Callus Induction and Plant Regeneration from Carum copticum and Assessment of Antioxidant Responses and Phytochemical Profiling by In Vitro Salinity Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Seed Culture and Preparation of Explants
2.2. Callus Induction and Plant Regeneration of C. copticum
2.3. Salt Treatment
2.4. Measuring Amount of Proline
2.5. Measuring Amount of Reducing Carbohydrates
2.6. Protein Extraction and Enzyme Assay
2.7. Measuring of Total Phenolic Content
2.8. Measuring of Anthocyanin Content
2.9. Determination of Essential Oils
2.10. Statistical Analysis
3. Results
3.1. Callus Induction and Plant Regeneration
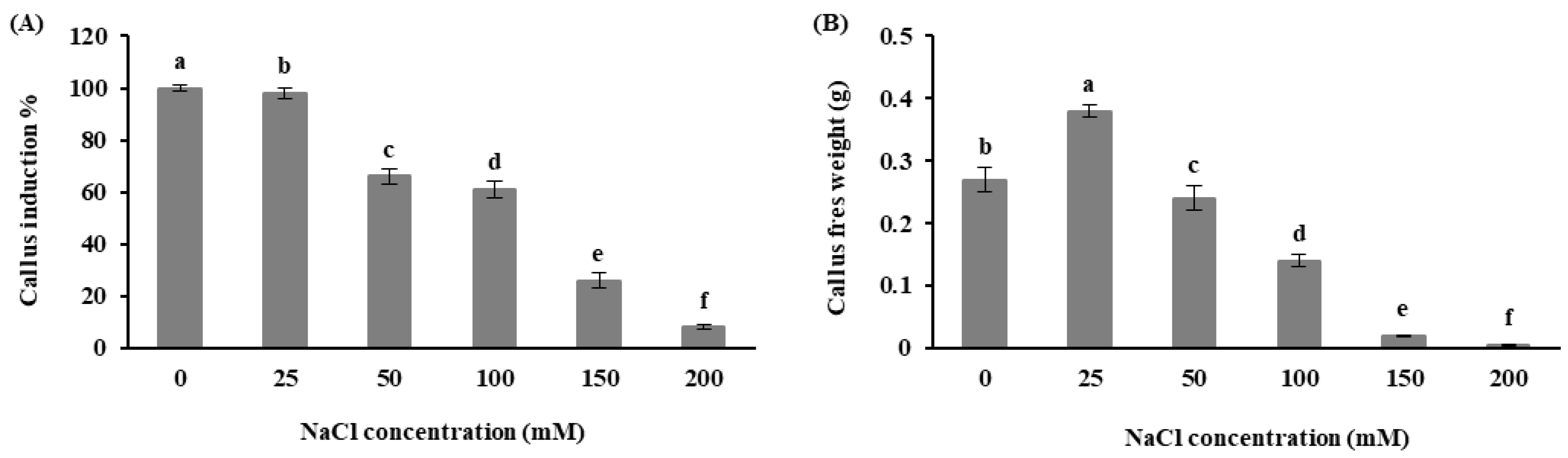
3.2. Effects of In Vitro Salt Stress on Callus Induction Percentage and Fresh Weight
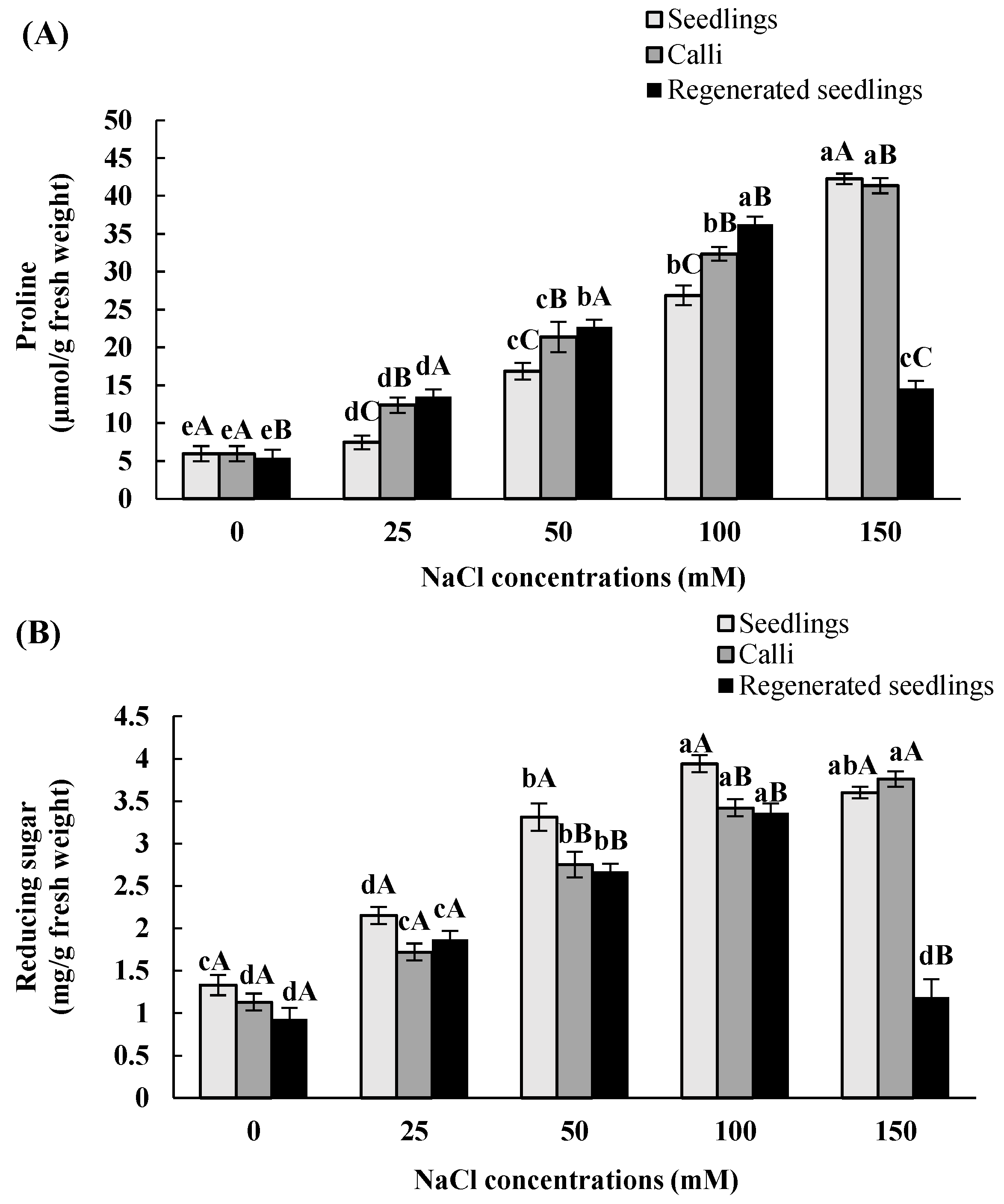
3.3. Effects of In Vitro Salt Stress on Accumulation of Proline and Reducing Carbohydrate
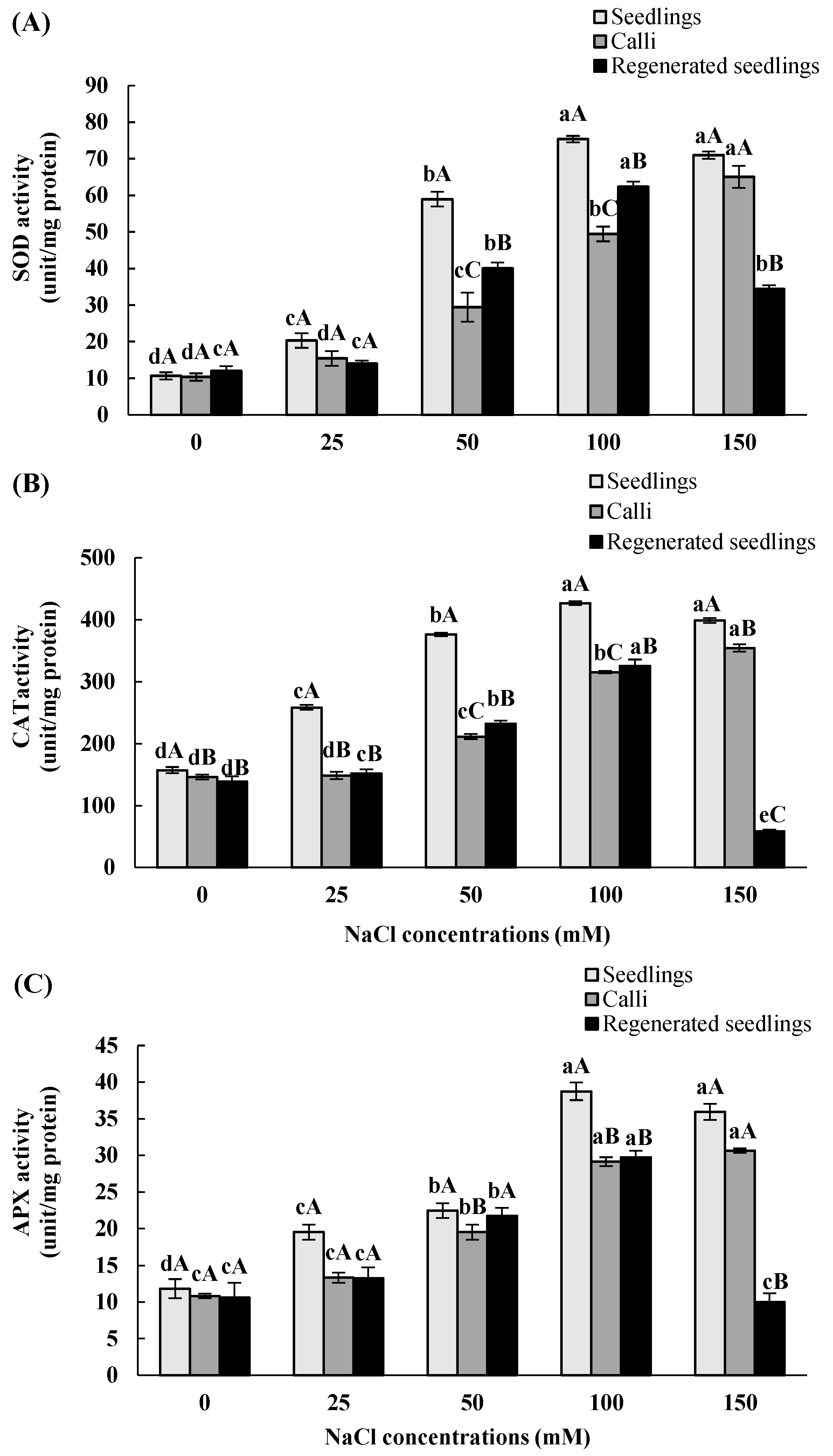
3.4. Effects of In Vitro Salt Stress on Antioxidant Enzyme Activities
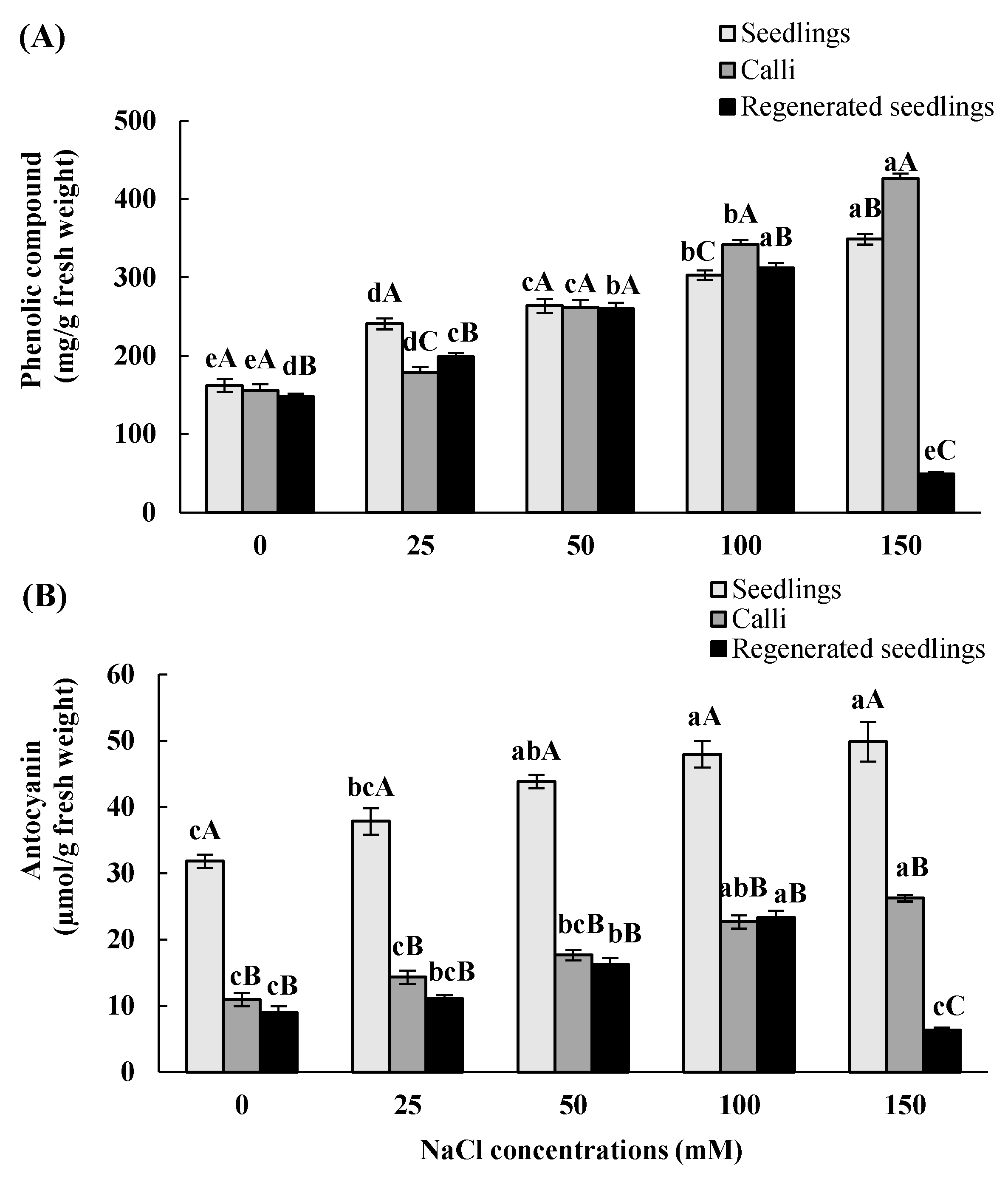
3.5. Effects of In Vitro Salt Stress on Accumulation of Total Phenolic and Anthocyanin Contents
3.6. Effects of In Vitro Salt Stress on Essential Oil Components
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lucini, L.; Borgognone, D.; Rouphael, Y.; Cardarelli, M.; Bernardi, J.; Colla, G. Mild potassium chloride stress alters the mineral composition, hormone network, and phenolic profile in artichoke leaves. Front. Plant. Sci. 2016, 7, 948. [Google Scholar] [CrossRef] [PubMed]
- Javed, H.; Nagoor Meeran, M.F.; Kumar Jha, N.; Ojha, S.H. Carvacrol, a plant metabolite targeting viral protease (Mpro) and ACE2 in host cells can be a possible candidate for COVID-19. Front. Plant. Sci. 2020, 11, 601335. [Google Scholar] [CrossRef] [PubMed]
- Isah, T. Stress and defense responses in plant secondary metabolites production. Biol. Res. 2019, 52, 39. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Leal, C.A.; Puente-Garza, C.A.; García-Lara, S. In vitro plant tissue culture: Means for production of biological active compounds. Planta 2018, 248, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Sytar, O.; Barki, S.; Zivcak, M.; Brestic, M. The involvement of different secondary metabolites in salinity tolerance of crops. In Salinity Responses and Tolerance in Plants; Kumat, V., Ed.; Springer: Cham, Switzerland, 2018; Volume 2, pp. 21–48. [Google Scholar]
- Bourgaud, F.; Gravot, A.; Milesi, S.; Gontier, E. Production of plant secondary metabolites: A historical perspective. Plant Sci. 2001, 161, 839–851. [Google Scholar] [CrossRef]
- Xu, C.; Tang, X.; Shao, H.; Wang, H. Salinity tolerance mechanism of economic halophytes from physiological to molecular hierarchy for improving food quality. Curr. Genom. 2016, 17, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, N.J.; Urwin, P.E. The interaction of plant biotic and abiotic stresses: From genes to the field. J. Exp. Bot. 2012, 63, 3523–3543. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Sharma, S.; Saxena, S. Effect of abiotic stress on growth parameters and steviol glycoside content in Stevia rebaudiana (Bertoni) raised in vitro. J. Appl. Res. Med. Aromat. Plants 2016, 3, 160–167. [Google Scholar] [CrossRef]
- Tari, I.; Kiss, G.; Deer, A.K.; Csiszar, J.; Erdei, L.; Galle, A.; Gémes, K.; Horváth, F.; Poór, P.; Szepesi, Á.; et al. Salicylic acid increased aldose reductase activity and sorbitol accumulation in tomato plants under salt stress. Biol. Plant 2010, 54, 677–683. [Google Scholar] [CrossRef]
- Altpeter, F.; Springer, N.M.; Bartley, L.E.; Blechl, A.E.; Brutnell, T.P.; Citovsky, V.; Conrad, L.; Gelvin, S.B.; Jackson, D.P.; Kausch, A.P.; et al. Advancing crop transformation in the era of genome editing. Plant Cell 2016, 28, 1510–1520. [Google Scholar] [CrossRef]
- Karakas, F.P.; Turker, A.U. Improvement of shoot proliferation and comparison of secondary metabolites in shoot and callus cultures of Phlomis armeniaca by LC-ESI-MS/MS analysis. In Vitro Cell Dev. Biol.-Plant 2016, 52, 608–618. [Google Scholar] [CrossRef]
- Zarshenas, M.M.; Moein, M.; Mohammadi Samani, M.; Petramfar, P. An overview on Ajwain (Trachyspermum ammi) pharmacological effects; modern and traditional. J. Nat. Remedies. 2013, 14, 98–105. [Google Scholar]
- Boskabady, M.H.; Alitaneh, S.; Alavinezhad, A. Carum copticum L.: A herbal medicine with various pharmacological effects. Biomed. Res. Int. 2014, 2014, 569087. [Google Scholar] [CrossRef] [PubMed]
- Cimanga, K.; Kambu, K.; Tona, L.; Apers, S.; De Bruyne, T.; Hermans, N. Correlation between chemical composition and antibacterial activity of essential oils of some aromatic medicinal plants growing in the Democratic Republic of Congo. J. Ethnopharmacol. 2002, 79, 213–220. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Plant. Physiol. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldern, R.P.; Teare, I.D. Rapid determination of free proline for water stress studies. Plant. Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Somogyi-Nelson, M. Notes on sugar determination. J. Biol. Chem. 1952, 195, 19–29. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of proteindye binding. Annu. Rev. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Giannotolitis, C.N.; Ries, S.K. Superoxide dismutase: II. Purification and quantitative relationship with water-soluble protein in seedling. Plant Physiol. 1997, 59, 315–318. [Google Scholar] [CrossRef]
- Aebi, H. Catalases. In Methods of Enzymatic Analysis; Bergmeyer, H.U., Ed.; Academic Press: New York, NY, USA, 1974; pp. 673–684. [Google Scholar]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Adabavazeh, F.; Pourseyedi, S.; Nadernejad, N.; Razavizadeh, R.; Mozafari, H. Evaluation of synthesized magnetic nanoparticles and salicylic acid effects on improvement of antioxidant properties and essential oils of Calotropis procera hairy roots and seedlings. Plant Cell Tiss. Organ Cul. 2022, 151, 133–148. [Google Scholar] [CrossRef]
- Wagner, G.J. Content and vacuole/extra vacuole distribution of neutral sugars, free amino acids and anthocyanins in protoplast. Plant Physiol. 1979, 64, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Li, X.M.; Tian, S.L.; Pang, Z.C. Extraction of Cuminum cyminum essential oil by combination technology of organic solvent with low boiling point and system distillation. Food Chem. 2009, 115, 1114–1119. [Google Scholar] [CrossRef]
- Davies, N.W. Gas chromatographic retention indices of monoterpenes and sesquiterpenes on methylsilicon and carbowax 20M Phases. J. Chromatogr. A 1990, 503, 1–24. [Google Scholar] [CrossRef]
- El-Hendawy, S.E.; Al-Suhaibani, N.A.; Hassan, W.M.; Dewir, Y.H.; Elsayed, S.; Ashkar, I.; Abdella, K.A.; Schmidhalter, U. Evaluation of wave lengths and spectral reflectance indices for high-throughput assessment of growth, water relations and ion contents of wheat irrigated with saline water. Agric. Water Manag. 2019, 212, 358–377. [Google Scholar] [CrossRef]
- Narayani, M.; Srivastava, S. Elicitation: A stimulation of stress in in vitro plant cell/tissue cultures for enhancement of secondary metabolite production. Phytochem. Rev. 2018, 16, 1227–1252. [Google Scholar] [CrossRef]
- Rejeb, I.B.; Pastor, V.; Mauch-Mani, B. Plant responses to simultaneous biotic and abiotic stress: Molecular mechanisms. Plants 2014, 3, 458–475. [Google Scholar] [CrossRef]
- Dogan, M. Effect of salt stress on in vitro organogenesis from nodal explant of Limnophila aromatica (Lamk.) Merr. And Bacopa monnieri (L.) Wettst. and their physio-morphological and biochemical responses. Physiol. Mol. Biol. Plants 2020, 26, 803–816. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Yu, J.; Zhang, H.; Wang, L.; Wang, S.; Guo, S.; Miao, Y.; Chen, S.; Li, Y.; et al. NaCl-responsive ROS scavenging and energy supply in alkaligrass callus revealed from proteomic analysis. BMC Genom. 2019, 20, 990. [Google Scholar] [CrossRef]
- Benderradji, L.; Brini, F.; Kellou, K.; Ykhlef, N.; Djekoun, A.; Masmoudi, K.; Bouzerzour, H. Callus induction, proliferation, and plantlets Regeneration of two bread wheat (Triticum aestivum L.) genotypes under saline and heat stress conditions. Int. Sch. Res. Notices 2012, 2012, 1–8. [Google Scholar] [CrossRef]
- Parihar, P.; Singh, S.; Singh, R.; Singh, V.P.; Prasad, S.M. Effect of salinity stress on plants and its tolerance strategies: A review. Environ. Sci. Poll. Res. 2015, 22, 4056–4075. [Google Scholar] [CrossRef]
- Tsukagoshi, H.; Busch, W.; Benfey, P.N. Transcriptional regulation of ROS controls transition from proliferation to differentiation in the root. Cell 2010, 143, 606–616. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.T.; Wan, Z.Y.; Li, S.; Zhang, Y. Spatiotemporal production of breactive oxygen species by NADPH oxidase is critical for tapetal programmed bcell death and pollen development in Arabidopsis. Plant Cell 2014, 26, 2007–2023. [Google Scholar] [CrossRef] [PubMed]
- Flowers, T.J.; Colmer, T.D. Salinity tolerance in halophytes. New Phytol. 2008, 179, 945–963. [Google Scholar] [CrossRef] [PubMed]
- Verslues, P.E.; Agarwal, M.; Katiyar-Agarwal, S.; Zhu, J.; Zhu, J.K. Methods and concepts in quantifying resistance to drought, salt and freezing, abiotic stresses that affect plant water status. Plant J. 2006, 45, 523–539. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Székely, G.; Abrahám, E.; Cséplo, A.; Rigó, G.; Zsigmond, L.; Csiszár, J.; Ayaydin, F.; Strizhov, N.; Jásik, J.; Schmelzer, E.; et al. Duplicated P5CS genes of Arabidopsis play distinct roles in stress regulation and developmental control of proline biosynthesis. Plant J. 2008, 53, 11–28. [Google Scholar] [CrossRef] [PubMed]
- Patade, V.Y.; Bhargava, S.; Suprasanna, P. Salt and drought tolerance of sugarcane under iso-osmotic salt and water stress: Growth, osmolytes accumulation, and antioxidant defense. J. Plant Interact. 2011, 6, 275–282. [Google Scholar] [CrossRef]
- Errabii, T.; Gandonou, C.B.; Essalmani, H.; Abrini, J.; Idaomar, M.; Senhaji, N.S. Effects of NaCl and mannitol induced stress on sugarcane Saccharum sp. callus cultures. Acta Physiol. Plant 2007, 29, 95–102. [Google Scholar] [CrossRef]
- Keunen, E.L.S.; Peshev, D.; Vangronsveld, J.; van den Ende, W.I.M.; Cuypers, A.N.N. Plant sugars are crucial players in the oxidative challenge during abiotic stress: Extending the traditional concept. Plant Cell Environ. 2013, 36, 1242–1255. [Google Scholar] [CrossRef]
- Hannachi, S.; Steppe, K.; Eloudi, M.; Mechi, L.; Bahrini, I.; Van Labeke, M.C. Salt stress induced changes in photosynthesis and metabolic profiles of one tolerant (‘Bonica’) and one sensitive (‘Black beauty’) eggplant cultivars (Solanum melongena L.). Plants 2022, 11, 590. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zhai, J.; Liu, Y.; Cheng, X.; Xia, Z.; Chen, F.; Cui, D.; Jiang, X. The response of Mo-hydroxylases and abscisic acid to salinity in wheat genotypes with differing salt tolerances. Acta Physiol. Plant 2012, 34, 1767–1778. [Google Scholar] [CrossRef]
- Dvořák, P.; Krasylenko, Y.; Zeiner, A.; Šamaj, J.; Takáč, T. Signaling toward reactive oxygen species-scavenging enzymes in plants. Front. Plant. Sci. 2021, 11, 618835. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Wang, R.; Yang, X.; Ju, Q.; Li, W.; Lü, S.; Tran, L.S.P.; Xu, J. The R2R3-MYB transcription factor AtMYB49 modulates salt tolerance in Arabidopsis bymodulating the cuticle formation and antioxidant defence. Plant Cell Environ. 2020, 43, 1925–1943. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, Y.; Deng, C.; Deng, S.; Li, N.; Zhao, C.; Zhao, R.; Liang, S.; Chen, S. The Arabidopsis Ca2+-dependent protein kinase CPK12 is involved in plant response to salt stress. Int. J. Mol. Sci. 2018, 19, 4062. [Google Scholar] [CrossRef]
- Liu, J.; Fu, C.; Li, G.; Khan, M.N.; Wu, H. ROS homeostasis and plant salt tolerance: Plant nanobiotechnology updates. Sustainability 2021, 13, 3552. [Google Scholar] [CrossRef]
- Feki, K.; Tounsi, S.; Brini, F. Comparison of an antioxidant system in tolerant and susceptible wheat seedlings in response to salt stress. Span. J. Agric. Res. 2017, 15, e0805. [Google Scholar] [CrossRef]
- Jdey, A.; Falleh, H.; Jannet, S.B.; Mkadmini Hammi, K.; Dauvergne, X.; Magné, C.; Ksouri, R. Anti-aging activities of extracts from Tunisian medicinal halophytes and their aromatic constituents. EXCLI J. 2017, 16, 755–769. [Google Scholar]
- Ben Ahmed, C.; Rouina, B.B.; Sensoy, S. Saline water ırrigation effects on fruit development, quality, and phenolic composition of virgin olive oils, cv. Chemlali. J. Agric. Food Chem. 2009, 57, 2803–2811. [Google Scholar] [CrossRef]
- Plaza, B.M.; Jimenez, S.; Segura, M.L. Physiological stress caused by salinity in Cordyline fruticosa and its indicators. Commun. Soil Sci. Plant Anal. 2009, 40, 473–484. [Google Scholar] [CrossRef]
- Daiponmak, W.; Theerakulpisut, P.; Thanonkao, P.; Vanavichit, A.; Prathepha, P. Changes of anthocyanin cyanidin-3- glucoside content and antioxidant activity in Thai rice varieties under salinity stress. Sci. Asia 2010, 36, 286–291. [Google Scholar] [CrossRef]
- Waskiewicz, A.; Muzolf-Panek, M.; Golinski, P. Phenolic content changes in plants under salt stress. In Ecophysiology and Responses of Plants under Salt Stress; Springer: New York, NY, USA, 2013; pp. 283–314. [Google Scholar]
- Ramakrishna, A.; Ravishankar, G.A. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal. Behav. 2011, 6, 1720–1731. [Google Scholar] [PubMed]
- Ksouri, R.; Megdiche, W.; Debez, A. Salinity effects on polyphenol content and antioxidant activities in leaves of the halophyte Cakile maritima. Plant Physiol. Biochem. 2007, 45, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Hanen, F.; Ksouri, R.; Megdiche, W. Effect of salinity on growth, leaf-phenolic content and antioxidant scavenging activity in Cynara cardunculus L. In Biosaline Agriculture and High Salinity Tolerance; Abdelly, C., Öztürk, M., Ashraf, M., Grignon, C., Eds.; Birkhäuser: Basel, Switzerland, 2008; pp. 335–343. [Google Scholar]
- Montanari, M.; Deglnnocenti, E.; Maggini, R. Effect of nitrate fertilization and saline stress on the contents of active constituents of Echinacea angustifolia DC. Food Chem. 2008, 107, 1461–1466. [Google Scholar] [CrossRef]
- Slama, I.; MʹRabet, R.; Ksouri, R.; Talbi, O.; Debez, A.; Abdelly, C. Effects of salt treatment on growth, lipid membrane peroxidation, polyphenol content, and antioxidant activities in leaves of Sesuvium portulacastrum L. Arid Land Res. Manag. 2017, 31, 404–417. [Google Scholar] [CrossRef]
- Eryılmaz, F. The relationships between salt stress and anthocyanin content in higher plants. Biotechnol. Biotechnol. Equip. 2006, 20, 47–52. [Google Scholar] [CrossRef]
- Valifard, M.; Mohsenzadeh, S.; Kholdebarin, B.; Rowshan, V. Effects of salt stress on volatile compounds, total phenolic content and antioxidant activities of Salvia mirzayanii. S. Afr. J. Bot. 2014, 93, 92–97. [Google Scholar] [CrossRef]
- El-Mashad, A.A.A.; Mohamed, H.I. Brassinolide alleviates salt stress and increases antioxidant activity of cowpea plants (Vigna sinensis). Protoplasma 2012, 249, 625–635. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Babbar, N.; Oberoi, H.S.; Sandhu, S.K. Therapeutic and nutraceutical potential of bioactive compounds extracted from fruit residues. Crit. Rev. Food Sci. Nutr. 2015, 55, 319–337. [Google Scholar] [CrossRef]
- Assaf, M.; Korkmaz, A.; Karaman, Ş.; Kulak, M. Effect of plant growth regulators and salt stress on secondary metabolite composition in Lamiaceae species. S. Afr. J. Bot. 2022, 144, 480–493. [Google Scholar] [CrossRef]
- Tounekti, T.; Vadel, A.M.; Oñate, M.; Khemira, H.; Munné-Bosch, S. Salt-induced oxidative stress in rosemary plants: Damage or protection? Environ. Exp. Bot. 2011, 71, 298–305. [Google Scholar] [CrossRef]
- Sarmoum, R.; Haid, S.; Biche, M.; Djazouli, Z.; Zebib, B.; Merah, O. Effect of salinity and water stress on the essential oil components of Rosemary (Rosmarinus officinalis L.). Agronomy 2019, 9, 214. [Google Scholar] [CrossRef]
- Pichersky, E.; Noel, J.P.; Dudareva, N. Biosynthesis of plant volatiles: Nature’s diversity and ingenuity. Science 2006, 311, 808–811. [Google Scholar] [CrossRef]
- Dubey, V.S.; Bhalla, R.; Luthra, R. Sucrose mobilization in relation to essential oil biogenesis during palmarosa (Cymbopogon martinii Roxb. Wats. Var. Motia) inflorescence development. J. Biosci. 2003, 28, 479–487. [Google Scholar] [CrossRef]
- Neffati, M.; Sriti, J.; Hamdaoui, G.; Kchouk, M.E.; Marzouk, B. Salinity impact on fruit yield, essential oil composition and antioxidant activities of Coriandrum sativum fruit extracts. Food Chem. 2011, 124, 221–225. [Google Scholar] [CrossRef]
- Karray-Bouraoui, N.; Ksouri, R.; Falleh, H.; Rabhi, M.; Grignon, C.; Lachaal, M. Effects of environment and development stage on phenolic content and antioxidant activities of Tunisian Mentha pulegium L. J. Food Biochem. 2010, 34, 79–89. [Google Scholar] [CrossRef]
- Ben Taarit, M.; Msaada, K.; Hosni, K.; Marzouk, B. Physiological changes and essential oil composition of clary sage (Salvia sclarea L.) rosette leaves as affected by salinity. Acta Physiol. Plant 2011, 33, 153–162. [Google Scholar] [CrossRef]
- Baatour, O.; Kaddour, R.; Aidi Wannes, W.; Lachaa, M.; Marzouk, B. Salt effects on the growth, mineral nutrition, essential oil yield and composition of marjoram (Origanum majorana). Acta Physiol. Plant 2010, 32, 45–51. [Google Scholar] [CrossRef]


| Callus Selection Medium (MS) | Days for Callus Initiation | Callus Induction (%) | Callus Fresh Weight (g) | Morphology | |
|---|---|---|---|---|---|
| 2,4-D mg L−1 | BAP mg L−1 | ||||
| 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | - |
| 0.0 | 0.25 | 0.0 | 0.0 | 0.0 | - |
| 0.0 | 0.5 | 0.0 | 0.0 | 0.0 | - |
| 0.0 | 1 | 0.0 | 0.0 | 0.0 | - |
| 0.0 | 1.5 | 0.0 | 0.0 | 0.0 | - |
| 0.0 | 2 | 0.0 | 0.0 | 0.0 | - |
| 0.25 | 0.0 | 0.0 | 0.0 | 0.0 | - |
| 0.25 | 0.25 | 11 | 24 ± 10 | 0.1 ± 0.1 | Greenish–Yellow Friable |
| 0.25 | 0.5 | 8 | 90 ± 10 | 0.4 ± 0.2 | Greenish–Yellow Friable |
| 0.25 | 1 | 8 | 100 ± 0.0 | 1.0 ± 0.2 | Greenish–Yellow Friable |
| 0.25 | 1.5 | 8 | 80 ± 34 | 0.9 ± 0.1 | Greenish–Yellow Friable |
| 0.25 | 2 | 9 | 93.3 ± 11 | 0.7 ± 0.2 | Greenish–Yellow Friable |
| 0.5 | 0.0 | 0.0 | 0.0 | 0.0 | - |
| 0.5 | 0.25 | 10 | 86.6 ± 6 | 0.2 ± 0.0 | Greenish–Yellow Friable |
| 0.5 | 0.5 | 9 | 90 ± 10 | 0.4 ± 0.0 | Greenish–Yellow Friable |
| 0.5 | 1 | 12 | 85 ± 13 | 0.7 ± 0.1 | Greenish–Yellow Friable |
| 0.5 | 1.5 | 15 | 100 ± 0.0 | 0.7 ± 0.1 | Greenish–Yellow Friable |
| 0.5 | 2 | 14 | 100 ± 0.0 | 0.8 ± 0.1 | Greenish–Yellow Friable |
| 1 | 0.0 | 0.0 | 0.0 | 0.0 | - |
| 1 | 0.25 | 11 | 100 ± 0.0 | 0.4 ± 0.1 | Yellowish–White Friable |
| 1 | 0.5 | 10 | 71.6 ± 24 | 0.4 ± 0.0 | Yellowish–White Friable |
| 1 | 1 | 9 | 75.3 ± 13 | 0.3 ± 0.1 | Yellowish–White Friable |
| 1 | 1.5 | 10 | 80 ± 0.0 | 0.5 ± 0.0 | Yellowish–White Friable |
| 1 | 2 | 10 | 100 ± 0.0 | 0.5 ± 0.2 | Yellowish–White Friable |
| 1.5 | 0.0 | 0.0 | 0.0 | 0.0 | - |
| 1.5 | 0.25 | 11 | 93.3 ± 11 | 0.6 ± 0.1 | Yellowish–White Compact |
| 1.5 | 0.5 | 9 | 80 ± 0.0 | 0.2 ± 0.0 | Yellowish–White Compact |
| 1.5 | 1 | 9 | 90 ± 10 | 0.4 ± 0.1 | Yellowish–White Compact |
| 1.5 | 1.5 | 9 | 80 ± 0.0 | 0.3 ± 0.0 | Yellowish–White Compact |
| 1.5 | 2 | 11 | 60 ± 26 | 0.2 ± 0.1 | Yellowish–White Compact |
| 2 | 0.0 | 0.0 | 0.0 | 0.0 | - |
| 2 | 0.25 | 10 | 83.3 ± 5.6 | 0.1 ± 0.1 | White Compact |
| 2 | 0.5 | 11 | 90 ± 10 | 0.1 ± 0.1 | White Compact |
| 2 | 1 | 8 | 90 ± 10 | 0.6 ± 0.1 | White Compact |
| 2 | 1.5 | 10 | 90 ± 10 | 0.4 ± 0.1 | White Compact |
| 2 | 2 | 13 | 66.6 ± 11 | 0.5 ± 0.0 | White Compact |
| Callus Selection Medium (MS) | Days for Callus Initiation | Callus Induction (%) | Callus Fresh Weight (g) | Morphology | |
|---|---|---|---|---|---|
| 2,4-D mg L−1 | BAP mg L−1 | ||||
| 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | - |
| 0.0 | 0.25 | 0.0 | 0.0 | 0.0 | - |
| 0.0 | 0.5 | 0.0 | 0.0 | 0.0 | - |
| 0.0 | 1 | 0.0 | 0.0 | 0.0 | - |
| 0.0 | 1.5 | 0.0 | 0.0 | 0.0 | - |
| 0.0 | 2 | 0.0 | 0.0 | 0.0 | - |
| 0.25 | 0.0 | 0.0 | 0.0 | 0.0 | - |
| 0.25 | 0.25 | 9 | 43.3 ± 5.7 | 0.2 ± 0.0 | Greenish–Yellow Friable |
| 0.25 | 0.5 | 8 | 51.6 ± 27 | 0.2 ± 0.1 | Greenish–Yellow Friable |
| 0.25 | 1 | 8 | 63.3 ± 32 | 0.3 ± 0.1 | Greenish–Yellow Friable |
| 0.25 | 1.5 | 8 | 80 ± 0.0 | 0.2 ± 0.2 | Greenish–Yellow Friable |
| 0.25 | 2 | 9 | 73.3 ± 5.7 | 0.2 ± 0.0 | Greenish–Yellow Friable |
| 0.5 | 0.0 | 0.0 | 0.0 | 0.0 | - |
| 0.5 | 0.25 | 0.0 | 0.0 | 0.0 | - |
| 0.5 | 0.5 | 10 | 23.3 ± 5.7 | 0.2 ± 0.2 | Greenish–Yellow Friable |
| 0.5 | 1 | 9 | 73.3 ± 11 | 0.1 ± 0.0 | Greenish–Yellow Friable |
| 0.5 | 1.5 | 11 | 40 ± 0.0 | 0.1 ± 0.0 | Greenish–Yellow Friable |
| 0.5 | 2 | 10 | 76.6 ± 5.7 | 0.3 ± 0.2 | Greenish–Yellow Friable |
| 1 | 0.0 | 0.0 | 0.0 | 0.0 | - |
| 1 | 0.25 | 0.0 | 0.0 | 0.0 | - |
| 1 | 0.5 | 10 | 53.3 ± 5.7 | 0.1 ± 0.1 | Yellowish–White Friable |
| 1 | 1 | 11 | 33.3 ± 5.7 | 0.003 ± 0.0 | Yellowish–White Friable |
| 1 | 1.5 | 9 | 53.3 ± 5.7 | 0.1 ± 0.0 | Yellowish–White Friable |
| 1 | 2 | 10 | 40 ± 0 | 0.2 ± 0.1 | Yellowish–White Compact |
| 1.5 | 0.0 | 0.0 | 0.0 | 0.0 | - |
| 1.5 | 0.25 | 10 | 56.6 ± 5.7 | 0.2 ± 0.1 | Yellowish–White Compact |
| 1.5 | 0.5 | 11 | 60 ± 0.0 | 0.2 ± 0.2 | Yellowish–White Compact |
| 1.5 | 1 | 10 | 66.6 ± 5.7 | 0.2 ± 0.0 | Yellowish–White Compact |
| 1.5 | 1.5 | 10 | 96.6 ± 3.7 | 0.2 ± 0.1 | Yellowish–White Compact |
| 1.5 | 2 | 11 | 40 ± 34 | 0.4 ± 0.1 | Yellowish–White Compact |
| 2 | 0.0 | 0.0 | 0.0 | 0.0 | - |
| 2 | 0.25 | 12 | 33.3 ± 5.7 | 0.1 ± 0.0 | White Compact |
| 2 | 0.5 | 12 | 66.6 ± 20 | 0.1 ± 0.0 | White Compact |
| 2 | 1 | 12 | 76.6 ± 20 | 0.6 ± 0.1 | White Compact |
| 2 | 1.5 | 12 | 56.6 ± 5.7 | 0.2 ± 0.1 | White Compact |
| 2 | 2 | 11 | 23.3 ± 5.7 | 0.2 ± 0.02 | White Compact |
| Culture Medium (MS) | Callus with Shoots (%) | Number of Shoots Per Explant | Shoot Length (cm) | |
|---|---|---|---|---|
| 2,4-D mg L−1 | BAP mg L−1 | |||
| 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 0.0 | 0.25 | 5 ± 1 | 4 ± 0.7 | 0.5 ± 0.01 |
| 0.0 | 0.5 | 25 ± 1 | 7 ± 0.7 | 0.6 ± 0.02 |
| 0.0 | 1 | 56 ± 3 | 19 ± 0.7 | 0.8 ± 0.01 |
| 0.0 | 1.5 | 47 ± 2 | 17 ± 1.0 | 0.7 ± 0.01 |
| 0.0 | 2 | 52 ± 3 | 18 ± 0.9 | 0.7 ± 0.02 |
| 0.25 | 0.0 | 0.0 | 0.0 | 0.0 |
| 0.25 | 0.25 | 7 ± 2 | 6 ± 0.8 | 0.9 ± 0.03 |
| 0.25 | 0.5 | 63 ± 2 | 22 ± 0.9 | 0.9 ± 0.4 |
| 0.25 | 1 | 75 ± 2 | 27 ± 1 | 1 ± 0.02 |
| 0.25 | 1.5 | 80 ± 1 | 31 ± 0.5 | 1.0 ± 0.02 |
| 0.25 | 2 | 50.1 ± 2 | 21 ± 1.1 | 0.9 ± 0.02 |
| 0.5 | 0.0 | 0.0 | 0.0 | 0.0 |
| 0.5 | 0.25 | 5 ± 1 | 6 ± 0.5 | 0.5 ± 0.02 |
| 0.5 | 0.5 | 8 ± 2 | 5 ± 0.2 | 0.7 ± 0.02 |
| 0.5 | 1 | 52 ± 3 | 19 ± 0.9 | 0.8 ± 0.02 |
| 0.5 | 1.5 | 49 ± 2 | 18 ± 0.7 | 0.7 ± 0.02 |
| 0.5 | 2 | 41 ± 3 | 16 ± 1.0 | 0.6 ± 0.02 |
| Culture Medium (MS) | Rooting (%) | Number of Roots Per Shoot | Root Length (cm) | |
|---|---|---|---|---|
| IBA mg L−1 | IAA mg L−1 | |||
| 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 0.0 | 0.5 | 45 ± 3 | 4 ± 1 | 0.5 ± 0.03 |
| 0.0 | 1 | 75 ± 3 | 9.9 ± 0.5 | 1.1 ± 0.02 |
| 0.5 | 0.0 | 31 ± 5 | 3.5 ± 0.4 | 0.8 ± 0.01 |
| 0.5 | 0.5 | 61 ± 4 | 7.4 ± 0.8 | 0.8 ± 0.01 |
| 0.5 | 1 | 45 ± 5 | 5.8 ± 0.3 | 0.7 ± 0.02 |
| 1 | 0.0 | 63 ± 3 | 7.7 ± 0.3 | 0.9 ± 0.01 |
| 1 | 0.5 | 8 ± 3 | 2 ± 0.3 | 0.3 ± 0.02 |
| 1 | 0.1 | 4 ± 1 | 2 ± 0.1 | 0.3 ± 0.02 |
| Source | df | Mean Square | Sig. | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Proline | Reducing Sugar | SOD | CAT | APX | Total Phenolic | Anthocyanin | |||
| Explants | 2 | 66.719 | 2.860 | 984.511 | 82,271.035 | 399.776 | 27,511.182 | 3717.216 | 0.000 |
| NaCl concentrations | 4 | 1316.920 | 8.237 | 4858.424 | 60,983.228 | 712.782 | 36,674.622 | 290.332 | 0.000 |
| Explants × NaCl concentrations | 8 | 195.899 | 1.073 | 344.638 | 19,213.986 | 147.201 | 23,056.463 | 73.106 | 0.000 |
| Error | 30 | 0.021 | 0.061 | 14.448 | 15.575 | 14.583 | 16.748 | 14.429 | |
| Proline | Reducing Sugar | |||||
|---|---|---|---|---|---|---|
| NaCl Concentrations (mM) | Seedlings | Calli | Regenerated Seedlings | Seedlings | Calli | Regenerated Seedlings |
| 0 | 5.6 ± 1eA | 5.7 ± 1eA | 5.3 ± 0.9 eB | 1.3 ± 0.12 cA | 1.1 ± 0.1 dA | 0.9 ± 0.13 dA |
| 25 | 7.5 ± 0.9 dC | 12.4 ±1 dB | 13.4 ± 0.8 dA | 2.1 ± 0.1 dA | 1.7 ± 0.1 cA | 1.9 ± 0.1cA |
| 50 | 16.9 ± 1.1 cC | 21.3 ± 2 cB | 22.7 ± 1.2 bA | 3.3 ± 0.16 bA | 2.8 ± 0.15 bB | 2. 7 ± 0.09 bB |
| 100 | 26.9 ± 1.3 bC | 32.4 ± 0.9 bB | 36.2 ± 0.8 aA | 4 ± 0.1 aA | 3.4 ± 0.1 aB | 3.7 ± 0.11aB |
| 150 | 42.2 ± 0.7 aA | 41.3 ± 1 aB | 14.6 ± 0.2 cC | 3.6 ± 0.07 abA | 3.8 ± 0.09 aA | 1.2 ± 0.21 dB |
| SOD | CAT | APX | |||||||
|---|---|---|---|---|---|---|---|---|---|
| NaCl Concentrations (mM) | Seedlings | Calli | Regenerated Seedlings | Seedlings | Calli | Regenerated Seedlings | Seedlings | Calli | Regenerated Seedlings |
| 0 | 10.6 ± 1 dA | 10.3 ± 1 dA | 12.1 ± 1.3 cA | 157.1 ± 5 dA | 146 ± 4 dB | 139 ± 8.2 dB | 11.8 ± 1.3 dA | 10.8 ± 0.3 cA | 10.7 ± 2 cA |
| 25 | 20.3 ± 2 cA | 15.4 ± 2 dA | 14 ± 0.8 cA | 258.4 ± 4 cA | 148.7 ± 6 dB | 152 ± 6.7 cB | 19.5 ± 1 cA | 13.3 ± 0.7 cA | 13.2 ± 1.5 cA |
| 50 | 58.9 ± 2 bA | 29.4 ± 4 cC | 40.1 ± 1.6 bB | 376.2 ± 3 bA | 211.4 ± 4 cC | 232.1 ± 4.9 bB | 22.4 ± 1 bA | 19.5 ± 1 bB | 21.7 ± 1.1 bA |
| 100 | 75.3 ± 0.9 aA | 49.4 ± 2 bC | 62.3 ± 1.4 aB | 426.9 ± 3 aA | 315.3 ± 2 bC | 325.5 ± 10.3 aB | 38.7 ± 1.2 aA | 29.1 ± 0.6 aB | 29.8 ± 0.9 aB |
| 150 | 70.9 ± 1 aA | 65 ± 3 aA | 34.4 ± 1 bB | 398.9 ± 4 aA | 354.6 ± 6 aB | 58.4 ± 3 eC | 35.9 ± 1.1 aA | 30.6 ± 0.3 aA | 10 ± 1.2 cB |
| Total Phenolic | Anthocyanin | |||||
|---|---|---|---|---|---|---|
| NaCl Concentrations (mM) | Seedlings | Calli | Regenerated Seedlings | Seedlings | Calli | Regenerated Seedlings |
| 0 | 162 ± 8 eA | 155.9 ± 8 eA | 147.9 ± 4 dB | 31.8 ± 1 cA | 11 ± 1 cB | 8.9 ± 1 cB |
| 25 | 240.8 ± 7 dA | 178.8 ± 7 dC | 198.8 ± 5 cB | 37.8 ± 2 bcA | 14.3± 1 cB | 11.03 ± 0.6 bcB |
| 50 | 263.9 ± 9 cA | 261.7 ± 9 cA | 259.8 ± 8 bA | 43.8 ± 1 abA | 17.6 ± 0.8 bcB | 16.2 ± 1 b B |
| 100 | 302.7 ± 6 bC | 341.6 ± 6 bA | 311.7 ± 7 aB | 47.9 ± 2 aA | 22.7 ± 1 abB | 23.4 ± 1 aB |
| 150 | 348.8 ± 7 aB | 425.8 ± 6 aA | 48.8 ± 3 eC | 49.8 ± 3 aA | 26.2 ± 0.5 aB | 6.2 ± 0.4 cC |
| Essential Oil Components | Retention Time (min) | Seedlings | Regenerated Seedlings | Callus | |||
|---|---|---|---|---|---|---|---|
| Control | 100 mM NaCl | Control | 100 mM NaCl | Control | 100 mM NaCl | ||
| 2-pentyl furan | 908 | 1.4 ± 0.2 | 0.8 ± 0.1 | 1.1 ± 0.1 | 1.4 ± 0.2 | - | - |
| Tricyclene | 922 | 0.9 ± 0.0 | 1.4 ± 0.1 | 0.9 ± 0.1 | - | - | - |
| α-thujene | 936 | 1.2 ± 0.1 | 1.0 ± 0.0 | 1.3 ± 0.1 | 1.0 ± 0.1 | 1.4 ± 0.1 | 1.3 ± 0.1 |
| α-pinene | 948 | 2.5 ± 0.1 | 4.2 ± 0.5 | 4.5 ± 0.2 | 1.8 ± 0.1 | 4.1 ± 0.2 | 1.7 ± 0.1 |
| Hexanol | 959 | 1.1 ± 0.2 | 0.9 ± 0.1 | 0.9 ± 0.0 | 1.2 ± 0.2 | - | 0.8 ± 0.1 |
| Sabinene | 971 | 0.9 ± 0.0 | 1.1 ± 0.1 | 1.6 ± 0.0 | 0.8 ± 0.02 | 0.8 ± 0.0 | 1.2 ± 0.1 |
| ß-pinene | 985 | 1.3 ± 0.2 | 1.0 ± 0.2 | 1.2 ± 0.1 | 1.3 ± 0.1 | 1.1 ± 0.1 | 2.1 ± 0.1 |
| 3-octanone | 993 | 0.9 ± 0.1 | 1.2 ± 0.0 | 1.1 ± 0.2 | 1.0 ± 0.1 | 3.8 ± 0.1 | 1.2 ± 0.1 |
| Myrcene | 998 | 2.2 ± 0.1 | 3.9 ± 0.1 | 1.8 ± 0.2 | 3.2 ± 0.2 | 2.0 ± 0.1 | 2.4 ± 0.2 |
| 2-caren | 1006 | 1.3 ± 0.1 | 1.0 ± 0.1 | 1.2 ± 0.2 | 1.0 ± 0.1 | 1.4 ± 0.1 | 3.5 ± 0.1 |
| a-phylandrene | 1011 | 0.8 ± 0.0 | 1.2 ± 0.2 | 0.9 ± 0.2 | 1.5 ± 0.2 | 4.1 ± 0.2 | 1.0 ± 0.1 |
| α-fenchen | 1016 | 1.7 ± 0.2 | 0.9 ± 0.1 | 1.1 ± 0.0 | 1.7 ± 1.1 | 1.1 ± 0.1 | 1.3 ± 0.2 |
| α-terpinene | 1027 | 1.1 ± 0.2 | 1.4 ± 0.2 | 1.4 ± 0.0 | 1.6 ± 0.0 | 2.2 ± 0.1 | 2.0 ± 0.1 |
| p-cymene | 1034 | 25.1 ± 4.2 | 15.4 ± 2.1 | 22.5 ± 7.7 | 19.1 ± 0.6 | 12.1 ± 1.4 | 12.8 ± 1.2 |
| β-phylandrene | 1043 | 1.3 ± 0.1 | 1.0 ± 0.0 | 1.3 ± 0.2 | 0.9 ± 0.0 | 1.8 ± 0.1 | 0.9 ± 1.2 |
| Limonene | 1050 | 1.1 ± 0.1 | 0.9 ± 0.0 | 1.0 ± 0.01 | 1.6 ± 0.2 | 1.4 ± 0.1 | 1.7 ± 0.6 |
| γ-terpinene | 1066 | 15.2 ± 1.4 | 21.5 ± 1.1 | 23.2 ± 1.3 | 17.1 ± 1.1 | 18.2 ± 2.7 | 28.1 ± 1.7 |
| Cis-sabinen hydrate | 1079 | 1.2 ± 0.1 | 0.9 ± 0.2 | 1.0 ± 0.07 | 1.4 ± 0.0 | 2.0 ± 0.1 | 1.9 ± 0.2 |
| Terpinolene | 1092 | 1.8 ± 0.2 | 1.2 ± 0.1 | 1.4 ± 0.1 | 1.3 ± 0.1 | 1.1 ± 0.1 | 2.1 ± 0.1 |
| Linalool | 1152 | 1.4 ± 0.2 | 1.1 ± 0.2 | 1.1 ± 0.0 | 0.9 ± 1.1 | 2.1 ± 0.1 | 1.0 ± 0.1 |
| Terpinene-4-ol | 1178 | 1.0 ± 0.0 | - | 0.8 ± 0.5 | 1.6 ± 0.2 | 1.9 ± 0.1 | 1.1 ± 0.1 |
| Thymol methyl ether | 1235 | 0.8 ± 0.1 | 0.9 ± 0.1 | 1.2 ± 1 | 2.0 ± 0.1 | - | - |
| Verbenone | 1260 | 0.9 ± 0.0 | 1.3 ± 0.2 | 1.6 ± 0.1 | 1.1 ± 0.0 | 1.2 ± 0.1 | 1.0 ± 0.1 |
| Bronyl acetate | 1281 | 1.3 ± 0.1 | 0.9 ± 0.2 | 1.1 ± 0.1 | 0.8 ± 0.1 | - | - |
| Thymol | 1296 | 19.3 ± 2.1 | 26.6 ± 2.2 | 14.6 ± 0.6 | 22.4 ± 2.6 | 17.0 ± 0.1 | 24.4 ± 4.5 |
| Carvacrol | 1310 | 1.2 ± 0.2 | 1.0 ± 0.1 | 0.9 ± 0.0 | 1.4 ± 0.1 | 7.7 ± 2.1 | 7.0 ± 2.2 |
| Candinol | 1338 | 0.9 ± 0.0 | 0.8 ± 0.2 | 1.3 ± 0.1 | 3.0 ± 0.5 | 0.9 ± 0.1 | 2.0 ± 0.1 |
| Hexadecanoeic acid | 1370 | 1.5 ± 0.2 | 1.0 ± 0.1 | 1.4 ± 0.1 | 1.6 ± 0.2 | 2.7 ± 0.1 | 3.1 ± 0.5 |
| Cedrene | 1398 | 0.9 ± 0.0 | 1.3 ± 0.0 | 0.8 ± 0.1 | 1.3 ± 0.8 | - | - |
| β-caryophyllene | 1418 | 1.1 ± 0.1 | 0.9 ± 0.0 | 0.9 ± 0.0 | 1.0 ± 0.1 | 0.8 ± 0.1 | 1.3 ± 0.1 |
| γ-elemene | 1440 | 0.9 ± 0.0 | 1.2 ± 0.4 | 1.2 ± 0.1 | 1.4 ± 0.1 | - | - |
| α-humulene | 1463 | 1.7 ± 0.1 | 1.0 ± 0.2 | - | 0.8 ± 0.1 | 1.0 ± 0.1 | 1.1 ± 0.1 |
| Germacren D | 1490 | 1.0 ± 0.0 | 0.9 ± 0.0 | 1.3 ± 0.0 | 1.1 ± 0.0 | - | - |
| β-bisabolene | 1537 | 0.8 ± 0.0 | - | 0.8 ± 0.0 | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Razavizadeh, R.; Adabavazeh, F.; Chermahini, M.R.; Komatsu, S. Callus Induction and Plant Regeneration from Carum copticum and Assessment of Antioxidant Responses and Phytochemical Profiling by In Vitro Salinity Stress. Horticulturae 2023, 9, 22. https://doi.org/10.3390/horticulturae9010022
Razavizadeh R, Adabavazeh F, Chermahini MR, Komatsu S. Callus Induction and Plant Regeneration from Carum copticum and Assessment of Antioxidant Responses and Phytochemical Profiling by In Vitro Salinity Stress. Horticulturae. 2023; 9(1):22. https://doi.org/10.3390/horticulturae9010022
Chicago/Turabian StyleRazavizadeh, Roya, Fatemeh Adabavazeh, Marzieh Rezaee Chermahini, and Setsuko Komatsu. 2023. "Callus Induction and Plant Regeneration from Carum copticum and Assessment of Antioxidant Responses and Phytochemical Profiling by In Vitro Salinity Stress" Horticulturae 9, no. 1: 22. https://doi.org/10.3390/horticulturae9010022
APA StyleRazavizadeh, R., Adabavazeh, F., Chermahini, M. R., & Komatsu, S. (2023). Callus Induction and Plant Regeneration from Carum copticum and Assessment of Antioxidant Responses and Phytochemical Profiling by In Vitro Salinity Stress. Horticulturae, 9(1), 22. https://doi.org/10.3390/horticulturae9010022







