Abstract
Persimmon (Diospyros kaki Thunb.) is an economically important tree with a long history of cultivation in China. So far, a total of approximately 1000 varieties have been found in China. To systematically evaluate the diversity of persimmon fruit quality, 22 quality measures of appearance, intrinsic, and sensory quality were evaluated using 61 typical persimmon fruit. According to the findings, the coefficient of variation (CV) of 15 appearance and intrinsic quality index values ranged from 13.81% (fruit shape index) to 165.80% (firmness), and the CV values of 7 intrinsic quality attributes were all higher than 50%, with the CV of total polyphenols and ironic soluble pectin contents (ISP) being as high as 159.82% and 143.80%, respectively. These findings showed that several persimmon germplasm resources had a highly diverse range of fruit quality, wide variation, and distribution. Insoluble tannin and soluble sugar were shown to have a substantial positive correlation with the sensory flavor indexes (p < 0.05), indicating their significance in influencing the flavor quality of persimmon fruit. Cluster analysis was performed utilizing 15 indexes of appearance, intrinsic quality, and 7 indexes of sensory quality. The samples were divided into two groups: group I consisted of 52 pollination−constant and astringent (PCA) and 1 pollination−-variant astringent (PVA) persimmon resources, and group II consisted of 6 pollination−constant non−astringent (PCNA) and 2 pollination−variant non−astringent (PVNA) persimmon resources. The results were consistent with the classification based on the mode of astringency loss, indicating that there was a significant difference in the quality of astringent and non−astringent persimmon fruit. This study provides theoretical references for the development and application of persimmon germplasm resources.
1. Introduction
The persimmon (Diospyros kaki Thunb.: Ebenaceae) is an important subtropical fruit in East Asia, where it is appreciated for its high quality and abundance of beneficial bioactive compounds (phenolics, pectin, soluble sugars, main minerals, etc.) [1]. According to pomology, there are four different types of persimmon cultivars: pollination−constant and non−astringent (PCNA), which is non−astringent whether or not there are seeds; pollination−constant and astringent (PCA), which is astringent whether or not there are seeds; pollination−variant and non−astringent (PVNA), which is astringent if there are few or no seeds; pollination−variant and astringent (PVA), which has non−astringent flesh around the seeds only [2,3,4]. Generally, we call PCNA and PVNA non−astringent persimmons because they are non−astringent and sweet at harvest maturity with firm flesh, and we call PCA and PVA astringent persimmons because they lose astringency when they become over−ripe with extremely soft flesh [4,5]. Persimmons are believed to have originated in China as a significant food source in prehistoric times, and an extensive history of cultivation has resulted in the development of roughly 1000 different varieties of D. kaki from subtropical regions to temperate regions, except Tibet, Sinkiang, Inner Mongolia, Qinghai, Heilongjiang, and Jilin [5,6,7]. However, besides a few PCNA germplasms found in Luotian County, Hubei Province, the rest are all PCA−type in China, and most PCNA and PVA varieties were introduced from Japan [7,8,9].
One of the most critical aspects that determines a fruit’s economic value and has a direct impact on its ability to compete in the market is fruit quality [10]. Both the intrinsic qualities and the appearance of the fruit are considered to be part of the fruit’s quality. The primary evaluation indices include the fruit weight, shape index, color, soluble sugars, firmness, tannin, pectin, polyphenols, and others. These indices are representative of the various aspects of the fruit’s characteristics. Sensory evaluation is the systematic review of the appearance, taste, texture, and aroma of a product, as well as other attributes that are traditionally evaluated by trained panels utilizing human sensory organs. The sensory quality of the fruit has a significant impact on the overall satisfaction of customers, and it is widely regarded as one of the most essential components of the fruit for customers [11,12].
China continues to be the global leader in persimmon fruit production and planting area [9]; however, the lack in fruit quality evaluation has resulted in unequal fruit quality, causing China’s exports to lag behind those of other countries. Systematic evaluation is necessary before breeding and processing because fruit quality traits fluctuate significantly among the various persimmon germplasms. In this study, 61 representative persimmon germplasm resources were screened for appearance, intrinsic quality, and sensory quality, to provide theoretical references for the development and application of persimmon germplasm resources.
2. Materials and Methods
2.1. Plant Material
In this study, 61 genotypes of Diospyros kaki were examined, including 52 PCA varieties (51 samples from China and 1 sample from Japan), 1 PVA variety, 6 PCNA varieties, and 2 PVNA varieties (all samples from Japan). The type composition and geographical distribution of samples are presented in Figure 1 and Table S1. All of the samples were collected from the Persimmon Germplasm Repository in Yuanyang, Henan, China (34°55′30″~34°56′45″ N, 113°46′24″~113°47′59″ E; Climate zones: temperate; Soil condition: sandy, pH 7.3~8.1; Mean annual precipitation: 571 mm; Mean annual temperature: 14.4 °C). Forty mature fruits without astringency were randomly collected from six 10−year−old healthy persimmon trees for each sample. Half of each cultivar’s fruit was utilized for appearance quality and sensory quality evaluations, while the other half was immediately peeled, frozen in liquid nitrogen, and kept in a refrigerator at −80 °C until processing for intrinsic attributes evaluation.
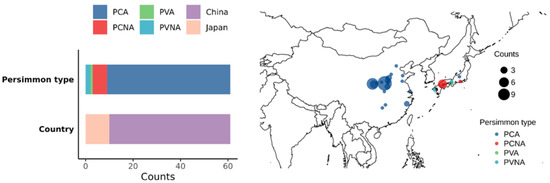
Figure 1.
Types and origins of the materials evaluated in this study.
2.2. Appearance Quality of Fruit Measurement
The fresh weight, transverse diameter, and vertical diameter were measured using an electronic balance and a vernier caliper (accurate to 0.01 g and 0.01 mm). The fruit shape index was calculated as vertical diameter/transverse diameter. The firmness of the fruit was determined using a firmness tester (GY−4; Zhejiang Tuopu, Ningbo, China) with an 8 mm plunger. The results are expressed as pressure (N). A colorimeter (CR−400, Minolta Co., Osaka, Japan) was employed to evaluate the color qualities of persimmon fruit. Each sample was assigned three measurements of a* (–a* = greenness, +a* = redness), L* (lightness; 0 = black, 100 = white), and b* (–b* = blueness, +b* = yellowness) [13,14].
2.3. Intrinsic Quality of Fruit Measurement
The amount of soluble sugar was determined utilizing anthrone−sulfuric acid colorimetry [15,16], the results were expressed as sucrose equivalents in milligrams per gram of persimmon fruit.
According to Oshida et al. [17], the Folin–Ciocalteu method was employed to determine the amounts of both soluble and insoluble tannin, the results were expressed as tannin acid equivalents in milligrams per gram of persimmon fruit.
The Folin–Ciocalteu method was utilized to measure the total phenolic content as described by Li et al., and the results were expressed as gallic acid equivalents in milligrams per gram of persimmon fruit [18].
The pectin components were extracted according to the previous method [14,19] with a few modifications. In brief, fruit flesh (5 g) was pulverized before being heated with 15 mL of ethanol (80%, v/v) for 30 min at 100 °C. The mixture was then allowed to cool to room temperature (RT, 25 ± 5 °C). After 15 min of centrifugation at 8000× g, the supernatant was drained from the mixture. Cell wall material (CWM) was obtained by soaking the residue for 15 h in a 90% dimethyl sulfoxide solution, centrifuging to remove the supernatant, and then washing the residue twice with 15 mL of acetone and 15 mL of ethanol (80%), respectively, before drying to a constant weight at 45 °C. The 100 mg of CWM was placed in distilled water (8 mL) and shaken vigorously at RT for 3 h before being centrifuged for 15 min at 8000× g. Water−soluble pectin (WSP) was extracted from the supernatants. The residue was then thoroughly agitated at RT for 6 h while being resuspended in 8 mL of trans −1,2−cyclohexane diamine tetraacetic acid (CDTA) (50 mmol L−1, pH 6.5 with acetate buffer). The CDTA−soluble pectin (ISP) was obtained by collecting the supernatants. The residue was then resuspended for 12 h at 4 °C in 8 mL of 50 Na2CO3 (mmol L−1) containing CDTA (2 mmol L−1). The covalent soluble pectin (CSP) was obtained from the supernatants. The carbazole colorimetry method was employed to determine the amount of pectin components utilizing galacturonic acid as the standard of reference.
2.4. Sensory Quality Evaluation
A total of 15 experienced judges evaluated the persimmon’s sensory qualities, including its appearance and taste, on a scale from 1 to 5, where 1 denotes extreme dislike and 5 indicates great liking [20,21]. Random numbers were utilized to generate the codes for the samples. The panelists were permitted to evaluate any sample twice or three times at RT and under standard laboratory lighting conditions. Spring water was used for palate cleansing between samples. The data were analyzed utilizing Reasonableness−Satisfaction and Multi−Value Theory. The Reasonableness−Satisfaction Theory refers to the reasonable degree to which characteristics can meet people’s needs, and it is expressed as a value between 0 and 1. The value of “1” indicates that the sample’s characteristics completely conform to the needs, while “0” indicates that they do not conform at all. Assuming that the satisfaction of a certain characteristic is M(bi), the maximum value is max(bi), and the minimum value is min(bi), the formula for calculating “reasonable satisfaction” using a single factor is as follows: (1).
The weights for color, skin thickness, sweetness, juice content, texture, pulp thickness, and flavor were determined to be 0.1, 0.05, 0.35, 0.1, 0.1, 0.1, and 0.2, respectively, based on the relevant data and the judgments of persimmon experts. The formula for determining each sample’s synthetic “reasonable−satisfaction” (V) is as follows: (2).
The greater the value, the higher this sample’s sensory score, and the more it satisfies people’s needs [22,23,24].
2.5. Statistical Analysis
The measurements were performed in triplicate and the data was collated by Microsoft Excel 2021, SPSS20.0 for Windows. SPSS, Chicago, IL, USA was used for the analysis (Pearson correlation; ANOVA, p ≤ 0.05). The data of appearance and intrinsic quality for cluster analysis were standardized by Z−score approach and cluster analysis was conducted according to WARD and Euclidean square distance method. R (Version 4.2), ggplot and ggtree were used to make charts.
3. Results
3.1. Diversity of Persimmon Fruit Appearance and Intrinsic Quality
The diversity of fruit appearance and intrinsic quality among 61 persimmon germplasm resources is illustrated in Figure 2 and Figure 3 and Table S2. The coefficients of variation (CV) of the eight appearance quality measures ranged from 13.81 to 165.80%. The following is a list of the CV in descending order: fruit firmness (165.80%) > a* (44.94%) > fresh fruit weight (36.86%) > b* (35.36%) > L* (16.72%) > transverse diameter (14.73%) > vertical diameter (14.11%) > fruit shape index (13.81%). The firmness, L*, and b* values of PCNA and PVNA persimmon fruit were higher than those of PCA and PVA, as illustrated in Figure 3. The CV of fruit firmness was as high as 165.80%, which was related to the fact that PCA and PVA persimmon fruit lose astringency until they become soft with over−ripening, whereas PCNA and PVNA persimmon fruit lose astringency when the fruit turns orange in color onwards.

Figure 2.
Fruit Profile diagram of four cultivated persimmon types. Note: PCA: (A–I). (A) Zhongshi No.5; (B) Huojing; (C) Boai_Bayuehuang; (D) Haian_Xiaofangshi; (E) Lishuishi; (F) Xinan_Niuxin; (G) Zhongshi No.6; (H) Lintong_Banshi; (I) Hongdenglong. PVA: (J) Tonewase. PVNA: (K) Zenjimaru. PCNA: (L–O). (L) Shinshuu; (M) Taishuu; (N) Kanshu; (O) Youhou.
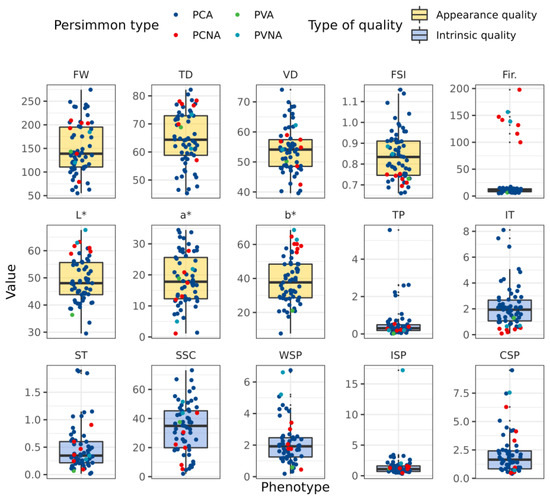
Figure 3.
The variation analysis on the indexes of different fruit quality among persimmon germplasms. Note: FW: Fresh weight; TD: Transverse diameter; VD: Vertical diameter; FSI: Fruit shape index; Fri.: Firmness; TP: Total polyphenols; IT: Insoluble tannin; ST: Soluble tannin; SSC: Soluble sugar content; WSP: Water soluble pectin; ISP: Ironic soluble pectin; CSP: Covalent soluble pectin.
Fruits with a shape index below 0.8 are thought to be oblate, those between 0.8 and 0.9 to be almost round, those between 0.9 and 1.0 to be oval or conical, and those over 1.0 to be oblong [25,26,27]. Figure 3 demonstrates that 50% of the fruit shape index for the germplasm resources for persimmons was concentrated between 0.75 and 0.91, indicating that the majority of the fruit had an oblate, round, or nearly round shape, and there were few oblong germplasm resources with a fruit shape index of more than 1. The fruit shape index for PCA and PVA ranged from 0.66 to 1.16, whereas those for PCNA and PVNA were all lower than 0.9, indicating that the persimmon fruit in those two samples were shaped oblate, round, or nearly round. The variance ranges for PCA and PVA persimmon fruit shapes were also high.
The inherent characteristics of total polyphenols, insoluble and soluble tannins, soluble sugar, WSP, ISP, and CSP are depicted in Figure 3 and Table S2. The CV values in descending order were as follows: total polyphenols (159.82%) > ISP (143.80%) > CSP (87.10%) > soluble tannin (86.94%) > insoluble tannin (76.19%) > WSP (65.90%) > soluble sugar (51.85%), and the CV values were all above 50%, indicating a high degree of variation and rich diversity. The distribution of intrinsic fruit quality differed significantly between ranges, showing that the variation of each component’s concentration is considerable, and the distribution range is broad.
The majority of germplasm resources had low−to−moderate amounts of total polyphenols, insoluble tannin, soluble tannin, soluble sugar, WSP, ISP, and CSP, while few germplasm resources had high levels. The extremely high values of total polyphenols, insoluble tannin, soluble tannin, and soluble sugar content were all PCA cultivars. PCA persimmon fruit had a greater insoluble tannin content than PCNA and PVNA.
Figure 4 shows the results of cluster analysis. A total of 61 samples were divided into two groups, group I consisted of 52 PCA and 1 PVA persimmon resources, and group II consisted of 6 PCNA and 2 PVNA persimmon resources. The results showed a significant difference between astringent and non−astringent persimmon in terms of fruit appearance and intrinsic quality, which was consistent with the classification made based on the mode of astringency loss.
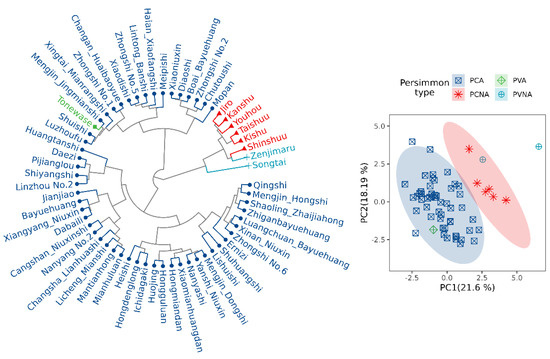
Figure 4.
Cluster analysis and principal component analysis of 61 different persimmon cultivars.
Figure 4 also illustrates the results of a principal components analysis that was conducted to examine the similarities and differences between all samples using 15 fruit quality indexes. The results demonstrated that samples were separated into two groups, with samples from the PCA and PVA grouped and samples from the PCNA and PVNA grouped, indicating that the mode of astringency reduction had a substantial impact on persimmon fruit quality.
3.2. Sensory Quality Analysis of Persimmon Fruit
Figure 5 and Table S2 both display the sensory quality evaluation score. Zhongshi No. 1, Huojing, Hongdenglong, Zhongshi No. 5, 6, and Tonewase had the highest synthetic “reasonable−satisfaction” values, with scores of 0.909, 0.863, 0.848, 0.843, 0.835, and 0.834, respectively. PCNA and PVNA persimmon germplasm resources had higher rankings for the synthetic “reasonable−satisfaction” scores of “Taishuu,” “Kishu,” “Youhou,” and “Jiro.”
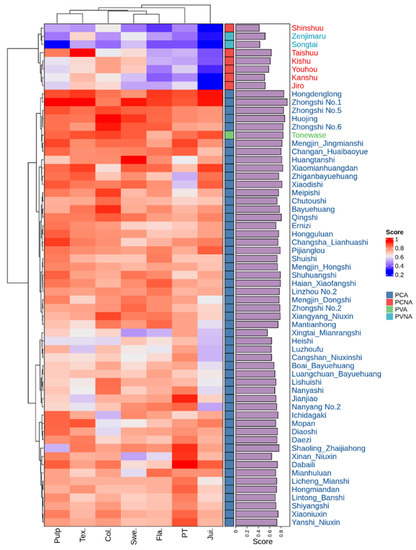
Figure 5.
The synthetic “Reasonable−satisfaction” scores and cluster analysis of sensory quality evaluation with different cultivars of persimmon fruit. Note: Pulp: Pulp; Tex.: Texture; Col.: Color; Swe.: Sweet; Fla.: Flavor; PT: Pericarp thickness; Jui.: Juice.
Systematic cluster analysis was conducted based on 15 fruit quality indexes of 61 samples as shown in Figure 5. The samples in this study were separated into two groups: group I consisted of 52 PCA and 1 PVA persimmon resources, while group II comprised 6 PCNA and 2 PVNA persimmon resources, demonstrating a significant difference in sensory quality between astringent and non−astringent persimmon fruit. In addition, as shown in Figure 6, taste and sweetness were grouped into a single class, and since soluble sugars constitute the material basis of fruit sweetness, the results further demonstrated that soluble sugar is the most influential component in determining the flavor of persimmon fruit.
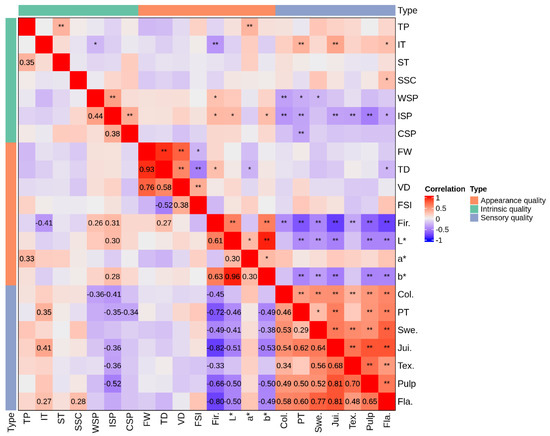
Figure 6.
Pearson correlation of appearance, intrinsic and sensory qualities. Note: TP: Total polyphenols; IT: Insoluble tannin; ST: Soluble tannin; SSC: Soluble sugar content; WSP: Water soluble pectin; ISP: Ironic soluble pectin; CSP: Covalent soluble pectin; FW: Fresh weight; TD: Transverse diameter; VD: Vertical diameter; FSI: Fruit shape index; Fri.: Firmness; Col.: Color; PT: Pericarp thickness; Swe.: Sweet; Jui.: Juice; Tex.: Texture; Pulp: Pulp; Fla.: Flavor. * and ** indicate significant difference at the level of 0.05 and 0.01, respectively.
3.3. Pearson Correlation and Cluster Analysis of Appearance, Intrinsic and Sensory Qualities
Figure 6 shows the findings of a Pearson correlation analysis performed on the appearance, intrinsic, and sensory qualities of fruit with various persimmon germplasm resources. The soluble sugar and insoluble tannin contents were positively correlated with the sensory flavor index (p < 0.05). The flavor and sweetness were clustered into a single class, indicating that sugar content was a significant factor influencing the sensory flavor of persimmon fruit. There was negative connection between insoluble tannin content and soluble pectin content (p < 0.05).
Additionally, there was a significant positive relationship between insoluble tannin content and sensory indexes, such as pericarp thickness, juice, and flavor (p < 0.05); this indicated that insoluble tannin content had a substantial impact on the fruit’s sensory quality.
Fruit firmness was also favorably correlated with the contents of WSP and ISP (p < 0.05) and negatively correlated with insoluble tannin (p < 0.05), demonstrating that persimmon fruit firmness was correlated with changes in tannin content as well as the composition and content of pectin.
4. Discussion
Color, shape, and size are examples of appearance qualities that can visually represent the external morphological characteristics of each species. These qualities are important indices for evaluating the quality of commodities and the characteristics of varieties, and they serve as basis for satisfying consumers’ demands for “good look,” which impacts their purchasing decisions [28]. China has a long history of persimmon cultivation and is one of the persimmon’s native regions. Numerous phenotypic variations have resulted from long−term natural evolution and human selection. In this study, it was determined that the diversity in persimmon fruit appearance quality was significant and widely distributed and that the majority of germplasm resources were in the medium level of fresh fruit weight, transverse diameter, and vertical diameter with normal distributions. Maede et al. [29] found that there was considerable shape diversity among different persimmon cultivars. Zhang et al. [30] compared the morphological diversity of 260 persimmon cultivars, found the CV values of longitudinal diameter, transverse diameter, lateral diameter, fruit shape index, stalk length, stalk diameter, pedicle length, pedicle width, and fresh weight per fruit ranged from 16.98% (fruit shape index) to 50.57% (fresh weight per fruit). The results of this study were consistent with these findings. The firmness, L*, and b* values of PCNA and PVNA persimmon fruit were greater than those of PCA and PVA, whereas the variation range of PCA and PVA persimmon fruit was greater than PCNA and PVNA. This is because PCA and PVA persimmon fruit lose astringency until they soften and turn red with over−ripening, whereas PCNA and PVNA persimmon fruit lose astringency when the fruit turns orange.
While consumers place a high value on appearance, they also have significant criteria for intrinsic quality, which serves as the basis for satisfying their needs for “good taste” and “health.” [31,32,33]. According to the findings of this study, the contents of total polyphenols, insoluble tannin, soluble tannin, soluble sugar, WSP, ISP, and CSP were highly variable and widely distributed, with the majority of the germplasm resources had low to medium levels, and only a few germplasm resources had high contents. These components serve as the material basis for the multiple healthcare functions of persimmon fruit [34,35]. Li et al. [36] found that astringent persimmons had significantly higher concentrations of phenolics and greater antioxidant capacities than non-astringent persimmons in either pulp or peel. The results of the study also showed that many PCA resources have a high potential research value because of their high levels of the active component, and it is required to screen good quality germplasm resources through evaluation, which will contribute positively to the development of persimmon.
The sensory quality of fruit is the primary reason why consumers purchase a specific variety of fruit [11], and sensory characteristics (appearance, aroma, flavor, and texture) contribute differentially to the acceptance of different fruit [37]. The sensory quality of several persimmon germplasm was found to be highly diverse in the current research. The synthetic “reasonable−satisfaction” scores of PCA and PVA persimmons were higher than those of PCNA and PVNA, primarily because astringent persimmons have been cultivated in China for more than 2000 years [7], whereas non−astringent persimmons were introduced to China in the late 1970s and have been cultivated for a relatively short time, and the consumers in China now prefer astringent persimmon fruit with traditional persimmon flavor. Sugar content was a significant factor influencing the sensory flavor of persimmon fruit and consumer’s acceptance increased with soluble sugar content, which was comparable with Chinese consumers’ desire for purely sweet flavors.
The findings of a cluster analysis based on 15 qualities and 7 sensory qualities both demonstrated that astringent persimmon resources (52 PCA and 1 PVA) grouped while non−astringent persimmon resources (6 PCNA and 2 PVNA) grouped, demonstrating a significant difference in fruit quality between astringent and non−astringent persimmon fruit. These findings were consistent with the classification based on the mode of astringency loss. In addition, flavor and sweetness of sensory quality were clustered into one class, and correlation analysis revealed that the content of soluble sugar was positively correlated with the sensory flavor index (p < 0.05), and that the content of insoluble tannin was positively correlated with the sensory pericarp thickness, juice, and flavor indices (p < 0.05), suggesting that insoluble tannin and sugar contents were important factors influencing the flavor of persimmon fruit.
The negative connection between insoluble tannin content and soluble pectin content (p < 0.05) may be due to the interaction of persimmon fruit tannins with pectin during astringency removal, resulting in the formation of the insoluble tannins–pectin complex [38,39,40]. There was a substantial inverse association between firmness and all sensory indexes, indicating that Chinese consumers are more receptive to PCA and PVA persimmons consumed until fruit softening, which is consistent with the traditional intake of soft ripe PCA persimmon fruit.
There was a significant difference in fruit quality between astringent and non−astringent persimmon resources, and insoluble tannin and sugar contents were significant factors affecting fruit quality; however, the reasons for the difference have not been systematically reported and must be investigated in depth. In this study, a systematic evaluation of typical persimmon germplasm was performed to investigate the degree of diversity and distribution of fruit quality. The outcomes of this study will contribute to the ongoing growth of the persimmon fruit industry and provide vital information for the continued utilization of persimmon germplasm resources.
5. Conclusions
In conclusion, there was a great diversity of appearance, intrinsic, and sensory qualities among several persimmon germplasm resources with a broad variation extent and distribution range. The CV of 15 appearance and intrinsic quality indexes ranged from 13.81 to 165.80%, fruit firmness, total polyphenols, and ISP were as high as 165.80%, 159.82% and 143.80%, respectively, whereas L*, transverse diameter, vertical diameter and fruit shape index were as low as 16.72%,14.73%, 14.11% and 13.81%, respectively, The samples were separated into astringent and non−astringent groups using cluster analysis based on 15 indexes of appearance, intrinsic quality, and 7 indexes of sensory quality, indicating that there was a significant difference between astringent and non−astringent persimmon fruit quality, and the results were consistent with the classification based on the mode of astringency loss. Significantly positive correlations (p < 0.05) were found between the contents of insoluble tannin and soluble sugar and the sensory flavor indexes, indicating that insoluble tannin and soluble sugar are essential factors influencing the flavor quality of persimmon fruit.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae9010024/s1, Table S1: The materials analyzed; Table S2: The variation analysis on the indexes of different fruit quality among persimmon germplasms; Table S3: Sensory quality evaluation scores of different persimmon cultivars.
Author Contributions
W.H. performed the majority of this study; Q.Z., T.P. and Y.W. helped to finish the experiments and analyze partial data; W.H. wrote this manuscript; H.L. and Y.L. participated in the sampling and investigation. T.L. and J.F. designed the experiments and helped draft and review the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the by National Key R&D Program of China (2019YFD1001200), the National Natural Science Foundation of China (32071801) and the National Key R&D Program of China (2019YFD1000600).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
There were no conflicts of interest in the submission of this manuscript.
References
- Ye, L.; Mai, Y.; Wang, Y.; Yuan, J.; Suo, Y.; Li, H.; Han, W.; Sun, P.; Diao, S.; Fu, J. Metabolome and Transcriptome Analysis Reveal the Accumulation Mechanism of Carotenoids and the Causes of Color Differences in Persimmon (Diospyros kaki Thunb.) Fruits. Agronomy 2022, 12, 2688. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Q.; Pu, T.; Suo, Y.; Han, W.; Diao, S.; Li, H.; Sun, P.; Fu, J. Transcriptomic profiling analysis to identify genes associated with PA biosynthesis and insolubilization in the late stage of fruit development in C−PCNA persimmon. Sci. Rep. 2022, 12, 19140. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, A. Retrospects and prospects on persimmon research. Acta Hortic. 2005, 685, 177–187. [Google Scholar] [CrossRef]
- Taira, S. Astringency in persimmon. In Fruit Analysis, 1st ed.; Linskens, H.F., Jackson, J.F., Eds.; Springer: Berlin/Heidelberg, Germany, 1995; Volume 18, pp. 97–110. [Google Scholar]
- Parfitt, D.E.; Yonemori, K.; Honsho, C.; Nozaka, M.; Kanzaki, S.; Sato, A.; Yamada, M. Relationships among Asian persimmon cultivars, astringent and non−astringent types. Tree Genet. Genomes 2015, 11, 24. [Google Scholar] [CrossRef]
- Yonemori, K.; Sugiura, A.; Yamada, M. Persimmon genetics and breeding. Plant Breed. Rev. 2000, 19, 191–225. [Google Scholar]
- Liang, Y.; Han, W.; Sun, P.; Liang, J.; Wuyun, T.; Li, F.; Fu, J. Genetic diversity among germplasms of Diospyros kaki based on SSR markers. Sci. Hortic. 2015, 186, 180–189. [Google Scholar] [CrossRef]
- Luo, Z.R.; Wang, R.Z. Persimmon in China: Domestication and traditional utilizations of genetic resources. Adv. Hortic. Sci. 2008, 22, 239–243. [Google Scholar]
- Han, W.; Cao, K.; Diao, S.; Sun, P.; Li, H.; Mai, Y.; Suo, Y.; Fu, J. Characterization of browning during CO2 deastringency treatment in astringent persimmon fruit. J. Food Meas. Charact. 2022, 16, 2273–2281. [Google Scholar] [CrossRef]
- Hou, J.; Wang, D.; Jia, W.; Pan, L. Commentary on application of data mining in fruit quality evaluation. In Computer and Computing Technologies in Agriculture IX, 1st ed.; Li, D., Li, Z., Eds.; Springer International Publishing AG: Gewerbestrasse, Switzerland, 2016; Volume 2, pp. 505–513. [Google Scholar]
- Wismer, W.V.; Harker, F.R.; Gunson, F.A.; Rossiter, K.L.; Lau, K.; Seal, A.G.; Lowe, R.G.; Beatson, R. Identifying flavour targets for fruit breeding: A kiwifruit example. Euphytica 2005, 141, 93–104. [Google Scholar] [CrossRef]
- Bavay, C.; Symoneaux, R.; Maître, I.; Kuznetsova, A.; Brockhoff, P.B.; Mehinagic, E. Importance of fruit variability in the assessment of apple quality by sensory evaluation. Postharvest Biol. Technol. 2013, 77, 67–74. [Google Scholar] [CrossRef]
- Chung, H.S.; Kim, H.S.; Lee, Y.G.; Seong, J.H. Effect of deastringency treatment of intact persimmon fruits on the quality of fresh−cut persimmons. Food Chem. 2015, 166, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Li, J.; Zong, W.; Sun, W.; Mo, W.; Li, S. Comparison of calcium and ultrasonic treatment on fruit firmness, pectin composition and cell wall−Related enzymes of postharvest apricot during storage. J. Food Sci. Technol. 2021, 59, 1588–1597. [Google Scholar] [CrossRef] [PubMed]
- Laurentin, A.; Edwards, C.A. A microtiter modification of the anthrone−Sulfuric acid colorimetric assay for glucose−Based carbohydrates. Anal. Biochem. 2003, 315, 143–145. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Jiang, W.; Zhao, Y. Physiological and Biochemical Experiment Guidance of Postharvest Fruits and Vegetables, 1st ed.; China Light Industry Press Ltd.: Beijing, China, 2007; pp. 54–59. [Google Scholar]
- Oshida, M.; Yonemori, K.; Sugiura, A. On the nature of coagulated tannins in astringent−Type persimmon fruit after an artificial treatment of astringency removal. Postharvest Biol. Technol. 1996, 8, 317–327. [Google Scholar] [CrossRef]
- Li, X.; Wu, X.; Huang, L. Correlation between antioxidant activities and phenolic contents of radix Angelicae sinensis (Danggui). Molecules 2009, 14, 5349–5361. [Google Scholar] [CrossRef]
- Zhi, H.; Liu, Q.; Xu, J.; Dong, Y.; Liu, M.; Zong, W. Ultrasound enhances calcium absorption of jujube fruit by regulating the cellular calcium distribution and metabolism of cell wall polysaccharides. J. Sci. Food. Agric. 2017, 97, 5202–5210. [Google Scholar] [CrossRef]
- Bibi, N.; Khattak, A.B.; Mehmood, Z. Quality improvement and shelf life extension of persimmon fruit (Diospyros kaki). J. Food Eng. 2007, 79, 1359–1363. [Google Scholar] [CrossRef]
- Sanchís, E.; Mateos, M.; Pérez−Gago, M.B. Effect of maturity stage at processing and antioxidant treatments on the physico−chemical, sensory and nutritional quality of fresh−Cut ‘Rojo Brillante’persimmon. Postharvest Biol. Technol. 2015, 105, 34–44. [Google Scholar] [CrossRef]
- Huang, S.; Yang, G.; Fang, C. Fruit quality evaluation of 40 pear cultivar by using multidimensional value theory. Hunan Forestry Sci. Technol. 2003, 30, 26–27. [Google Scholar]
- Wei, J.; Ma, J.; Chen, J.; Wang, X.; Ren, X. Quality differences and comprehensive evaluation of Korla Fragrant Pear from different Habitats. Food sci. 2017, 38, 87–91. [Google Scholar]
- Zhang, Q.; Pang, P.; Xiong, B.; Zhou, Z.; Jiang, X.; Han, Y.; Wang, H.; Li, H.; Xia, X.; Chen, Z.; et al. Comparative screening test of three Peach varieties in Jiangyou. IOP Conf. Ser. Earth Environ. Sci. 2020, 474, 032015. [Google Scholar] [CrossRef]
- Sun, H.H.; Zhao, Y.B.; Li, C.M.; Chen, D.M.; Wang, Y.; Zhang, X.Y.; Han, Z.H. Identification of markers linked to major gene loci involved in determination of fruit shape index of apples (Malus domestica). Euphytica 2012, 185, 185–193. [Google Scholar] [CrossRef]
- Liu, R. Analysis and Evaluation on Quality of Apple Cultivars in Different Maturation. Ph.D. Thesis, Northwest A&F University, Yangling, China, 2014. [Google Scholar]
- Bai, S.; Bi, J.; Fang, F.; Wang, P.; Gong, L. Current research progress and prospects of technologies for apple quality evaluation. Food sci. 2011, 32, 286–290. [Google Scholar]
- Yamamoto, K.; Ninomiya, S.; Kimura, Y.; Hashimoto, A.; Yoshioka, Y.; Kameoka, T. Strawberry cultivar identification and quality evaluation on the basis of multiple fruit appearance features. Comput. Electron. Agr. 2015, 110, 233–240. [Google Scholar] [CrossRef]
- Maeda, H.; Akagi, T.; Tao, R. Quantitative characterization of fruit shape and its differentiation pattern in diverse persimmon (Diospyros kaki) cultivars. Sci. Hortic. 2018, 228, 41–48. [Google Scholar] [CrossRef]
- Zhang, Y.; Suo, Y.; Sun, P.; Han, W.; Diao, S.; Li, H.; Zhang, J.; Fu, J.; Li, F. Analysis on fruit morphological diversity of persimmon germplasm resources. Acta Hortic. 2022, 47, 1473–1490. [Google Scholar]
- Magwaza, L.S.; Opara, U.L.; Nieuwoudt, H.; Cronje, P.J.R.; Saeys, W.; Nicolaï, B. NIR spectroscopy applications for internal and external quality analysis of citrus fruit−a review. Food Bioprocess Technol. 2012, 5, 425–444. [Google Scholar] [CrossRef]
- Xu, Q.; Hao, Y.; Huang, S.; Deng, X. Advances in fruit quality researches. CHN Basic Sci. 2016, 18, 55–62. [Google Scholar]
- Kader, A.A. Flavor quality of fruits and vegetables. J. Sci. Food Agr. 2008, 88, 1863–1868. [Google Scholar]
- Butt, M.S.; Sultan, M.T.; Aziz, M.; Nazc, A.; Ahmeda, W.; Kumard, N.; Imran, M. Persimmon (Diospyros kaki) fruit: Hidden phytochemicals and health claims. EXCLI J. 2015, 14, 542–561. [Google Scholar]
- Redpath, S.; George, A.P. Health and medicinal benefits of persimmon fruit: A review. Adv. Hort.Sci. 2008, 22, 244–249. [Google Scholar]
- Li, P.M.; Du, G.R.; Ma, F.W. Phenolics concentration and antioxidant capacity of different fruit tissues of astringent versus non−Astringent persimmons. Sci. Hortic. 2011, 129, 710–714. [Google Scholar] [CrossRef]
- Bayarri, S.; Costell, E. Sensory evaluation of fruit and vegetable flavors. In Handbook of Fruit and Vegetable Flavors, 1st ed.; Hui, Y.H., Ed.; John Wiley & Sons Inc: Hoboken, America, 2010; Volume 3, pp. 45–57. [Google Scholar]
- Taira, S.; Ono, M.; Matsumoto, N. Reduction of persimmon astringency by complex formation between pectin and tannins. Postharvest Biol. Technol. 1997, 12, 265–271. [Google Scholar] [CrossRef]
- Guan, C.; Chen, L.; Chen, W.; Mo, R.; Zhang, Q.; Du, X.; Liu, J.; Luo, Z. SSAP analysis reveals candidate genes associated with deastringency in persimmon (Diospyros kaki Thunb.) treated with 40 °C water. Tree Genet. Genomes 2015, 11, 20. [Google Scholar] [CrossRef]
- Wang, Y.; Li, K.; Li, C. Effects of interaction between pectin and tannin on the deastringency of different varieties of persimmons during maturing. Modern Food Sci. Technol. 2019, 35, 87–94. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).