Pectin, Lignin and Disease Resistance in Brassica napus L.: An Update
Abstract
1. Introduction
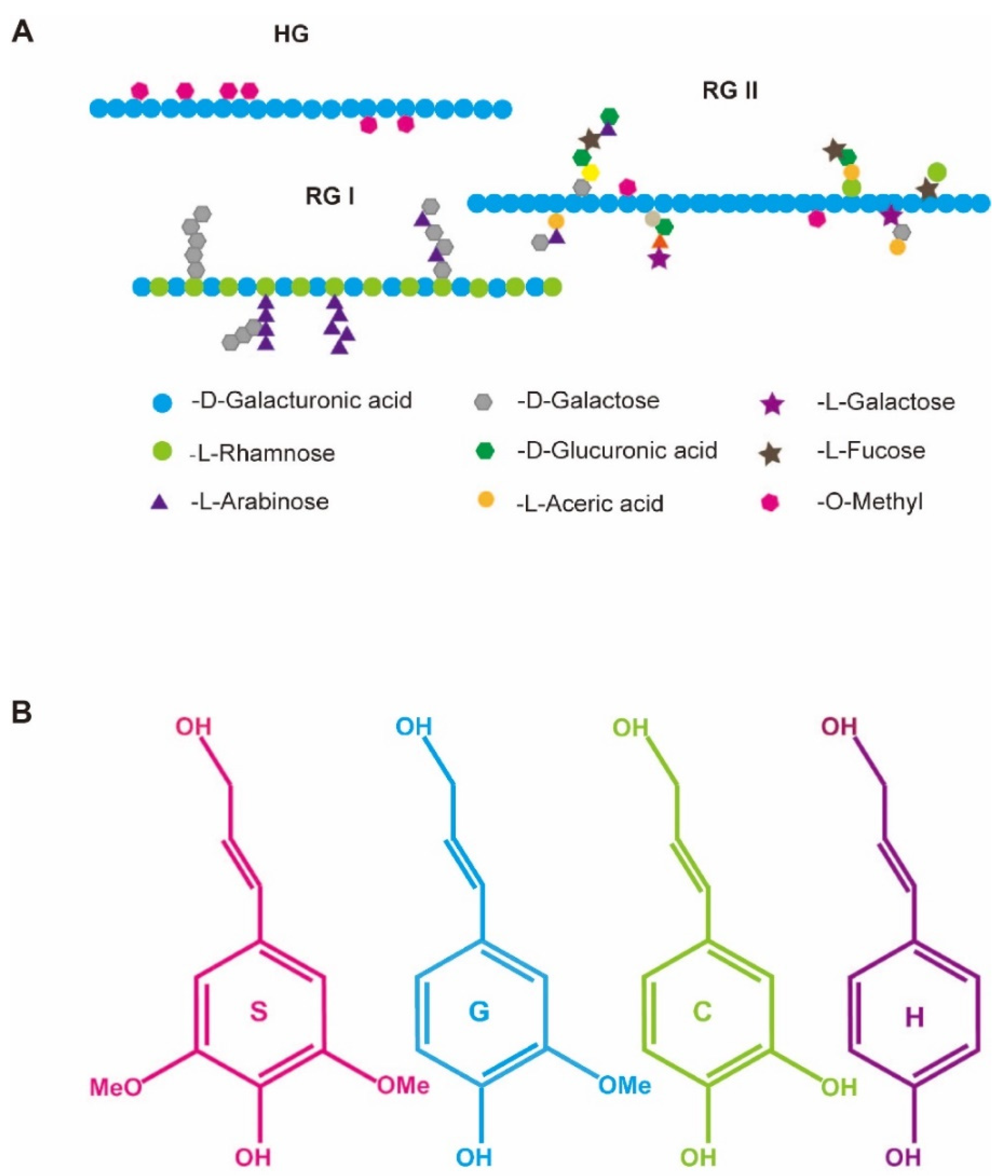
2. Pectin Metabolism Is Closely Associated with Disease Resistance in B. napus
3. The Content and Composition of Lignin Determine Disease Resistance of B. napus
4. Pectin and Lignin Act Synergistically to Defend against Pathogens in B. napus
5. Transcriptional Regulation of Cell-Wall-Mediated Immunity in B. napus
6. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Glossary
| Middle lamella (ML) | A layer rich in pectic polysaccharides that glues adjacent cells together. |
| Primary cell wall (PCW) | A layer surrounding plant cells and mainly composed of polysaccharides including cellulose, hemicellulose and pectin. |
| Secondary cell wall (SCW) | Synthesized by specialized plant cells when cell growth ceases, mainly composed of polysaccharides (about 65%) and lignin (about 35%). |
| Lignin | A principal structural component of secondary cell walls in higher terrestrial plants, which strengths cell wall rigidity. Lignin is typically composed of p-hydroxyphenyl (H), guaiacyl (G) and syringyl (S) units that derive from the polymerization of various aromatic monomers. |
| Xyloglucan | The main component of hemicellulose, which is composed of a 1,4-β-glucan linked backbone. Side chains such as galactosyl and fucosyl-galactosyl residues are linked to the backbone via xylose residues. |
| Cellulose | One of the three main cell wall polysaccharides, which is made up of β-1,4-linked glucan chains. Multiple linear celluloses are usually organized into crystalline microfibrils in the plant primary cell wall, acting as a major load-bearing cell wall component. |
| Pectin | A predominant cell wall polysaccharide of the plant primary cell wall and middle lamella, which is abundant in galacturonic acid and is classified into three major types depending on the composition of the backbone and side chains, including homogalacturonan (HG), rhamnogalacturonan I (RG-I) and rhamnogalacturonan II (RG-II). |
| Cell wall integrity (CWI) | The structural and functional integrity of the plant cell wall. |
| Microbe-associated molecular pattern (PAMP) | Molecules derived from the microbe itself, which can trigger defense responses. |
| Damage-associated molecular patterns (DAMPs) | Endogenous signaling molecules that are derived and released from damaged host cellular structures, which can be perceived by cell surface localized receptors and activate host immune responses. |
| Pattern recognition receptors (PRRs) | Localized on cell surface such as receptor-like kinases/proteins (RLKs/RLPs) with extracellular ligand-binding domain that can monitor and detect nonself and self-derived signaling molecules, triggering immune responses. |
| Pattern-triggered immunity (PTI) | The first layer of the plant immunity, involving a set of induced defenses when pattern recognition receptors perceive signals derived from pathogens or damaged host cellular structures. |
| Effector-triggered immunity (ETI) | The second layer of the plant immunity, involving a series of immune responses that are more sustained and robust than PTI when intracellular receptors recognize pathogen-secreted effectors. |
| Galacturonosyltransferase (GAUT) | An enzyme that is involved in the synthesis of homogalacturonan (HG) by catalyzing the elongation of HG oligogalacturonides in an α-1, 4-configuration. |
| Pectin methylesterase (PME) | A pectin-related enzyme that can decrease the degree of methylesters on the pectin backbone by removing methyl groups from esterified homogalacturonan. |
| Pectin methylesterase inhibitor (PMEI) | Cell wall proteins that can regulate the degree of the methylesterification of pectins by inhibiting the activity of pectin methylesterase. |
| Polygalacturonase-inhibiting protein (PGIP) | Plant cell wall proteins with leucine-rich repeat, which inhibits pectin depolymerization by inactivating the enzymatic activity of polygalacturonase. |
| Ferulate 5-hydroxylase (F5H) | One of the key enzymes that regulate the S/G lignin composition in plants. |
References
- Neik, T.X.; Amas, J.; Barbetti, M.; Edwards, D.; Batley, J. Understanding Host–Pathogen Interactions in Brassica napus in the Omics Era. Plants 2020, 9, 1336. [Google Scholar] [CrossRef]
- Bacete, L.; Mélida, H.; Miedes, E.; Molina, A. Plant cell wall-mediated immunity: Cell wall changes trigger disease resistance responses. Plant J. 2018, 93, 614–636. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Ngou, B.P.M.; Ding, P.; Xin, X.-F. PTI-ETI crosstalk: An integrative view of plant immunity. Curr. Opin. Plant Biol. 2021, 62, 102030. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Liu, Z.; Shen, H.; Wu, D. Damage-Associated Molecular Pattern-Triggered Immunity in Plants. Front. Plant Sci. 2019, 10, 646. [Google Scholar] [CrossRef]
- Meents, M.J.; Watanabe, Y.; Samuels, A.L. The cell biology of secondary cell wall biosynthesis. Ann. Bot. 2018, 121, 1107–1125. [Google Scholar] [CrossRef]
- Molina, A.; Miedes, E.; Bacete, L.; Rodríguez, T.; Mélida, H.; Denancé, N.; Sánchez-Vallet, A.; Rivière, M.-P.; López, G.; Freydier, A.; et al. Arabidopsis cell wall composition determines disease resistance specificity and fitness. Proc. Natl. Acad. Sci. USA 2021, 118, e2010243118. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Tang, Y.; Wu, J.; Chen, F.; Yang, Y.; Pan, X.; Dong, X.; Jin, X.; Liu, S.; Du, X. Brassica napus Mediator Subunit16 Induces BnMED25- and BnWRKY33-Activated Defense Signaling to Confer Sclerotinia sclerotiorum Resistance. Front. Plant Sci. 2021, 12, 663536. [Google Scholar] [CrossRef]
- Sun, Q.; Lin, L.; Liu, D.; Wu, D.; Fang, Y.; Wu, J.; Wang, Y. CRISPR/Cas9-Mediated Multiplex Genome Editing of the BnWRKY11 and BnWRKY70 Genes in Brassica napus L. Int. J. Mol. Sci. 2018, 19, 2716. [Google Scholar] [CrossRef]
- Wang, Z.; Fang, H.D.; Chen, Y.; Chen, K.P.; Li, G.Y.; Gu, S.L.; Tan, X.L. Overexpression of BnWRKY33 in oilseed rape enhances resistance to Sclerotinia sclerotiorum. Mol. Plant Pathol. 2014, 15, 677–689. [Google Scholar] [CrossRef]
- Liu, F.; Li, X.; Wang, M.; Wen, J.; Yi, B.; Shen, J.; Ma, C.; Fu, T.; Tu, J. Interactions of WRKY15 and WRKY33 transcription factors and their roles in the resistance of oilseed rape to Sclerotinia infection. Plant Biotechnol. J. 2018, 16, 911–925. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, F.; Wang, Z.; Zhuo, C.; Hu, K.; Li, X.; Wen, J.; Bin Yi, B.; Shen, J.; Ma, C.; et al. Transcription factor WRKY28 curbs WRKY33-mediated resistance to Sclerotinia sclerotiorum in Brassica napus. Plant Physiol. 2022, 190, 2757–2774. [Google Scholar] [CrossRef]
- Jiang, J.; Liao, X.; Jin, X.; Tan, L.; Lu, Q.; Yuan, C.; Xue, Y.; Yin, N.; Lin, N.; Chai, Y. MYB43 in Oilseed Rape (Brassica napus) Positively Regulates Vascular Lignification, Plant Morphology and Yield Potential but Negatively Affects Resistance to Scle-rotinia sclerotiorum. Genes 2020, 11, 581. [Google Scholar] [CrossRef]
- Wang, D.; Yang, L.; Silva, C.; Sarwar, R.; Chen, Y.; Blanco-Ulate, B. Pectin-mediated plant immunity. Annu. Plant Rev. 2022, 5, 55–80. [Google Scholar]
- Round, A.N.; Rigby, N.M.; MacDougall, A.J.; Morris, V.J. A new view of pectin structure revealed by acid hydrolysis and atomic force microscopy. Carbohydr. Res. 2010, 345, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Paniagua, C.; Kirby, A.R.; Gunning, A.P.; Morris, V.J.; Matas, A.J.; Quesada, M.A.; Mercado, J.A. Unravelling the nanostructure of strawberry fruit pectins by endo-polygalacturonase digestion and atomic force microscopy. Food Chem. 2017, 224, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Gong, Q.; Su, X.; Cheng, Y.; Wu, H.; Huang, Z.; Xu, A.; Dong, J.; Yu, C. Microscopic and Transcriptomic Comparison of Powdery Mildew Resistance in the Progenies of Brassica carinata × B. napus. Int. J. Mol. Sci. 2022, 23, 9961. [Google Scholar] [CrossRef] [PubMed]
- Engelsdorf, T.; Gigli-Bisceglia, N.; Veerabagu, M.; Mckenna, J.F.; Augstein, F.; Does, D.V.D.; Zipfel, C.; Hamann, T. Pat-tern-Triggered Immunity and Cell Wall Integrity Maintenance Jointly Modulate Plant Stress Responses. Cold Spring Harb. Lab. 2017, 11, eaao3070. [Google Scholar]
- Lionetti, V.; Fabri, E.; De Caroli, M.; Hansen, A.R.; Willats, W.G.; Piro, G.; Bellincampi, D. Three Pectin Methylesterase Inhibitors Protect Cell Wall Integrity for Arabidopsis Immunity to Botrytis. Plant Physiol. 2017, 173, 1844–1863. [Google Scholar] [CrossRef]
- Manabe, Y.; Nafisi, M.; Verhertbruggen, Y.; Orfila, C.; Gille, S.; Rautengarten, C.; Cherk, C.; Marcus, S.E.; Somerville, S.; Pauly, M.; et al. Loss-of-Function Mutation of REDUCED WALL ACETYLATION2 in Arabidopsis Leads to Reduced Cell Wall Acetylation and Increased Resistance to Botrytis cinerea. Plant Physiol. 2011, 155, 1068–1078. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, M.; Pontiggia, D.; Raggi, S.; Cheng, Z.; Scaloni, F.; Ferrari, S.; Ausubel, F.M.; Cervone, F.; De Lorenzo, G. Plant immunity triggered by engineered in vivo release of oligogalacturonides, damage-associated molecular patterns. Proc. Natl. Acad. Sci. USA 2015, 112, 5533–5538. [Google Scholar] [CrossRef] [PubMed]
- Kalunke, R.M.; Tundo, S.; Benedetti, M.; Cervone, F.; De Lorenzo, G.; D’Ovidio, R. An update on polygalacturonase-inhibiting protein (PGIP), a leucine-rich repeat protein that protects crop plants against pathogens. Front. Plant Sci. 2015, 6, 146. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wan, L.; Zhang, X.; Xin, Q.; Song, Y.; Hong, D.; Sun, Y.; Yang, G. Interaction between Brassica napus polygalac-turonase inhibition proteins and Sclerotinia sclerotiorum polygalacturonase: Implications for rapeseed resistance to fungal in-fection. Planta 2021, 253, 34. [Google Scholar] [CrossRef]
- Bashi, Z.D.; Rimmer, S.R.; Khachatourians, G.G.; Hegedus, D.D. Brassica napus polygalacturonase inhibitor proteins inhibit Sclerotinia sclerotiorum polygalacturonase enzymatic and necrotizing activities and delay symptoms in transgenic plants. Can. J. Microbiol. 2013, 59, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Coculo, D.; Lionetti, V. The Plant Invertase/Pectin Methylesterase Inhibitor Superfamily. Front. Plant Sci. 2022, 13, 863892. [Google Scholar] [CrossRef]
- Komarova, T.V.; Sheshukova, E.V.; Dorokhov, Y.L. Cell wall methanol as a signal in plant immunity. Front. Plant Sci. 2014, 5, 101. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Jin, S.; Chen, Z.; Shan, Y.; Li, L. Genome-wide identification of the pectin methylesterase inhibitor genes in Brassica napus and expression analysis of selected members. Front. Plant Sci. 2022, 13, 940284. [Google Scholar] [CrossRef]
- Galletti, R.; Ferrari, S.; De Lorenzo, G. Arabidopsis MPK3 and MPK6 Play Different Roles in Basal and Oligogalacturonide- or Flagellin-Induced Resistance against Botrytis cinerea. Plant Physiol. 2011, 157, 804–814. [Google Scholar] [CrossRef]
- Gravino, M.; Savatin, D.V.; Macone, A.; De Lorenzo, G. Ethylene production in Botrytis cinerea-and oligogalacturonide-induced immunity requires calcium-dependent protein kinases. Plant J. 2015, 84, 1073–1086. [Google Scholar] [CrossRef]
- Ferrari, S.; Galletti, R.; Denoux, C.; De Lorenzo, G.; Ausubel, F.M.; Dewdney, J. Resistance to Botrytis cinerea induced in Ara-bidopsis by elicitors is independent of salicylic acid, ethylene, or jasmonate signaling but requires PHYTOALEXIN DEFI-CIENT3. Plant Physiol. 2007, 144, 367–379. [Google Scholar] [CrossRef]
- Tronchet, M.; Balaguã, C.; Kroj, T.; Jouanin, L.; Roby, D. Cinnamyl alcohol dehydrogenases-C and D, key enzymes in lignin biosynthesis, play an essential role in disease resistance in Arabidopsis. Mol. Plant Pathol. 2010, 11, 83–92. [Google Scholar] [CrossRef]
- Yang, Q.; He, Y.; Kabahuma, M.; Chaya, T.; Kelly, A.; Borrego, E.; Bian, Y.; El Kasmi, F.; Yang, L.; Teixeira, P.; et al. A gene encoding maize caffeoyl-CoA O-methyltransferase confers quantitative resistance to multiple pathogens. Nat Genet. 2017, 49, 1364–1372. [Google Scholar] [CrossRef]
- Ma, Q.-H.; Zhu, H.-H.; Han, J.-Q. Wheat ROP proteins modulate defense response through lignin metabolism. Plant Sci. 2017, 262, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Liu, Z.; Zhu, L.; Zhang, C.; Chen, Y.; Zhou, Y.; Li, F.; Li, X. Overexpression of cotton (Gossypium hirsutum) dirigent1 gene enhances lignification that blocks the spread of Verticillium dahliae. Acta Biochim. Biophys. Sin. 2012, 44, 555–564. [Google Scholar] [CrossRef]
- Liu, D.; Wu, J.; Lin, L.; Li, P.; Li, S.; Wang, Y.; Li, J.; Sun, Q.; Liang, J.; Wang, Y. Overexpression of Cinnamoyl-CoA Reductase 2 in Brassica napus Increases Resistance to Sclerotinia sclerotiorum by Affecting Lignin Biosynthesis. Front Plant Sci. 2021, 12, 732–733. [Google Scholar] [CrossRef] [PubMed]
- Lahlali, R.; Song, T.; Chu, M.; Yu, F.; Kumar, S.; Karunakaran, C.; Peng, G. Evaluating Changes in Cell-Wall Components Associated with Clubroot Resistance Using Fourier Transform Infrared Spectroscopy and RT-PCR. Int. J. Mol. Sci. 2017, 18, 2058. [Google Scholar] [CrossRef]
- Höch, K.; Koopmann, B.; von Tiedemann, A. Lignin Composition and Timing of Cell Wall Lignification Are Involved in Brassica napus Resistance to Stem Rot Caused by Sclerotinia sclerotiorum. Phytopathology 2021, 111, 1438–1448. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Luo, L.; Zheng, L.Q. Lignins: Biosynthesis and Biological Functions in Plants. Int. J. Mol. Sci. 2018, 19, 335. [Google Scholar] [CrossRef]
- Gallego-Giraldo, L.; Posé, S.; Pattathil, S.; Peralta, A.G.; Hahn, M.G.; Ayre, B.G.; Sunuwar, J.; Hernandez, J.; Patel, M.; Shah, J.; et al. Elicitors and defense gene induction in plants with altered lignin compositions. New Phytol. 2018, 219, 1235–1251. [Google Scholar] [CrossRef] [PubMed]
- Bhuiyan, N.H.; Selvaraj, G.; Wei, Y.; King, J. Gene expression profiling and silencing reveal that monolignol biosynthesis plays a critical role in penetration defence in wheat against powdery mildew invasion. J. Exp. Bot. 2008, 60, 509–521. [Google Scholar] [CrossRef]
- Eynck, C.; Séguin-Swartz, G.; Clarke, W.E.; Parkin, I.A. Monolignol biosynthesis is associated with resistance to Sclerotinia sclerotiorum in Camelina sativa. Mol. Plant Pathol. 2012, 13, 887–899. [Google Scholar] [CrossRef]
- Cao, Y.; Yan, X.; Ran, S.; Ralph, J.; Smith, R.A.; Chen, X.; Qu, C.; Li, J.; Liu, L. Knockout of the lignin pathway gene BnF5H decreases the S/G lignin compositional ratio and improves Sclerotinia sclerotiorum resistance in Brassica napus. Plant Cell Environ. 2022, 45, 248–261. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.S.; Jang, E.; Kim, J.; Kim, S.H.; Lee, M.-H.; Nam, M.H.; Tobimatsu, Y.; Park, O.K. Pathogen-induced autophagy regulates monolignol transport and lignin formation in plant immunity. Autophagy 2022, 9, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Islam, T.; Lee, B.-R.; Park, S.-H.; La, V.H.; Jung, W.-J.; Bae, D.-W.; Kim, T.-H. Hormonal regulations in soluble and cell-wall bound phenolic accumulation in two cultivars of Brassica napus contrasting susceptibility to Xanthomonas campestris pv. campestris. Plant Sci. 2019, 285, 132–140. [Google Scholar] [CrossRef]
- Larkan, N.J.; Ma, L.; Haddadi, P.; Buchwaldt, M.; Parkin, I.A.P.; Djavaheri, M.; Borhan, M.H. The Brassica napus wall-associated kinase-like (WAKL) gene Rlm9 provides race-specific blackleg resistance. Plant J. 2020, 104, 892–900. [Google Scholar] [CrossRef] [PubMed]
- Gallego-Giraldo, L.; Liu, C.; Pose-Albacete, S.; Pattathil, S.; Peralta, A.G.; Young, J.; Westpheling, J.; Hahn, M.G.; Rao, X.; Knox, J.P.; et al. ARABIDOPSIS DEHISCENCE ZONE POLYGALACTURONASE 1 (ADPG1) releases latent defense signals in stems with reduced lignin content. Proc. Natl. Acad. Sci. USA 2020, 117, 3281–3290. [Google Scholar] [CrossRef]
- Xiao, C.; Barnes, W.J.; Zamil, M.S.; Yi, H.; Puri, V.M.; Anderson, C.T. Activation tagging of Arabidopsis POLYGALAC-TURONASE INVOLVED IN EXPANSION2 promotes hypocotyl elongation, leaf expansion, stem lignification, mechanical stiffening, and lodging. Plant J. 2017, 89, 1159–1173. [Google Scholar] [CrossRef]
- Bacete, L.; Hamann, T. The Role of Mechanoperception in Plant Cell Wall Integrity Maintenance. Plants 2020, 9, 574. [Google Scholar] [CrossRef]
- Miedes, E.; Vanholme, R.; Boerjan, W.; Molina, A. The role of the secondary cell wall in plant resistance to pathogens. Front. Plant Sci. 2014, 5, 358. [Google Scholar] [CrossRef]
- Yadav, V.; Wang, Z.; Wei, C.; Amo, A.; Ahmed, B.; Yang, X.; Zhang, X. Phenylpropanoid Pathway Engineering: An Emerging Approach towards Plant Defense. Pathogens 2020, 9, 312. [Google Scholar] [CrossRef]
- Jiang, J.J.; Ma, S.H.; Ye, N.H.; Jiang, M.; Cao, J.S.; Zhang, J.H. WRKY transcription factors in plant responses to stresses. J. Integr. Plant Biol. 2017, 59, 86–101. [Google Scholar] [CrossRef]
- Zhou, J.; Lee, C.; Zhong, R.; Ye, Z.-H. MYB58 and MYB63 Are Transcriptional Activators of the Lignin Biosynthetic Pathway during Secondary Cell Wall Formation in Arabidopsis. Plant Cell 2009, 21, 248–266. [Google Scholar] [CrossRef] [PubMed]
- Zhong, R.; Richardson, E.A.; Ye, Z.-H. The MYB46 Transcription Factor Is a Direct Target of SND1 and Regulates Secondary Wall Biosynthesis in Arabidopsis. Plant Cell 2007, 19, 2776–2792. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, R.L.; Zhong, R.; Ye, Z.-H. MYB83 Is a Direct Target of SND1 and Acts Redundantly with MYB46 in the Regulation of Secondary Cell Wall Biosynthesis in Arabidopsis. Plant Cell Physiol. 2009, 50, 1950–1964. [Google Scholar] [CrossRef] [PubMed]
- Chezem, W.R.; Memon, A.; Li, F.S.; Weng, J.K.; Clay, N.K. SG2-Type R2R3-MYB Transcription Factor MYB15 Controls Defense-Induced Lignification and Basal Immunity in Arabidopsis. Plant Cell. 2017, 29, 1907–1926. [Google Scholar] [CrossRef]
- Nafisi, M.; Fimognari, L.; Sakuragi, Y. Interplays between the cell wall and phytohormones in interaction between plants and necrotrophic pathogens. Phytochemistry 2015, 112, 63–71. [Google Scholar] [CrossRef]
- Denness, L.; McKenna, J.F.; Segonzac, C.; Wormit, A.; Madhou, P.; Bennett, M.; Mansfield, J.; Zipfel, C.; Hamann, T. Cell wall damage-induced lignin biosynthesis is regulated by a reactive oxygen species- and jasmonic acid-dependent process in Ara-bidopsis. Plant Physiol. 2011, 156, 1364–1374. [Google Scholar] [CrossRef] [PubMed]

| Gene Name | Phenotype | Pathogen Tested | Reference |
|---|---|---|---|
| PMR5, PGIP1, PMEI9, RWA2, PDCB1, C/VIF2 | R | powdery mildew | [16] |
| WAK1, WAKL10 | S | powdery mildew | [16] |
| BnPGIP2, BnPGIP5 | R | Sclerotinia sclerotiorum | [22] |
| BnMED16 | R | Sclerotinia sclerotiorum | [7] |
| BnaC.CCR2.b, CCR | R | Sclerotinia sclerotiorum | [34] |
| PAL, BnCCR2, BnCAD5, Bn4CL | R | Sclerotinia sclerotiorum | [36] |
| Rcr1 | R | Plasmodiophora brassicae Woronin | [35] |
| MYB43 | S | Sclerotinia sclerotiorum | [12] |
| CHS, CAD, COMT1 | R | Xanthomonas campestrispv. campestris | [43] |
| F5H | S | Sclerotinia sclerotiorum | [41] |
| Rlm9, WAKL | R | Leptosphaeria maculans | [44] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Lu, Q.; Jin, S.; Fan, X.; Ling, H. Pectin, Lignin and Disease Resistance in Brassica napus L.: An Update. Horticulturae 2023, 9, 112. https://doi.org/10.3390/horticulturae9010112
Wang D, Lu Q, Jin S, Fan X, Ling H. Pectin, Lignin and Disease Resistance in Brassica napus L.: An Update. Horticulturae. 2023; 9(1):112. https://doi.org/10.3390/horticulturae9010112
Chicago/Turabian StyleWang, Duoduo, Qianhui Lu, Shunda Jin, Xiangyun Fan, and Hui Ling. 2023. "Pectin, Lignin and Disease Resistance in Brassica napus L.: An Update" Horticulturae 9, no. 1: 112. https://doi.org/10.3390/horticulturae9010112
APA StyleWang, D., Lu, Q., Jin, S., Fan, X., & Ling, H. (2023). Pectin, Lignin and Disease Resistance in Brassica napus L.: An Update. Horticulturae, 9(1), 112. https://doi.org/10.3390/horticulturae9010112






