biofortification. Antioxidants 2019, 8, 519. [Google Scholar] [CrossRef]
- Osmolovskaya, N.; Shumilina, J.; Kim, A.; Didio, A.; Grishina, T.; Bilova, T.; Keltsieva, O.A.; Zhukov, V.; Tikhonovich, I.; Tarakhovskaya, E.; et al. Methodology of Drought Stress Research: Experimental Setup and Physiological Characterization. Int. J. Mol. Sci. 2018, 19, 4089. [Google Scholar] [CrossRef]
- Koyama, R.; Itoh, H.; Kimura, S.; Morioka, A.; Uno, Y. Augmentation of antioxidant constituents by drought stress to roots in leafy vegetables. HortTechnology 2012, 22, 121–125. [Google Scholar] [CrossRef]
- Del Carmen Martinez-Ballesta, M.; Moreno, D.A.; Carvajal, M. The physiological importance of glucosinolates on plant response to abiotic stress in Brassica. Int. J. Mol. Sci. 2013, 14, 11607–11625. [Google Scholar] [CrossRef] [PubMed]
- Brazel, S.; Barickman, T.; Sams, C. Short-term waterlogging of kale (Brassica oleracea L. var. acephala) plants causes a decrease in carotenoids and chlorophylls while increasing nutritionally important glucosinolates. In Proceedings of the VIII International Symposium on Human Health Effects of Fruits and Vegetables-FAVHEALTH, Stuttgart, Germany, 3 December 2021; pp. 175–180. [Google Scholar]
- Mezgebe, A.; Azerefegne, F. Effect of water stress on glucosinolate content of Brassica carinata and performance of Brevicoryne brassicae and Myzus persicae. Int. J. Trop. Insect Sci. 2020, 41, 953–960. [Google Scholar] [CrossRef]
- Vanegas Torres, A.; Tish, N.; Rodov, V. Enhancement of Glucosinolate Formation in Broccoli Sprouts by Hydrogen Peroxide Treatment. Foods 2022, 11, 655. [Google Scholar] [CrossRef]


| Treatments | De-1 | De-2 | De-3 |
|---|---|---|---|
| Chl a (mg·g−1 FW) | 1.07 ± 0.01 a | 1.08 ± 0.01 a | 1.02 ± 0.01 b |
| Chl b (mg·g−1 FW) | 0.67 ± 0.03 a | 0.65 ± 0.02 a | 0.57 ± 0.01 b |
| Chl (a + b) (mg·g−1 FW) | 1.75 ± 0.04 a | 1.72 ± 0.02 a | 1.59 ± 0.02 b |
| Car (mg·g−1 FW) | 0.16 ± 0.01 b | 0.17 ± 0.01 b | 0.19 ± 0.00 a |
| Chl a/b | 1.60 ± 0.05 b | 1.71 ± 0.02 ab | 1.82 ± 0.02 a |
| Treatments | De-1 | De-2 | De-3 |
|---|---|---|---|
| N (g·kg−1 DW) | 51.57 ± 0.29 a | 49.81 ± 0.01 b | 50.51 ± 0.27 b |
| P (g·kg−1 DW) | 6.19 ± 0.01 a | 6.09 ± 0.02 b | 5.93 ± 0.02 c |
| K (g·kg−1 DW) | 60.21 ± 0.04 a | 54.75 ± 0.19 b | 54.33 ± 0.36 b |
| Ca (g·kg−1 DW) | 39.14 ± 0.01 b | 40.67 ± 0.07 a | 38.71 ± 0.06 c |
| Mg (g·kg−1 DW) | 5.61 ± 0.09 a | 5.76 ± 0.01 a | 5.74 ± 0.01 a |
| S (g·kg−1 DW) | 17.74 ± 0.06 b | 18.47 ± 0.01 a | 14.91 ± 0.06 c |
| Zn (mg·kg−1 DW) | 58.21 ± 0.34 a | 43.04 ± 0.30 b | 30.33 ± 0.22 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, J.; Jiang, H.; Li, Y.; He, R.; Liu, K.; Chen, Y.; He, X.; Liu, X.; Liu, H. Growth, Phytochemicals, and Antioxidant Activity of Kale Grown under Different Nutrient-Solution Depths in Hydroponic. Horticulturae 2023, 9, 53. https://doi.org/10.3390/horticulturae9010053
Tan J, Jiang H, Li Y, He R, Liu K, Chen Y, He X, Liu X, Liu H. Growth, Phytochemicals, and Antioxidant Activity of Kale Grown under Different Nutrient-Solution Depths in Hydroponic. Horticulturae. 2023; 9(1):53. https://doi.org/10.3390/horticulturae9010053
Chicago/Turabian StyleTan, Jiehui, Haozhao Jiang, Yamin Li, Rui He, Kaizhe Liu, Yongkang Chen, Xinyang He, Xiaojuan Liu, and Houcheng Liu. 2023. "Growth, Phytochemicals, and Antioxidant Activity of Kale Grown under Different Nutrient-Solution Depths in Hydroponic" Horticulturae 9, no. 1: 53. https://doi.org/10.3390/horticulturae9010053
APA StyleTan, J., Jiang, H., Li, Y., He, R., Liu, K., Chen, Y., He, X., Liu, X., & Liu, H. (2023). Growth, Phytochemicals, and Antioxidant Activity of Kale Grown under Different Nutrient-Solution Depths in Hydroponic. Horticulturae, 9(1), 53. https://doi.org/10.3390/horticulturae9010053










