Seasonal Change in Phytochemical Composition and Biological Activities of Carissa macrocarpa (Eckl.) A. DC. Leaf Extract
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant-Material Collection
2.2. Preparation of Plant Material for Extraction
2.3. Extraction of C. Macrocarpa Leaves
2.4. Evaporation of Extracts
2.5. Phytochemical Screening
2.5.1. Detection of Alkaloids
2.5.2. Detection of Tannins
2.5.3. Detection of Phenols
2.5.4. Detection of Quinones
2.5.5. Detection of Flavonoids
2.5.6. Detection of Saponins and Steroids
Saponins
Steroids
2.5.7. Detection of Proteins
2.5.8. Detection of Carbohydrates
2.5.9. Detection of Mucilage and Gum
2.5.10. Detection of Resins
2.6. Antibacterial Activity
2.7. DDPH Scavenging Activity
2.8. Statistical Analysis
3. Results and Discussion
3.1. Yield of Extract and Screening of Phytochemicals
3.2. Antibacterial Activity
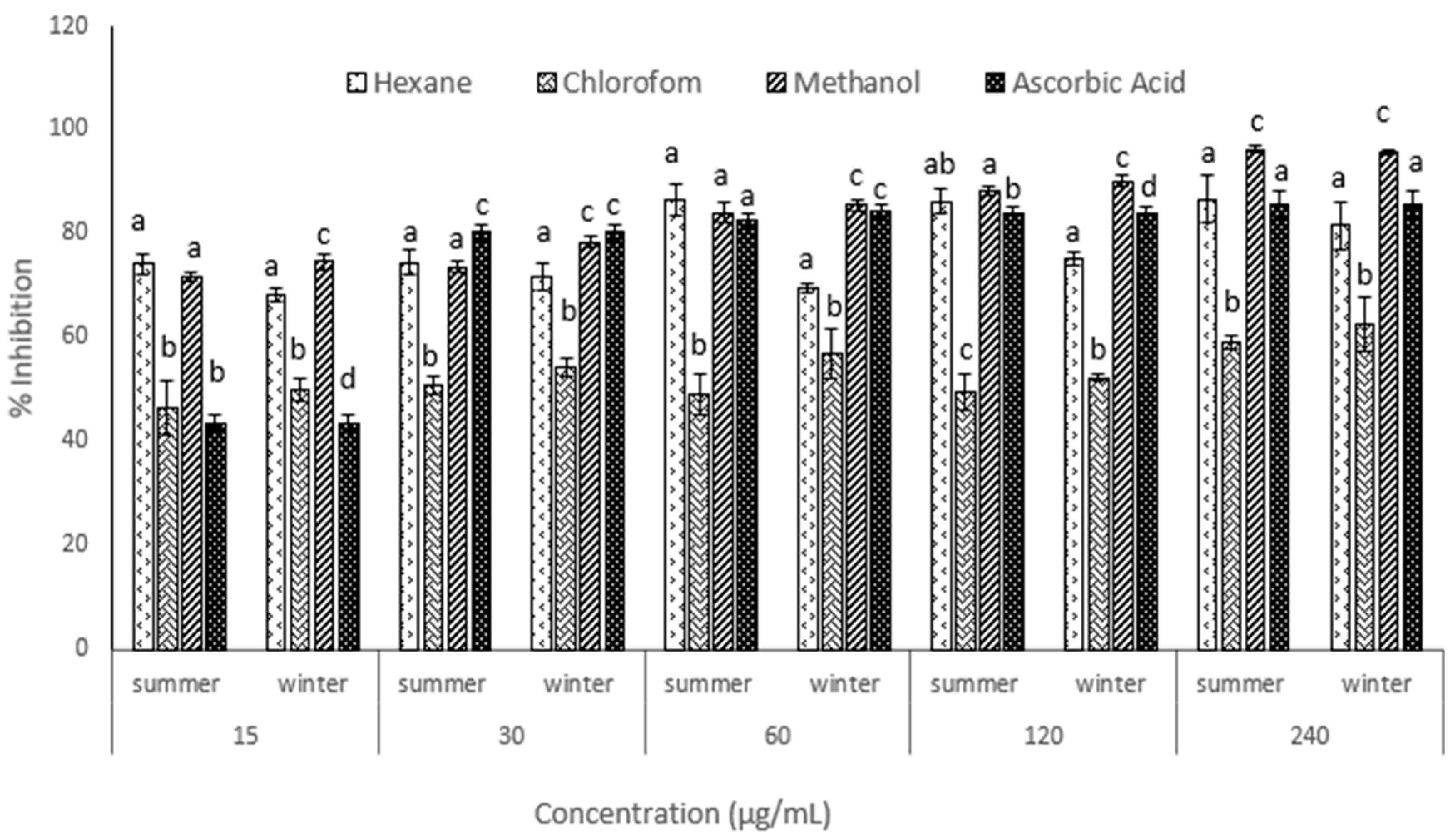
3.3. Antioxidant Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shanley, P.; Luz, L. The Impacts of Forest Degradation on Medicinal Plant Use and Implications for Health Care in Eastern Amazonia. BioScience 2003, 53, 573. [Google Scholar] [CrossRef]
- Sheldon, J.W.; Balick, M.J.; Laird, S.A.; Milne, G.M. Medicinal plants: Can utilization and conservation coexist? Adv. Econ. Bot. 1997, 12, 1–104. [Google Scholar]
- Mbatha, N.; Street, R.A.; Ngcobo, M.; Gqaleni, N. Sick certificates issued by South African traditional health practitioners: Current legislation, challenges and the way forward: Issues in medicine. South Afr. Med. J. 2012, 102, 129–131. [Google Scholar] [CrossRef] [PubMed]
- Svoboda, K.P.; Svoboda, T.G.; Syred, A.D. Secretory Structures of Aromatic and Medicinal Plants: A Review and Atlas of Micrographs; Microscopix Publications: Hoddesdon, UK, 2000. [Google Scholar]
- Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G.; Scheffer, J.J. Factors affecting secondary metabolite production in plants: Volatile components and essential oils. Flavour Fragr. J. 2008, 23, 213–226. [Google Scholar] [CrossRef]
- Yadav RN, S.; Agarwala, M. Phytochemical analysis of some medicinal plants. J. Phytol. 2011, 3, 10–14. [Google Scholar]
- Wakeel, A.; Jan, S.A.; Ullah, I.; Shinwari, Z.K.; Xu, M. Solvent polarity mediates phytochemical yield and antioxidant capacity of Isatis tinctoria. PeerJ 2019, 7, e7857. [Google Scholar] [CrossRef] [PubMed]
- Nchabeleng, L.; Mudau, F.N.; Mariga, I.K. Effects of chemical composition of wild bush tea (Athrixia phylicoides DC.) growing at locations differing in altitude, climate and edaphic factors. J. Med. Plants Res. 2012, 6, 1662–1666. [Google Scholar] [CrossRef]
- Jayanthy, A.; Prakash, K.U.; Remashree, A.B. Seasonal and geographical variations in cellular characters and chemical contents in Desmodium gangeticum (L.) DC.—An ayurvedic medicinal plant. Int. J. Herb. Med. 2013, 1, 34–37. [Google Scholar]
- Odjegba, V.J.; Alokolaro, A.A. Simulated drought and salinity modulate the production of phytochemicals in Acalypha wilkesiana. J. Plant Stud. 2013, 2, 105. [Google Scholar] [CrossRef]
- Kale, V.S.; Shitole, M.G. Variable rates of primary and secondary metabolites during different seasons and physiological stages in Datura metel L. J. Maharashtra Agric. Univ. 2010, 35, 371–374. [Google Scholar]
- Gololo, S.S.; Shai, L.J.; Agyei, N.M.; Mogale, M.A. Effect of seasonal changes on the quantity of phytochemicals in the leaves of three medicinal plants from Limpopo province, South Africa. J. Pharmacogn. Phytother. 2016, 8, 168–172. [Google Scholar]
- Valgas, C.; Souza SM, D.; Smânia, E.F.; Smânia, A., Jr. Screening methods to determine antibacterial activity of natural products. Braz. J. Microbiol. 2007, 38, 369–380. [Google Scholar] [CrossRef]
- Babu, H.R.; Savithramma, N. Screening of secondary metabolites of underutilized species of Cyperaceae. Int. J. Pharm. Sci. Rev. Res. 2014, 24, 182–187. [Google Scholar]
- Lim, T.K. Carissa macrocarpa. In Edible Medicinal and Non-Medicinal Plants; Springer: Dordrecht, The Netherlands, 2012; pp. 237–239. [Google Scholar]
- Halliwell, B.; Gutteridge, J.M. Free Radicals in Biology and Medicine; Oxford University Press: New York, NY, USA, 2015. [Google Scholar]
- Erkan, N. Antioxidant activity and phenolic compounds of fractions from Portulaca oleracea L. Food Chem. 2012, 133, 775–781. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Mayne, S.T. Antioxidant nutrients and chronic disease: Use of biomarkers of exposure and oxidative stress status in epidemiologic research. J. Nutr. 2003, 133, 933S–940S. [Google Scholar] [CrossRef] [Green Version]
- Rakesh, S.U.; Patil, P.R.; Mane, S.R. Use of natural antioxidants to scavenge free radicals: A major cause of diseases. Int. J. PharmTech Res. 2010, 2, 1074–1081. [Google Scholar]
- Kapadiya, D.B.; Dabhi, B.K.; Aparnathi, K.D. Spices and herbs as a source of natural antioxidants for food. Int. J. Curr. Microbiol. Appl. Sci. 2016, 5, 280–288. [Google Scholar] [CrossRef]
- Tadhani, M.B.; Patel, V.H.; Subhash, R. In vitro antioxidant activities of Stevia rebaudiana leaves and callus. J. Food Compos. Anal. 2007, 20, 323–329. [Google Scholar] [CrossRef]
- Bhadane, B.S.; Patil, M.P.; Maheshwari, V.L.; Patil, R.H. Ethnopharmacology, phytochemistry, and biotechnological advances of family Apocynaceae: A review. Phytother. Res. 2018, 32, 1181–1210. [Google Scholar] [CrossRef]
- El-Taher, A.M.; EL-Gendy, A.G.; Lila, M.I. Morphological and anatomical studies on some taxa of family Apocynaceae. Al-Azhar J. Agric. Res. 2019, 44, 136–147. [Google Scholar] [CrossRef]
- The Plant List. Carissa. 2022. Available online: http://www.theplantlist.org/tpl1.1/record/kew-34192 (accessed on 8 August 2022).
- Moodley, R.; Koorbanally, N.; Jonnalagadda, S.B. Elemental composition and fatty acid profile of the edible fruits of Amatungula (Carissa macrocarpa) and impact of soil quality on chemical characteristics. Anal. Chim. Acta 2012, 730, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Khalil, H.E.; Aljeshi, Y.M.; Saleh, F.A. Authentication of Carissa macrocarpa cultivated in Saudi Arabia; botanical, phytochemical and genetic study. J. Pharm. Sci. Res. 2015, 7, 497. [Google Scholar]
- Moodley, R.; Chenia, H.; Jonnalagadda, S.B.; Koorbanally, N. Antibacterial and anti-adhesion activity of the pentacyclic triterpenoids isolated from the leaves and edible fruits of Carissa macrocarpa. J. Med. Plants Res. 2011, 5, 4851–4858. [Google Scholar]
- Alsudani AA AA, Z.; Altameme HJ, M. A Taxonomical study of Carissa macrocarpa (Eckl.) A. Dc (Apocynaceae) in Iraq. Rev. Int. Geogr. Educ. 2021, 11, 1328–1341. [Google Scholar]
- National Research Council. Lost Crops of Africa: Volume III: Fruits; National Academies Press: Washington, DC, USA, 2008; Volume 3, pp. 262–269. [Google Scholar]
- Ibrahim, H.; Abdurahman, E.M.; Shok, M.; Ilyas, N.; Bolaji, R. Preliminary phytochemical and antimicrobial studies of the leaves of Carissa edulis Vahl. In Standardization and Utilization of Herbal Medicines: Challenges of the 21st Century, Proceedings of the 1st International Workshop on Herbal Medicinal Products, Ibadan, Nigeria, 22–24 November 1998; University of Ibadan, Department of Pharmacognosy: Ibadan, Nigeria, 1999; pp. 217–222. [Google Scholar]
- Khalil, H.E.; Mohamed, M.E.; Morsy, M.A.; Kandeel, M. Flavonoid and phenolic compounds from Carissa macrocarpa: Molecular docking and cytotoxicity studies. Pharmacogn. Mag. 2018, 14, 304. [Google Scholar] [CrossRef]
- Abbas, M.; Rasool, N.; Riaz, M.; Zubair, M.; Abbas, M.; Noor Ul, H.; Hayat, N. GC-MS profiling, antioxidant, and antimicrobial studies of various parts of Carissa grandiflora. Bulg. Chem. Commun. 2014, 46, 831–839. [Google Scholar]
- Dhatwalia, J.; Kumari, A.; Verma, R.; Upadhyay, N.; Guleria, I.; Lal, S.; Thakur, S.; Gudeta, K.; Kumar, V.; Chao, J.C.C.; et al. Phytochemistry, pharmacology, and nutraceutical profile of Carissa species: An updated review. Molecules 2021, 26, 7010. [Google Scholar] [CrossRef]
- Souilem, F.; Dias, M.I.; Barros, L.; Calhelha, R.C.; Alves, M.J.; Harzallah-Skhiri, F.; Ferreira, I.C. Phenolic profile and bioactive properties of Carissa macrocarpa (Eckl.) A. DC.: An in vitro comparative study between leaves, stems, and flowers. Molecules 2019, 24, 1696. [Google Scholar] [CrossRef]
- Souilem, F.; El Ayeb, A.; Djlassi, B.; Ayari, O.; Chiboub, W.; Arbi, F.; Ascizzi, R.; Flamini, G.; Harzallah-Skhiri, F. Chemical composition and activity of essential oils of Carissa macrocarpa (Eckl.) A. DC. Cultivated in Tunisia and its anatomical features. Chem. Biodivers. 2018, 15, e1800188. [Google Scholar] [CrossRef]
- Surendra, T.V.; Roopan, S.M.; Arasu, M.V.; Al-Dhabi, N.A.; Sridharan, M. Phenolic compounds in drumstick peel for the evaluation of antibacterial, hemolytic and photocatalytic activities. J. Photochem. Photobiol. B Biol. 2016, 161, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Sofowara, A. Medicinal Plants and Traditional Medicine in Africa; Spectrum Books Ltd.: Ibadan, Nigeria, 1993. [Google Scholar]
- Tamilselvi, N.; Krishnamoorthy, P.; Dhamotharan, R.; Arumugam, P.; Sagadevan, E. Analysis of total phenols, total tannins and screening of phytocomponents in Indigofera aspalathoides (Shivanar Vembu) Vahl EX DC. J. Chem. Pharm. Res. 2012, 4, 3259–3262. [Google Scholar]
- Trease, G.E.; Evans, W.C. Pharmacognosy; Bahiv Tinal: London, UK, 1985. [Google Scholar]
- Khandelwal, K. Practical Pharmacognosy; Pragati Books Pvt. Ltd.: Nagpur, India, 2008. [Google Scholar]
- Harborne, A.J. Phytochemical Methods a Guide to Modern Techniques of Plant Analysis; Springer Science & Business Media: Dordrecht, The Netherlands, 1998. [Google Scholar]
- Bargah, R.K. Preliminary test of phytochemical screening of crude ethanolic and aqueous extract of Moringa pterygosperma Gaertn. J. Pharmacogn. Phytochem. 2015, 4, 7–9. [Google Scholar]
- Kumar, R.S.; Balasubramanian, P.; Govindaraj, P.; Krishnaveni, T. Preliminary studies on phytochemicals and antimicrobial activity of solvent extracts of Coriandrum sativum L. roots (Coriander). J. Pharmacogn. Phytochem. 2014, 2, 74–78. [Google Scholar]
- Perez, C. Antibiotic assay by agar-well diffusion method. Acta Biol. Med. Exp. 1990, 15, 113–115. [Google Scholar]
- Braca, A.; Sortino, C.; Politi, M.; Morelli, I.; Mendez, J. Antioxidant activity of flavonoids from Licania licaniaeflora. J. Ethnopharmacol. 2002, 79, 379–381. [Google Scholar] [CrossRef]
- Doss, A.; Mubarack, H.M.; Dhanabalan, R. Antibacterial activity of tannins from the leaves of Solanum trilobatum Linn. Indian J. Sci. Technol. 2009, 2, 41–43. [Google Scholar] [CrossRef]
- Ruther, J.; Meiners, T.; Steidle, J.L.M. Allelochemical Reactions Involving Heterotrophic Microorganisms. Biogeochem. Inland Waters 2010, 22, 436. [Google Scholar]
- Geetha, B.; Gowda, K.P.; Kulkarni, G.T.; Badami, S. Microwave assisted fast extraction of mucilages and pectins. Indian J. Pharm. Educ. Res. 2009, 43, 260–265. [Google Scholar]
- Sungthongjeen, S.; Pitaksuteepong, T.; Somsiri, A.; Sriamornsak, P. Studies on pectins as potential hydrogel matrices for controlled-release drug delivery. Drug Dev. Ind. Pharm. 1999, 25, 1271–1276. [Google Scholar] [CrossRef]
- Rahal, A.; Kumar, A.; Singh, V.; Yadav, B.; Tiwari, R.; Chakraborty, S.; Dhama, K. Oxidative stress, prooxidants, and antioxidants: The interplay. BioMed Res. Int. 2014, 2014, 761264. [Google Scholar] [CrossRef] [PubMed]
- Rabi, T.; Bishayee, A. Terpenoids and breast cancer chemoprevention. Breast Cancer Res. Treat. 2009, 115, 223–239. [Google Scholar] [CrossRef] [PubMed]
- Krishnaiah, D.; Devi, T.; Bono, A.; Sarbatly, R. Studies on phytochemical constituents of six Malaysian medicinal plants. J. Med. Plants Res. 2009, 3, 67–72. [Google Scholar]
- Shah, B.A.; Qazi, G.N.; Taneja, S.C. Boswellic acids: A group of medicinally important compounds. Nat. Prod. Rep. 2009, 26, 72–89. [Google Scholar] [CrossRef]
- Peter, H.; Pavel, T.; Marie, S. Flavonoids-potent and versatile biologically active compounds interacting with cytochrome P450. Chem. Biol. Interact. 2002, 139, 1–21. [Google Scholar]
- Killedar, S.G.; More, H.N. Estimation of tannins in different parts of Memecylon umbellatum Burm. J. Pharm. Res. 2010, 3, 554–556. [Google Scholar]
- Ganguly, T.; Sainis, K.B. Inhibition of cellular immune responses by Tylophora indica in experimental models. Phytomedicine 2001, 8, 348–355. [Google Scholar] [CrossRef]
- Staerk, D.; Lykkeberg, A.K.; Christensen, J.; Budnik, B.A.; Abe, F.; Jaroszewski, J.W. In Vitro Cytotoxic Activity of Phenanthroindolizidine Alkaloids from Cynanchum vincetoxicum and Tylophora tanakae against Drug-Sensitive and Multidrug-Resistant Cancer Cells. J. Nat. Prod. 2002, 65, 1299–1302. [Google Scholar] [CrossRef]
- Just, M.J.; Recio, M.C.; Giner, R.M.; Cuéllar, M.J.; Máñez, S.; Bilia, A.R.; Ríos, J.L. Anti-inflammatory activity of unusual lupane saponins from Bupleurum fruticescens. Planta Med. 1998, 64, 404–407. [Google Scholar] [CrossRef]
- Estrada, A.; Katselis, G.S.; Laarveld, B.; Barl, B. Isolation and evaluation of immunological adjuvant activities of saponins from Polygala senega L. Comp. Immunol. Microbiol. Infect. Dis. 2000, 23, 27–43. [Google Scholar] [CrossRef]
- Nikaido, H.; Vaara, M. Molecular basis of bacterial outer membrane permeability. Microbiol. Rev. 1985, 49, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Rabe, T.; Van Staden, J. Antibacterial activity of South African plants used for medicinal purposes. J. Ethnopharmacol. 1997, 56, 81–87. [Google Scholar] [CrossRef]
- Sekirov, I.; Russell, S.L.; Antunes LC, M.; Finlay, B.B. Gut microbiota in health and disease. Physiol. Rev. 2010, 90, 859–904. [Google Scholar] [CrossRef]
- Silva, C.D.; Herdeiro, R.S.; Mathias, C.J.; Panek, A.D.; Silveira, C.S.; Rodrigues, V.P.; Renno, M.N.; Falcao, D.Q.; Cerqueira, D.M.; Minto AB, M.; et al. Evaluation of antioxidant activity of Brazilian plants. Pharmacol. Res. 2005, 52, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Kasote, D.M.; Katyare, S.S.; Hegde, M.V.; Bae, H. Significance of antioxidant potential of plants and its relevance to therapeutic applications. Int. J. Biol. Sci. 2015, 11, 982. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, J.; Kumar, S.; Vankar, P.S. Correlation of antioxidant activity and phytochemical profile in native plants. Nutr. Food Sci. 2012, 42, 71–79. [Google Scholar] [CrossRef]
- Kessler, M.; Ubeaud, G.; Jung, L. Anti-and pro-oxidant activity of rutin and quercetin derivatives. J. Pharm. Pharmacol. 2003, 55, 131–142. [Google Scholar] [CrossRef]
- Talla, E.; Tamfu, A.N.; Gade, I.S.; Yanda, L.; Mbafor, J.T.; Laurent, S.; Elst, L.V.; Popova, M.; Bankova, V. New mono-ether of glycerol and triterpenes with DPPH radical scavenging activity from Cameroonian propolis. Nat. Prod. Res. 2017, 31, 1379–1389. [Google Scholar] [CrossRef]
- Miao, J.; Li, X.; Zhao, C.; Gao, X.; Wang, Y.; Gao, W. Active compounds, antioxidant activity and α-glucosidase inhibitory activity of different varieties of Chaenomeles fruits. Food Chem. 2018, 248, 330–339. [Google Scholar] [CrossRef]
- Akwu, N.A.; Naidoo, Y.; Singh, M.; Nundkumar, N.; Lin, J. Phytochemical screening, in vitro evaluation of the antimicrobial, antioxidant and cytotoxicity potentials of Grewia lasiocarpa E. Mey. ex Harv. S. Afr. J. Bot. 2019, 123, 180–192. [Google Scholar] [CrossRef]
- Assimopoulou, A.N.; Zlatanos, S.N.; Papageorgiou, V.P. Antioxidant activity of natural resins and bioactive triterpenes in oil substrates. Food Chem. 2005, 92, 721–727. [Google Scholar] [CrossRef]
- Iqbal, S.; Bhanger, M.I. Effect of season and production location on antioxidant activity of Moringa oleifera leaves grown in Pakistan. J. Food Compos. Anal. 2006, 19, 544–551. [Google Scholar] [CrossRef]
- Kaplan, F.; Kopka, J.; Sung, D.Y.; Zhao, W.; Popp, M.; Porat, R.; Guy, C.L. Transcript and metabolite profiling during cold acclimation of Arabidopsis reveals an intricate relationship of cold-regulated gene expression with modifications in metabolite content. Plant J. 2007, 50, 967–981. [Google Scholar] [CrossRef] [PubMed]
| Solvent | Yield (Summer) | Yield (Winter) | ||
|---|---|---|---|---|
| (g) | (%) | (g) | (%) | |
| Hexane | 0.82 | 8.2 | 0.98 | 9.8 |
| Chloroform | 0.48 | 4.8 | 0.65 | 6.5 |
| Methanol | 1.5 | 15 | 2.43 | 24.3 |
| Phytochemical Constituent | Type of Test | Winter | Summer | ||||
|---|---|---|---|---|---|---|---|
| H | C | M | H | C | M | ||
| Alkaloids | Wagner’s | - | - | + | - | ++ | ++ |
| Meyer’s | ++ | + | - | ++ | + | - | |
| Tannins | Ferric chloride | + | + | ++ | + | + | ++ |
| Phenols | Lead acetate | + | + | ++ | ++ | ++ | ++ |
| Tannins (naphthoquinone) | Gelatine | ++ | - | + | + | - | + |
| Flavonoids | Alkaline-reagent test | + | - | + | + | - | + |
| Acid-hydrolysis test | + | - | ++ | + | - | ++ | |
| Saponins | Foam test | - | - | + | - | - | + |
| Olive oil test | - | ++ | ++ | - | + | ++ | |
| Steroids (terpenoids) | Salkowski’s test | + | - | + | + | - | + |
| Lieberman–Bouchard test | + | - | + | + | - | + | |
| Proteins | Biuret test | + | + | + | + | + | + |
| Carbohydrates | Molisch’s test | - | ++ | ++ | - | + | + |
| Mucilage + gums | Precipitation test | + | + | - | + | + | - |
| Ruthenium-red test | - | + | + | - | + | + | |
| Resin | Acetone test | + | ++ | - | + | ++ | - |
| Bacterial Strain | Concentrations (mg/mL) | Positive Control | ||||
|---|---|---|---|---|---|---|
| 10 | 5 | 2.5 | 1.25 | 0.625 | ||
| Summer | ||||||
| S. aureus | 7.75 ± 1.77 | 7.25 ± 0.35 | 6.75 ± 0.35 | 7.0 ± 0.00 | 7.0 ± 0.00 | 10 ± 1.41 |
| E. coli | 6.75 ± 0.35 | 6.75 ± 0.35 | 6.5 ± 0.35 | 6.5± 0.00 | 6.5 ± 0.00 | 11 ± 1.41 |
| Winter | ||||||
| S. aureus | 8.17 ± 1.04 | 8.17 ± 1.04 | 7.25 ± 0.35 | 7.0 ± 0.00 | 7.0 ± 0.00 | 10 ± 1.32 |
| E. coli | 6.83 ± 0.58 | 6.83 ± 0.58 | 6.75 ± 0.00 | 6.5± 0.00 | 6.5 ± 0.00 | 11 ± 1.00 |
| Sample | IC50 (µg/mL) | |
|---|---|---|
| Summer | Winter | |
| Hexane | 0.15 | 0.29 |
| Chloroform | 44.76 | 13.06 |
| Methanol | 1.72 | 0.67 |
| Ascorbic acid | 8.26 | 8.26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramasar, R.; Naidoo, Y.; Dewir, Y.H.; El-Banna, A.N. Seasonal Change in Phytochemical Composition and Biological Activities of Carissa macrocarpa (Eckl.) A. DC. Leaf Extract. Horticulturae 2022, 8, 780. https://doi.org/10.3390/horticulturae8090780
Ramasar R, Naidoo Y, Dewir YH, El-Banna AN. Seasonal Change in Phytochemical Composition and Biological Activities of Carissa macrocarpa (Eckl.) A. DC. Leaf Extract. Horticulturae. 2022; 8(9):780. https://doi.org/10.3390/horticulturae8090780
Chicago/Turabian StyleRamasar, Reshika, Yougasphree Naidoo, Yaser Hassan Dewir, and Antar Nasr El-Banna. 2022. "Seasonal Change in Phytochemical Composition and Biological Activities of Carissa macrocarpa (Eckl.) A. DC. Leaf Extract" Horticulturae 8, no. 9: 780. https://doi.org/10.3390/horticulturae8090780
APA StyleRamasar, R., Naidoo, Y., Dewir, Y. H., & El-Banna, A. N. (2022). Seasonal Change in Phytochemical Composition and Biological Activities of Carissa macrocarpa (Eckl.) A. DC. Leaf Extract. Horticulturae, 8(9), 780. https://doi.org/10.3390/horticulturae8090780







