1. Introduction
Wild herbs have been a source of food or bioactive compounds for humans since ancient times. Wild leafy vegetables are part of the diet of many people around the world. Wild edible species are often underutilized and can be grown to increase the biodiversity of cultivated vegetables and herbs in the human diet [
1]. These wild species are collected from nature and used as food. Some herbs have been cultivated to provide an innovation in food production chain. Most wild species in agricultural systems are weeds and can be found in wild lands, along roads, and in urban and peri-urban areas [
2]. In cropping systems, they are removed with mechanical or agrochemical treatments. However, the majority of wild species can survive in hostile conditions through the adaptation of their metabolism. These plants can grow by exploiting the limited resources available, such as nutrients and water. These growing conditions increase the accumulation of some defense metabolites that can also have beneficial effects on human health [
1]. The screening and selection of these wild species can increase the biodiversity of cultivated vegetables and expand market opportunities for leafy vegetables or baby leaf production. From an agronomic point of view, these species often have a high tolerance against biotic and abiotic stresses, and for this reason, they are very interesting considering the climate change scenario. These traits are also particularly important for reducing fertilizers or agrochemical applications, as requested by the EU Green Deal strategies by 2030 [
3].
There are several wild species which have been studied for different proprieties, but just a few have been effectively adopted as crops in horticulture and successfully included in commercial catalogs. Hedge mustard (
Sisymbrium officinale (L.) Scop.), often referred to by the synonym
Erisymum officinale, has some traits that could be exploited for the vegetable market [
4]. This plant species can be found in Europe, North Africa, and Western Asia. It is an herb famous for its therapeutic properties, which have been used for treating aphonia and hoarseness [
5]. For these pharmaceutical proprieties, hedge mustard is also known as “the singers’ plant”.
Sisymbrium officinale is commonly used by professional singers as an infuse, or in the form of several herbal preparations such as extracts, tablets, or drops, to prevent or treat voice discomfort [
5]. This species belongs to the Brassiceae family, and the main bioactive compounds are represented by glucosinolates and sulfur-containing compounds such as glucoputranjivine, glucojiabutina, napoleiferina, sinalbina, and sinigrin [
6]. The concentration of glucosinolates can vary in different plant organs, and glucosinolate chemical species are differentially accumulated in roots, stems, and fruit [
7]. These compounds contribute to the antioxidant powder of this species and can have scavenger activity, reducing reactive oxygen species (ROS) and helping plants to adapt and survive in different environments. However, these chemical compounds are also known to have beneficial effects on human health [
8]. The successful cultivation of wild species depends on the market demands and the adaptation to agronomic practices. Hedge mustard has been studied as a potential leaf vegetable and its responses to different fertilization levels have been noted [
9]. The reduction in fertilization has been found to improve some quality traits, such as pigment and nitrate accumulation. For cultivation purpose, it is very important to improve the germination of seeds. Several studies have been carried out on germination using nitrate and light treatments [
10]. Another important parameter that is considered important for cultivated plants is their tolerance to drought.
Therefore, the objective of this work was to evaluate the yield and the way in which quality parameters change when plants are shifted from well-watered conditions to a water reduction regime. The present work is based on the hypothesis that high concentrations of bioactive compounds in this species may be able to confer drought tolerance. The results of this work can be useful for providing agricultural information for growing this species in geographical areas with limited water availability, or during winter, when watering is reduced to avoid disease development.
2. Materials and Methods
2.1. Plant Materials and Water Reduction Management
Two hedge mustard (
Sisymbrium officinale (L.) Scop.) populations were harvested in the wild near Milan and Bergamo, and were, therefore, been named MI (Milan) and BG (Bergamo), respectively. Plants were grown for seed production and then used for the water reduction experiments [
4,
9]. Plants were cultivated in greenhouses equipped with sensors for control of the environmental parameters. The greenhouse was located in the Department of Agricultural and Environmental Science of the University of Milan (GPS 45.47688876073164, 9.227201084659612). Seeds were sown in panels filled with commercial fertilized substrate (6 February and 20 February 2018). Seedlings were transplanted into pots when they reached 7–10 cm height (19 April 2018), and cultivation was carried out using complete substrate (Vigorplant, Italy) containing the following components: 13% volcanic peat, 18% calibrated peat, 21% Baltic peat, 22% dark peat, and 26% Irish peat, with pH (H
2O) 6.5–7.5 and electric conductivity (EC) 0.35–0.45 dS m
−1. The pH at the beginning of the experiment was 6.5, and plants were placed in plastic pots of 14 cm diameter and 2 L volume.
Ten plants of each pot were fertilized with NPK granular fertilizer (14:7:17) at 4 g/pot, providing 13 g/m
2 N; 7 g/m
2 P
2O
5; 8 g/m
2 K
2O [
9].
Water reduction (WR) was performed by gravitropic determination with the full water availability (100%) for the well-watered plants and half that weight of water supplied to the well-water plants (50% WR). During the cultivation period, the pots were weighed in order to restore the same level of water availability that was present at the beginning of the experiment.
The harvest was performed at the 13 BBCH growth stage for each cultivation cycle. Sampling was randomized by casually choosing plants from each pot.
The yields of leaf biomass of the two wild populations were determined to measure total leaf biomass production (3 July 2018). On leaves, the following parameters—anthocyanins, carotenoids, phenols, nitrate, and total sugars—were measured for quality evaluation.
2.2. Chlorophyll a Fluorescence
At harvest, the chlorophyll a fluorescence was determined using a fluorometer (Handy PEA, Hansatech, United Kingdom). Leaves were covered with leaf clips to fully oxidase the photosystem II. Dark incubation was carried out for 30 min. The chlorophyll a fluorescence induction curve was measured using high-intensity light of 3000 µmol m−2 s−1 (600 W m−2). Chlorophyll a fluorescence-derived parameters were automatically calculated, including the variable fluorescence (Fv) to maximum fluorescence (Fm) and their ratio, Fv/Fm. Induction curve data were used for JIP analyses and the following parameters were reported: performance index (PI), dissipation of energy per reaction center (DIo/RC), and density of reaction centers at the Fm stage (RC/CSm).
2.3. Analytical Determinations
The quality of the hedge mustard populations under water stress conditions was determined. Approximately 1 g of leaves was harvested at the end of the cultivation cycle. Fresh plant matter was determined at the end of the growing cycle. All determinations were performed at harvest.
2.3.1. Chlorophylls and Carotenoids Concentrations
Leaf pigments such as total chlorophylls and total carotenoids were obtained by the extraction of 5 leaf disks of 5 mm diameter, containing 25–40 mg of leaf mix. Extraction was carried out using 5 mL of methanol (99.9%) as solvent, and the disks were kept in a dark, cold room at 4 °C for 24 h. Chlorophyll a, b, and total chlorophylls were immediately determined after extraction. Th readings were performed at 665.2 and 652.4 nm for leaf chlorophylls and 470 nm for total carotenoids. The total chlorophylls and carotenoids were determined by Lichtenthaler’s formula [
11].
2.3.2. Secondary Metabolites Such as Anthocyanins or Phenolic Compounds Index
Phenolics in leaves were spectrophotometrically determined by direct measurement of the leaf extract. Leaf disks weighing approximately 25–40 mg were extracted using 3 mL methanolic HCl (1%). The mixture was incubated overnight and the supernatant was used for total phenolic determination using the Folin–Ciocalteu method [
12]. Data are reported as gallic acid equivalent mg/100 g of fresh weight.
Anthocyanins were spectrophotometrically determined. Leaf disks (5 mm diameter) of approximately 20–30 mg were extracted by 3 mL of methanolic HCl (1%). Mixtures were stored overnight at 4 °C in dark conditions. The anthocyanins were expressed as cyanidin-3-glucoside equivalents spectrophotometrically determined at 535 nm, and quantification was performed using an extinction coefficient (ε) of 29,600 [
12].
2.3.3. Leaf Nitrate Concentration
The accumulation of nitrate was spectrophotometrically determined using the salicylic–sulfuric acid method [
13]. Nitrate was collected once at the end of the growing cycle when plants reached the growth stage of 13 BBCH.
About 1 g of fresh leaves was ground in 5 mL of distilled water. Extracts were purified using a centrifuge at 4000 rpm for 15 min. The supernatant was used for colorimetric determinations. Samples of 20 µL were taken, as well as 80 µL (5% w/v) of salicylic acid in concentrated sulfuric acid and 3 mL of NaOH 1.5 N. Samples were cooled, and the absorbance was measured at 410 nm. Nitrate quantification was carried out using to the KNO3 standard calibration curve.
2.3.4. Total Sugar Determination
The sugar concentration of the fresh leaves was extracted as explained for the determination of nitrate levels. Sugars were determined according the anthrone’s assay with slight modification [
14]. The reagent (anthrone) was prepared by dissolving 0.1 g of anthrone in 50 mL of 95% H
2SO
4, and it was left for 40 min before use. The extract (200 µL) was transferred to 1 mL of anthrone. Samples were placed on ice for 5 min and then mixed by a vortex. Samples was heated at 95 °C to create the reaction. After 5 min of incubation, samples were cooled, and absorbance was assessed at 620 nm. The total sugar concentration was calculated referring to the glucose standard calibration curve.
2.4. Statistical Analyses
The experimental design was organized as follow: two wild populations (MI and BG); two different water availability treatments, 100% or 50%; ten plants/pots for each water level, for a total number of 20 plants for the MI wild population and 20 for the BG wild population; and two cultivation cycles.
Biological replicates (n = 4) were taken for each water regime level and for each wild population for the measurement of chlorophyll a fluorescence, while three biological replicates (n = 3) were taken for each water regime level of each wild population.
Two-way ANOVA (water availability and wild population) was also performed, and differences among the means were determined using Tukey’s post-test (p < 0.05). The principal component analysis (PCA) was carried out among parameters measured, with eigenvalues > 1.
3. Results
The hedge mustard plants were subjected to water irrigation reduction up to 50% of crop availability. The yield did not significantly change between the two water regimes or the two wild populations. Values ranged from 26 to 40 g plant
−1 (fresh weight). The MI population grown at 100% WR showed a yield of 26 g plant
−1 FW and 22.3 g plant
−1 FW at 50% WR. The BG population showed a yield of 35 g plant
−1 FW at 100% WR and 40 g plant
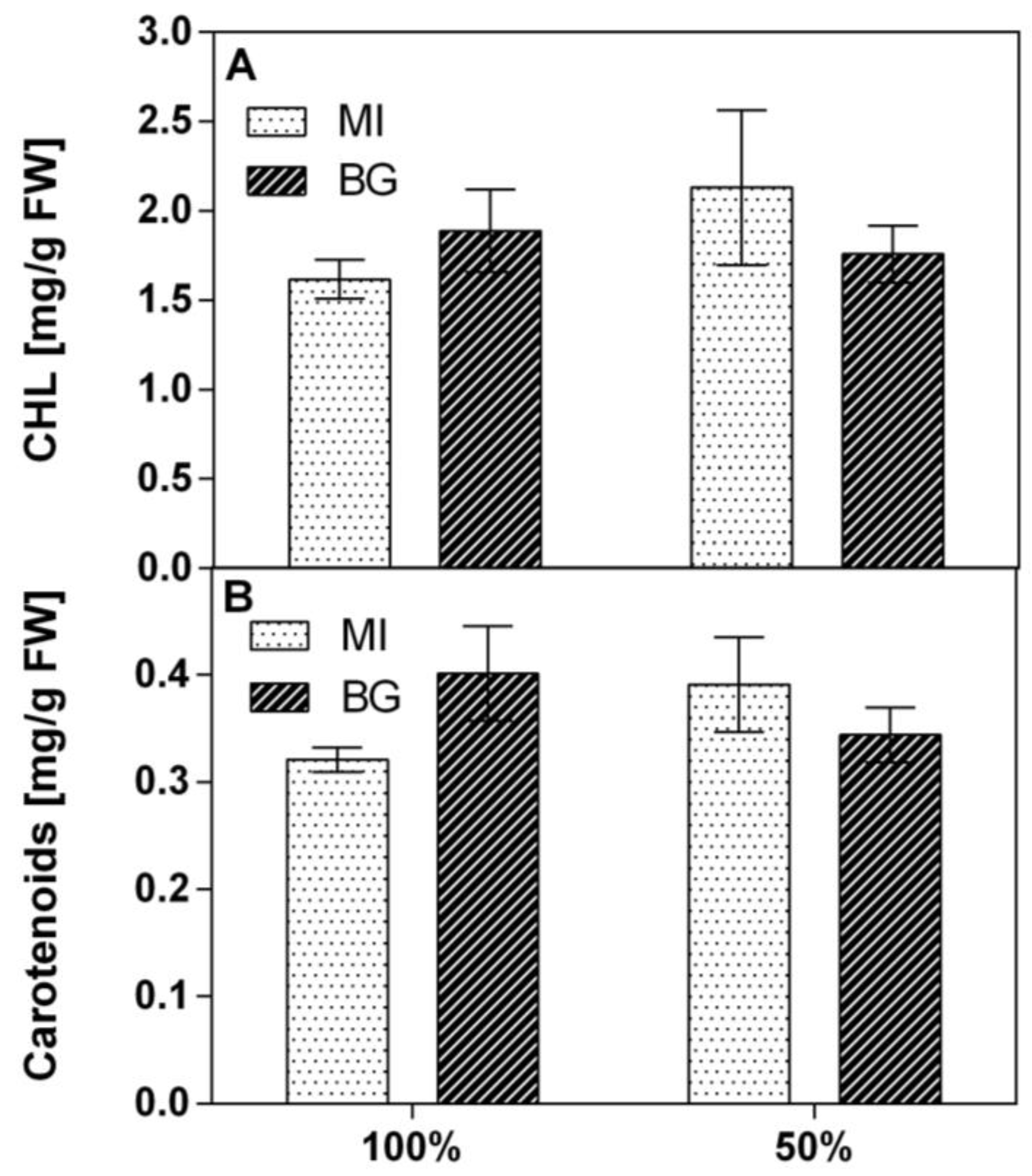
−1 FW at 50% WR. The chlorophyll and carotenoid concentrations did not significantly change according to the different experimental conditions. Chlorophyll values were comprised from 1.6 to 2.1 mg g
−1 FW (
Figure 1A), while total carotenoids ranged from 0.32 to 0.40 mg g
−1 FW (
Figure 1B).
The chlorophyll
a fluorescence was measured at harvest, and relative parameters were calculated. The results showed that no significant differences were found between wild populations and water regimes. The maximum efficiency values of PSII (Fv/Fm) were from 0.81 to 0.82. The performance index (PI) ranged from 2.02 to 2.52, while the dissipation energy was distributed from 0.46 to 0.57. The reaction centers for the cross-section ranged from 2061 to 2270 (
Table 1).
The electron flux per reaction center (ETo/RC) is an index that measures the flux of electrons transferred to the photosynthesis machinery, and it is closely associated with the photosynthetic activity of plants. Data measured indicated that there were no significant differences, and values were of 1.2–1.3 a.u. (
Table 1).
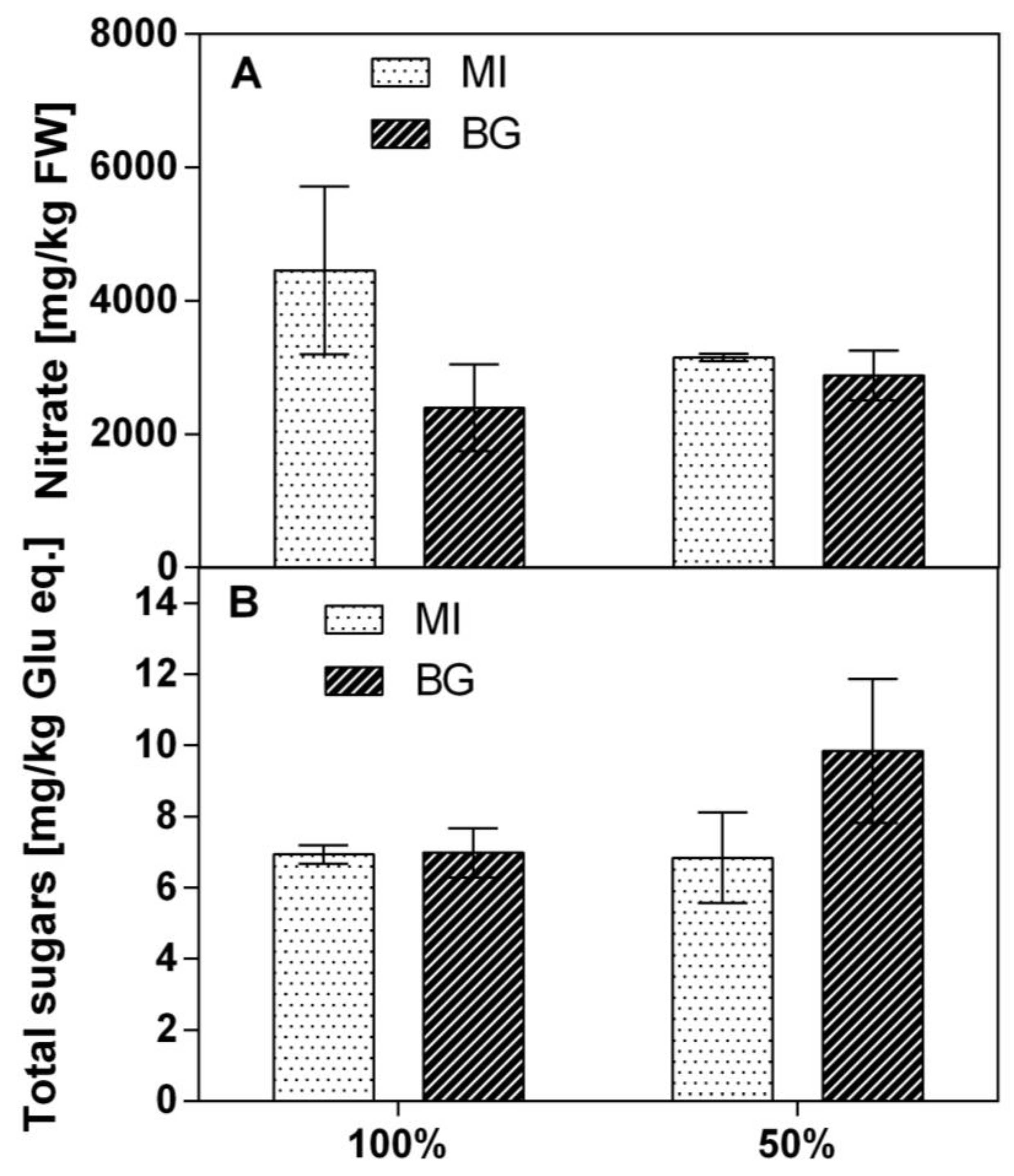
Of the two populations grown with limited water availability, the nitrate concentration in the leaves was higher in MI compared to BG in normal conditions, with values that ranged from 3200 to 5800 mg/kg FW. Under limited water availability, both populations showed the same values. However, the BG population did not show any changes in concentration between normal and drought conditions, while the MI population showed a nitrate reduction (
Figure 2A).
The total sugars did not significantly change between water availability regimes and populations. The concentration ranged from 7 to 10 mg kg
−1 FW on average, even if in the BG population, data showed a wider variability with higher means at 50% water availability (
Figure 2B).
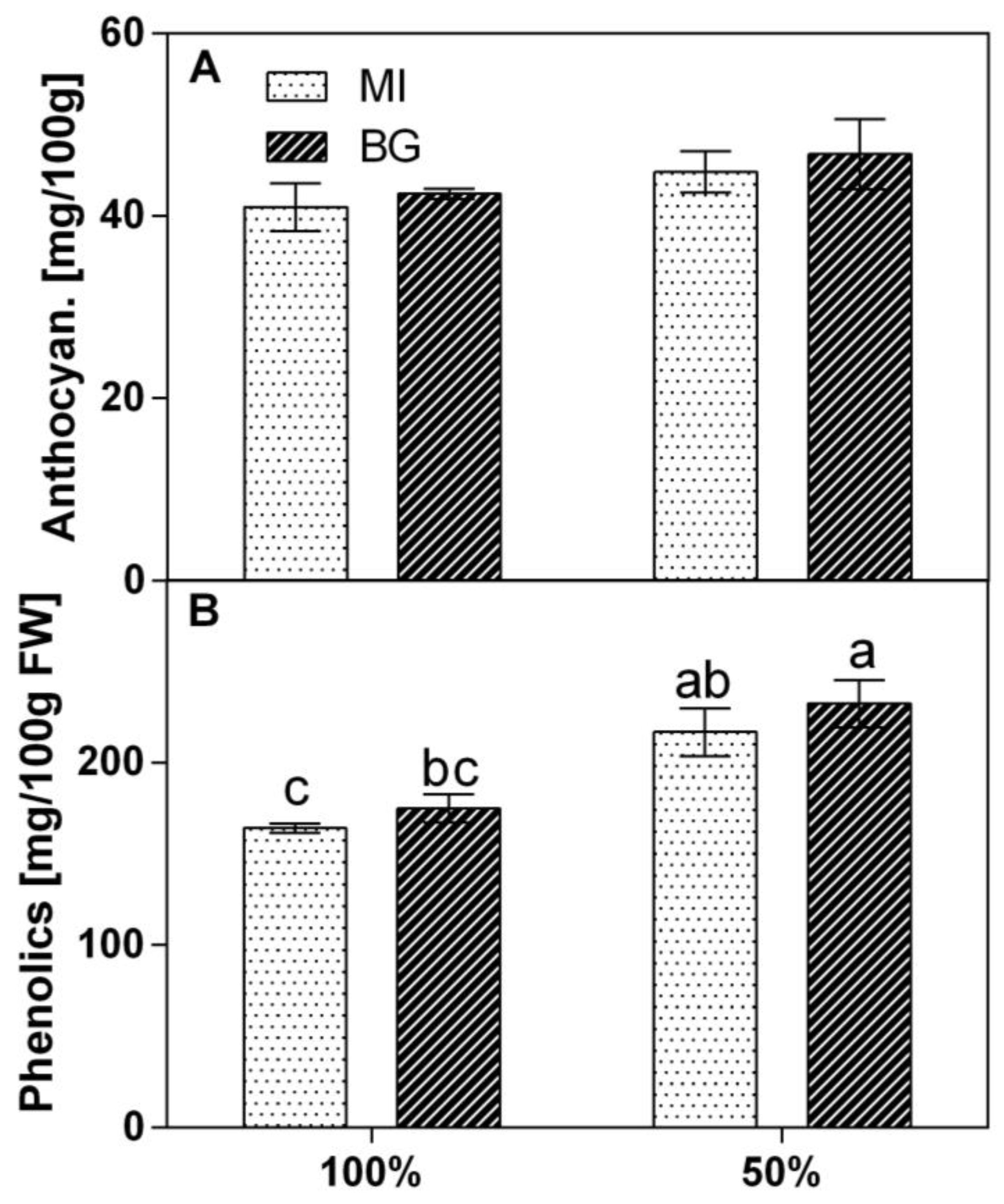
The effect of water stress was evaluated on the basis of the secondary metabolism compound changes. Anthocyanin concentrations ranged from 41 to 47 mg 100
−1 FW, and differences were not significant between water regimes and wild populations (
Figure 3A). Phenolic compounds significantly increased under 50% water reduction in both populations. Statistical analysis revealed that the interaction of WR × Population was not significant (
p = 0.82), while the WR factor was significant, at
p = 0.0006. The population factor, instead, was not significant, with a
p = 0.23. At 100% water availability, the phenolic compounds ranged from 164 to 174 mg 100 g
−1 FW, while at 50%, they ranged from 217 to 233 mg 100 g
−1 FW (
Figure 3B). In both populations, the reduction in water induced an increase of 25% in total phenolics.
Significant correlations were found among chlorophyll and chlorophyll a fluorescence parameters, anthocyanins, and phenols. Anthocyanins were also significantly correlated with the number of reaction centers, the electron flux, and dissipation energy through the reaction centers (
Table 2).
The PCA performed using all analytical data revealed that there was no clear separation of components among the parameters measured (
Figure 4). The results indicated that there was no significant separation between the wild population and WR regimes.
4. Discussion
In the cultivation of leafy vegetables, the water availability for fresh-cut or minimally processed production chains is very important. The reduction in water supply can prevent excessive leaching and loss of nutrients into the soil, avoiding underground water pollution. At the agronomic level, during winter growing cycles, the reduction in the irrigation water can limit the incidence of fungal diseases. However, the reduction in water supply can increase the nitrate concentration, with a negative effect on quality [
15]. In the present study, the yield of hedge mustard was different between populations, but it was not affected by water reduction, indicating the ability of this species to grow under water shortage. Wild species are usually more tolerant than their cultivated relatives. This evidence has been reported for cotton, potato, maize, rice, and soybean [
16,
17] plants. It is well-known that wild relatives are a good source of tolerant traits against abiotic stresses, which is useful in genetic improvement programs. However, transferring a trait from a wild species to cultivated ones is not easy and requires many years of work. Therefore, direct screening of suitable new wild species for agricultural purposes can provide a fast product innovation. In the fresh-cut industry, the identification of new crops with high nutritional quality is highly desired [
18]. Several studies have been carried out to provide information on the potential use of wild or underutilized species as baby leaves for the fresh-cut industries. The effect of water reduction availability was evaluated on both the primary and secondary metabolism. The influence of water limitation on the primary metabolism was evaluated at harvesting time by measuring the chlorophyll concentration and the chlorophyll
a fluorescence [
19]. The chlorophyll concentration is important in leafy vegetables because it is connected to the photosynthetic machinery and the light harvesting complex, but also to the visual appearance of the produce, along with anthocyanins [
20]. Chlorophyll
a fluorescence measurements are important for understanding the stress conditions of crops, and they allow us to estimate the light use efficiency [
21]. The obtained results were similar to those reported in previous studies focused on the comparison of hedge mustard in fertilization experiments [
9] and wild populations [
4]. The total carotenoids showed the same trend as chlorophyll, and did not change. Carotenoids are antioxidants, and have chlorophyll protection functions. However, they can contribute to the production of total antioxidants and enhance the nutritional quality of the product.
The nitrate concentration was measured since, for leafy vegetable production, there are limits imposed by European Union [
22] for their commercialization. The EU regulation n. 1258/2011 reported that for some Brassiceae, including the
Sisymbrium tenuifolium, the limits were differentiated among crops and in different cultivation periods. In winter (1 October to 31 March) the limits are 7000 mg kg
−1 FW, and 6000 mg kg
−1 FW in summer (1 April to 30 September). The results demonstrated that the two populations have different nitrate accumulation abilities, but neither nitrate concentrations overcame the EU limits under water stress. The MI showed higher leaf nitrate concentrations, and these findings confirm previous data [
9]. However, the leaf pigment concentration can vary with growing periods [
4,
9]. Nitrates, as sugars, can contribute to osmotic adjustment under drought stress. A slight increase in total sugars was observed under 50% water reduction conditions in BG population. The lack of increase in these two parameters suggests that the 50% water reduction did not induce significant stress in the hedge mustard.
Total sugars are important, because they represent the energy source of plants for their metabolism and are required for respiration after harvest. The amount of total sugars is important for the post-harvest storage duration and shelf-life of leafy vegetables.
Among the secondary metabolites, anthocyanins and phenolic compounds were monitored, and the results indicated that lower water availability increased the phenolic compounds. Since glucosinolates and phenolic compounds contribute to beneficial effects on human health, many of the secondary metabolites have pharmacological proprieties [
8].
Sisymbrium officinale (L.) has been widely studied for its potential pharmaceutical applications. It has been found that its extracts are able to inhibit mutagenicity in vitro [
23]. The isopropylisothiocyanate and 2-buthylisothiocyanate isolated from hedge mustard were tested in vitro and found to be potent agonists of TRPA1 [
24]. Glucoputranjivin has been also found to be a selective agonist of the T2R16 receptor [
25]. Anti-arthritic activity was also observed for the dichloromethane extracts in vitro using rat liver microsomal cells.
S. officinale extracts were able to reduce the production of the pro-inflammatory mediator nitric oxide, as well as lipid peroxidation [
6]. The efficacy of
S. officinale extracts depends on the concentration of bioactive compounds. The obtained results suggest that a water reduction of up to 50% of the water availability does not affect the yield, and even induces an increase in bioactive compounds of this species. Similar results have been reported for rapeseed (
Brassica napus L.), which, grown under drought stress, showed an increase in chlorophylls, carotenoids, total pigment, phenolic compounds, flavonoids, and antioxidant activities [
26]. The effects of water reduction was also studied in wild rocket (
Diplotaxis tenuifolia L.) for which the water supply was 50% of the evapotranspiration, and it was found to increase total phenols, total carotenoids, and nitrates [
15].
The increase in antioxidant compounds under abiotic stress is a crop defense mechanism that invests energy for the biosynthesis of secondary metabolites. Antioxidant compounds reduce the ROS accumulation and increase crop tolerance to abiotic stress. Crops with fast and positive responses to the abiotic stress have a higher adaptation ability and a higher nutritional quality.