A Study on the Characteristics of Buds and Flowers in Pomegranate: Differences among Cultivars
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site and Cultivars
2.2. Buds Observation, Collection, and Analysis
2.3. Flowers Observation, Collection, and Measurements
2.4. Statistical Analysis
3. Results and Discussion
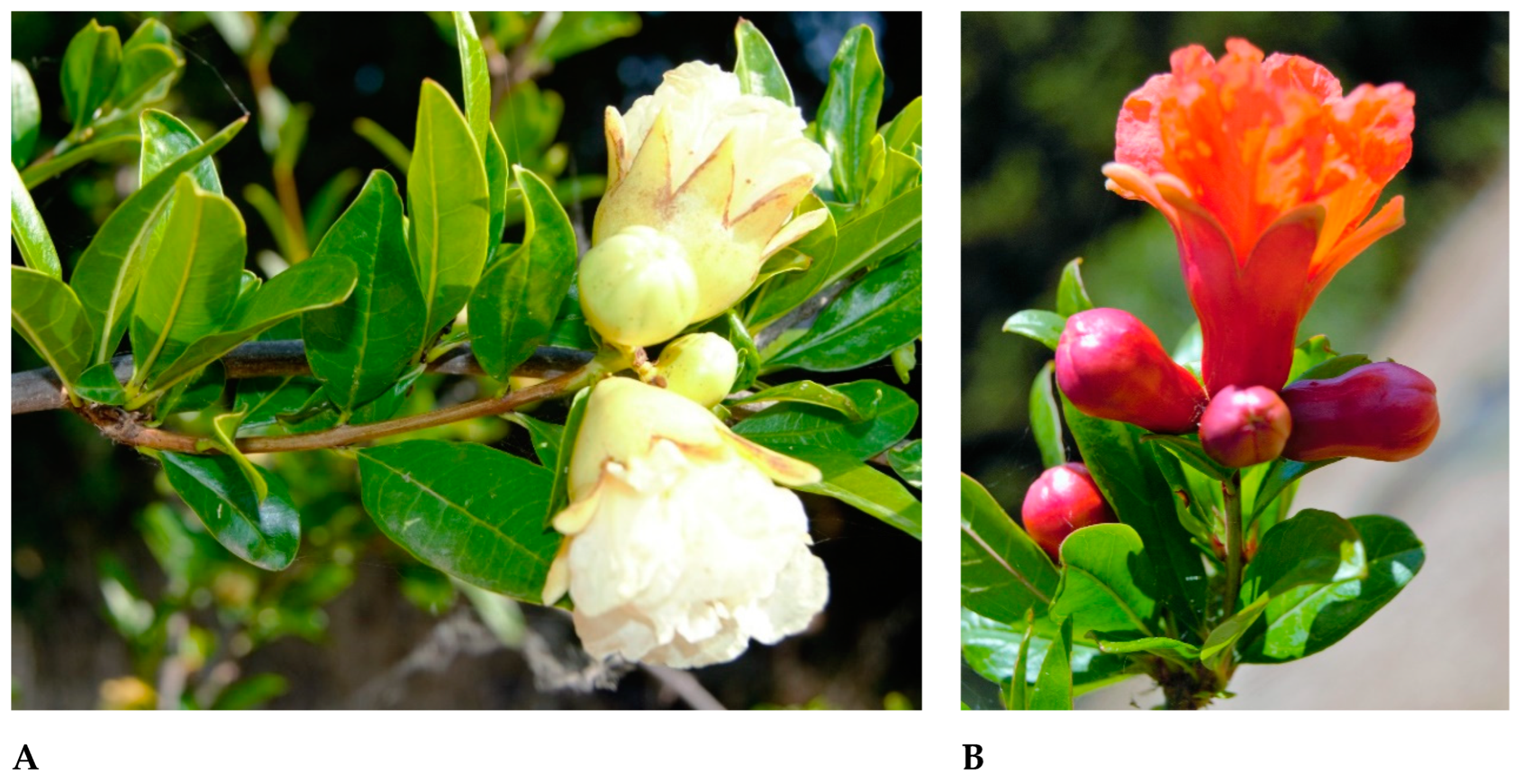
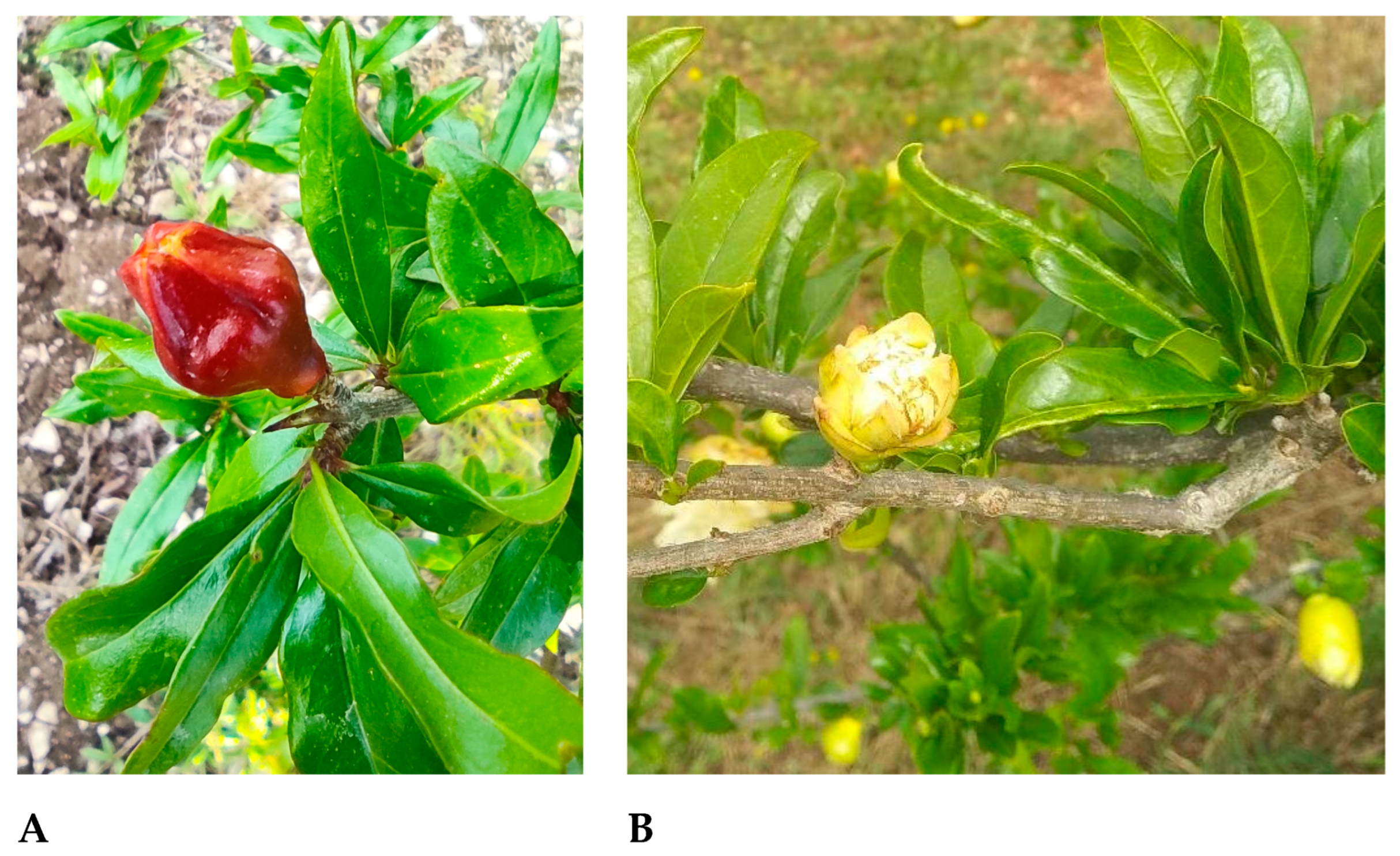
3.1. Field Observations: Buds and Flowers
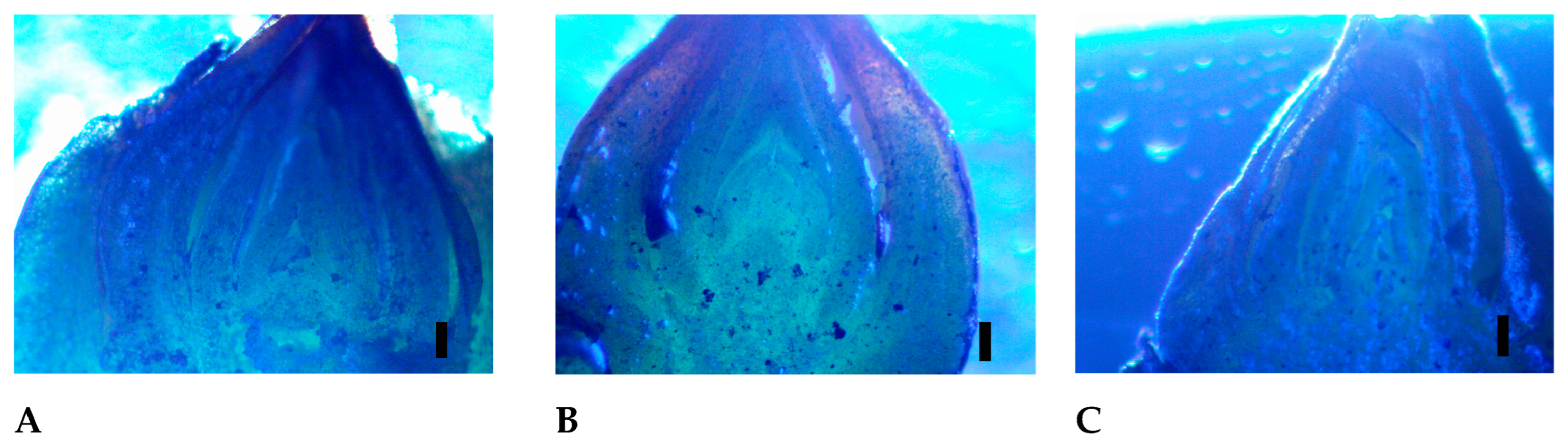
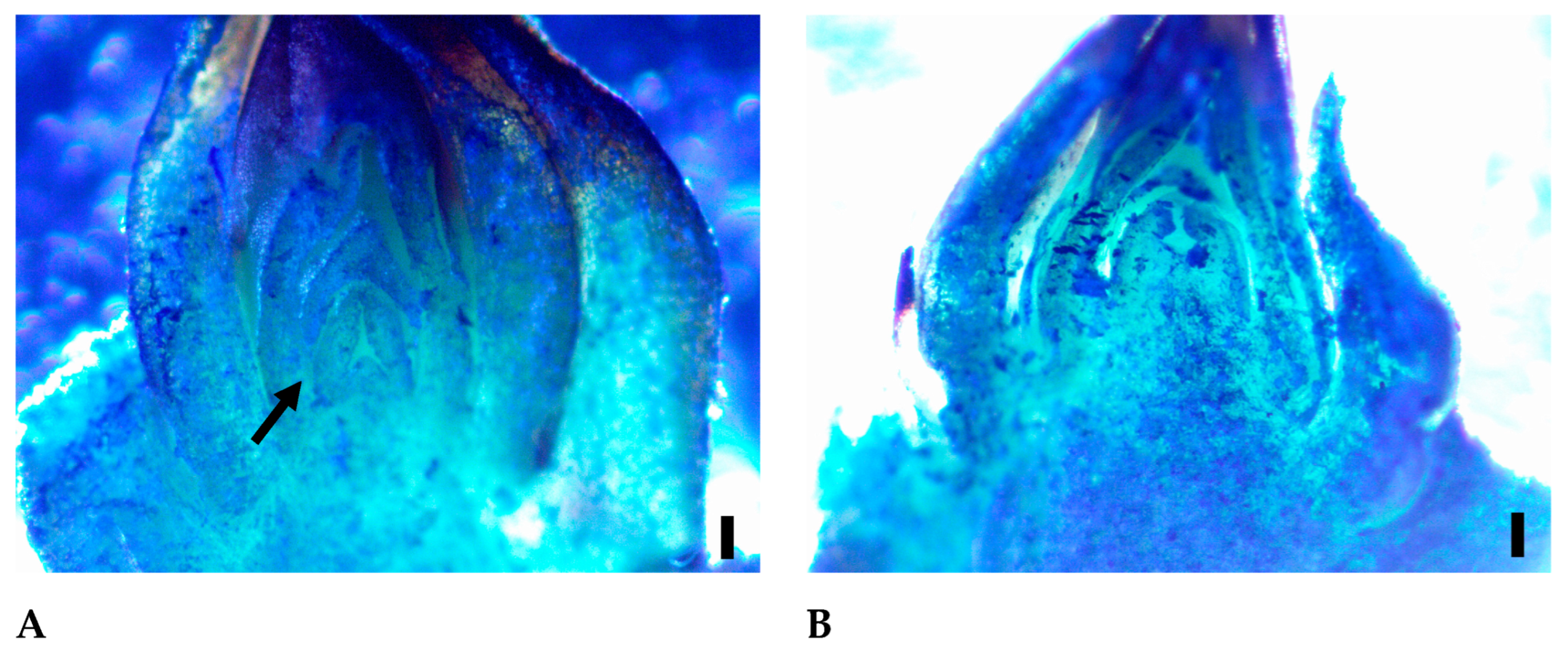
3.2. Microscopic Analysis of the Buds
3.3. Lab Observations of the Flowers
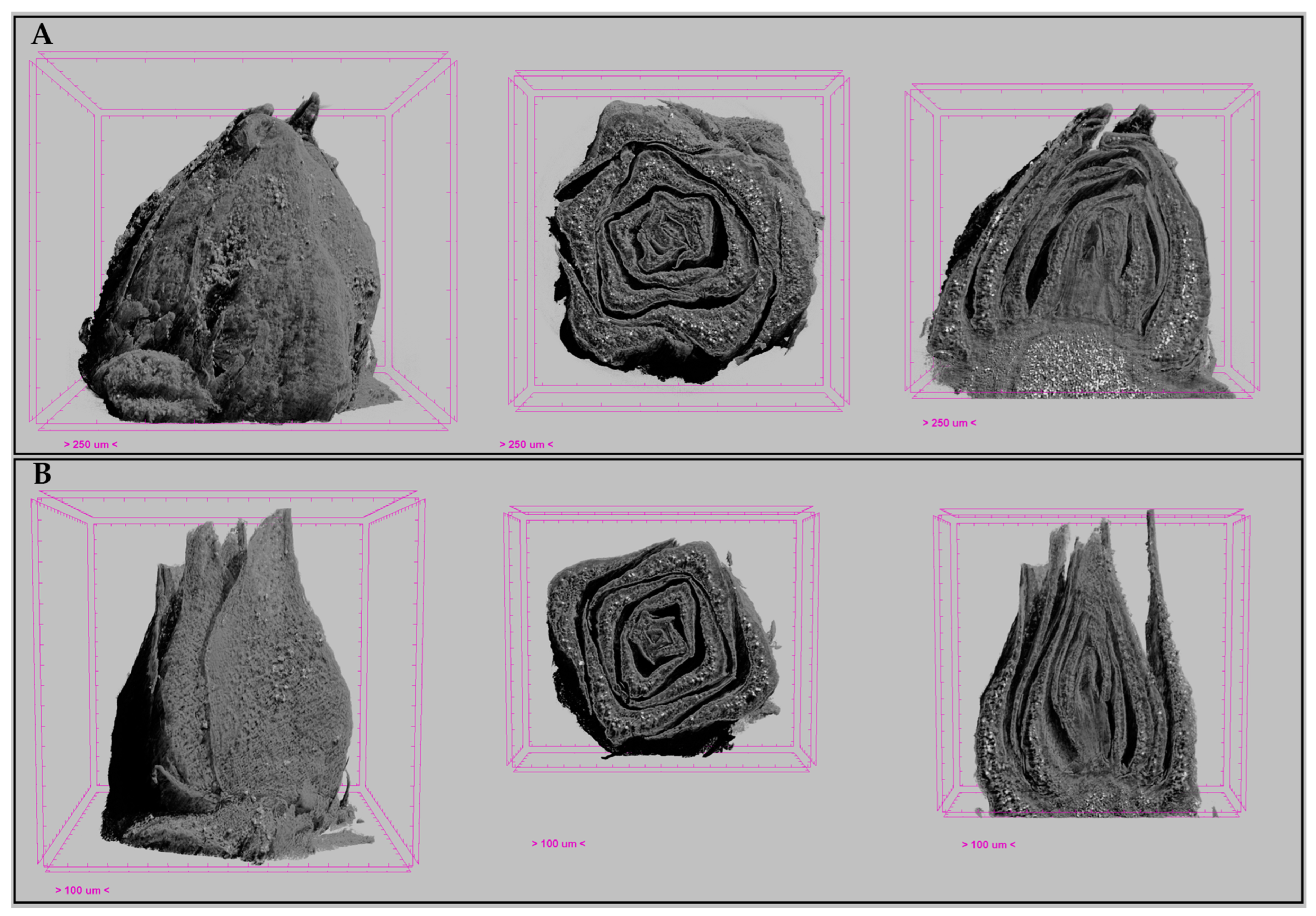
3.4. X-ray Analysis
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sarkhosh, A.; Yavari, A.; Zamani, Z. The Pomegranate: Botany, Production and Uses; CABI Publishing: Wallingford, Oxfordshire, UK, 2020; p. 600. [Google Scholar]
- Istituto Nazionale Di Statistica, ISTAT. Available online: https://www.istat.it/en/ (accessed on 30 October 2022).
- Pontonio, E.; Montemurro, M.; Pinto, D.; Marzani, B.; Trani, A.; Ferrara, G.; Mazzeo, A.; Gobbetti, M.; Rizzello, C.G. Lactic acid fermentation of pomegranate juice as a tool to improve antioxidant activity. Front. Microbiol. 2019, 10, 1550. [Google Scholar] [CrossRef] [PubMed]
- Ryugo, K. Fruit Culture–Its Science and Art; Wiley: New York, NY, USA, 1988; p. 352. [Google Scholar]
- Levin, G.M. Pomegranate; Third Millennium Publishing: Tempe, AZ, USA, 2006; p. 162. [Google Scholar]
- Da Silva, J.A.T.; Rana, T.S.; Narzary, D.; Verma, N.; Meshram, D.T.; Ranade, S.A. Pomegranate biology and biotechnology: A review. Sci. Hortic. 2013, 160, 85–107. [Google Scholar] [CrossRef]
- Rajaei, H.; Yazdanpanah, P. Buds and leaves in pomegranate (Punica granatum L.): Phenology in relation to structure and development. Flora 2015, 214, 61–69. [Google Scholar] [CrossRef]
- Ashton, R. Meet the pomegranate. In The Incredible Pomegranate Plant & Fruit; Ashton, R., Baer, B., Silverstein, S., Eds.; Third Millennium Press: Tempe, AZ, USA, 2006; pp. 3–8. [Google Scholar]
- Fahan, A. The Leaf, the Flower, the Seed. In Plant Anatomy; Hakkibutz Hameuhad Publ House Ltd.: Jerusalem, Israel, 1976; pp. 171–212. [Google Scholar]
- Babu, K.D. Floral biology of pomegranate (Punica granatum L.). Fruit Veg. Cereal Sci. Biotechnol. 2010, 4, 45–50. [Google Scholar]
- Wetzstein, H.Y.; Ravid, N.; Wilkins, E.; Martinelli, A.P. A morphological and histological characterization of bisexual and male flower types in pomegranate. J. Am. Soc. Hortic. Sci. 2011, 136, 83–92. [Google Scholar] [CrossRef]
- Wetzstein, H.Y.; Yi, W.; Porter, J.A.; Ravid, N. Flower position and size impact ovule number per flower, fruit set, and fruit size in pomegranate. J. Am. Soc. Hortic. Sci. 2013, 138, 159–166. [Google Scholar] [CrossRef]
- Wetzstein, H.Y.; Porter, J.A.; Ravid, N. Reproductive biology of pomegranate from flowering to fruit development. Acta Hortic. 2015, 1089, 21–28. [Google Scholar] [CrossRef]
- Holland, D.; Hatib, K.; Bar-Ya’akov, I. Pomegranate: Botany, horticulture, breeding. Hortic. Rev. 2009, 35, 127–191. [Google Scholar]
- Burmistrov, L.A. Pomegranate Culture in Central Asia. WANATCA (West Australian Nut and Tree Crop Association), Yearbook 17. 1993. Available online: https://www.growables.org/information/TropicalFruit/PomWANATCA.htm (accessed on 30 October 2022).
- El Sese, A.M. Physiological studies on flowering and fruiting habits of some pomegranate cultivars under Assiut conditions. Assiut J. Agric. Sci. 1988, 19, 320–336. [Google Scholar]
- Chaudhari, S.M.; Desai, U.T. Effect of plant growth regulators on flower sex in pomegranate. Indian J. Agric. Sci. 1993, 63, 34–35. [Google Scholar]
- Reddy, Y.N. Certain new approaches to the production problems of pomegranate. Acta Hortic. 2011, 890, 287–293. [Google Scholar] [CrossRef]
- Assaf, R.; Bar-Ya’akov, I.; Dagan, M.; Fahima, M.; Hatib, K. Pomegranate floral biology and trials to increase productivity. Alon Hanotea 1991, 45, 461–471. [Google Scholar]
- Gozlekci, S.; Kaynak, L. Physical and chemical changes during fruit development and flowering in pomegranate (Punica granatum L.) cultivar Hicaznar grown in Antalya region. Options Méditerranéennes Série A Séminaires Méditerranéens 2000, 42, 79–85. [Google Scholar]
- Nalawadi, U.G.; Farooqui, A.A.; Dasappa, N.; Reddy, M.A.; Gubbaiah Sulikeri, G.S.; Nalini, A.S. Studies on the floral biology of pomegranate (Punica granatum L.). Mysore J. Agric. Sci. 1973, 7, 213–225. [Google Scholar]
- Ferrara, G.; Mazzeo, A. Potential and actual bud fruitfulness: A tool for predicting and managing the yield of table grape varieties. Agronomy 2021, 11, 841. [Google Scholar] [CrossRef]
- Gallotta, A.; Palasciano, M.; Mazzeo, A.; Ferrara, G. Pollen production and flower anomalies in apricot (Prunus armeniaca L.) cultivars. Sci. Hortic. 2014, 172, 199–205. [Google Scholar] [CrossRef]
- Boselli, M.; Bahouaoui, M.A.; Lachhab, N.; Sanzani, S.M.; Ferrara, G.; Ippolito, A. Protein hydrolysates effects on grapevine (Vitis vinifera L., cv. Corvina) performance and water stress tolerance. Sci. Hortic. 2019, 258, 108784. [Google Scholar] [CrossRef]
- Ferrara, G.; Mazzeo, A.; Matarrese, A.M.S.; Pacucci, C.; Trani, A.; Fidelibus, M.W.; Gambacorta, G. Ethephon as a potential abscission agent for table grapes: Effects on pre-harvest abscission, fruit quality and residue. Front. Plant Sci. 2016, 7, 620. [Google Scholar] [CrossRef]
- Bedbabis, S.; Ben Rouina, B.; Boukhris, M.; Ferrara, G. Effects of irrigation with treated wastewater on root and fruit mineral elements of Chemlali olive cultivar. Sci. World J. 2014, 2014, 973638. [Google Scholar] [CrossRef]
- Marcotuli, I.; Mazzeo, A.; Colasuonno, P.; Terzano, R.; Nigro, D.; Porfido, C.; Tarantino, A.; Cigliano, R.A.; Sanseverino, W.; Gadaleta, A.; et al. Fruit development in Ficus carica L.: Morphological and genetic approaches to fig buds for an evolution from monoecy toward dioecy. Front. Plant Sci. 2020, 11, 1208. [Google Scholar] [CrossRef]
- Mazzeo, A.; Palasciano, M.; Gallotta, A.; Camposeo, S.; Pacifico, A.; Ferrara, G. Amount and quality of pollen grains in four olive (Olea europaea L.) cultivars as affected by ‘on’ and ‘off’ years. Sci. Hortic. 2014, 170, 89–93. [Google Scholar] [CrossRef]
- Singh, R.P.; Kar, P.L.; Dhuria, H.S. Studies on behaviour of flowering and sex expression in some pomegranate cultivars. Plant Sci. 1978, 10, 29–31. [Google Scholar]
- Meena, K.K.; Singh, R.; Pareeka, S.; Kashyap, P. Evaluation of pomegranate (Punica granatum L.) genotypes for morphological and flowering characteristics under semiarid climate. Acta Hortic. 2011, 890, 233–238. [Google Scholar] [CrossRef]
- Babu, K.D.; Chandra, R.; Jadhav, V.T.; Sharma, J. Blossom biology of pomegranate cv. ‘Bhagawa’ under semiarid tropics of Western India. Acta Hortic. 2011, 890, 227–232. [Google Scholar] [CrossRef]
- Vivian, F.I. Evolution of petal identity. J. Exp. Bot. 2009, 60, 2517–2527. [Google Scholar] [CrossRef]
- Santiago-Mejía, H.; Zavaleta-Mancera, H.A.; Cortés-Flores, J.I.; Turrent-Fernández, A.; Albino-Garduño, R. Vascularization and starch grains during the morphogenesis of peach floral buds on different flowering date. Agrociencia 2018, 52, 1121–1135. [Google Scholar]
- Fadón, E.; Herrero, M.; Rodrigo, J. Dormant flower buds actively accumulate starch over winter in sweet cherry. Front. Plant Sci. 2018, 9, 171. [Google Scholar] [CrossRef] [PubMed]
- Felker, F.C.; Robitaille, H.A.; Hess, F.D. Morphological and ultrastructural development and starch accumulation during chilling of sour cherry flower buds. Am. J. Bot. 1983, 70, 376–386. [Google Scholar] [CrossRef]






| Cultivar | Bud/Node 1 | Bud Evolution 2 | Bud Size 3 | Flower Type 4 | Flower 5 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | V | M | F | Sm | B | H | M | S | C | |
| Comune S. Giorgio | • | •••• | ••••• | ••• | • | ••• | • | ••• | •• | • | ••• | |
| Wonderful | • | •••• | • | ••••• | ••• | • | ••• | • | •••• | • | • | ••• |
| Haku Botan | •• | •••• | •••• | ••• | •• | ••• | •• | •••• | • | •• | ••• | |
| Ki-Zakuro | •• | •••• | • | •••• | ••• | •• | ••• | •• | •••• | • | ••• | ••• |
| Treatment | Number of Sepals | Number of Petals | Number of Stamens | Flower Height (mm) | Flower Width (mm) | Style Length (mm) | Stigma Area (mm2) | Ovary Width (mm) | Ovary Height (mm) |
|---|---|---|---|---|---|---|---|---|---|
| Cultivar | |||||||||
| ‘Comune S. Giorgio’ | 5.9a | 5.9b | 192.2a | 26.8a | 12.2c | 5.2a | 0.7b | 2.5b | 3.5a |
| ‘Wonderful’ | 5.8a | 5.7b | 168.6b | 24.1a | 13.2c | 4.5a | 0.4b | 2.0b | 2.0b |
| ‘Haku-Botan’ | 6.3a | 44.4a | 134.9c | 27.0a | 19.6a | 4.7a | 0.6b | 2.3b | 1.4b |
| ‘Ki-Zakuro’ | 5.9a | 47.4a | 121.9c | 26.3a | 17.1b | 2.7b | 4.2a | 3.8a | 1.8b |
| Flower | |||||||||
| Hermaphrodite | 6.0a | 22.8a | 164.7a | 31.1a | 17.6a | 8.2a | 1.7a | 3.7a | 3.4a |
| Male | 6.0a | 25.9a | 149.4b | 21.6b | 13.4b | 1.0b | 1.0b | 1.5b | 1.1b |
| Bud | |||||||||
| Mixed | 6.0a | 22.6a | 157.3a | 24.7b | 14.6a | 4.2a | 0.8b | 1.9b | 1.9a |
| Flower | 5.9a | 26.0a | 155.9a | 27.2a | 16.1a | 4.5a | 1.8a | 3.1a | 2.4a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrara, G.; Porfido, C.; Terzano, R.; Sarkhosh, A.; Mazzeo, A. A Study on the Characteristics of Buds and Flowers in Pomegranate: Differences among Cultivars. Horticulturae 2023, 9, 117. https://doi.org/10.3390/horticulturae9010117
Ferrara G, Porfido C, Terzano R, Sarkhosh A, Mazzeo A. A Study on the Characteristics of Buds and Flowers in Pomegranate: Differences among Cultivars. Horticulturae. 2023; 9(1):117. https://doi.org/10.3390/horticulturae9010117
Chicago/Turabian StyleFerrara, Giuseppe, Carlo Porfido, Roberto Terzano, Ali Sarkhosh, and Andrea Mazzeo. 2023. "A Study on the Characteristics of Buds and Flowers in Pomegranate: Differences among Cultivars" Horticulturae 9, no. 1: 117. https://doi.org/10.3390/horticulturae9010117
APA StyleFerrara, G., Porfido, C., Terzano, R., Sarkhosh, A., & Mazzeo, A. (2023). A Study on the Characteristics of Buds and Flowers in Pomegranate: Differences among Cultivars. Horticulturae, 9(1), 117. https://doi.org/10.3390/horticulturae9010117







