High-Relative-Humidity Storage Reduces the Chilling Injury Symptoms of Red Sweet Peppers in the Breaker Stage
Abstract
1. Introduction
2. Materials and Methods
2.1. Fruit Materials and Storage Conditions
2.2. Weight Loss, Chilling Injury (CI) Index and Calyx Browning Index
2.3. Respiration and Ethylene Production Rates
2.4. Electrolyte Leakage and Malondialdehyde Content (MDA)
2.5. Color Change
2.6. Soluble Solids Content and Firmness
2.7. DPPH Radical Scavenging Activity and Vitamin C
2.8. Microbiological Analysis
2.9. Statistical Analysis
3. Results and Discussion
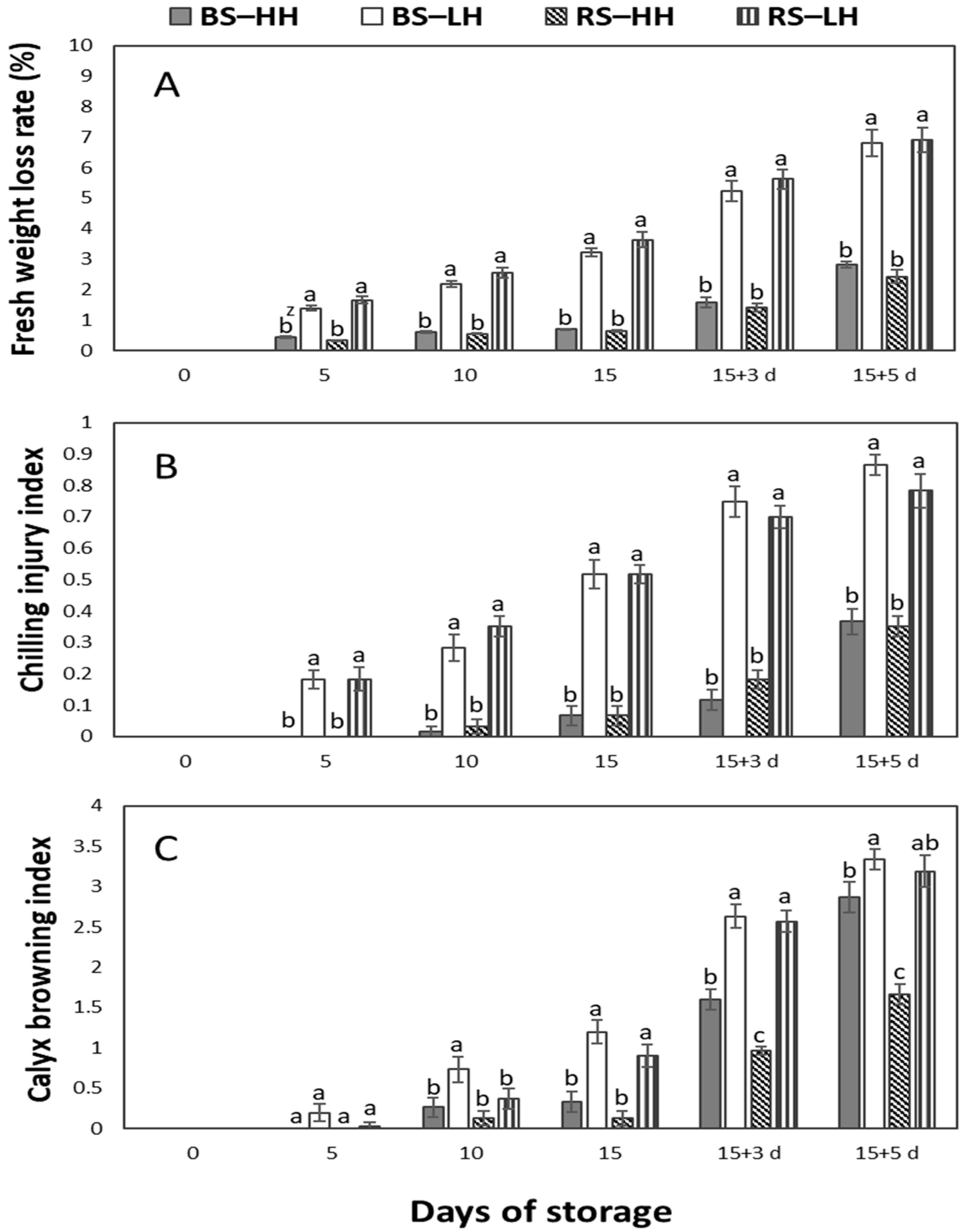
3.1. Fruit Water Loss Rate, Chilling Injury Index and Calyx Browning Index
3.2. Storage Humidity Effect on Soluble Solids Content and Firmness of the Fruit
3.3. Effect of Storage Humidity on Fruit Respiration and Ethylene Production Rates
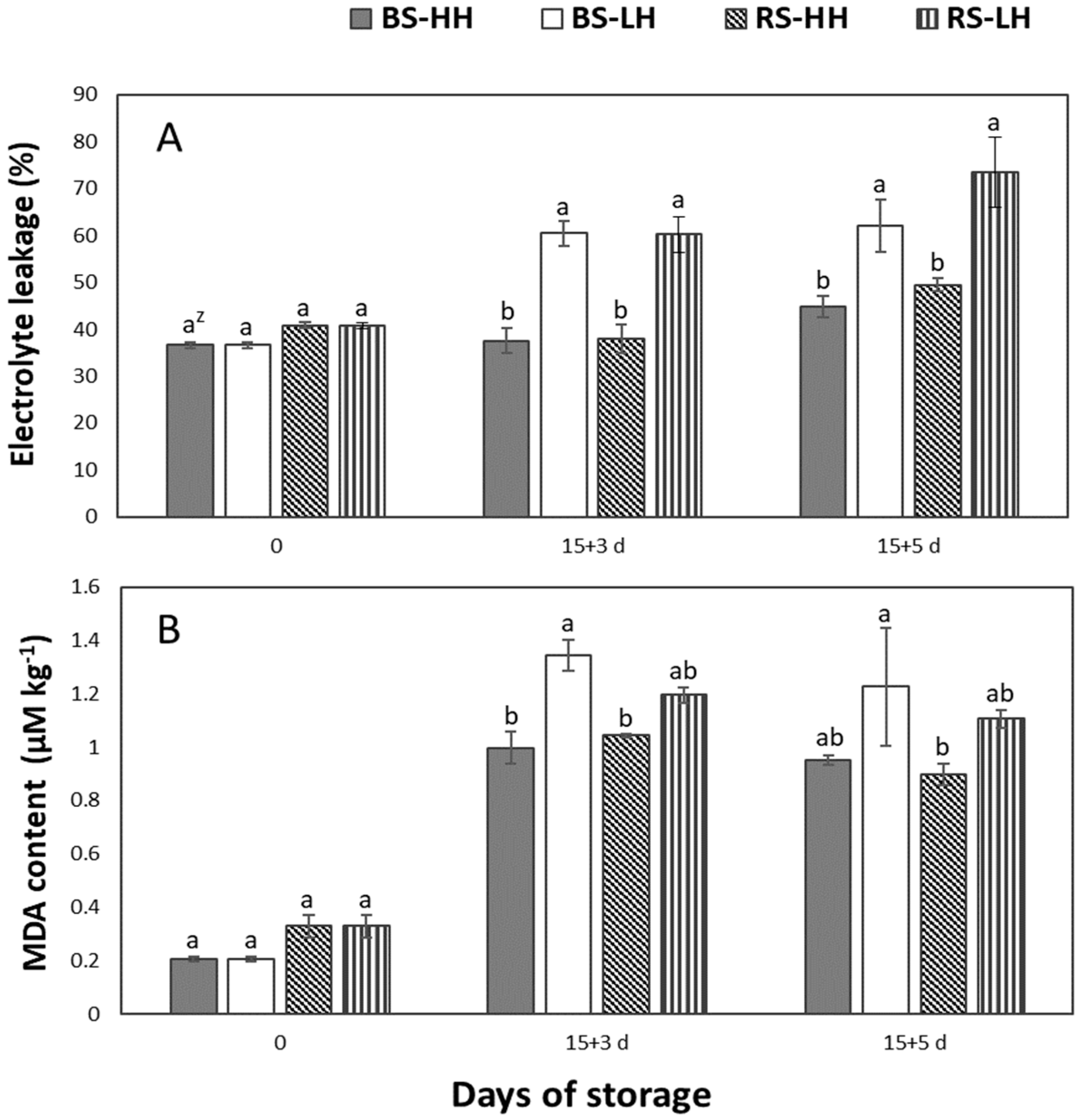
3.4. Storage Humidity Effect on Electrolyte Leakage and Malondialdehyde Content (MDA)
3.5. The Effect of Fruit Storage Humidity on Color
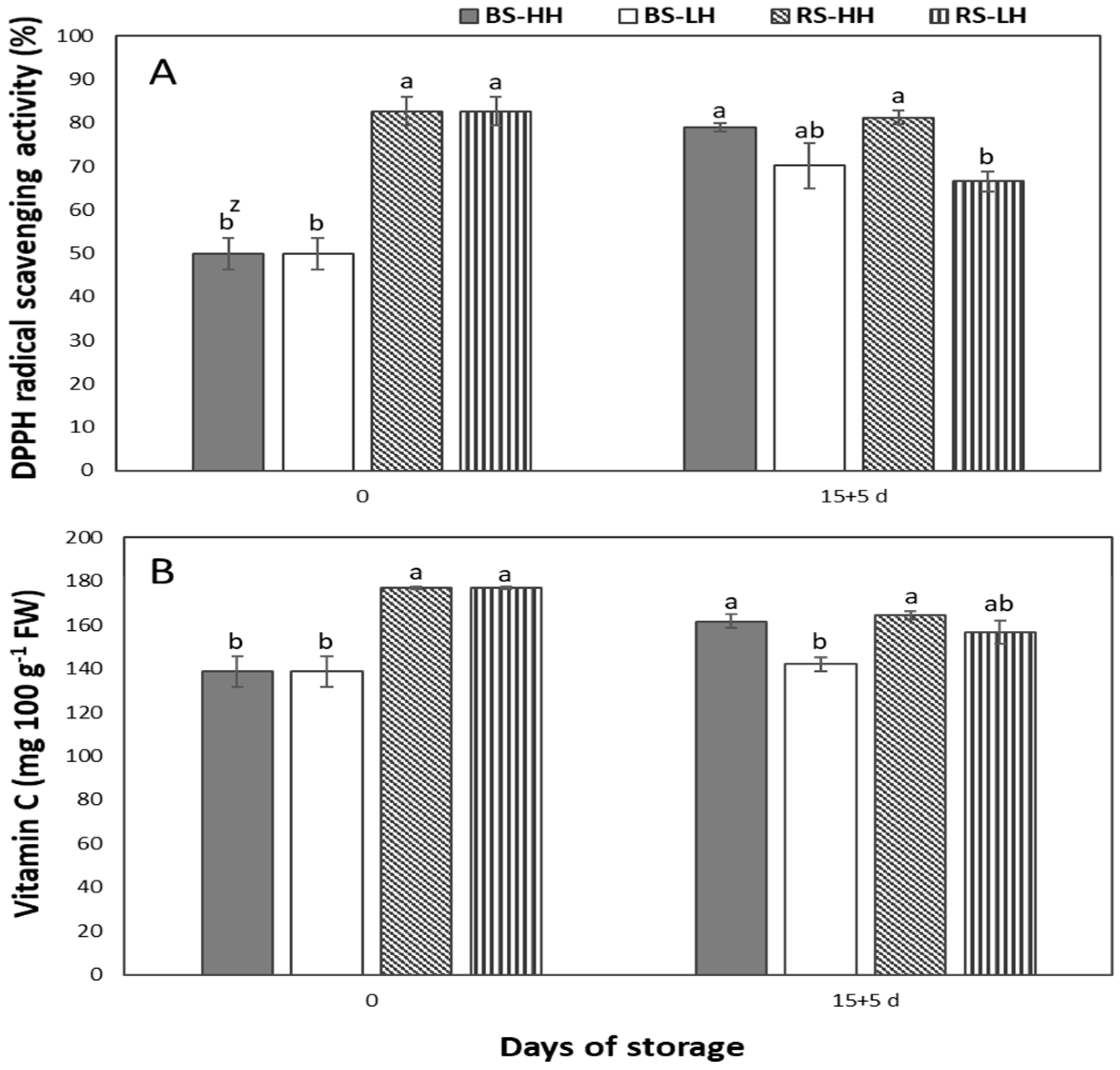
3.6. Storage Humidity Effect on DPPH Radical Scavenging Activity and Vitamin C of the Fruit
3.7. Microbiological Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lim, C.S.; Kang, S.M.; Cho, J.L.; Gross, K.C.; Woolf, A.B. Bell pepper (Capsicum annuum L.) fruits are susceptible to chilling injury at the breaker stage of ripeness. HortScience 2007, 42, 1659–1664. [Google Scholar] [CrossRef]
- Maalekuu, K.; Elkind, Y.; Fallik, E.; Shalom, Y.; Tuvia-Alkalai, S. Quality evaluation of three sweet pepper cultivars after prolonged storage. Adv. Hortic. Sci. 2003, 17, 1000–1005. [Google Scholar]
- Mercado, J.; Quesada, M.; Reid, M.; Valpuesta, V.; Cantwell, M. Storage of bell peppers in controlled atmospheres at chilling and nonchilling temperatures. Acta Hortic. 1995, 412, 134–142. [Google Scholar] [CrossRef]
- Babellahi, F.; Paliwal, J.; Erkinbaev, C.; Amodio, M.L.; Chaudhry, M.M.A.; Colelli, G. Early detection of chilling injury in green bell peppers by hyperspectral imaging and chemometrics. Postharvest Biol. Technol. 2020, 162, 111100. [Google Scholar] [CrossRef]
- Liu, L.; Wei, Y.; Shi, F.; Liu, C.; Liu, X.; Ji, S. Intermittent warming improves postharvest quality of bell peppers and reduces chilling injury. Postharvest Biol. Technol. 2015, 101, 18–25. [Google Scholar] [CrossRef]
- Vicente, A.R.; Pineda, C.; Lemoine, L.; Civello, P.M.; Martinez, G.A.; Chaves, A.R. UV-C treatments reduce decay, retain quality and alleviate chilling injury in pepper. Postharvest Biol. Technol. 2005, 35, 69–78. [Google Scholar] [CrossRef]
- González-Aguilar, G.; Gayosso, L.; Cruz, R.; Fortiz, J.; Báez, R.; Wang, C. Polyamines induced by hot water treatments reduce chilling injury and decay in pepper fruit. Postharvest Biol. Technol. 2000, 18, 19–26. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, L.; Wang, Q.; Zuo, J. Low temperature conditioning combined with methyl jasmonate can reduce chilling injury in bell pepper. Sci. Hortic. 2019, 243, 434–439. [Google Scholar] [CrossRef]
- Wang, Q.; Ding, T.; Gao, L.; Pang, J.; Yang, N. Effect of brassinolide on chilling injury of green bell pepper in storage. Sci. Hortic. 2012, 144, 195–200. [Google Scholar] [CrossRef]
- Purvis, A.C. Diphenylamine reduces chilling injury of green bell pepper fruit. Postharvest Biol. Technol. 2002, 25, 41–48. [Google Scholar] [CrossRef]
- Serrano, M.; Martínez-Madrid, M.C.; Pretel, M.T.; Riquelme, F.; Romojaro, F. Modified atmosphere packaging minimizes increases in putrescine and abscisic acid levels caused by chilling injury in pepper fruit. J. Agric. Food Chem. 1997, 45, 1668–1672. [Google Scholar] [CrossRef]
- Miller, W.; Hale, P.; Spalding, D.; Davis, P. Quality and decay of mango fruit wrapped in heat-shrinkable film. HortScience 1983, 18, 957–958. [Google Scholar] [CrossRef]
- Zuo, X.; Cao, S.; Zhang, M.; Cheng, Z.; Cao, T.; Jin, P.; Zheng, Y. High relative humidity (HRH) storage alleviates chilling injury of zucchini fruit by promoting the accumulation of proline and ABA. Postharvest Biol. Technol. 2021, 171, 111344. [Google Scholar] [CrossRef]
- Fahmy, K.; Nakano, K. Influence of relative humidity on development of chilling injury of cucumber fruits during low temperature storage. Asia Pac. J. Sustain. Agric. Food Energy 2013, 1, 1–5. [Google Scholar]
- Meng, X.; Huang, Z.; Bi, F.; Jin, W. Dry-fog controlled humidity system and its application in fruit & vegetable storage. Trans. Chin. Soc. Agric. Eng. 2016, 32, 271–276. [Google Scholar]
- Singh, R.; Giri, S.; Kotwaliwale, N. Shelf-life enhancement of green bell pepper (Capsicum annuum L.) under active modified atmosphere storage. Food Packag. Shelf Life 2014, 1, 101–112. [Google Scholar] [CrossRef]
- Nunes, M.; Delgado, A.; Emond, J. Quality curves for green bell pepper (Capsicum annuum L.) stored at low and recommended relative humidity levels. Acta Hortic. 2012, 945, 71–78. [Google Scholar] [CrossRef]
- Li, Y.; Golding, J.B.; Arcot, J.; Wills, R.B. Continuous exposure to ethylene in the storage environment adversely affects ‘Afourer’ mandarin fruit quality. Food Chem. 2018, 242, 585–590. [Google Scholar] [CrossRef]
- Wang, L.-X.; Choi, I.-L.; Kang, H.-M. Effect of High CO2 Treatment and MA packaging on sensory quality and physiological-biochemical characteristics of green asparagus (Asparagus officinalis L.) during postharvest storage. Horticulturae 2020, 6, 84. [Google Scholar] [CrossRef]
- Oboh, G. Effect of blanching on the antioxidant properties of some tropical green leafy vegetables. LWT-Food Sci. Technol. 2005, 38, 513–517. [Google Scholar] [CrossRef]
- Afolabi, A.S.; Choi, I.-L.; Lee, J.H.; Beom, K.Y.; Kang, H.-M. Comparison of Storability and Quality of Sweet Pepper (Capsicum annum L.) Grown in Two Different Hydroponics Media. Korean J. Packag. Sci. Technol. 2022, 28, 39–46. [Google Scholar] [CrossRef]
- Dargie, T.; Bizuayehu, T.; Ali, M.; Haddis, Y.; Net, B. Effects of harvesting stage and storage duration on postharvest quality and shelf life of sweet bell pepper (Capsicum annuum L.) varieties under passive refrigeration system. Int. J. Biotechnol. Mol. Biol. Res. 2013, 4, 98–104. [Google Scholar]
- Agüero, M.; Ponce, A.; Moreira, M.; Roura, S. Lettuce quality loss under conditions that favor the wilting phenomenon. Postharvest Biol. Technol. 2011, 59, 124–131. [Google Scholar] [CrossRef]
- Díaz-Pérez, J.C. Transpiration rates in eggplant fruit as affected by fruit and calyx size. Postharvest Biol. Technol. 1998, 13, 45–49. [Google Scholar] [CrossRef]
- Lallu, N. Studies Relating Browning at the Calyx Surface to Handling of ‘Royal Gala’ Apples in Water Dumps. VI Int. Postharvest Symp. 2009, 877, 483–490. [Google Scholar] [CrossRef]
- Kissinger, M.; Tuvia-Alkalai, S.; Shalom, Y.; Fallik, E.; Elkind, Y.; Jenks, M.A.; Goodwin, M.S. Characterization of physiological and biochemical factors associated with postharvest water loss in ripe pepper fruit during storage. J. Am. Soc. Hortic. Sci. 2005, 130, 735–741. [Google Scholar] [CrossRef]
- Vazquez-Ochoa, R.I.; Colinas-Leon, M.T. Changes in guavas of three maturity stages in response to temperature and relative humidity. HortScience 1990, 25, 86–87. [Google Scholar] [CrossRef]
- Valenzuela, J.L.; Manzano, S.; Palma, F.; Carvajal, F.; Garrido, D.; Jamilena, M. Oxidative stress associated with chilling injury in immature fruit: Postharvest technological and biotechnological solutions. Int. J. Mol. Sci. 2017, 18, 1467. [Google Scholar] [CrossRef]
- Bhandari, S.R.; Jung, B.-D.; Baek, H.-Y.; Lee, Y.-S. Ripening-dependent changes in phytonutrients and antioxidant activity of red pepper (Capsicum annuum L.) fruits cultivated under open-field conditions. HortScience 2013, 48, 1275–1282. [Google Scholar] [CrossRef]
- Cuvi, M.J.A.; Vicente, A.R.; Concellón, A.; Chaves, A.R. Changes in red pepper antioxidants as affected by UV-C treatments and storage at chilling temperatures. LWT-Food Sci. Technol. 2011, 44, 1666–1671. [Google Scholar] [CrossRef]
- Akram, N.; Hasan, M.; Rehman, R.; Ateeq, R.; Ahmad, Z.; Khan, U.; Hayat, F. Combined application of methyl salicylate and l-arginine alleviates chilling injury, potentiates antioxidant system and maintains quality of sweet pepper (Capsicum annum L.) fruits cv. ‘Winner’. J. Hortic. Sci. Technol. 2021, 4, 68–75. [Google Scholar] [CrossRef]
- Lee, S.K.; Kader, A.A. Preharvest and postharvest factors influencing vitamin C content of horticultural crops. Postharvest Biol. Technol. 2000, 20, 207–220. [Google Scholar] [CrossRef]
- Martínez, S.; López, M.; González-Raurich, M.; Bernardo Alvarez, A. The effects of ripening stage and processing systems on vitamin C content in sweet peppers (Capsicum annuum L.). Int. J. Food Sci. Nutr. 2005, 56, 45–51. [Google Scholar] [CrossRef]

| Maturity Stage | Storage Conditions | Firmness (n) | Soluble Solids Content (°Brix) | ||
|---|---|---|---|---|---|
| Initial | Final | Initial | Final | ||
| BS | HH | 50.17 ± 2.70 a Z | 43.80 ± 1.07 a | 6.84 ± 0.02 b | 7.06 ± 0.06 c |
| LH | 39.52 ± 0.67 ab | 7.08 ± 0.06 c | |||
| RS | HH | 38.80 ± 0.66 b | 38.52 ± 1.36 ab | 7.48 ± 0.04 a | 7.40± 0.03 b |
| LH | 36.11 ± 1.24 b | 8.26 ± 0.27 a | |||
| Maturity stage (A) | *** | *** | *** | *** | |
| Storage conditions (B) | NS | *** | NS | *** | |
| A × B | NS | * | NS | *** | |
| Maturity | Storage Conditions | Respiration Rate (CO2 mg kg−1 h−1) | Ethylene Production Rate (µL kg−1 h−1)h | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 15 | 15 + 3 d | 15 + 5 d | 0 | 15 | 15 + 3 d | 15 + 5 d | ||
| BS | HH | 9.52 ± 0.68 a Z | 14.18 ± 0.40 a | 7.87 ± 0.28 a | 3.70 ± 0.30 a | 2.37 ± 0.12 a | 0.90 ± 0.07 a | 0.53 ± 0.03 a | 0.66 ± 0.10 a |
| LH | 11.24 ± 0.90 b | 7.08 ± 0.44 a | 4.82 ± 1.61 a | 1.15 ± 0.12 ab | 0.68 ± 0.07 a | 0.76 ± 0.04 a | |||
| RS | HH | 8.34 ± 0.76 a | 9.63 ± 1.21 b | 6.03 ± 0.03 a | 2.30 ± 0.30 b | 2.21 ± 0.09 a | 0.87 ± 0.08 ab | 0.67 ± 0.05 a | 0.55 ± 0.03 a |
| LH | 9.84 ± 0.51 b | 8.19 ± 0.58 a | 3.71 ± 0.12 a | 0.73 ± 0.03 b | 0.63 ± 0.03 a | 0.67 ± 0.06 a | |||
| Maturity stage (A) | NS | *** | NS | * | NS | ** | NS | NS | |
| Storage conditions (B) | NS | *** | * | *** | NS | *** | *** | *** | |
| A × B | NS | * | * | * | NS | NS | NS | NS | |
| Maturity Stage | Storage Conditions | Storage Days | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 5 | 10 | 15 | 15 + 3 d | 15 + 5 d | |||
| a* | BS | HH | 3.28 ± 0.3 b Z | 2.96 ± 1.4 b | 6.82 ± 2.0 b | 10.16 ± 2.1 b | 33.60 ± 1.5 b | 34.90 ± 1.4 a |
| LH | 3.38 ± 0.3 b | 2.36 ± 0.8 b | 3.90 ± 1.0 b | 5.18 ± 1.1 c | 33.08 ± 0.7 b | 34.48 ± 3.2 a | ||
| RS | HH | 27.94 ± 0.4 a | 39.78 ± 0.4 a | 40.70 ± 0.4 a | 38.94 ± 0.6 a | 37.16 ± 0.3 a | 36.38 ± 0.7 a | |
| LH | 30.40 ± 0.6 a | 44.02 ± 1.1 a | 42.54 ± 1.1 a | 39.60 ± 0.7 a | 38.36 ± 0.6 a | 34.14 ± 1.7 a | ||
| h° | BS | HH | 66.14 ± 2.9 a | 81.08 ± 4.6 a | 75.62 ± 4.6 a | 68.24 ± 4.9 a | 41.08 ± 2.1 a | 31.62 ± 1.3 a |
| LH | 64.08 ± 4.4 a | 82.76 ± 3.7 a | 74.92 ± 3.5 a | 72.60 ± 4.2 a | 39.04 ± 2.3 a | 29.06 ± 2.4 a | ||
| RS | HH | 23.24 ± 0.6 b | 28.70 ± 0.8 b | 28.52 ± 0.8 b | 27.20 ± 0.9 b | 25.26 ± 0.4 b | 23.06 ± 0.6 a | |
| LH | 23.78 ± 0.7 b | 31.42 ± 0.8 b | 29.58 ± 0.6 b | 27.08 ± 0.6 b | 24.56 ± 0.3 b | 21.52 ± 0.9 a | ||
| Maturity stage (A) | *** | *** | *** | * | * | NS | ||
| Storage conditions (B) | NS | ** | * | ** | *** | *** | ||
| A × B | NS | *** | *** | *** | *** | *** | ||
| Maturity Stage | Storage Conditions | Number of Microorganisms (log CFU g−1) | |||||
|---|---|---|---|---|---|---|---|
| Aerobic Count | E. coli | Yeast and Mold | |||||
| Initial | Final | Initial | Final | Initial | Final | ||
| BS | HH | 3.86 ± 0.11 a Z | 3.15 ± 0.15 c | 3.52 ± 0.13 a | 3.87 ± 0.09 a | 3.27 ± 0.16 a | 3.49 ± 0.12 a |
| LH | 4.57 ± 0.15 a | 3.74 ± 0.16 a | 3.00 ± 0.00 a | ||||
| RS | HH | 4.08 ± 0.06 a | 3.90 ± 0.14 b | 4.03 ± 0.08 a | 3.64 ± 0.22 a | 3.39 ± 0.05 a | 3.00 ± 0.00 a |
| LH | 3.19 ± 0.12 c | 3.87 ± 0.17 a | 3.15 ± 0.15 a | ||||
| Maturity (A) | NS | * | NS | NS | NS | NS | |
| Storage conditions (B) | NS | *** | NS | NS | NS | NS | |
| A × B | NS | *** | NS | NS | NS | NS | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afolabi, A.S.; Choi, I.-L.; Lee, J.H.; Kwon, Y.B.; Kang, H.-M. High-Relative-Humidity Storage Reduces the Chilling Injury Symptoms of Red Sweet Peppers in the Breaker Stage. Horticulturae 2023, 9, 116. https://doi.org/10.3390/horticulturae9010116
Afolabi AS, Choi I-L, Lee JH, Kwon YB, Kang H-M. High-Relative-Humidity Storage Reduces the Chilling Injury Symptoms of Red Sweet Peppers in the Breaker Stage. Horticulturae. 2023; 9(1):116. https://doi.org/10.3390/horticulturae9010116
Chicago/Turabian StyleAfolabi, Abiodun Samuel, In-Lee Choi, Joo Hwan Lee, Yong Beom Kwon, and Ho-Min Kang. 2023. "High-Relative-Humidity Storage Reduces the Chilling Injury Symptoms of Red Sweet Peppers in the Breaker Stage" Horticulturae 9, no. 1: 116. https://doi.org/10.3390/horticulturae9010116
APA StyleAfolabi, A. S., Choi, I.-L., Lee, J. H., Kwon, Y. B., & Kang, H.-M. (2023). High-Relative-Humidity Storage Reduces the Chilling Injury Symptoms of Red Sweet Peppers in the Breaker Stage. Horticulturae, 9(1), 116. https://doi.org/10.3390/horticulturae9010116






