Application of Morphological and Physiological Markers for Study of Drought Tolerance in Lilium Varieties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Site and Plant Materials
2.2. Experimental Design
2.3. Soil Water Status
2.4. Determination of Leaf Morphological Indices
2.5. Determination of Photosynthetic Index
2.6. Determination of Physiological Index
2.7. Data Processing and Statistical Analysis
3. Results
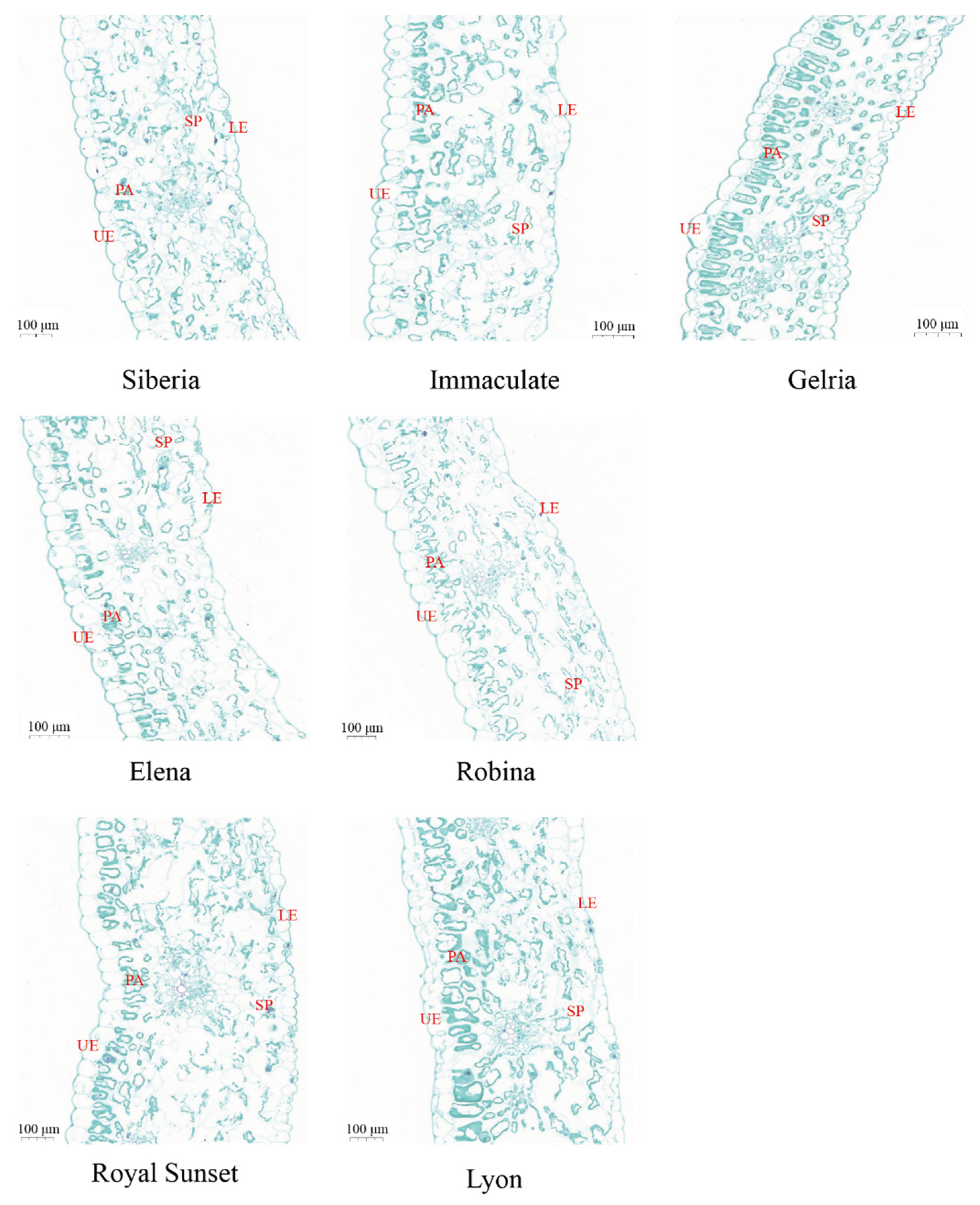
3.1. Leaf Morphology and Anatomical Structure of Seven Lily Species
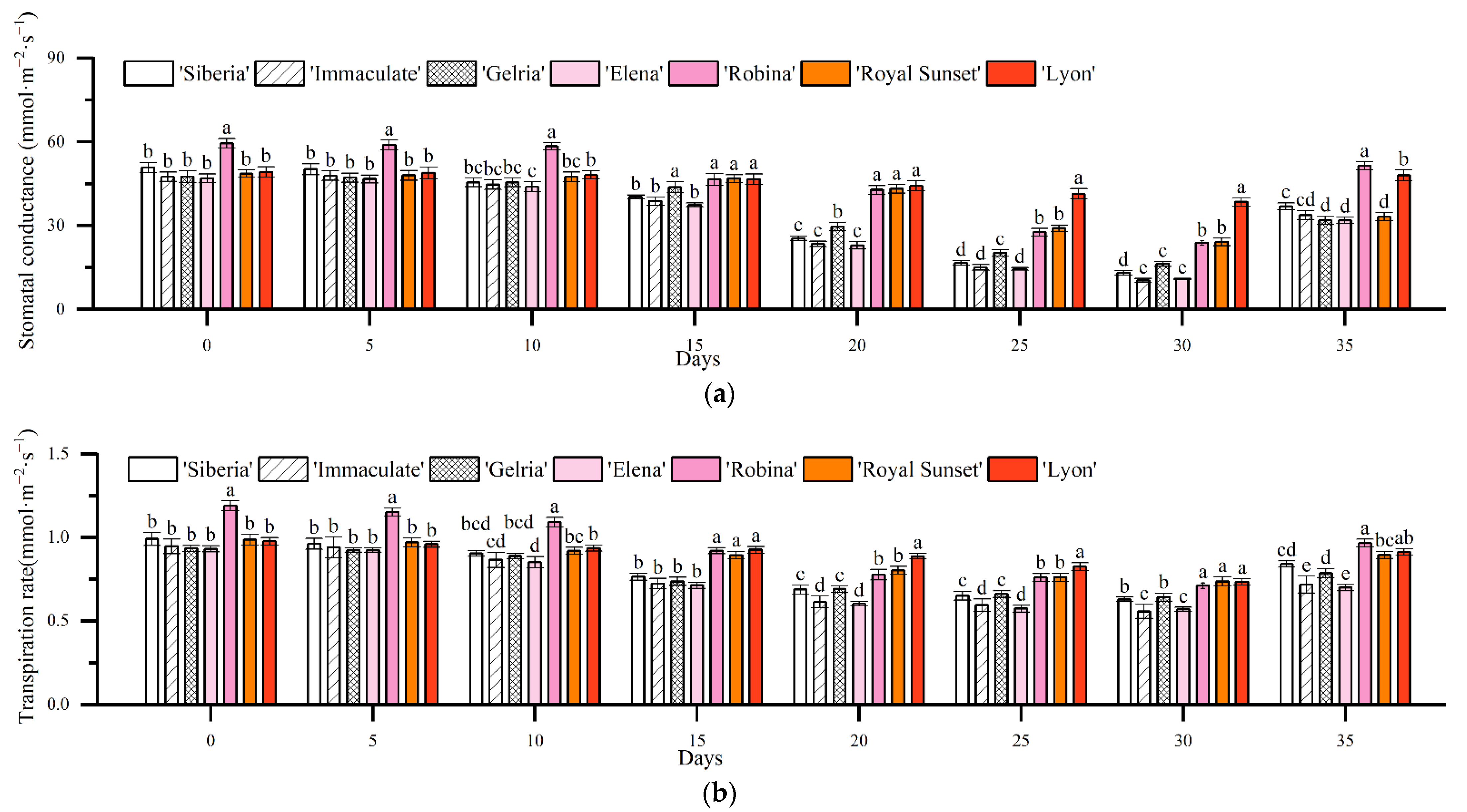
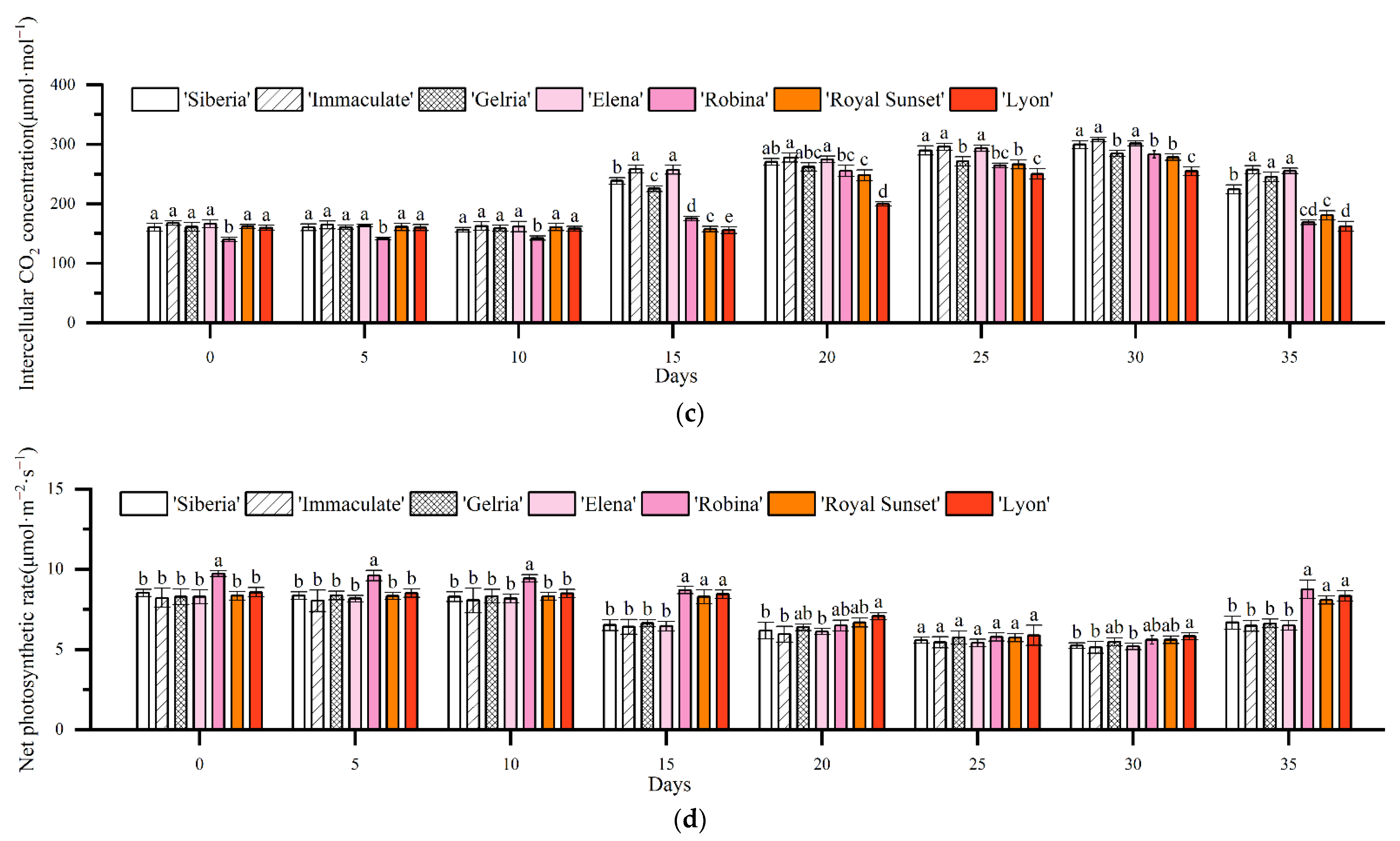
3.2. Effects of Drought Stress on Photosynthesis in Lily Leaves
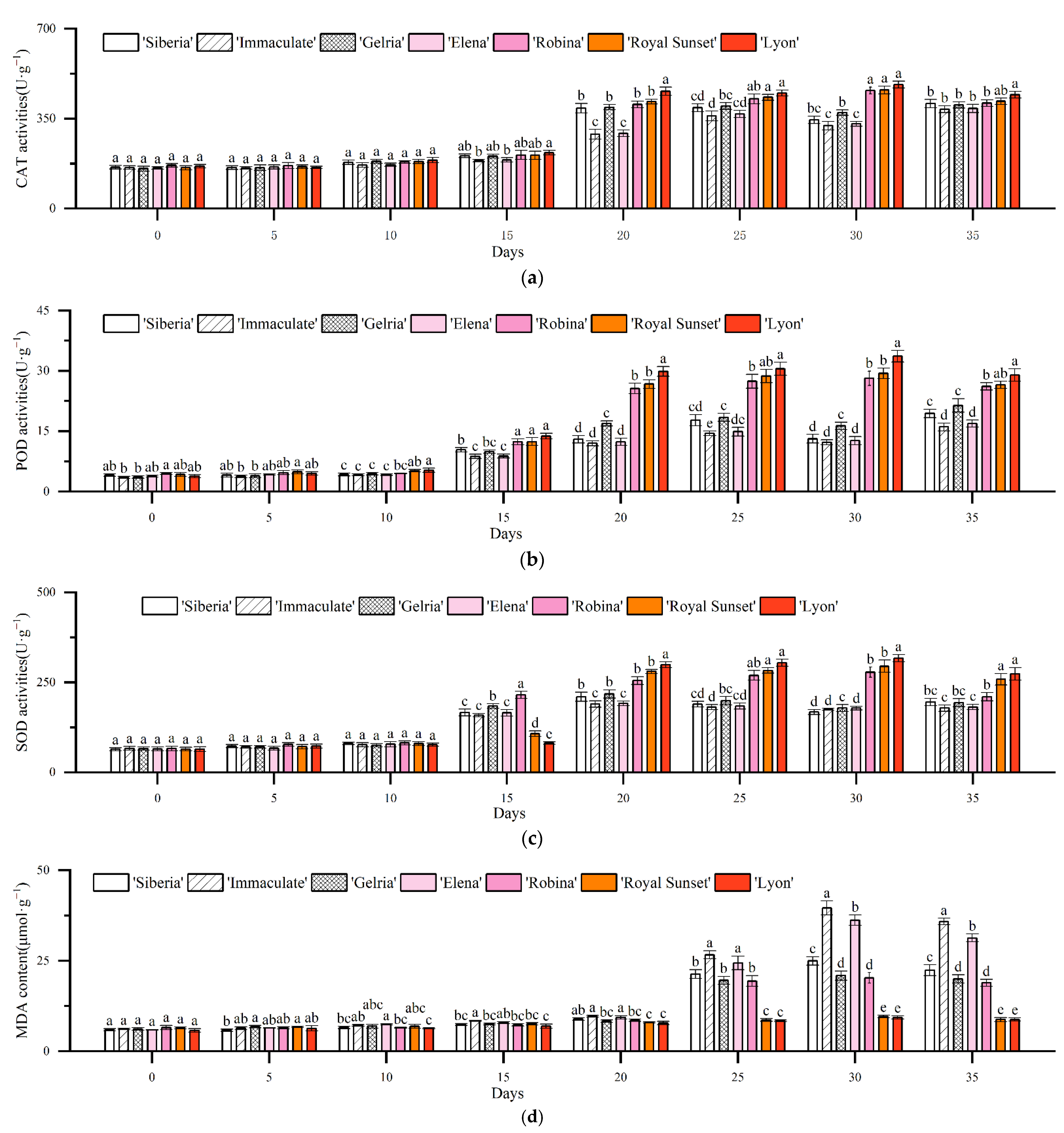
3.3. Effects of Drought Stress on Antioxidant Enzyme Activity in Lily Leaves
3.4. Effects of Drought Stress on the Physiological Metabolism of Lily Leaves
3.5. Evaluation of Drought Resistance in Lily Varieties
3.6. Canonical Discriminant Analysis
3.7. Correlation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Trenberth, K.E.; Dai, A.; van der Schrier, G.; Jones, P.D.; Barichivich, J.; Briffa, K.R.; Sheffield, J. Global warming and changes in drought. Nat. Clim. Chang. 2014, 4, 17–22. [Google Scholar] [CrossRef]
- Chaudhry, S.; Sidhu, G.P.S. Climate change regulated abiotic stress mechanisms in plants: A comprehensive review. Plant Cell Rep. 2022, 41, 1–31. [Google Scholar] [CrossRef]
- Kapoor, D.; Bhardwaj, S.; Landi, M.; Sharma, A.; Ramakrishnan, M.; Sharma, A. The impact of drought in plant metabolism: How to exploit tolerance mechanisms to increase Crop Production. Appl. Sci. 2020, 10, 5692. [Google Scholar] [CrossRef]
- Bakhshaie, M.; Khosravi, S.; Azadi, P.; Bagheri, H.; van Tuyl, J.M. Biotechnological advances in Lilium. Plant Cell Rep. 2016, 35, 1799–1826. [Google Scholar] [CrossRef]
- Khoyerdi, F.F.; Shamshiri, M.H.; Estaji, A. Changes in some physiological and osmotic parameters of several pistachio genotypes under drought stress. Sci. Hortic. 2016, 198, 44–51. [Google Scholar] [CrossRef]
- Li, W.; Wang, Y.; Zhang, Y.; Wang, R.; Guo, Z.; Xie, Z. Impacts of drought stress on the morphology, physiology, and sugar content of Lanzhou lily (Lilium davidii var. unicolor). Acta Physiol. Plant. 2020, 42, 127. [Google Scholar] [CrossRef]
- Shi, L.; Wang, Z.; Kim, W.S. Effect of drought stress on shoot growth and physiological response in the cut rose ‘charming black’ at different developmental stages. Hortic. Environ. Biotechnol. 2019, 60, 1–8. [Google Scholar] [CrossRef]
- Giordano, M.; Petropoulos, S.A.; Cirillo, C.; Rouphael, Y. Biochemical, physiological, and molecular aspects of ornamental plants adaptation to deficit irrigation. Horticulturae 2021, 7, 107. [Google Scholar] [CrossRef]
- Kuppler, J.; Wieland, J.; Junker, R.R.; Ayasse, M. Drought-induced reduction in flower size and abundance correlates with reduced flower visits by bumble bees. AoB Plants 2021, 13, 1–8. [Google Scholar] [CrossRef]
- Ennajeh, M.; Vadel, A.M.; Cochard, H.; Khemira, H. Comparative impacts of water stress on the leaf anatomy of a drought-resistant and a drought-sensitive olive cultivar. J. Hortic. Sci. Biotechnol. 2010, 85, 289–294. [Google Scholar] [CrossRef]
- Zhang, F.-J.; Zhang, K.-K.; Du, C.-Z.; Li, J.; Xing, Y.-X.; Yang, L.-T.; Li, Y.-R. Effect of drought stress on anatomical structure and chloroplast ultrastructure in leaves of sugarcane. Sugar Tech. 2015, 17, 41–48. [Google Scholar] [CrossRef]
- Westerband, A.C.; Bialic-Murphy, L.; Weisenberger, L.A.; Barton, K.E. Intraspecific variation in seedling drought tolerance and associated traits in a critically endangered, endemic Hawaiian shrub. Plant Ecol. Divers. 2020, 13, 159–174. [Google Scholar] [CrossRef]
- Bhargava, S.; Sawant, K. Drought stress adaptation: Metabolic adjustment and regulation of gene expression. Plant Breed. 2013, 132, 21–32. [Google Scholar] [CrossRef]
- Yang, P.M.; Huang, Q.C.; Qin, G.Y.; Zhao, S.P.; Zhou, J.G. Different drought-stress responses in photosynthesis and reactive oxygen metabolism between autotetraploid and diploid rice. Photosynthetica 2014, 52, 193–202. [Google Scholar] [CrossRef]
- McLaughlin, J.E.; Boyer, J.S. Sugar-responsive gene expression, invertase activity, and senescence in aborting maize ovaries at low water potentials. Ann. Bot. 2004, 94, 675–689. [Google Scholar] [CrossRef]
- Laxa, M.; Liebthal, M.; Telman, W.; Chibani, K.; Dietz, K.-J. The role of the plant antioxidant system in drought tolerance. Antioxidants 2019, 8, 94. [Google Scholar] [CrossRef]
- Fang, Y.; Xiong, L. General mechanisms of drought response and their application in drought resistance improvement in plants. Cell. Mol. Life Sci. 2015, 72, 673–689. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Mittler, R.; Balfagón, D.; Arbona, V.; Gómez-Cadenas, A. Plant adaptations to the combination of drought and high temperatures. Physiol. Plant. 2018, 162, 2–12. [Google Scholar] [CrossRef]
- Xu, Z.; Zhou, G.; Shimizu, H. Plant responses to drought and rewatering. Plant Signal. Behav. 2010, 5, 649–654. [Google Scholar] [CrossRef]
- Bo, W.; Fu, B.; Qin, G.; Xing, G.; Wang, Y. Evaluation of drought resistance in Iris germanica L. based on subordination function and principal component analysis. Emir. J. Food Agric. 2017, 29, 770–778. [Google Scholar] [CrossRef] [Green Version]
- Yi-ling, Y.; Chun-hui, H.; Qing-qing, G.; Xue-yan, Q.; Xiao-biao, X. Evaluation of drought-resistance traits of citrus rootstock seedlings by multiple statistics analysis. Acta Hortic. 2015, 1065, 379–386. [Google Scholar] [CrossRef]
- Liu, N.; Liu, S.; Gan, Y.; Zhang, Q.; Wang, X.; Liu, S.; Dai, J. Evaluation of mercury resistance and accumulation characteristics in wheat using a modified membership function. Ecol. Indic. 2017, 78, 292–300. [Google Scholar] [CrossRef]
- Wassie, M.; Zhang, W.; Zhang, Q.; Ji, K.; Chen, L. Effect of heat stress on growth and physiological traits of Alfalfa (Medicago sativa L.) and a comprehensive evaluation for heat tolerance. Agronomy 2019, 9, 597. [Google Scholar] [CrossRef]
- Gholizadeh, A.; Dehghani, H.; Akbarpour, O.; Amini, A.; Sadeghi, K.; Hanifei, M.; Sharifi-Zagheh, A. Assessment of Iranian wheat germplasm for salinity tolerance using analysis of the membership function value of salinity tolerance (MFVS). J. Crop Sci. Biotechnol. 2022, 1–9. [Google Scholar] [CrossRef]
- Ji, X.; Tang, J.; Fan, W.; Li, B.; Bai, Y.; He, J.; Pei, D.; Zhang, J. Phenotypic differences and physiological responses of salt resistance of walnut with four rootstock types. Plants 2022, 11, 1557. [Google Scholar] [CrossRef]
- Bittelli, M. Measuring soil water content: A review. HortTechnology 2011, 21, 293–300. [Google Scholar] [CrossRef]
- Kramer, D.M.; Johnson, G.; Kiirats, O.; Edwards, G.E. New Fluorescence Parameters for the Determination of QA Redox State and Excitation Energy Fluxes. Photosynth. Res. 2004, 79, 209. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef]
- de Azevedo Neto, A.D.; Prisco, J.T.; Enéas-Filho, J.; Medeiros, J.-V.R.; Gomes-Filho, E. Hydrogen peroxide pre-treatment induces salt-stress acclimation in maize plants. J. Plant Physiol. 2005, 162, 1114–1122. [Google Scholar] [CrossRef]
- Tsikas, D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal. Biochem. 2017, 524, 13–30. [Google Scholar] [CrossRef]
- Buysse, J.; Merckx, R. An Improved Colorimetric Method to Quantify Sugar Content of Plant Tissue. J. Exp. Bot. 1993, 44, 1627–1629. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Harb, A.; Krishnan, A.; Ambavaram, M.M.R.; Pereira, A. Molecular and physiological analysis of drought stress in Arabidopsis reveals early responses leading to acclimation in plant growth. Plant Physiol. 2010, 154, 1254–1271. [Google Scholar] [CrossRef] [PubMed]
- Deka, D.; Singh, A.K.; Singh, A.K. Effect of drought stress on crop plants with special reference to drought avoidance and tolerance mechanisms: A review. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 2703–2721. [Google Scholar] [CrossRef]
- Wang, G.; Yuan, Z.; Zhang, P.; Liu, Z.; Wang, T.; Wei, L. Genome-wide analysis of NAC transcription factor family in maize under drought stress and rewatering. Physiol. Mol. Biol. Plants 2020, 26, 705–717. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wang, Z.; Li, S.; Lü, X.; Wang, X.; Han, X. Changes in specific leaf area of dominant plants in temperate grasslands along a 2500-km transect in northern China. Sci. Rep. 2017, 7, 10780. [Google Scholar] [CrossRef]
- Luković, J.; Maksimović, I.; Zorić, L.; Nagl, N.; Perčić, M.; Polić, D.; Putnik-Delić, M. Histological characteristics of sugar beet leaves potentially linked to drought tolerance. Ind. Crops Prod. 2009, 30, 281–286. [Google Scholar] [CrossRef]
- Guha, A.; Sengupta, D.; Kumar Rasineni, G.; Ramachandra Reddy, A. An integrated diagnostic approach to understand drought tolerance in mulberry (Morus indica L.). Flora-Morphol. Distrib. Funct. Ecol. Plants 2010, 205, 144–151. [Google Scholar] [CrossRef]
- Dalal, V.K.; Tripathy, B.C. Water-stress induced downsizing of light-harvesting antenna complex protects developing rice seedlings from photo-oxidative damage. Sci. Rep. 2018, 8, 5955. [Google Scholar] [CrossRef] [Green Version]
- Abdolahi, M.; Maleki Farahani, S. Seed quality, water use efficiency and eco physiological characteristics of Lallemantia (Lallemantia sp.) species as effected by soil moisture content. Acta Agric. Slov. 2019, 113, 307. [Google Scholar] [CrossRef]
- Hossain, M.A.; Wani, S.H.; Bhattacharjee, S.; Burritt, D.J.; Tran, L.-S.P. (Eds.) Drought Stress Tolerance in Plants; Springer International Publishing: Cham, Switzerland, 2016; Volume 1, ISBN 978-3-319-28897-0. [Google Scholar]
- Flexas, J.; Ribas-Carbó, M.; Bota, J.; Galmés, J.; Henkle, M.; Martínez-Cañellas, S.; Medrano, H. Decreased Rubisco activity during water stress is not induced by decreased relative water content but related to conditions of low stomatal conductance and chloroplast CO2 concentration. New Phytol. 2006, 172, 73–82. [Google Scholar] [CrossRef]
- Heilmeier, H.; Wartinger, A.; Erhard, M.; Zimmermann, R.; Horn, R.; Schulze, E.-D. Soil drought increases leaf and whole-plant water use of Prunus dulcis grown in the Negev Desert. Oecologia 2002, 130, 329–336. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, D.; Wang, J.; Ding, Y.; Song, X. Effects of elevated CO2 and drought on plant physiology, soil carbon and soil enzyme activities. Pedosphere 2017, 27, 846–855. [Google Scholar] [CrossRef]
- Lawson, T.; Simkin, A.J.; Kelly, G.; Granot, D. Mesophyll photosynthesis and guard cell metabolism impacts on stomatal behaviour. New Phytol. 2014, 203, 1064–1081. [Google Scholar] [CrossRef]
- Naeem, M.; Naeem, M.S.; Ahmad, R.; Ahmad, R.; Ashraf, M.Y.; Ihsan, M.Z.; Nawaz, F.; Athar, H.-R.; Ashraf, M.; Abbas, H.T.; et al. Improving drought tolerance in maize by foliar application of boron: Water status, antioxidative defense and photosynthetic capacity. Arch. Agron. Soil Sci. 2018, 64, 626–639. [Google Scholar] [CrossRef]
- Naser, L.; Kourosh, V.; Bahman, K.; Reza, A. Soluble sugars and proline accumulation play a role as effective indices for drought tolerance screening in Persian walnut (Juglans regia L.) during germination. Fruits 2010, 65, 97–112. [Google Scholar] [CrossRef]
- García-Caparrós, P.; de Filippis, L.; Gul, A.; Hasanuzzaman, M.; Ozturk, M.; Altay, V.; Lao, M.T. Oxidative stress and antioxidant metabolism under adverse environmental conditions: A review. Bot. Rev. 2021, 87, 421–466. [Google Scholar] [CrossRef]
- Petrov, V.; Hille, J.; Mueller-Roeber, B.; Gechev, T.S. ROS-mediated abiotic stress-induced programmed cell death in plants. Front. Plant Sci. 2015, 6, 69. [Google Scholar] [CrossRef]
- Liao, T.; Wang, Y.; Xu, C.P.; Li, Y.; Kang, X.Y. Adaptive photosynthetic and physiological responses to drought and rewatering in triploid Populus populations. Photosynthetica 2018, 56, 578–590. [Google Scholar] [CrossRef]
- Li, W.-D.; Biswas, D.K.; Xu, H.; Xu, C.-Q.; Wang, X.-Z.; Liu, J.-K.; Jiang, G.-M. Photosynthetic responses to chromosome doubling in relation to leaf anatomy in Lonicera japonica subjected to water stress. Funct. Plant Biol. 2009, 36, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, J.; Liu, H.; Qiu, R.; Gao, Y.; Duan, A. Role of hydraulic signal and ABA in decrease of leaf stomatal and mesophyll conductance in Soil Drought-Stressed Tomato. Front. Plant Sci. 2021, 12, 653186. [Google Scholar] [CrossRef] [PubMed]
- Batool, M.; El-Badri, A.M.; Hassan, M.U.; Haiyun, Y.; Chunyun, W.; Zhenkun, Y.; Jie, K.; Wang, B.; Zhou, G. Drought stress in Brassica napus: Effects, tolerance mechanisms, and management strategies. J. Plant Growth Regul. 2022, 1–25. [Google Scholar] [CrossRef]
- Osório, M.L.; Osório, J.; Vieira, A.C.; Gonçalves, S.; Romano, A. Influence of enhanced temperature on photosynthesis, photooxidative damage, and antioxidant strategies in Ceratonia siliqua L. seedlings subjected to water deficit and rewatering. Photosynthetica 2011, 49, 3–12. [Google Scholar] [CrossRef]
- do Rosário Rosa, V.; Farias Dos Santos, A.L.; Da Alves Silva, A.; Peduti Vicentini Sab, M.; Germino, G.H.; Barcellos Cardoso, F.; de Almeida Silva, M. Increased soybean tolerance to water deficiency through biostimulant based on fulvic acids and Ascophyllum nodosum (L.) seaweed extract. Plant Physiol. Biochem. 2021, 158, 228–243. [Google Scholar] [CrossRef]
- Akitha Devi, M.K.; Giridhar, P. Variations in physiological response, lipid peroxidation, antioxidant enzyme activities, proline and isoflavones content in soybean varieties subjected to drought stress. Proc. Natl. Acad. Sci. India Sect. B Boil. Sci. 2015, 85, 35–44. [Google Scholar] [CrossRef]



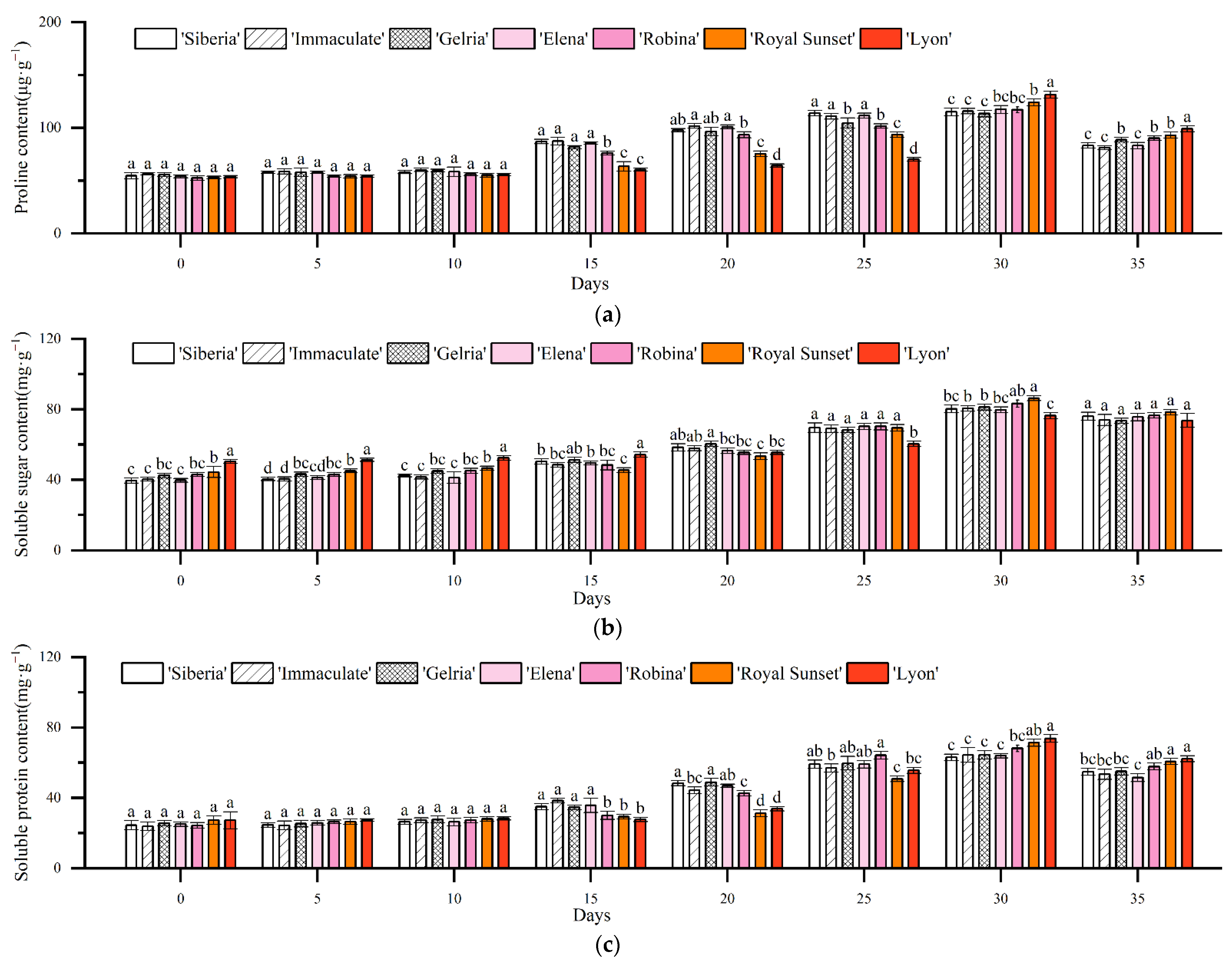
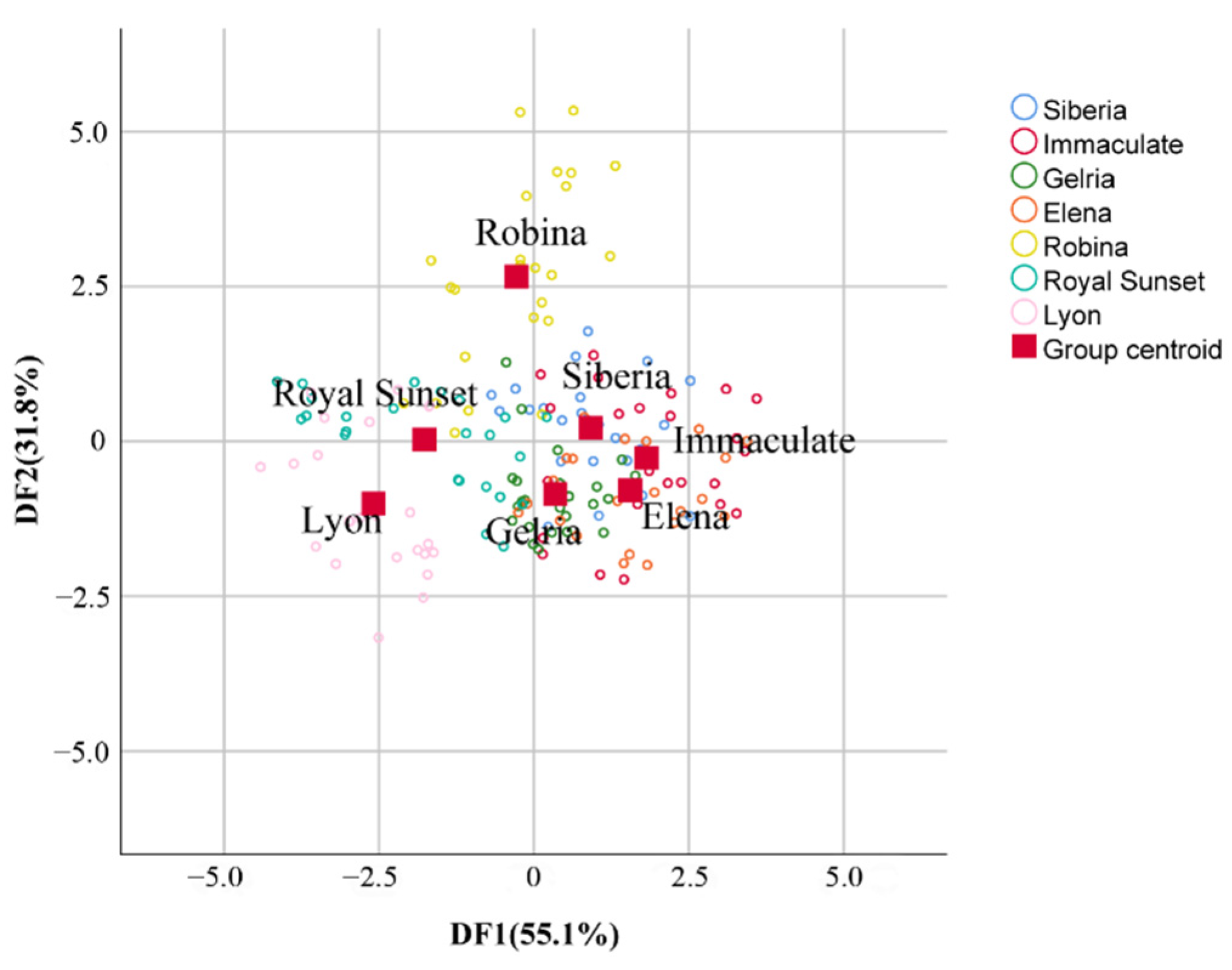

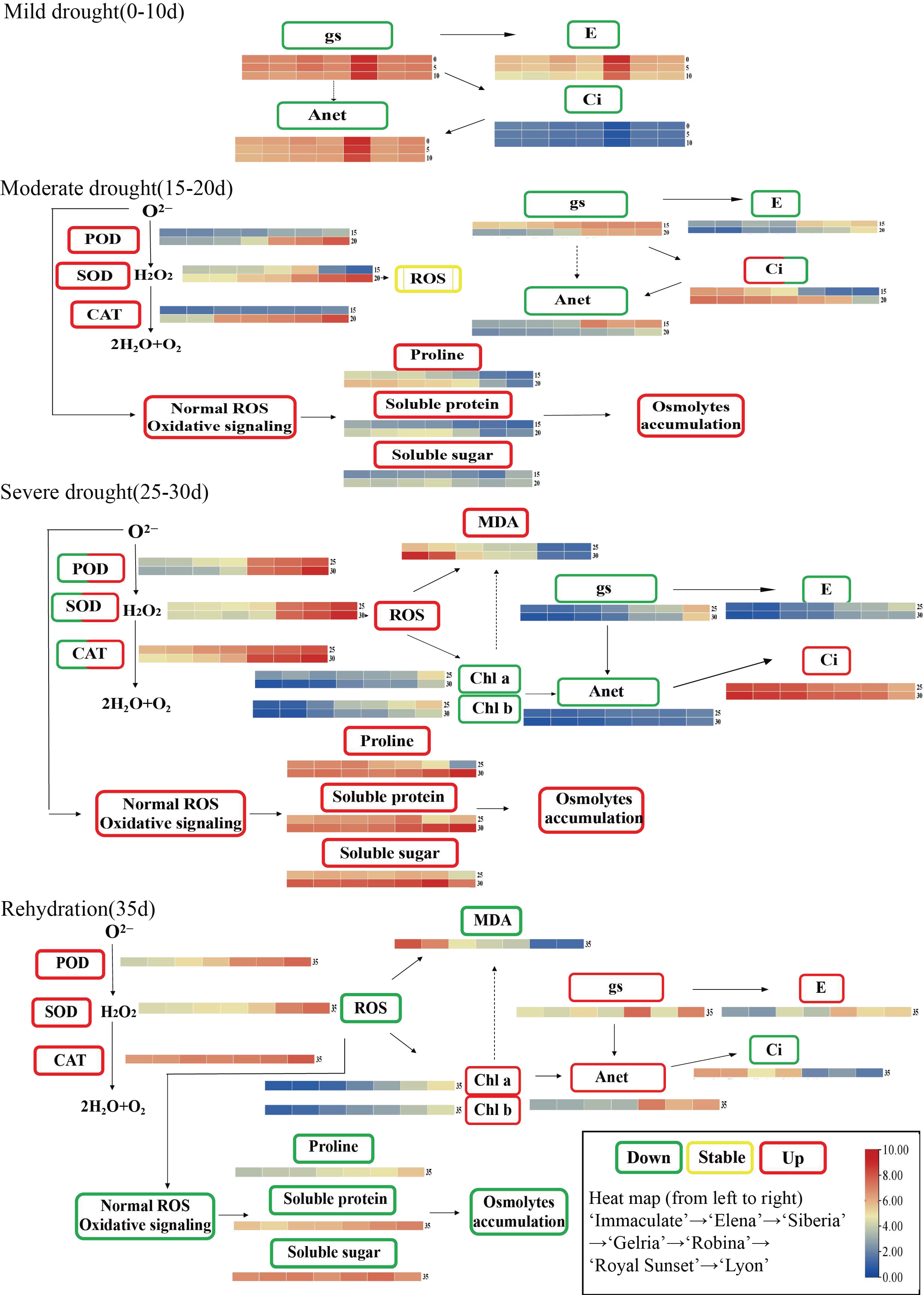
| 0 d | 5 d | 10 d | 15 d | 20 d | 25 d | 30 d | 35 d | |
|---|---|---|---|---|---|---|---|---|
| GWC (%) | 19.67 | 14.86 | 13.11 | 10.12 | 8.98 | 7.06 | 5.67 | 20.34 |
| RWC (%) | 84.78 | 64.05 | 56.51 | 43.62 | 38.71 | 30.43 | 24.44 | 87.67 |
| Varieties | Leaf Length (cm) | Leaf Width (cm) | Single Leaf Area (cm2) | Specific Leaf Area (cm2·g−1) | Total Leaf Area (cm2) |
|---|---|---|---|---|---|
| ‘Siberia’ | 10.71 ± 0.50 d | 2.27 ± 0.05 d | 16.33 ± 0.53 c | 114.46 ± 20.63 ab | 1148.30 ± 54.22 c |
| ‘Immaculate’ | 10.79 ± 0.58 c | 2.49 ± 0.06 c | 18.56 ± 0.50 b | 123.01 ± 9.82 ab | 1236.83 ± 43.93 b |
| ‘Gelria’ | 10.55 ± 0.62 d | 1.41 ± 0.03 e | 11.58 ± 0.80 d | 129.48 ± 38.72 a | 817.86 ± 27.39 e |
| ‘Elena’ | 10.79 ± 0.58 d | 2.55 ± 0.07 b | 17.55 ± 0.68 c | 117.04 ± 18.87 ab | 1209.21 ± 49.55 b |
| ‘Robina’ | 14.28 ± 0.65 a | 2.61 ± 0.09 a | 25.61 ± 0.50 a | 103.72 ± 22.06 c | 1826.84 ± 49.09 a |
| ‘Royal Sunset’ | 12.44 ± 0.67 b | 1.22 ± 0.04 f | 11.30 ± 0.46 d | 101.58 ± 15.75 c | 1138.22 ± 60.03 c |
| ‘Lyon’ | 9.37 ± 0.52 e | 0.85 ± 0.05 g | 6.38 ± 0.33 e | 81.31 ± 15.03 d | 992.79 ± 21.23 d |
| Varieties | Blade Thickness (μm) | Palisade Tissue Thickness (μm) | Spongy Tissue Thickness (μm) | Palisade Tissue/Spongy Tissue | Upper Epidermis Thickness (μm) | Lower Epidermis Thickness (μm) |
|---|---|---|---|---|---|---|
| ‘Siberia’ | 452.65 ± 11.24 c | 61.4 ± 4.29 c | 306.61 ± 10.53 b | 0.20 ± 0.01 cd | 66.13 ± 2.39 b | 46.23 ± 3.05 bc |
| ‘Immaculate’ | 446.88 ± 11.38 c | 46.44 ± 3.67 d | 298.15 ± 12.54 b | 0.16 ± 0.01 e | 65.15 ± 3.36 b | 49.57 ± 2.32 ab |
| ‘Gelria’ | 330.04 ± 9.86 e | 61.5 ± 4.19 c | 182.57 ± 6.91 d | 0.34 ± 0.04 a | 23.87 ± 1.29 c | 36.08 ± 2.81 d |
| ‘Elena’ | 407.86 ± 9.69 d | 53.3 ± 4.48 cd | 254.80 ± 8.14 c | 0.21 ± 0.02 c | 63.27 ± 3.68 b | 34.98 ± 1.87 d |
| ‘Robina’ | 484.25 ± 8.47 b | 86.14 ± 3.88 a | 280.81 ± 11.17 bc | 0.31 ± 0.02 b | 77.10 ± 3.57 a | 42.74 ± 1.92 c |
| ‘Royal Sunset’ | 615.74 ± 12.28 a | 77.80 ± 5.41 ab | 437.49 ± 12.23 a | 0.18 ± 0.02 de | 64.46 ± 3.18 b | 37.97 ± 3.15 d |
| ‘Lyon’ | 638.25 ± 11.97 a | 75.83 ± 3.96 b | 453.48 ± 11.96 a | 0.17 ± 0.00 e | 77.98 ± 2.38 a | 51.68 ± 4.85 a |
| Days | Siberia | Immaculate | Gelria | Elena | Robina | Royal Sunset | Lyon | |
|---|---|---|---|---|---|---|---|---|
| Chla | 30 | 0.209 | 0.148 | 0.401 | 0.177 | 0.479 | 0.464 | 0.697 |
| 35 | 0.256 | 0.213 | 0.390 | 0.210 | 0.524 | 0.675 | 0.815 | |
| Chlb | 30 | 0.366 | 0.202 | 0.497 | 0.198 | 0.495 | 0.633 | 0.731 |
| 35 | 0.396 | 0.225 | 0.494 | 0.266 | 0.566 | 0.703 | 0.846 | |
| CAT | 30 | 0.220 | 0.104 | 0.362 | 0.139 | 0.801 | 0.811 | 0.918 |
| 35 | 0.434 | 0.186 | 0.373 | 0.219 | 0.452 | 0.533 | 0.820 | |
| SOD | 30 | 0.042 | 0.087 | 0.108 | 0.107 | 0.707 | 0.806 | 0.941 |
| 35 | 0.216 | 0.085 | 0.198 | 0.108 | 0.324 | 0.695 | 0.811 | |
| POD | 30 | 0.066 | 0.030 | 0.200 | 0.048 | 0.691 | 0.741 | 0.918 |
| 35 | 0.280 | 0.075 | 0.401 | 0.124 | 0.693 | 0.717 | 0.866 | |
| MDA | 30 | 0.508 | 0.067 | 0.632 | 0.168 | 0.652 | 0.977 | 0.984 |
| 35 | 0.503 | 0.035 | 0.588 | 0.193 | 0.625 | 0.978 | 0.978 | |
| gs | 30 | 0.117 | 0.029 | 0.219 | 0.048 | 0.465 | 0.478 | 0.943 |
| 35 | 0.302 | 0.169 | 0.088 | 0.089 | 0.924 | 0.144 | 0.778 | |
| E | 30 | 0.444 | 0.171 | 0.496 | 0.217 | 0.757 | 0.853 | 0.847 |
| 35 | 0.548 | 0.173 | 0.380 | 0.119 | 0.929 | 0.712 | 0.770 | |
| Ci | 30 | 0.190 | 0.064 | 0.413 | 0.162 | 0.432 | 0.503 | 0.852 |
| 35 | 0.358 | 0.069 | 0.176 | 0.084 | 0.850 | 0.744 | 0.909 | |
| Anet | 30 | 0.353 | 0.269 | 0.527 | 0.311 | 0.619 | 0.624 | 0.794 |
| 35 | 0.198 | 0.139 | 0.180 | 0.145 | 0.797 | 0.603 | 0.675 | |
| Proline | 30 | 0.227 | 0.254 | 0.162 | 0.317 | 0.304 | 0.585 | 0.879 |
| 35 | 0.178 | 0.085 | 0.408 | 0.174 | 0.469 | 0.590 | 0.845 | |
| Soluble protein | 30 | 0.202 | 0.284 | 0.289 | 0.258 | 0.513 | 0.705 | 0.854 |
| 35 | 0.384 | 0.290 | 0.399 | 0.172 | 0.580 | 0.767 | 0.868 | |
| Soluble sugar | 30 | 0.432 | 0.454 | 0.503 | 0.397 | 0.645 | 0.869 | 0.159 |
| 35 | 0.668 | 0.483 | 0.441 | 0.626 | 0.709 | 0.867 | 0.456 | |
| Average membership function | 0.311 | 0.169 | 0.359 | 0.195 | 0.615 | 0.684 | 0.806 | |
| Rank | 5 | 7 | 4 | 6 | 3 | 2 | 1 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Jia, W.; Zheng, J.; Ma, L.; Duan, Q.; Du, W.; Cui, G.; Wang, X.; Wang, J. Application of Morphological and Physiological Markers for Study of Drought Tolerance in Lilium Varieties. Horticulturae 2022, 8, 786. https://doi.org/10.3390/horticulturae8090786
Li X, Jia W, Zheng J, Ma L, Duan Q, Du W, Cui G, Wang X, Wang J. Application of Morphological and Physiological Markers for Study of Drought Tolerance in Lilium Varieties. Horticulturae. 2022; 8(9):786. https://doi.org/10.3390/horticulturae8090786
Chicago/Turabian StyleLi, Xiang, Wenjie Jia, Jie Zheng, Lulin Ma, Qing Duan, Wenwen Du, Guangfen Cui, Xiangning Wang, and Jihua Wang. 2022. "Application of Morphological and Physiological Markers for Study of Drought Tolerance in Lilium Varieties" Horticulturae 8, no. 9: 786. https://doi.org/10.3390/horticulturae8090786
APA StyleLi, X., Jia, W., Zheng, J., Ma, L., Duan, Q., Du, W., Cui, G., Wang, X., & Wang, J. (2022). Application of Morphological and Physiological Markers for Study of Drought Tolerance in Lilium Varieties. Horticulturae, 8(9), 786. https://doi.org/10.3390/horticulturae8090786








