Abstract
Drought-tolerant plant growth-promoting rhizobacteria (PGPR) may promote plant development under limited water supply conditions, when plant’s water demand is not completely satisfied under rain-fed conditions or when irrigation water availability is limited. The aim of this study was to examine the effects of two inoculation treatments (B2: Alcaligenes sp. 3573, Bacillus sp. BAR16, and Bacillus sp. PAR11 strains and B3: Pseudomonas sp. MUS04, Rhodococcus sp. BAR03, and Variovorax sp. BAR04 strains) and compare those to a control (B0) without artificial inoculation on chlorophyll fluorescence, leaf chlorophyll content (SPAD value), canopy temperature, and the yield of the processing tomato cultivar H-1015 F1 grown under field conditions. The young seedlings of the hybrid tomato variety H-1015 F1 were immersed in 1% of B2 or B3 products (BAY-BIO, Szeged Hungary) for 5 min. Inoculated and untreated seedlings were grown under three irrigation treatments [regular irrigation (RI), deficit irrigation (DI), and no irrigation (I0)], to reveal the effect of PGPR under different levels of water stress. In the dry year (2018), higher canopy temperature and chlorophyll fluorescence (Fv/Fm) were measured during flowering in plants treated with bacteria than in untreated plants. In the stage of flowering and fruit setting, the B3 treatment led to a significant decrease in the Fv/Fm value, canopy temperature remained high, and the SPAD value was statistically the same in all treatments. Under limited water supply, in most cases, PGPR led to a significantly greater total yield but more unripe green berries compared to untreated plants. Under moderate water shortage (dry year + deficit irrigation), the B3 treatment resulted in 26% more ripe, marketable fruit and 49% less unripe fruit compared to the B2 treatment. On the other hand, in the wet year (2020), the bacterial treatments generally did not affect physiological properties, though the B2 treatment produced a higher marketable yield while the amount of green and diseased fruits did not differ statistically, compared to the B3 treatment under deficit irrigation. Based on our study, we recommend the application of the B3 PGPR product as it positively affected key physiological processes, leading to a higher marketable yield particularly under water shortage.
1. Introduction
Tomato (Lycopersicon esculentum Mill.) is a worldwide strategic crop. Recently, there has been an increase in the consumption and production of various tomato products. The growth and quality of tomato are influenced by several factors such as genotype, maturity stage, environmental factors and nutrient and water supply conditions. The greatest factor that limits yield in field-grown tomato is water shortage, which is increasingly prevalent due to ongoing climate change. The adverse effect of such factors on yield can be managed by adopting appropriate irrigation techniques, the use of bio-fertilizers, and the use of drought-resistant varieties. The application of different irrigation model systems provides the possibility to determine irrigation timing and irrigation water dose for crops [1,2,3,4], though this process requires knowledge of the response of the varieties to water-deficit stress. Under water shortage, plants respond by decreasing water loss as a result of stomata closing, which reduces transpiration as well as the inflow and delivery of atmospheric CO2 to the cells [5] but increases stomata resistance, therefore influencing the efficacy of water consumption [6]. Transpiration efficiency (TE) is an important factor in water use efficiency (WUE) and is defined as the quantity of water used (WU) or the evaporation devoted to biomass production [7,8]. Under water shortage, a significant positive correlation was found between fruit yield per plant and TE in sesame plants [9] and between WU, yield, biomass, and WUE in green bean [6].
Under water shortage, plant growth is compromised following the decrease in transpiration, the increase in canopy temperature, low stomata conductance [10] and reduced chlorophyll fluorescence and photosynthesis activity [11]. Photosynthetic activity can be assessed through the effectiveness of photochemical systems (PSI and PSII) and by measuring leaf photosynthetic pigments (e.g., chlorophyll content). The maximum photochemical quantum quantity (Fv/Fm) of the PSII photochemical system, as chlorophyll fluorescence, is indicative of the degree to which photosynthesis is undisturbed [12]. Under dry conditions, the quantity of photosynthetic pigments and chlorophyll fluorescence (Fv/Fm) change, suggesting their importance for assessing drought tolerance in different tomato genotypes [13,14].
Tomato is generally considered to have a high water demand [15], though it shows different levels of sensitivity during development. If water shortage occurs in the early phases of flowering, it causes flower drop [16]; if it occurs during fruit setting, then smaller berries are formed [10]; and although the quantity of marketable fruit will be less, their soluble dry matter content would be higher under water abundance [17]. Yield loss associated with drought stress can reach 40–60% in cultivated crops [18]. Under such stress conditions, the distribution and extent of the root system in dry soil are severely compromised, thereby inhibiting water and nutrient uptake [9,19]. Genotypes with deep-reaching, developed root systems or that are able to develop an interaction with the microorganisms living in the soil may be able to withstand periods of dryness.
Recently, interest in soil microbes has significantly increased, with studies examining the role of various bacteria in plant development and the reactions to salt and water stress. In the ScienceDirect database (www.sciencedirect.com, accessed on 1 June 2022), using soil microbe as keywords, the number of published research items has increased sharply from 1271 to 7974 during the last decade (2012–2022), suggesting the importance of such factors for sustainable agriculture under changing climatic conditions. In the rhizosphere, fungi, bacteria form the largest group of soil microorganisms which interact with plants fine tuning, and therefore key plant physiological processes [20]. Low soil moisture levels (10–14%) stimulate increased root hair density and length compared to other soil moisture levels, depending mainly on genetic [21] and hormonal regulation [22,23,24]. The bacterial community alters the root’s hormonal state, improving not only the plant’s nutrient and water uptake and thereby its development, but also leading to the secretion of various biochemical substances on the root, which results in microbial colonization and a symbiotic relationship [25,26]. Under water shortage, these PGPRs increase the biosynthesis of indole-acetic acid (IAA) and abscisic acid (ABA), leading to a significant growth extension of roots [24,27]. Bacillus species specifically boosted the generation of antioxidant substances under salt stress [28]. Various microbes exhibited a significant role in mitigating the adverse effects of abiotic stress, which represents a new eco-friendly approach for sustainable agricultural production [29,30]. Rhizobacteria are used as bio-fertilizer, plant conditioners, plant stimulants, and bio-pesticides [30,31]. PGPR inoculation with four strains has been shown to help minimize fertilizer inputs by maintaining the photosynthetic efficiency and seed yield of durum wheat under especially dry conditions [32]. Tomato seedling inoculation with PGPR had a positive effect on the process of photosynthesis, resulting in enhanced chlorophyll fluorescence parameters due to increased evapotranspiration in the thylakoid membrane. Consequently, improvements in marketable fruit yield, biomass quantity, and water use efficiency were observed [33]. Additionally, other authors [34] noticed that certain PGPR products also increased the soluble dry matter (Brix°) and vitamin C content of tomato berries. Despite the fact that microbes have been successfully used in a number of plant species [35,36,37], to our knowledge, no bio-fertilizer provides a full range of nutrients or is suitable for all plant species under changing climatic conditions [38]. There is still a large gap between the potential of PGPR and its practical use as an organic fertilizer in plant production [39] as far as the assembly, composition, and structure of rhizospheric microbes; and plant–microbe interactions occurring under drought stress conditions remain mostly unknown [40]. There is also a considerable lack of knowledge not only about the physiological effects of PGPR preparations suitable for tomato production, but also suitable bacterial strain combinations and the ripening stage at which the treatment should be applied. Since the frequency of heatwaves and drought periods is increasing, and the availability of irrigation water is limited, stress-mitigating solutions should be researched and applied, such as the application of PGPRs, but its effect might vary under different water supply levels, so the research should cover that field as well.
The aim of the experiments was to examine the effect of new preparations containing different rhizobacterial strains on the physiological characteristics, productivity, and quality of processing tomatoes under different water supply conditions.
2. Materials and Methods
2.1. Research Materials and Planning
The tomato variety H-1015 F1 hybrid (Heinz Seeds Company, Germany) was used in the 2018 and 2020 growing seasons. The experimental plot was located at the horticultural experimental farm of the Hungarian University of Agriculture and Life Sciences in Gödöllő, Hungary. The H-1015 F1 tomato variety is a medium-sized plant with early ripeness (approx. 114 days), resistant to plant pathogens and characterized by long berries of 75–80 g as well as a high Brix° (6.2–6.8). The cultivation was performed in a slightly alkaline, clayey brown forest soil, containing 40% sand, 47.5% silt and 11.5% clay in the 0–60 cm soil layer, neutral in pH. Soil mineral content was: P2O5 281 mg kg−1, K2O 203 mg kg−1, and NH4 2.5 mg kg−1 with humus content of 1.6%. Sowing was performed in a greenhouse using a Klasmann TS3 (Klasmann-Deilmann GmbH, Geeste, Germany) substrate. Before being transplanted to the field, the four-week-old seedlings were inoculated with a PGPR product and then grown in the field.
2.2. Rhizobacteria Inoculation
The seedlings of the hybrid tomato variety H-1015 F1 were treated with a mixture of two bacterial strains (colony-forming unit: 109 CFU/mL) provided by Zoltán Bay Research Institute (BAY-BIO, Szeged, Hungary) right before the transplantation to the field. Two bacterial treatments were used: the B2 treatment consisting of Alcaligenes sp. 3573, Bacillus sp. BAR16, and Bacillus sp. PAR11 strains, the B3 treatment consisting of Pseudomonas sp. MUS04, Rhodococcus sp. BAR03, and Variovorax sp. BAR04 strains and the untreated plants (control) were marked as B0. Before planting, the seedlings were immersed for 5 min in 20 litres of water containing 1% of bacterial solution. Seedlings were transplanted in a plant density of ~35,000 plants ha−1, meaning single row distance of 150 cm and 19 cm distance between plants. The two factors (irrigation and PGPR inoculation) were designed in split-blocks. The size of each plot was 75 m2. Samples were taken randomly from each plot in 4 replications. One replication consisted of 10 plants.
2.3. Climatic Data and Irrigation Treatments during Tomato Development
Three irrigation treatments were applied: regular irrigation (RI), where the evapotranspiration (ETc 100%) amount was replenished; deficit irrigation (DI), where only 50% of the ETc value was replenished to provide a moderate water shortage; and the I0 treatment, where ETc was not replenished. Under I0 treatment, the plants received natural precipitation during development along with nutrients after transplantation. The software AquaCrop 6.0 (FAO Rome, Italy) was used to calculate the RI, i.e., the plant evapotranspiration (ETc) value. A drip irrigation system was used to provide the necessary water.
Based on the precipitation quantities and temperatures of the years, 2018 can be considered to be moderately dry and 2020 to be wet (Table 1). In 2018, temperatures were relatively higher during tomato development than in 2020, with a significant difference in precipitation abundance. In 2018, a significant amount of rain fell prior to flowering; however, comparatively small amount of rain fell during flowering (ST1) and fruit setting and fruit development (ST2 and ST3, respectively) and the temperatures that rose above 30 °C impacted yield. In contrast, with the exception of a few days, 2020 was characterized by an even distribution of precipitation and temperatures of 30 °C during the development phase (ST1–ST3) (Figure 1).

Table 1.
Temperature (°C), precipitation (mm) and irrigation (mm) during the 2018 and 2020 growing years under deficit (DI), regular (RI) and no-irrigation (I0) conditions.
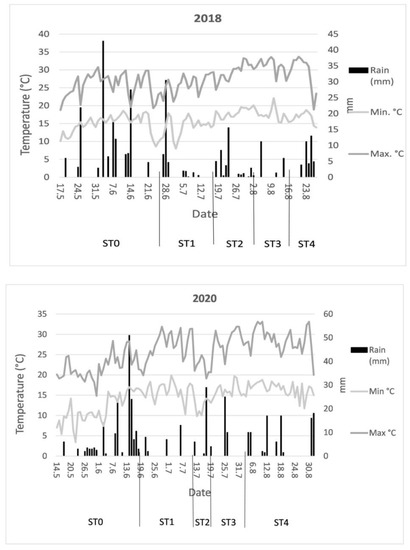
Figure 1.
Temperature (°C) and precipitation (mm) data recorded during the growth stages of the hybrid tomato variety H-1015 F1: ST0 = plantation–flowering, ST1 = flowering, ST2 = flowering and fruit setting, ST3 = early fruit development, and ST4 = fruit ripening.
2.4. Field Measurements
Ten plants were selected in each plot to physiological properties measurement. Leaf chlorophyll content, chlorophyll fluorescence, and canopy temperature were measured on four occasions between 10:00 a.m. and 1:00 p.m. using selected plants, at the beginning of flowering (ST1), during flowering and fruit setting (ST2), during early fruit development (ST3), and during fruit ripening (ST4). Leaf chlorophyll content was measured with a portable chlorophyll meter (SPAD 502 Konica Minolta, Warington, UK). The instrument measures leaf photosynthetic light adsorption percentage of leaves in red and near-infrared range, with the specified calculated SPAD value correlating to the leaf chlorophyll content. Chlorophyll fluorescence was measured using a PAM 2500 (Heinz, Walz, GmbH, Effeltrich, Germany) portable chlorophyll fluorometer. The fluorometer measures initial (F0) and maximum (Fm) fluorescence in samples adjusted against the dark. Chlorophyll fluorescence, expressed as the percentage of Fv/Fm, was specified using the following formula:
where F0 = initial fluorescence, Fm = maximum fluorescence, and Fv = variable fluorescence (Fm − F0).
Canopy temperature was measured using a Raytek MX4 (Raytek Corporation, Santa Cruz, CA, USA) portable infrared thermometer, simultaneously with the SPAD and chlorophyll fluorescence measurements.
2.5. Yield Analysis
The harvested fruits of the specified plants were weighed and sorted in different groups: group 1 (healthy, red-ripe marketable fruits), group 2 (healthy, green-unripe fruits, and group 3 (rotten, damaged and unmarketable fruits). The soluble dry matter content of the red-ripe, marketable fruits was measured using a Krüss DR201-95 handheld refractometer (A. Krüss Optronic GmbH, Hamburg, Germany) and expressed as degrees Brix (°Brix).
2.6. Statistical Analysis
The data were analyzed using one-way and two-way analysis of variance using SPSS 20 (IBM Hungary Ltd., Budapest, Hungary) Windows software. The homogeneity of the variance was assessed using Levene’s test. Duncan’s multi-range test was also used for means separation among treatment at the p ˂ 0.05 level.
3. Results
Previously, we demonstrated that water shortage occurring during tomato plant’s generative phase, from flowering to early berry ripening, negatively affected water consumption and photosynthesis-related processes [11] as well as fruit quality. In a moderately dry growing season (2018), canopy temperature and chlorophyll fluorescence were higher with respect to the wet and humid growing season (2020). Nevertheless, leaf relative chlorophyll content (SPAD values) (Figure 2, Figure 3 and Figure 4) remained unaffected by the growing seasons. The effect of PGPR products varied with the development stages and climatic conditions of the growing years. Under dry conditions (2018 growing season), the canopy temperature of untreated plants was the lowest (26 °C) during flowering (ST1) but increased significantly followed by the B2 and B3 treatments, attaining values ≥30 °C at the early fruit development stage (ST3) particularly following the B3 treatment (Figure 2). In the wet growing year (2020), the low canopy temperature (22.5–23.7 °C) was recorded during flowering (ST1) increased by 13–15% during the flowering and fruit setting (ST2) as well as the early fruit development stages (ST3). Under these particular conditions, the effect of the bacterial treatment on canopy temperature was not revealed statistically (Figure 2).
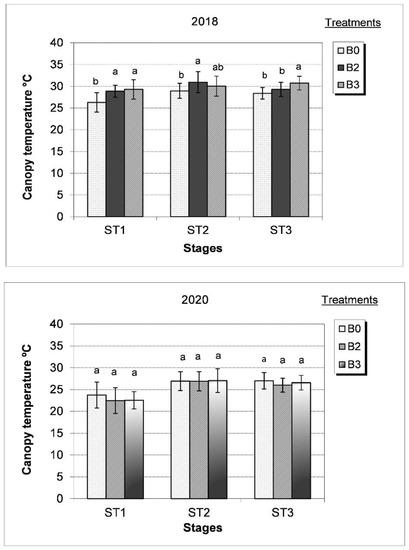
Figure 2.
Effect of different bacterial treatments (B0 = control, B2 = Alcaligenes sp. 3573, Bacillus sp. BAR16, and Bacillus sp. PAR11 strains and B3 = Pseudomonas sp. MUS04, Rhodococcus sp. BAR03, and Variovorax sp. BAR04 strains) on canopy temperature during development stages (ST1 = during flowering, ST2 = flowering with fruit setting, and ST3 = early fruit development) in the dry (2018) and wet (2020) growing years. Values represent the mean ± SD of four replicates. Bars marked with different letters are significantly different at the p ˂ 0.05 level.
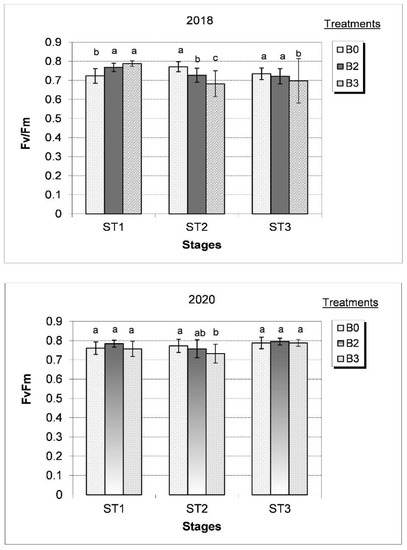
Figure 3.
Effect of different bacterial treatments (B0 = control, B2 = Alcaligenes sp. 3573, Bacillus sp. BAR16, and Bacillus sp. PAR11 strains and B3 = Pseudomonas sp. MUS04, Rhodococcus sp. BAR03, and Variovorax sp. BAR04 strains) on chlorophyll fluorescence (Fv/Fm) during development stages (ST1 = during flowering, ST2 = flowering with fruit setting, and ST3 = early fruit development) in the dry (2018) and wet (2020) growing years. Values represent the mean ± SD of four replicates. Bars marked with different letters are significantly different at the p ˂ 0.05 level.
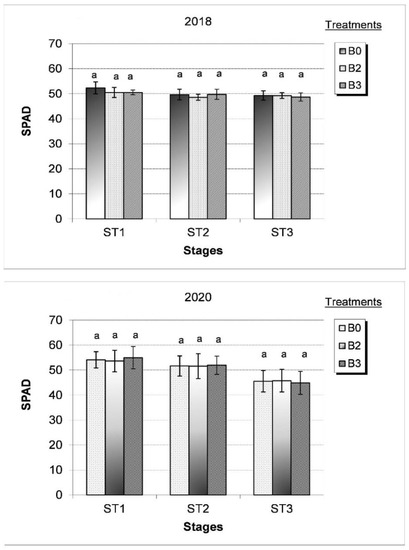
Figure 4.
Effect of different bacterial treatments (B0 = control, B2 = Alcaligenes sp. 3573, Bacillus sp. BAR16, and Bacillus sp. PAR11 strains and B3 = Pseudomonas sp. MUS04, Rhodococcus sp. BAR03, and Variovorax sp. BAR04 strains) on SPAD values during development stages (ST1 = during flowering, ST2 = flowering with fruit setting, and ST3 = early fruit development) in the dry (2018) and wet (2020) growing years. Values represent the mean ± SD of four replicates. Bars marked with different letters are significantly different at the p ˂ 0.05 level.
Photosynthesis remains unchanged and plant development is not impeded in the case of high chlorophyll fluorescence (Fv/Fm) and low SPAD value. Under dry growing conditions, leaf chlorophyll content decreased, leading to reduced light absorption (i.e., the utilization of light energy), increased light reflection, and a decreased Fv/Fm ratio. In a moderately dry growing season (2018), B2- and B3-treated plants exhibited a significant increase in chlorophyll fluorescence (0.77–0.79 Fv/Fm) during tomato flowering (ST1), which subsequently decreased significantly during the flowering and fruit setting phase (ST2). The largest decrease in Fv/Fm values was measured in B3-treated plants with respect to untreated plants (Figure 3).
In the wet growing year (2020), photosynthesis was well balanced from flowering until early berry development (ST1–ST3) with a Fv/Fm value between 0.76 and 0.79 in untreated plants (Figure 3). Under these conditions, the Fv/Fm value was not significantly affected by bacterial treatments, except for B3-treated plants, where values decreased significantly during the flowering and fruit setting (ST2) stage. Regardless of the changes in climatic conditions between the two years, the highest chlorophyll fluorescence (Fv/Fm) decrease was measured during flowering and fruit setting (ST2) in the B3 treatment, thus influencing photosynthetic activity and the ripening process.
Under moderate dry growing conditions (2018), the leaf SPAD index ranged between 49 and 52 during the ST1–ST3 phase. The SPAD value measured in H 1015 F1 tomato plants with bacterial treatment was only 4% less than values in untreated plants during flowering stage (ST1) and remained unchanged with advancing maturity (Figure 4). However, in the wet growing year (2020), the SPAD index gradually decreased during the ST1–ST3 phase. The SPAD value was the highest during flowering (54), 6% lower during flowering and fruit setting (ST2), and reached its lowest value (45 SPAD) during early fruit ripening (ST3); however, the effects of the bacteria treatment were not statistically significant (Figure 4).
Results indicate that during the wet growing year, the effect of PGPR was not obvious on physiological properties during plant’s generative phase. However, the impact was with differing extent during the dry growing year and depending on water supply (Figure 5). Under water shortage (I0) associated with moderately dry growing conditions (2018), the canopy temperature of untreated plants (B0) was high and significantly decreased by irrigation. Without irrigation (I0), canopy temperature remained unchanged around 30 °C and was not significantly influenced by PGPR treatments but under irrigated conditions, (DI, RI) the canopy temperature of bacterial treatments significantly increased with respect to untreated plants (Figure 5a).
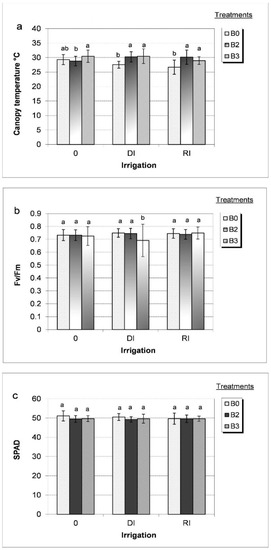
Figure 5.
Effect of different bacterial treatments (B0 = control, B2 = Alcaligenes sp. 3573, Bacillus sp. BAR16, and Bacillus sp. PAR11 strains and B3 = Pseudomonas sp. MUS04, Rhodococcus sp. BAR03, and Variovorax sp. BAR04 strains) on canopy temperature (a), chlorophyll fluorescence (b) and SPAD (c) from flowering to early fruit development under different water supplies (I0 = non irrigation, DI = deficit irrigation and RI = regular irrigation) during the dry growing year (2018). Values represent the mean ± SD of four replicates. Bars marked with different letters are significantly different at the p ˂ 0.05 level.
Regardless of water supply and in the dry growing season, chlorophyll fluorescence ranged from 0.73 to 0.75 Fv/Fm during the generative phase and was not significantly affected by bacterial treatments, except for B3, which caused a significant decrease in chlorophyll fluorescence (0.69 Fv/Fm) during deficit irrigation (Figure 5b). A similar result was observed during flowering and fruit setting (ST2), indicating that, at this stage, water shortage (DI) and bacterial treatments significantly affected photosynthetic activity. In the dry year growing year (2018), during flowering (ST1) and under non-irrigated (I0) conditions, the leaf SPAD value was slightly reduced in the treatments treated with PGPR, with no significant difference between PGPR-treated and untreated plants for leaf SPAD values during the generative stage or under irrigated (DI, RI) conditions (Figure 4 and Figure 5c).
Under moderate water shortage (dry year + deficit irrigation), PGPR treatments (B2, B3) significantly affected plant water balance. Canopy temperature increased as transpiration decreased, and photosynthetic activity (Fv/Fm) was satisfactory with the exception of B3, as reflected in the crop’s quality traits. Under water shortage, PGPR treatments significantly increased fruit quantity compared to untreated plants, though the percentage of healthy green fruits increased considerably among the distribution of the fruit types (Table 2). Depending on the degree of water shortage and different bacterial formulations was associated with different effects on the crop.

Table 2.
Effects of different water supply (I0 = non irrigation, DI = deficit irrigation and RI = regular irrigation) and bacterial treatments (PGPR) (B0 = control, B2 = Alcaligenes sp. 3573, Bacillus sp. BAR16, and Bacillus sp. PAR11 strains and B3 = Pseudomonas sp. MUS04, Rhodococcus sp. BAR03, and Variovorax sp. BAR04 strains) on the total yield (TY, t.ha−1), marketable yield (MY, t.ha−1), green yield (GY, t.ha−1), diseased/unmarketable yield (DY, t.ha−1) and green biomass (GBm, t.ha−1) as well as soluble solids (°Brix) of the processing tomato variety H-1015 F1 grown during the dry (2018) and wet (2020) growing years.
In the dry growing season and I0 treatment, B3-treated plants produced 32% less green berries than those B2-treated plants. A significant difference between the PGPR treatments under moderate water shortage (dry year + deficit irrigation) was noticed regarding green fruit yields. In fact, B3 treatment resulted in 26% more ripe marketable fruits and 49% less unripe fruits compared to the B2 treatment.
Soluble solid content was the highest under dry conditions (I0) and decreased significantly with irrigation. In the moderately dry growing year (2018), PGPR treatments had no effect on the Brix° value, though both water supply and PGPR treatments exhibited a negative impact under wet growing year (2020) (Table 2).
In the wet growing year (2020), there was a significant difference between the PGPR treatments. In fact, the B2 treatment resulted in a greater marketable yield and a higher Brix° value, but with higher percentage of diseased fruit compared to B3-treated plants and this effect was more pronounced under regular irrigation (RI) (Table 2).
4. Discussion
The frequency of high temperatures and dry periods caused by climate change negatively affects the yield of major field-grown horticultural crops. It is widely recognized that yield loss can be mitigated by irrigation management and bio-fertilizer application. However, limited bacterial strains establish a symbiotic relationship with horticultural crops. PGPR excretes various phytohormones and osmolytes which contribute to improve plant stress tolerance by altering key physiological processes such as stomata closing, transpiration and photosynthesis [10,22,41,42,43,44]. Nevertheless, the determination of the suitable application period of the PGPR treatment during plant development might be crucial for efficient output.
During tomato development stages, the PGPR treatments indirectly affected canopy temperature and chlorophyll fluorescence. In a moderate dry growing year, canopy temperature and chlorophyll fluorescence (Fv/Fm) significantly increased prior to flowering in the B2 and B3 treatments, compared to the untreated control plants. However, during flowering and fruit setting stages, chlorophyll fluorescence (Fv/Fm) underwent the greatest decrease in B3-treated plants, regardless of the considered growing year (dry or humid). Similar results were reported previously as a result of mycorrhiza inoculation, where chlorophyll fluorescence (Fv/Fm) increased in tomato until flowering and fruit setting but decreased with advancing maturity stages, especially under dry growing conditions [45,46]. Some PGPR strains (Bacillus safensis (FV46), and Brevibacillus lateosporus (C9F)) reduced the Fv/Fm value compared to control according to the finding of another study [47], which is similar to our experiment in 2018 (ST2 and ST3) and in 2020 (ST2).
Tahir et al. [48] found that PGPR strains increased relative chlorophyll content (SPAD) in leaves under good water supply and dry growing conditions compared to untreated plants. Bacillus strains were reported to improve plant growth by affecting physiological processes, such as increasing plant relative water and chlorophyll content under drought stress conditions [49,50] and increasing chlorophyll production in maize during salt stress [51]. Phylazonit bio-fertilizer containing Bacillus strains resulted in significant increases in SPAD values in Uno Rosso F1 tomato cultivar with respect to untreated plants [45]. In contrast, our results showed that the chlorophyll content of PGPR-treated H-1015 F1 tomato plants remained unchanged during the phenological stages under different water supply conditions. The different values are likely attributed to the different interactions of tomato cultivars X PGPR strains.
Altered photosynthesis-related traits such as leaf chlorophyll content and chlorophyll fluorescence arising from water shortage during flowering and fruit setting affected plant development, tomato berry weight, and consequently marketable yield [11]. The physiological processes underlying plant tolerance to water shortage and the deleterious effects on yield depend on various factors such as the considered plant species, the PGPR strain [51,52] and the developmental stage at which their application is more efficient.
Pseudomonas species were found to be particularly suitable during tomato flowering and fruit setting stages [53] while the beneficial effects of Bacillus bacteria were mostly reported for soybean [54]. Bacillus-based bio-fertilizer increased tomato berry weight under moderate water shortage [45]. Results indicated that under moderate water shortage (dry year + deficit irrigation) occurring during the tomato flowering to early fruit development stages, PGPR treatments resulted in improved water balance in H-1015 F1 tomato while photosynthetic activity remained unchanged. The application of the B3 treatment under these conditions led to a significant decrease in chlorophyll fluorescence and thus a reduction in photosynthetic activity. The B2 product contains Bacillus bacteria strains, leading to the highest photosynthetic activity, greater green mass, and an extended ripening process. This is in accordance with the findings of others, who reported that Bacillus-based products successfully forms colonies in tomato rhizosphere and contribute to improved growth and yield [55,56]. Root colonization and tomato plant growth were observed after 15 and 60 days, respectively [57].
The intense photosynthetic activity in leaves of B2-treated plants may have delayed the translocation of assimilates into the berries, which was reflected in the distribution of the fruit quality fractions and in a higher proportion of immature green berry formation. Under moderate water shortage (dry year + deficit irrigation), the B3 treatment resulted in greater quantities of marketable fruit and less green berries than the B2 treatment. This proves that under water shortage, plant physiological processes, yield quantities, and qualitative distribution are not only influenced by the bacterial composition of the applied products but also by their interaction with the host plant. Based on our results, under water shortage, both B2- and B3-treated plants produce better yields and more healthy green berries than untreated plants (Table 2). A significant difference could be detected between the two bacterial treatments under moderate water shortage (dry year + deficit irrigation). In fact, the B3 treatment resulted in 26% more marketable and 49% less unripe fruit than the B2 treatment. Contrary to expectations, the PGPR treatments did not impact either the number of diseased berries or their soluble dry matter content (Brix°) under moderate water shortage. Other authors [34] reported that certain PGPR bio-fertilizers decreased the quantity of diseased tomato berries and improved the Brixº value.
In the humid growing year, plants treated with B2 produced a greater marketable yield and improved the Brix° value but also lead to more rotten berries than in B3-treated plants. The results showed that the B2 bio-fertilizer, containing Alcaligenes strains in addition to Bacillus, resulted in prolonged ripening and a greater quantity of unripe green fruits via regulation of key physiological processes. However, the bio-fertilizer B3, containing three different rhizobacteria strains, resulted in more concentrated ripening stages and less unripe green fruits under water shortage conditions.
5. Conclusions
Our results show that in the case of water shortage, PGPR products influence plant physiological processes and increase yield quantities. Under water shortage occurring during tomato flowering and fruit setting, PGPR products significantly affected canopy temperature, chlorophyll fluorescence (Fv/Fm), and plant water balance and photosynthesis. The quali-quantitative distribution of fruit depends on the composition of the applied products, variety X bacterial strain interaction, and the water supply regime. Under water shortage, the Bacillus-species-containing B2 product resulted in undisturbed photosynthesis, prolonged ripening, a higher percentage of unripe green fruit, and more diseased fruit in good water supply. The B3 product, which has a different composition, was found to be more effective than the B2 product because the decrease in chlorophyll fluorescence led to a drop in photosynthetic activity and accelerated ripening processes, which contributed to the generation of a greater marketable yield and less unripe green berries. Based on our data, we recommend immersing young tomato seedlings, prior to transplantation, in a 1% solution of B3 combination product consisting of Pseudomonas sp. MUS04, Rhodococcus sp. BAR03, and Variovorax sp. BAR04 strains to improve the efficiency of the treatment. In the dry year, the examined PGPR products did not influence the Brix° value of the tomato fruit but had a negative effect in the wet year.
Author Contributions
Conceptualization, E.N. and L.H.; investigations and analysis, K.Z.H., B.A. and A.N.; methodology, Z.P.; writing—draft preparation, E.N.; write—review and editing, L.H., R.I. and S.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the EFOP-3.6.3-VEKOP-16-2017-00008 project. This work was supported by the ÚNKP-21-4 New National Excellence Program of the Ministry for Innovation and Technology from the source of the National Research, Development and Innovation Fund.
Acknowledgments
The authors would like to thank the staff of the Horticultural research farm at Gödöllő and also to everyone who contributed to this research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Agbna, G.H.D.; Donglia, S.; Zhipeng, L.; Elshaikha, N.A.; Guangchenga, S.; Timm, L.C. Effects of deficit irrigation and biochar addition on the growth, yield, and quality of tomato. Sci. Hortic. 2017, 222, 90–101. [Google Scholar] [CrossRef]
- Du, Y.D.; Cao, H.-X.; Liu, S.-Q.; Gu, X.-B.; Cao, Y.-X. Response of yield, quality, water and nitrogen use efficiency of tomato to different levels of water and nitrogen under drip irrigation in Northwestern China. J. Integr. Agric. 2017, 16, 1153–1161. [Google Scholar] [CrossRef] [Green Version]
- Lorenzi, F.; Alfieri, S.M.; Monaco, E.; Bonfante, A.; Basile, A.; Patanè, C.; Menenti, M. Adaptability to future climate of irrigated crops: The interplay of water management and cultivars responses. A case study on tomato. Biosyst. Eng. 2017, 157, 45–62. [Google Scholar] [CrossRef]
- Takács, S.; Csengeri, E.; Pék, Z.; Bíró, T.; Szuvandzisev, P.; Palotás, G.; Helyes, L. Performance evaluation of AquaCrop model in processing tomato biomass, fruit yield and water stress indicator modelling. Water 2021, 13, 3587. [Google Scholar] [CrossRef]
- Sing, S.K.; Reddy, K.R. Regulation of photosynthesis, fluorescence, stomatal conductance and water-use efficiency of cowpea (Vigna unguiculata [L.] Walp.) under drought. J. Photoch. Photobiol. B Biol. 2011, 105, 40–50. [Google Scholar] [CrossRef]
- Nemeskéri, E.; Molnár, K.; Pék, Z.; Helyes, L. Effect of water supply on water use related physiological traits and yield of snap beans in dry seasons. Irrig. Sci. 2018, 36, 143–158. [Google Scholar] [CrossRef]
- Vadez, V. Root hydraulics: The forgotten side of roots in drought adaptation. Field Crops Res. 2014, 165, 15–24. [Google Scholar] [CrossRef] [Green Version]
- Ratnakumar, P.; Vadez, V. Tolerant groundnut (A. hypogaea L.) genotypes to intermittent drought maintains high harvest index and has small leaf canopy under stress. Funct. Plant Biol. 2011, 38, 1016–1023. [Google Scholar] [CrossRef] [Green Version]
- Pasala, R.; ·Pandey, B.B.; Gandi, S.L.; Kulasekaran, R.; Guhey, A.; Reddy, A.V. An insight into the mechanisms of intermittent drought adaptation in sesame (Sesamum indicum L.): Linking transpiration efficiency and root architecture to seed yield. Acta Physiol. Plant 2021, 43, 148. [Google Scholar] [CrossRef]
- Helyes, L.; Bőcs, A.; Pék, Z. Effect of water supply on canopy temperature, stomatal conductance and yield quantity of processing tomato (Lycopersicon esculentum Mill.). Int. J. Hortic. Sci. 2010, 16, 13–15. [Google Scholar] [CrossRef]
- Nemeskéri, E.; Neményi, A.; Bőcs, A.; Pék, Z.; Helyes, L. Physiological factors and their relationship with the productivity of processing tomato under different water supplies. Water 2019, 11, 586. [Google Scholar] [CrossRef] [Green Version]
- Lobos, G.; Retamales, J.B.; Hancock, J.F.; Flore, J.A.; Cobo, N.; del Pozo, A. Spectral irradiance, gas exchange characteristics and leaf traits of Vaccinium corymbosum L. ‘Elliott’ grown under photo-selective nets. Environ. Exp. Bot. 2012, 75, 142–149. [Google Scholar] [CrossRef]
- Ogweno, J.O.; Song, X.S.; Hu, W.H.; Shi, K.; Zhou, Y.H.; Yu, J.Q. Detached leaves of tomato differ in their photosynthetic physiological response to moderate high and low temperature stress. Sci. Hortic. 2009, 123, 17–22. [Google Scholar] [CrossRef]
- Mishra, K.B.; Iannacone, R.; Petrozza, A.; Mishra, A.; Armentano, N.; La Vecchia, G.; Trtilek, M.; Cellini, F.; Nedbal, L. Engineered drought tolerance in tomato plants is reflected in chlorophyll fluorescence emission. Plant Sci. 2012, 182, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Huang, G.; Jia, D.; Wang, J.; Mota, M.; Pereira, L.S.; Huang, Q.; Xu, X.; Liu, H. Responses of drip irrigated tomato (Solanum lycopersicum L.) yield, quality and water productivity to various soil matric potential thresholds in an arid region of Northwest China. Agric. Water Manag. 2013, 129, 181–193. [Google Scholar] [CrossRef]
- Bahadur, A.; Chatterjee, A.; Kumar, R.; Singh, M.; Naik, P.S. Physiological and biochemical basis of drought tolerance in vegetables. Veg. Sci. 2011, 38, 1–16. [Google Scholar]
- Pék, Z.; Szuvandzsiev, P.; Neményi, A.; Helyes, L. Effect of season and irrigation on yield parameters and soluble solids content of processing cherry tomato. Acta Hortic. 2015, 1081, 197–202. [Google Scholar] [CrossRef]
- Shao, H.B.; Chu, L.Y.; Jaleel, A.; Zhao, C.X. Water-deficit stress-induced anatomical changes in higher plants. CR Biol. 2008, 331, 215–225. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, G.; Long, W.; Zou, X.; Li, F.; Nishio, T. Recent progress in drought and salt tolerance studies in Brassica crops. Breed. Sci. 2014, 64, 60–73. [Google Scholar] [CrossRef] [Green Version]
- Barriuso, J.; Solano, B.R.; Mañero, F.J.G. Protection against pathogen and salt stress by four plant growth-promoting rhizobacteria isolated from Pinus sp. on Arabidopsis thaliana. Phytopathology 2008, 98, 666–672. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Yang, X.G.; Batchelor, W.D.; Liu, Z.J.; Zhang, Z.T.; Wan, N.H.; Sun, S.; He, B.; Gao, J.Q.; Bai, F.; et al. A case study of climate-smart management in foxtail millet (Setaria italica) production under future climate change in Lishu county of Jilin, China. Agric. For. Meteorol. 2020, 292–293, 108131. [Google Scholar] [CrossRef]
- Sauter, A.; Dietz, K.J.; Hartung, W. A possible stress physiological role of abscisic acid conjugates in root-to-shoot signalling. Plant Cell Environ. 2002, 25, 223–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, A.C.; Travaglia, C.N.; Bottini, R.; Piccoli, P.N. Participation of abscisic acid and gibberellins produced by endophytic Azospirillum in the alleviation of drought effects in maize. Botany 2009, 87, 455–462. [Google Scholar] [CrossRef]
- Sharifi, R.; Ryu, C.M. Chatting with a Tiny Belowground Member of Holobiome: Communication Between Plants and Gowth-Promoting Rhizobacteria. Adv. Bot. Res. 2017, 82, 135–160. [Google Scholar]
- Kamilova, F.; Kravchenko, L.V.; Shaposhnikov, A.I.; Azarova, T.; Makarova, N.; Lugtenberg, B. Organic acids, sugars and L-tryptophane in exudates of vegetables growing on stonewool and their effects on activities of rhizosphere bacteria. Mol. Plant Microbe Interact. 2006, 9, 250–256. [Google Scholar] [CrossRef] [Green Version]
- Oku, S.; Komatsu, A.; Tajima, T.; Nakashimada, Y.; Kato, J. Identification of chemotaxis sensory proteins for amino acids in Pseudomonas fluorescens Pf0-1 and their involvement in chemotaxis to tomato root exudate and root colonization. Microbes Environ. 2012, 27, 462–469. [Google Scholar] [CrossRef] [Green Version]
- Dodd, I.C.; Zinovkina, N.Y.; Safronova, V.I.; Belimov, A.A. Rhizobacterial mediation of plant hormone status. Ann. Appl. Biol. 2010, 157, 361–379. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, G.; Zhang, W.; Lang, D.; Zhixian, L.; Zhang, X. Bacillus sp. G2 improved the growth of Glycyrrhiza uralensis Fisch. related to antioxidant metabolism and osmotic adjustment. Acta Physiol. Plant 2021, 43, 152. [Google Scholar] [CrossRef]
- Nadeem, S.M.; Ahmad, M.; Zahir, Z.A.; Javaid, A.; Ashraf, M. The role of mycorrhizae and plant growth promoting rhizobacteria (PGPR) in improving crop productivity under stressful environments. Biotechnol. Adv. 2014, 32, 429–448. [Google Scholar] [CrossRef]
- Singh, V.K.; Singh, A.K.; Singh, P.P.; Kumar, A. Interaction of plant growth promoting bacteria with tomato under abiotic stress: A review. Agric. Ecosyst. Environ. 2018, 267, 129–140. [Google Scholar] [CrossRef]
- Berg, G. Plant-microbe interactions promoting plant growth and health: Perspectives for controlled use of microorganisms in agriculture. Appl. Microbiol. Biotechnol. 2009, 84, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Khanghahi, M.Y.; Leoni, B.; Crecchio, C. Photosynthetic responses of durum wheat to chemical/microbiological fertilization management under salt and drought stresses. Acta Physiol. Plant 2021, 43, 123. [Google Scholar] [CrossRef]
- Le, A.T.; Pék, Z.; Takács, S.; Neményi, A.; Helyes, L. The effect of plant growth-promoting rhizobacteria on yield, water use efficiency and Brix Degree of processing tomato. Plant Soil Environ. 2018, 64, 523–529. [Google Scholar] [CrossRef] [Green Version]
- Andryei, B.; Horváth, K.Z.; Agyemang Duah, S.; Takács, S.; Égei, M.; Szuvandzsiev, P.; Neményi, A. Use of plant growth promoting rhizobacteria (PGPRs) in the mitigation of water deficiency of tomato plants (Solanum lycopersicum L.). J. Cent. Eur. Agric. 2021, 22, 167–177. [Google Scholar]
- Zhou, Y.; Sang, T.; Tian, M.; Jahan, M.S.; Wang, J.; Li, X.; Guo, S.; Liu, H.; Wang, Y.; Shu, S. Effects of Bacillus cereus on photosynthesis and antioxidant metabolism of cucumber seedlings under salt stress. Horticulturae 2022, 8, 463. [Google Scholar] [CrossRef]
- Papoui, E.; Bantis, F.; Kapoulas, N.; Ipsilantis, I.; Koukounaras, A. A sustainable intercropping system for organically produced lettuce and green onion with the use of arbuscular mycorrhizal inocula. Horticulturae 2022, 8, 466. [Google Scholar] [CrossRef]
- Sneha, S.; Anitha, B.; Sahair, R.A.; Raghu, N.; Gopenath, T.S.; Chandrashekrappa, G.K.; Basalingappa, K.M. Biofertilizer for crop production and soil fertility. Acad. J. Agric. Res. 2018, 6, 299–306. [Google Scholar]
- Kaur, R.; Kaur, S.; Kaur, G. Molecular and physiological manipulations in rhizospheric bacteria. Acta Physiol. Plant 2021, 43, 77. [Google Scholar] [CrossRef]
- Vejan, P.; Abdullah, R.; Khadiran, T.; Ismail, S.; Boyce, A.N. Role of plant growth promoting rhizobacteria in agricultural sustainability: A review. Molecules 2016, 21, 573. [Google Scholar] [CrossRef]
- Aslam, M.M.; Okal, E.J.; Idris, A.L.; Qian, Z.; Xu, W.; Karanja, J.K.; Wani, S.H.; Yuan, W. Rhizosphere microbiomes can regulate plant drought tolerance. Pedosphere 2022, 32, 61–74. [Google Scholar] [CrossRef]
- Yang, J.; Kloepper, J.W.; Ryu, C.M. Rhizosphere bacteria help plants tolerate abiotic stress. Trends Plant Sci. 2009, 14, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Forni, C.; Duca, D.; Glick, B.R. Mechanisms of plant response to salt and drought stress and their alteration by rhizobacteria. Plant Soil 2017, 410, 335–356. [Google Scholar] [CrossRef]
- Davies, W.J.; Zhang, J. Root signals and the regulation of growth and development of plants in drying soil. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1991, 42, 55–76. [Google Scholar] [CrossRef]
- Bresson, J.; Varoquaux, F.; Bontpart, T.; Touraine, B.; Vile, D. The PGPR strain Phyllobacterium brassicacearum STM196 induces a reproductive delay and physiological changes that result in improved drought tolerance in Arabidopsis. N. Phytol. 2013, 200, 558–569. [Google Scholar] [CrossRef]
- Nemeskéri, E.; Horváth, K.; Pék, Z.; Helyes, L. Effect of mycorrhizal and bacterial products on the traits related to photosynthesis and fruit quality of tomato under water deficiency conditions. Acta Hortic. 2019, 1233, 61–66. [Google Scholar] [CrossRef]
- Horvath, K.Z.; Andryei, B.; Helyes, L.; Pék, Z.; Neményi, A.; Nemeskéri, E. Effect of mycorrhizal inoculations on physiological traits and bioactive compounds of tomato under water scarcity in field conditions. Not. Bot. Hortic. Agrobot Cluj Napoca 2020, 48, 1233–1247. [Google Scholar] [CrossRef]
- Costa-Santos, M.; Mariz-Ponte, N.; Dias, M.C.; Moura, L.; Marques, G.; Santos, C. Effect of Bacillus spp. and Brevibacillus sp. on the Photosynthesis and Redox Status of Solanum lycopersicum. Horticulturae 2021, 7, 24. [Google Scholar] [CrossRef]
- Tahir, M.; Khalid, U.; Khan, M.B.; Shahid, M.; Ahmad, I.; Akram, M.; Ijaz, M.; Hussain, M.; Farooq, A.B.; Naeem, M.A.; et al. Auxin and 1-aminocyclopropane-1-carboxylate deaminase activity exhibiting rhizobacteria enhanced maize quality and productivity under water deficit conditions. Int. J. Agric. Biol. 2019, 21, 943–954. [Google Scholar]
- Gagné-Bourque, F.; Bertrand, A.; Claessens, A.; Aliferis, K.A.; Jabaji, S. Alleviation of drought stress and metabolic changes in timothy (Phleum pratense L.) colonized with Bacillus subtilis B26. Front. Plant Sci. 2016, 7, 584. [Google Scholar] [CrossRef] [Green Version]
- Zhou, C.; Ma, Z.; Zhu, L.; Xiao, X.; Xie, Y.; Zhu, J.; Wang, J. Rhizobacterial strain Bacillus megaterium BOFC15 induces cellular polyamine changes that improve plant growth and drought resistance. Int. J. Mol. Sci. 2016, 17, 976. [Google Scholar] [CrossRef]
- Chen, L.; Liu, Y.; Wu, G.; Veronican, N.; Jeri, K.; Shen, Q.; Zhang, N.; Zhang, R. Induced maize salt tolerance by rhizosphere inoculation of Bacillus amyloliquefaciens SQR9. Physiol. Plant 2016, 158, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Kloepper, J.W.; Lifshitz, R.; Zablotowich, R.K. Free-living bacterial inocula for enhancing crop productivity. Trends Biotechnol. 1989, 7, 39–43. [Google Scholar] [CrossRef]
- Bona, E.; Cantamessa, S.; Massa, N.; Manassero, P.; Marsano, F.; Copetta, A.; Lingua, G.; D’Agostino, G.; Gamalero, E.; Berta, G. Arbuscular mycorrhizal fungi and plant growth-promoting pseudomonads improve yield, quality and nutritional value of tomato: A field study. Mycorrhiza 2017, 27, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mondani, F.; Khani, K.; Honarmand, S.L.; Saeidi, M. Evaluating effects of plant growth-promoting rhizobacteria on the radiation use efficiency and yield of soybean (Glycine max) under water deficit stress condition. Agric. Water Manag. 2019, 213, 707–713. [Google Scholar] [CrossRef]
- Vaikuntapu, P.R.; Dutta, S.; Samudrala, R.B.; Rao, V.R.V.N.; Kalam, S.; Podile, A.R. Preferential promotion of Lycopersicon esculentum (tomato) growth by plant growth promoting bacteria associated with tomato. Indian J. Microbiol. 2014, 54, 403–412. [Google Scholar] [CrossRef] [Green Version]
- Zhou, D.; Huang, X.F.; Chaparro, J.M.; Badri, D.V.; Manter, D.K.; Vivanco, J.M.; Guo, J. Root and bacterial secretions regulate the interaction between plants and PGPR leading to distinct plant growth promotion effects. Plant Soil 2016, 401, 259–272. [Google Scholar] [CrossRef]
- Kalam, S.; Basu, A.; Podile, A.R. Functional and molecular characterization of plant growth promoting Bacillus isolates from tomato rhizosphere. Heliyon 2020, 6, e04734. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).