Abstract
In this study, we analyzed and compared the concentrations of selected metals/metalloids and the antioxidant response of Salix alba L. (white willow) bark in the highly polluted area around the Kosovo A and B thermal power plants. The antioxidant capacity of Salix alba bark was evaluated in terms of the total phenolics, flavonoids, chlorophylls, and carotenoids, while the metal content in the soil and willow bark was analyzed by ICP-OES (inductively coupled plasma optical emission spectroscopy). For total antioxidant level assessment, FRAP, DPPH, and CUPRAC assays were conducted. The mean concentrations of selected elements in soil and willow dry mass range from 15,698.4 mg kg−1 dry mass (soil) to 371.1 mg kg−1 (willow bark) for Al; 37.676 mg kg−1 (soil) to <2 ppb (willow bark) for As; 14.8 mg kg−1 (soil) to 0.62 mg kg−1 (willow bark) for Cd; 24.2 mg kg−1 (soil) to 1.2 mg kg−1 (willow bark) for Cr; 58.8 mg kg−1 (soil) to 9.1 mg kg−1 (willow bark) for Cu; 16,975.68 mg kg−1 (soil) to 385.4 mg kg−1 (willow bark) for Fe; 95.0 mg kg−1 (soil) to 7.9 mg kg−1 (willow bark) for Ni; 185.2 mg kg−1 (soil) to <1 ppb (willow bark) for Pb; and 226.7 mg kg−1 (soil) to 87.7 mg kg−1 (willow bark) for Zn. Additionally, the Salix alba bark samples presented mean values of 12,191.6 mg kg−1 for Ca, 1306.0 mg kg−1 for Mg, and 123.1363 mg kg−1 for Mn. The mean phenolic content was 39.292 mg GAE g−1 DW, being 28.222 mg CE g−1 DW for flavonoids, 38.099 mg g−1 FW for CHLa, 49.240 mg g−1 FW for CHLb, and 94.976 mg g−1 FW for CAR. The results of this study indicate that the bark of Salix alba contains significant amounts of phenolic compounds, and strong positive and moderate negative correlations are revealed between total phenolic compounds and iron, and total phenolics and nickel and manganese, respectively.
1. Introduction
From ancient times, members of the genus Salix (Family Salicaceae) have been used in traditional medicine for the treatment of various health conditions, especially muscular and rheumatic disorders [1]. The analgesic, antipyretic, anti-inflammatory, anti-rheumatic, antiseptic, and astringent properties of willow are well-known, while species of the genus Salix have also been used for their diuretic, febrifuge, hypnotic, and sedative effects [2]. Considering these effects, willow bark has been used in the preparation of pharmaceutical formulations to treat many different types of pain, as well as to relieve sore throats and fevers associated with infections of the upper respiratory system and flu-like symptoms [3]. White willow—otherwise known as salicin willow—has been used for thousands of years due to its impact on health [4]. According to the European Pharmacopoeia (2005), willow bark is defined as the dry bark (whole or fragmented) of young or current-year branches of different species of the genus Salix. At present, the use of willow bark, both in traditional medicine and in the pharmaceutical industry, is supported by a considerable number of publications that provide data regarding its effects on health [2].
Willow (Salix spp.) is a highly diverse genera containing more than 450 species distributed worldwide, from tropical to cold-temperate climates. Salix alba L. is one of the most widely distributed and popular willows. It appears from sea level up to 2400 m and generally grows near water on the banks of rivers and lakes, usually surviving for only 20–30 years [5]. Salix alba is a native plant in Europe, Central Asia, and North Africa, while in the Americas it was introduced in the late 17th and early 18th centuries. Today, except for in Australia and Antarctica, Salix alba is found in all other continents, occurring in pure stands or in a mixture with other willows and poplars [6].
White willow tolerates a broad range of soil types. From a botanical point of view, it is fast-growing and is one of the tallest willow species, with heights up to 30 m and diameters of approximately 1 m in its native habitat [7]. It is characterized by thin, horizontally extended branches that are easily broken, with deeply fissured bark colored dark grey to brown/yellow brown with corky ridges. Its root system is highly developed, with a deep central root and numerous lateral roots.
Heavy metals can be found in surface water, soil, and plants [8]. They are non-biodegradable, have long biological half-lives and have the potential to accumulate. Some of these metals play important roles in the normal development of biological processes in living organisms. Even though plants take nutrients from water, soil, and air, they are not completely selective for basic components, and may take up elements such as metals, which are unsafe even in low amounts [9]. Different plant species in particular have specific tolerance levels for heavy metals/metalloids, such that some plants have adapted to grow in soils rich in heavy metals/metalloids. Studies have confirmed critical differences in the norm of absorption and distribution of these elements and shown crucial changes in the concentration of heavy metals in various parts of the plant. The age of the plant, soil concentration, climate, family, and genetic structure change the ability of a plant to absorb and synthesize chemical content [10]. A few metals are commonly immobilized in the roots within the tree (e.g., lead, chromium, and copper), while cadmium, nickel, and zinc are more easily transported to the shoots; however, the soil properties in different areas, different climatic conditions, and different sampling times during individual studies must be taken into account [11]. As metals compete and replace the essential elements in plants, thus disrupting their normal function, they can be toxic, consequently damaging biodiversity and making the environment unsuitable for plant growth. The damage observed in the presence of toxic concentrations of heavy metals can be attributed to various causes, which usually interact and may include direct and indirect harmful effects (e.g., through oxidation-induced stress) [12]. Toxic levels of heavy metals can cause cell membrane injury and the breakdown of biomolecules and cell organelles due to increased reactive oxygen species (ROS) production in plants [13].
Considerable studies in recent years have established the important connection between air, water, soil, and plant pollution and toxic element accumulation. The zone around the Kosovo thermal power plants (KTPPs), energy producers, is the greatest concern of the Republic of Kosovo in the context of environmental pollution [14]. From a pollution point of view, the most important activities are those in coal (lignite) mines and the production of electric power. Fly and bottom ash from the KTPPs are the main components of this environmental pollution, and these industrial components are rich in elements with high toxic potential, such as Pb, Cd, As, and Ni [15]. A survey conducted during 2005, regarding fly ash release from the KTPPs, showed that EU standards were exceeded in very high percentages, approximately 400–500 times [15,16].
Plants are subjected to a variety of stressors in the natural world, all of which contribute to the over-production of reactive oxygen species [14]. Heavy metals, as abiotic stress factors, also initiate various processes in plants, some of which may even be harmful to their anatomical, morphological, physiological, and biochemical function, causing the production of reactive oxygen species and oxidative stress in plants. Plants have evolutionarily developed mechanisms to defend themselves against the harmful impact of heavy metals, involving the activation of antioxidant systems to neutralize reactive oxygen. Several definitions of chemical, biological, and biomedical antioxidants are known. Generally, any substance which, when existing in low concentrations compared to those of an oxidizable substrate, substantially delays or inhibits the oxidation of this substrate [17], or “any substance that delays, prevents or removes oxidative damage to a target molecule” [18], is defined as an antioxidant. Plants are generally known for their antioxidant properties, as they are rich in substances with antioxidant activity, commonly referred to as polyphenols [19]. A plant’s antioxidant composition facilitates radical scavenging, supporting the conversion of radicals into less-reactive species [20]. Antioxidants usually act as reducing agents and neutralize free radicals by oxidizing themselves; examples include thiols, ascorbic acid, and polyphenols [21].
Antioxidants have become an essential part of our lives and, as a result of their action in the body to neutralize or break up ROS, it has been shown that different parts of plants are rich sources of a range biologically active agents—usually phenolics—and phytochemicals, which are plant constituents that possess different biological potentials, such as antioxidant and antimicrobial activities [22,23,24,25]. To determine the antioxidant capacity, many different methods based on free radical scavenging have been developed in recent decades; in some cases, these mechanisms have not yet been clearly differentiated. An antioxidant capacity test may involve electron-based transfer (ET)- or hydrogen atom transfer (HAT)-based analyses [26]. The ferric-reducing/antioxidant power (FRAP) method (referring to ferric-reducing ability) measures the potential of antioxidants to reduce ferric iron, and has been presented as a new method for the evaluation of antioxidant capacity [27]. Cupric-reducing antioxidant capacity (CUPRAC), which involves measuring the reduction of Cu (II) to Cu (I) by antioxidants, is an uncomplicated method for extensively relevant analysis of antioxidant capacity, and is beneficial for a broad class of polyphenols, including phenolic acids, flavonoids, carotenoids, hydroxycinnamic acids, thiols, synthetic antioxidants, and vitamins C and E [28,29]. The DPPH (2,2-diphenyl-1-picrylhydrazyl) method measures the ability of antioxidants to reduce 2,2-diphenyl-1-picrylhydrazyl (DPPH), an organic radical, through spectrophotometry [30]. The DPPH assay is the oldest non-direct assay for antioxidant activity determination, based on the potential of the 2,2-diphenyl-1-picrilhydrazyl free radical to interact with phenol hydrogen donors [31].
Considering the importance of Salix alba in traditional and modern medicine, in this study, we aim to analyze and evaluate the influence of selected heavy metals in the antioxidant response of Salix alba bark from the highly polluted area around the Kosovo thermal power plants. The antioxidant capacity of Salix alba bark was evaluated in terms of total phenolics, flavonoids, chlorophylls, and carotenoids, while the metal content in soil and willow bark was analyzed by ICP-OES. For total antioxidant level assessment, FRAP, DPPH, and CUPRAC assays were carried out.
2. Materials and Methods
2.1. Sampling Points and Material
Thermal power plants Kosovo A and Kosovo B, as a part of the local heavy industry and major contributors to environmental pollution in the area, are located about 6.5 km northwest of capital of Kosovo, Pristina, near the municipality of Obiliq. Ninety water, soil, and plant samples (thirty of each) were collected along 30 km of the Sitnica river, which passes near the Kosovo thermal power plants, prior to the determination of heavy metal contents and other parameters. Fifteen samples each of water, soil, and Salix alba bark were taken from a starting point near Lipjan municipality, about 17 km from the source of pollution, up to nearby the KTPPs, and fifteen additional samples of each were collected, beginning near the thermal power plants, up to the ending point where the river exits the municipality of Vushtrri, approximately 15 km from the source of pollution. There was an average distance of 1 km between sampling points, the locations of which are shown in Figure 1.
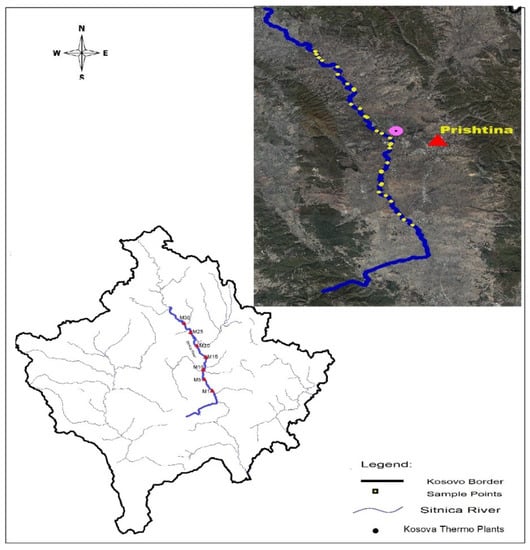
Figure 1.
The flow of the river Sitnica in Kosovo and the sampling points.
2.2. Plant Material
Bark samples (200–250 g) were taken from Salix alba located along the river (Figure 2), collected at a height of 1.5–2 m above the ground and packed in paper bags. To eliminate any surface particles or impurities, the willow bark samples were washed with deionized water and then dried in an oven for 24 h at 65 °C. Then, 6 mL concentrated nitric acid and 2 mL concentrated hydrogen peroxide were added into a microwave digestion vessel containing 500 mg of minced willow bark samples, which were digested in the acid solution and carefully shaken. The vessels were sealed and heated in a microwave and, after cooling to room temperature (20 °C), the solution was filtered and diluted to 50 mL in a volumetric flask with deionized water.

Figure 2.
Salix alba at sampling site 14 near the KTPPs.
2.3. Soil Material
At a depth of 30–40 cm around the willow tree, 2000 g of soil samples were collected and packed in plastic bags. Air-dried and milled samples (2.5 g) were digested with 10 mL aqua regia (concentrated nitric acid and hydrochloric acid in a 1:3 molar ratio) and carefully shaken. The vessels were closed after 20 min, and the solution was heated in a microwave. The solution was filtered and diluted with deionized water to 100 mL in a volumetric flask after the vessels were cooled to room temperature (20 °C).
2.4. Determination of Total Phenols
Powdered and dried plant material was used to extract phenolic compounds (0.1 g) with 80% (v/v) CH3OH in an ultrasonic bath for 30 min at 4 °C [32,33]. After that, the methanolic extracts were centrifuged at 12,000 rpm for 15 min, and the supernatants were used for the determination of phenolic compound and chlorophyll contents, as well as antioxidant activities. The total phenolic contents were determined by mixing plant methanolic extracts with Folin–Ciocalteu reagent and 0.7 M Na2CO3 [34]. The samples were incubated at 50 °C for 15 min, then cooled at room temperature. The absorbance was measured spectrophotometrically at 765 nm. The total phenolic content data are expressed as milligrams of gallic acid equivalent (GAE) per gram of dry weight (mg GAE g−1 DW).
2.5. Determination of Total Flavonoids
The assay described by Zhishen et al. (1999) was used to determine the total flavonoid content in plant extracts. Properly diluted extract was mixed with 5% NaNO2 and allowed to react for 5 min. After that, 10% AlCl3 was added and the mixture stood for another 5 min. Finally, 1 M NaOH and distilled water were added to the reaction mixture and the absorbance was measured spectrophotometrically at 510 nm [35]. Total flavonoid data are expressed as milligrams of catechin equivalent (CE) per gram of dry weight (mg CE g−1 DW).
2.6. Determination of Total Chlorophylls and Carotenoids
The chlorophyll and carotenoid contents in plant extracts were determined using the method of Lichtenthaler and Wellburn (1983) [36]. The absorbance of an acetone extract was measured at 666 nm (chlorophyll a), 653 nm (chlorophyll b), and 470 nm (carotenoids). The chlorophyll and carotenoid concentrations are expressed as mg chlorophyll/carotenoid per gram of dry weight (mg g−1 DW) [36].
2.7. Cupric-Reducing Antioxidant Capacity (CUPRAC)
The CUPRAC assay of plant extracts was carried out using the method of Apak et al. (2004). The reaction mixture consisted of plant extract, 10 mM CuCl2, 7.5 mM neocuproine, and 1 M CH3COONH4 buffer (pH 7.0). The samples were incubated for 30 min at room temperature and the absorbance was measured at 450 nm. The molar extinction coefficient of Trolox (ε535 = 1.67 × 104 Lmol−1·cm−1) was used for CUPRAC determination. The CUPRAC values of plant extracts are expressed as micromoles of Trolox equivalent (TE) per gram of dry weight (μmol TE g−1 DW).
2.8. Ferric-Reducing Antioxidant Power (FRAP)
The FRAP assay of plant extracts was conducted according to the method of Benzie and Strain (1996) [37]. The FRAP reagent consisted of 300 mM CH3COONa buffer (pH 3.6), 10 mM TPTZ (2,4,6-tri(2-pyridyl)-s-triazine) in 40 mM HCl, and 20 mM FeCl3 (10:1:1, v/v/v). An aliquot of plant extract was mixed with FRAP reagent and incubated for 30 min at 37 °C under dark conditions. The absorbance of samples was measured at 593 nm. Data are expressed as micromoles of Fe2+ equivalent per gram of dry weight (μM Fe2+ g−1 DW).
2.9. DPPH Radical Scavenging Activity
Using the method of Brand-Williams et al. (1995), we determined the ability of plant extracts to scavenge the stable free radical 2,2-diphenyl-1-picrylhydrazyl (DPPH). The reaction mixture consisted of plant extract and 0.25 mM DPPH in CH3OH. The extract was substituted with CH3OH in the control sample. The DPPH scavenging reaction was performed in the dark for 10 min at room temperature, and the decrease in absorbance was measured at 517 nm. Data are expressed as micromoles of Trolox equivalent (TE) per gram of dry weight (μM TE g−1 DW) [38].
2.10. Reagents and Equipment
The amounts of metals in the sample were determined by inductively coupled plasma optical emission spectrometry (ICP-OES), using a Perkin Elmer Optima 2100 DV (Optical Emission Spectrometer), which was set up and optimized according to the manufacturer’s instructions. Blank samples were prepared in the same manner. A high-quality KERN ABJ analytical balance was used for weight measurement, and samples were milled in a Retsch miller (GM 200; plant samples). The samples were dried in an oven (Mega Term M-160) and heated in a Berghof Microwave (MWS 3+).
The following solvents (of analytical grade) were used: methanol (Sigma-Aldrich, Steinheim, Germany) and acetone (Carlo Erba, Milan, Italy). The following chemicals (of analytical grade) were used: Folin–Ciocalteu reagent (Carlo Erba, Milan, Italy); and sodium carbonate, sodium acetate, sodium nitrite, sodium hydroxide, ammonium acetate, aluminum chloride, cupric chloride, neocuproine, catechin hydrate, (±)-6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid, gallic acid, 2,4,6-tri(2-pyridyl)-s-triazine, ferric chloride, and 2,2-diphenyl-1-picrylhydrazyl (Sigma-Aldrich, Steinheim, Germany). Spectrophotometric analyses of antioxidant parameters were carried out using a SpectraMax 190 Microplate Reader (Molecular Devices Corp., Sunnyvale, CA, USA) with SoftMax Pro (v. 5.4.1) software.
2.11. Statistical Analysis
All parameters were analyzed using the data analysis tools in VassarStats (http://vassarstats.net/ accessed on 4 February 2022) and Microsoft Excel 2016. A Pearson correlation test was used to analyze the correlation between variables in soil and Salix alba bark samples. The values indicate statistical significance at p < 0.05. The obtained data from the analysis of total phenols, flavonoids, and chlorophylls and carotenoids are expressed as mean values ± SD (n = 3). The correlations between the analyzed parameters were calculated according to Pearson’s correlation coefficient, and details are presented in the Results and Discussion sections. Principal component analysis (PCA) was performed to determine the variance of selected parameters. Cluster analysis (CA) was conducted to determine the similarity and variance of influencing factors across various data sets [39].
3. Results
3.1. Selected Elements (Metals/Metalloids) in Soil
The content of heavy metals in soil is influenced by a variety of factors. Due to its negative ecological impacts, the heavy metal contamination of soil is a major environmental concern. Heavy metals occur naturally in low concentrations in soils; however, due to their acute and chronic toxicity, they are considered soil pollutants at high concentrations [40].
On both sides of the Sitnica river are several rivers and ravines, some of which pass near the KTPPs, having an obvious impact on it being one of the most polluted rivers in Kosovo. Water samples were taken at points from 30 km along the Sitnica river (approximately 15 km in both directions from the source of pollution: KTPPs), and were analyzed for the accumulation of aluminum, arsenic, cadmium, chromium, copper, iron, nickel, lead, and zinc. The values (mg L−1) of selected elements in water samples varied, depending on the location from which the samples were taken and the distance from the main source of pollution (i.e., KTPPs). The results are presented in Table 1, showing the minimum, maximum, and average values for the analyzed metals/metalloids.

Table 1.
Mean, minimum, and maximum contents (mg L−1) of analyzed metals/metalloids in Sitnica river.
The mean value of aluminum in the analyzed water samples was 0.346 mg L−1, with the highest concentration in water (W) sample W6 (0.99 mg L−1) and the lowest in sample W11 (0.163 mg L−1). The average amount of arsenic found in sampling sites was 0.01 mg L−1 (ranging from 0.0019 mg L−1 (W23) to 0.022 mg L−1 (W18)), while cadmium concentrations in the analyzed samples were all below the detection limit of the method used (i.e., 0.001 mg L−1). Chromium values in all analyzed Sitnica river water samples were in the interval from 0.006 mg L−1 (W13) to 0.037 mg L−1 (W1). The mean copper value in the selected water samples was 0.014 mg L−1, with a minimum concentration of 0.011 mg L−1 in sample W9 and the highest concentration in sample W24 (0.024 mg L−1). The mean concentration of iron in the analyzed water samples from Sitnica river was 0.314 mg L−1, with values ranging from 0.069 mg L−1 in sample W27 to 1.571 mg L−1, detected in sample W6. Nickel is a very hazardous and dangerous element, and its content in the study area varied from 0.029 mg L−1 to 0.078 mg L−1. During this research, traces of lead were determined only in four water samples, with concentrations ranging from 0.299 mg L−1 (W15) to 0.642 mg L−1 (W12). The mean detected value in water samples from Sitnica river for zinc was 0.093 mg L−1, with values ranging from 0.071 mg L−1 in sample W28 to the maximum concentration in sample W2 (0.122 mg L−1) [41]. These results indicate that the mean levels (expressed in mg L−1) of the selected metals/metalloids in water samples covering 30 km of Sitnica river (which passes near the KTPPs) were in the following order: Pb ˃ Al ˃ Fe ˃ Zn ˃ Ni ˃ Cu ˃ Cr ˃ As.
Soils are not considered contaminated, from human, animal, and plant standpoints, until a threshold concentration is surpassed and the metal(s) begin to influence biological processes [42]. The maximum permissible concentrations of heavy metals in soil are defined differently, mainly based on regulations at the national level, with some regional differences. All findings from soil samples collected along the Sitnica river are expressed on the basis of mg kg−1 dry mass; the data are summarized in Table 2. Selected mineral and trace elements were determined in close proximity to Salix alba roots at the same sampling sites. The soil pH was generally alkaline, ranging from 7.4 in sample S1 (S: soil sample) to 8.5 in sample S30, with a mean pH value of 8.08.

Table 2.
Mean, minimum, and maximum concentrations (mg kg−1) of analyzed metals/metalloids in soil.
Aluminum was the largest constituent of inorganic soil fractions, typically ranging from 1% to 30% (i.e., 10,000 to 300,000 mg kg−1) [43,44]. The obtained data suggest that the average aluminum concentration in analyzed samples in the KTPP region was in line with these criteria, where the highest concentration was observed in sample S9 and the lowest concentration in sample S29. The arsenic concentration ranged from 20.29 mg kg−1 (sample S18) to the highest determined level of 78.36 mg kg−1 in sample S10, which was collected within 10 km of the KTPPs. This is higher than the results obtained for the Anadrinia area (Kosovo), where the arsenic values ranged from 16.49 mg kg−1 to 62.44 mg kg−1 [45]. Cadmium is one of the most environmentally hazardous metals, harmfully impacting all biological processes in living organisms [42]. The cadmium concentration varied from the lowest content of 9.83 mg kg−1, which was practically identical to the lowest content for the Anadrinia area (9.36 mg kg−1) [45], to the highest content of 22.23 mg kg−1, which is higher than the intervention value when compared with data of Dutch Target and Intervention Values (2000). The mean cadmium content was estimated to be 14.85 mg kg−1, and the highest and lowest concentrations appeared at the same sampling points, presenting the highest and lowest concentrations of arsenic (sample S10 and S18), respectively. In all soil samples, the chromium content was less than the target value (100 mg kg−1) [46], ranging from 18.07 mg kg−1 in sample S1 to 35.85 mg kg−1 in sample S22. The average copper concentration was measured to be 58.85 mg kg−1, with the lowest (29.88 mg kg−1) and highest (100.02 mg kg−1) concentrations in sampling locations close to each other (samples S27 and S29). The average concentration of copper in the samples taken along Sitnica river was about 2.5 times higher compared with the average values of 20 to 30 mg kg−1 reported by Hooda (2010) [47]. Iron was the heavy metal with the highest average concentration (16,975.67 mg kg−1) in the analyzed soil samples along Sitnica river and, at the same time, was the element with the highest concentration in all types of analyzed samples. The lowest concentration (13,216.97 mg kg−1) was detected in sample S18, which was within 10 km of the source of pollution, while the highest concentration (20,399 mg kg−1) was detected in sample S28. Nickel is most commonly found in soil and plants absorb nickel quickly and easily [48]. An average value of 25 mg kg−1 for nickel has been reported in the region surrounding Tunçbilek thermal power plant in Turkey, which is lower than the average value (95.04 mg kg−1) found in samples around the KTPPs [49]. The measured lead content ranged from 84.20 mg kg−1 (sample S18) to 667.97 mg kg −1 (sample S29), thus ranging from the target value’s margin (85 mg kg−1) to above the intervention value (530 mg kg−1) [50]. This content was higher than that reported in records for the Anadrinia region, which ranged from 16.27 mg kg−1 to 42.58 mg kg−1 [45]. In terms of distance from the source of contamination, there were also differences in lead concentration between sampling points, with the highest concentration at sample point S29, located north of the KTPPs. Zinc, which is toxic to plants, occurs in soil mainly due to industrial contamination. The mean content in soil samples taken along the Sitnica river was more than four times higher, compared with data from the U.S., where the zinc concentration in agricultural soil ranges from 1.5 to 264 mg kg−1 [51]. The highest concentration of zinc (948.71 mg kg−1) in the analyzed soil samples was detected in the same sample with the highest level of cadmium (sample S10), whereas the lowest concentration was observed in sample S27. Based on the average concentrations of the selected elements in soil samples taken along the Sitnica river, the following order was observed: Fe ˃ Al ˃ Zn ˃ Pb ˃ Ni ˃ Cu ˃ As ˃ Cr ˃ Cd.
3.2. Selected Elements (Metals/Metalloids) in Willow Bark Samples of Salix alba
Different types of plants accumulate different minerals and trace elements, based on the environmental state, type of element, and plant potential for heavy metals/metalloids [52]. Table 3 shows the concentrations of selected elements in willow bark samples obtained in the KTPP-polluted area along the Sitnica river, with a summary of mean, maximum, and minimum concentrations of the analyzed elements.

Table 3.
Mean, minimum, and maximum contents (mg kg−1) of analyzed metals/metalloids in Salix alba bark samples.
All 10 of the selected mineral and trace elements—aluminum, calcium, cadmium, chromium, copper, iron, magnesium, manganese, nickel, and zinc—were found in the Salix alba bark samples, while arsenic, lead, and molybdenum contents were below the detection limits for the analysis methods used.
It has been shown that aluminum is more soluble and that its bioavailability increases in acidic soil and water (pH < 5.5), while it tends to be biologically inactive in neutral alkaline media (pH 5.5–8.0); however, its solubility increases in a strongly alkaline medium (pH > 8.0), but its bioavailability in plants is not known [53]. Aluminum was found in all plant samples, ranging from 67.79 mg kg−1 in sample P29 (P: plant) to 894.77 mg kg−1 in sample P17; standard recorded values range from 10,000 to 300,000 mg kg−1 [42]. Calcium was detected in relatively high quantities, ranging from 5260 mg kg−1 to 22,280 mg kg−1, in all plant samples tested [53]. The presence of calcium in high amounts restricts plant communities on calcareous soils [54]. The highest concentration of calcium was detected in sample P10 of willow bark, taken on the southern side of the KTPPs, while the lowest concentration of calcium was detected in sample P27, taken north of the KTPPs along the Sitnica river [55]. It has been shown that the genus Salix can accumulate large amounts of toxic elements, especially cadmium [56]. The cadmium content varied from 0.09 mg kg−1 to 4.49 mg kg−1, with maximum detected concentrations similar to those reported by Vandecasteele et al. (2002) [57] (1.3–3.6 mg kg−1), and significantly higher than the WHO-recommended level (<0.02 mg kg−1) [46]. Cd affects the biochemical, physical, and genetic levels in plants, and is highly toxic even at low concentrations, but it has an affinity for concentrating in plants and marine species [58]. Interest in the study of chromium has recently increased, mainly due to the importance of chromium as an essential trace element. The soluble content of chromium in the soil controls the amount of chromium in plants. In plants, the concentration of chromium varied from 0.006 to 18 mg kg−1 d.w. and in the analyzed samples, the level of chromium ranged from 0.85 mg kg−1 (in sample P27) to 1.89 mg kg−1 (in sample P17), and was mostly (in 20 willow samples) within the WHO recommendation for plants (1.30 mg kg−1) [46]; namely, the mean content of chromium (1.28 mg kg−1) was within the suggested concentration. The copper concentration ranged from 5.09 mg kg−1 (sample P12) to 28.66 mg kg−1 (sample P7), the latter being higher than the WHO-recommended acceptable amount (10 mg kg−1) in medicinal plants. Copper is an important micronutrient, for which the level of critical deficiency ranges from 1 to 5 mg kg−1, the suitable level is 6–12 mg kg−1, and the toxic level is above 20–30 mg kg−1 dry weight [59]. In this study, the mean content of copper (9.1 mg kg−1) was within the range of suitable values for plants. The iron level in the willow bark samples ranged from 66.79 mg kg−1 (in sample P29) to 910.75 mg kg−1 (in sample P10), with an average concentration of 385.47 mg kg−1. These findings were higher than the WHO-recommended iron concentration (20 mg kg−1) in medicinal plants. The concentration of magnesium in plant samples ranged from 840 mg kg−1 (in the starting point sample, P1) to 1680 mg kg−1 (in sample P7). The values of Mg in willow bark, with an average concentration of 1360 mg kg−1, were higher than the acceptable level (200 mg g−1) for plants, according to the WHO report (1996) [46]. Exposure to magnesium for long periods may inhibit the accumulation of large amounts of ammonium in plants, so the Mg/Mn ratio can also be taken as an indicator of manganese toxicity in plants [60]. The manganese content in willow bark samples varied between 19.68 mg kg−1 (in sample P13, the entering point near the KTPPs) and 392.75 mg kg−1 (from the ending sample point, P30), which was above the acceptable level of manganese in medicinal plants recommended by the WHO (200 mg kg−1). The mean content of manganese (123.13 mg kg−1) was within the allowable range. The nickel content in willow bark samples varied from 6.49 mg kg−1 (in sample P27) to 10.09 mg kg−1 (in sample P11), which was very close to the highest allowed concentration (10 mg/kg) recommended by the WHO [61]. The acceptable concentration of nickel in plant tissues ranged between 0.5 and 5 mg kg−1, and the mean content observed in willow bark samples (7.69 mg kg−1) exceeded this limit [62]. The zinc content in all analyzed willow bark samples was higher than the recommended content in medicinal plants (50 mg kg−1), ranging from 56.39 mg kg−1 (sample P22) to 140.94 mg kg−1 (P11). Sample P11, where the maximum content of zinc was detected, was collected very close to the KTPPs. The special motility system of zinc possessed by plants allows for its absorption from the soil, while a high content of zinc limits the growth of roots [63,64]. The data indicate that the concentrations of the selected elements in willow bark were in the following order (mean results in mg kg−1): Ca > Mg > Fe > Al > Mn > Zn > Cu > Ni > Cr > Cd.
3.3. Phenolic Content and Antioxidant Properties of Salix alba
Plants are potential sources of antioxidants, such as tannins, terpenoids, saponins, flavonoids, phenols, ascorbic acid, and many more compounds having the potential to scavenge free radicals [65]. In Table 4, the values obtained at all the sampling points, in terms of the minimum, maximum, and mean values for the total phenols, total flavonoids, and chlorophylls and carotenoids, as well as mean values for CUPRAC, FRAP, and DPPH, are presented.

Table 4.
Summary of results regarding total phenols, total flavonoids, chlorophylls and carotenoids, and antioxidant activities (CUPRAC, FRAP, and DPPH) of Salix alba bark.
Total phenolic content was determined using the Folin–Ciocalteu method and are expressed as milligrams of gallic acid equivalent (GAE) per gram of dry weight. The range of total phenolics was from 12.63 to 78.53 mg GAE g−1 DW (Figure 3).
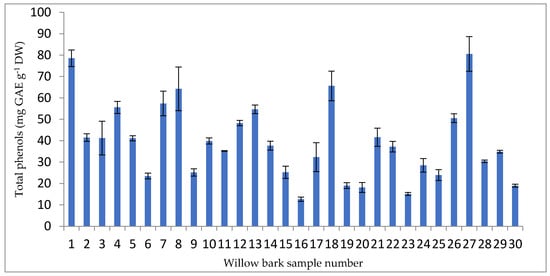
Figure 3.
Total phenols in willow bark samples (mg GAE g −1 DW).
The highest total phenolic content (80.537 mg GAE g−1 DW) was determined at sample point P27, while the lowest content was determined at sample point P16 (12.633 mg GAE g−1 DW). The mean value of total phenolics was 39.282 mg GAE g−1 DW, higher than the values reported by Tahirović and Bašić (2017) in Salix alba leaves (8.75 ± 0.006 mg GAE g−1) and Salix alba stem (7.02 ± 0.007 mg GAE g−1) [66].
It has been estimated that willow bark contains up to 20% of condensed tannins and flavonoids [67]. In willow bark samples, the flavonoids are expressed as milligrams of catechin equivalent (CE) per gram of dry weight (mg CE g−1 DW), and the results ranged from 9.25 mg CE g−1 DW (sample point P16) to 67.47 mg CE g−1 DW (sample point P1). The lowest result for phenolics and flavonoids in willow bark was detected at the same sample point (P16), which was the sampling site nearest to the KTPPs (Figure 4).
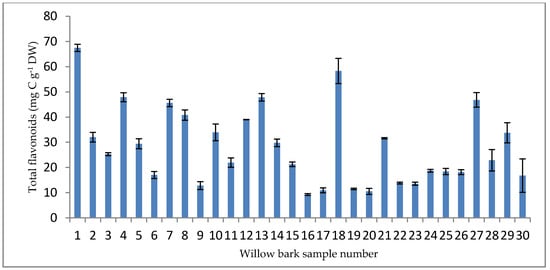
Figure 4.
Total flavonoids in willow bark samples (mg CE g −1 DW).
The concentrations of CHLa, CHLb, and CARs are presented as mg chlorophyll/carotenoid per gram of dry weight (mg g−1 DW). CHLa concentrations were in the range 13.73–86.28 mg g−1 DW (sample points P15 and P21, respectively), CHLb concentrations were in the range 26.06–71.50 mg g−1 DW (sample points P9 and P21, respectively), and carotenoids were in the range 42.26–152.58 mg g−1 DW (sample points P9 and P1, respectively); see Figure 5.
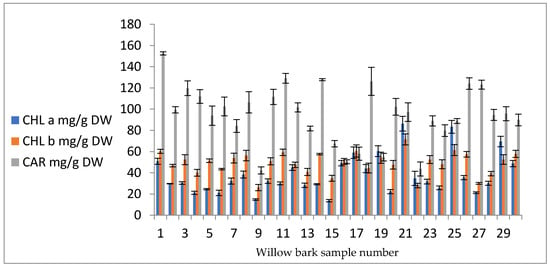
Figure 5.
CHLa, CHLb, and carotenoids (CARs) in willow bark samples (mg g−1 DW).
3.4. Antioxidant Activities of Salix alba
At present, no method can be considered reliable for determining antioxidant activity/capacity; therefore, a series of analyses is typically needed to assess the antioxidant potential. The antioxidant activity/capacity of the willow bark extracts were investigated using three methods: CUPRAC, FRAP, and DPPH. The CUPRAC method is an uncomplicated and flexible antioxidant capacity assay that can be used to measure phenolic acids, flavonoids, carotenoids, and other polyphenols [28]. It is a more favorable method due to the fact that, compared to other electron transfer methods, the working pH is physiological (pH = 7), compared to the acidic pH used in the FRAP method and the alkaline pH used in the Folin method [68]. The results of CUPRAC are expressed as μmol TE g−1 DW, and ranged from 92.927 μmol TE g−1 DW (sample point P16) to 454.651 μmol TE g−1 DW (sample point P27), as presented in Figure 6.
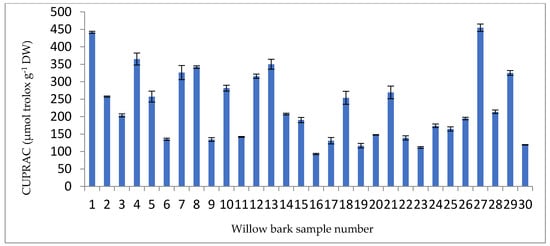
Figure 6.
CUPRAC results for willow bark samples (μmol TE g−1 DW).
The FRAP assay is an uncomplicated automated test that measures the ferric-reducing potential of plasma, and has been presented as a new method for assessing antioxidant power. This assay measures an antioxidant’s ability to reduce ferric iron. At a low pH, a complex of ferric iron and 2,3,5-triphenyl-1,3,4-triaza-2-azoniacyclopenta-1,4-diene chloride (TPTZ) is reduced to the ferrous form. The FRAP results are presented as micromoles of Fe2+ equivalent per gram of dry weight (μM Fe2+ g−1 DW) and, as presented in Figure 7, ranged from 89.61 μmol Fe2+ g−1 DW (sample P16) to 504.36 μmol Fe2+ g−1 DW (sample P18).
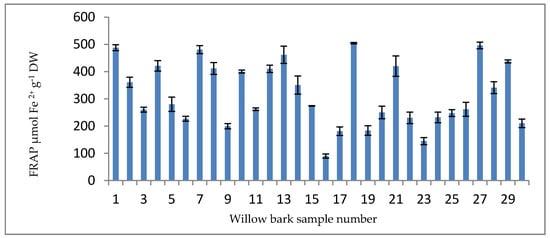
Figure 7.
FRAP results for willow bark samples (μmol Fe2+ g−1 DW).
The DPPH method spectrophotometrically measures the capacity of antioxidants to reduce 2,2-diphenyl-1-picrylhydrazyl (DPPH), an additional uncommon radical determined in biological systems. The final results are expressed in terms of μmol of Trolox equivalent (TE) per gram of dry sample. The DPPH scavenging activity, as presented in Figure 8, ranged from 31.28 μmol TE g−1 DW (sample P16) to 164.26 μmol TE g−1 DW (sample P1).
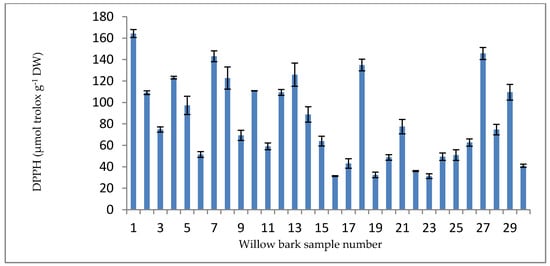
Figure 8.
DPPH results for willow bark samples (μmol TE g−1 DW).
Compared with the mean values presented by Tahirović and Bašić (2017), the mean value in our willow bark samples (82.742 μmol Trolox g−1 DW) was higher than in leaves (31.25 ± 0.19 μmol TE g−1) and Salix alba stem (30.13 ± 0.21 μmol TE g−1 DW). Furthermore, the mean value of DPPH was lower than that reported by Sulaiman et al. (2013) for Salix alba bark [69].
The antioxidant activity decreased in the following order: FRAP ˃ CUPRAC ˃ DPPH. The lowest results were detected mostly in samples taken from the area very close to the KTPPs (willow bark sample P16 for TP, TF, CUPRAC, FRAP, and DPPH, and CHLa in sample P15), and at sample point P9 (for CHLb and CAR). Meanwhile, the highest results were detected at sample P1 (TF, CAR, and DPPH), sample P18 (FRAP), sample P21 (CHLa and CHLb), and sample P27 (TP and CUPRAC).
3.5. Correlation Analysis between Selected Elements in Soil and Salix alba Bark
Pearson correlation analysis indicated moderate correlations between copper in water with nickel in soil (r = 0.382), as well as nickel in water to arsenic (r = 0.400), cadmium (r = 0.439), and chromium (r = 0.392) in soil. Regarding the correlations between the analyzed elements in soil and Salix alba bark, aluminum showed a moderate correlation with calcium in willow bark samples (r = 0.436); arsenic in soil with cadmium in willow bark (r = 0.653); cadmium in soil with its content in willow bark (r = 0.561); chromium in soil showed moderate correlations with copper and manganese in willow bark (r = 0.343 and r = 0.385, respectively); iron in soil with calcium (r = 0.513), magnesium (r = 0.449), and manganese (r = 0.438) in willow bark; nickel in soil showed moderate positive correlations with zinc (r = 0.558) and nickel (r = 0.340) in willow bark; and zinc in soil with cadmium in willow bark (r = 0.642).
The Pearson correlation matrix, provided in Table 5, shows significant correlations between most of the metals/metalloids determined in willow bark. Note the strong positive correlations between aluminum and chromium (r = 0.893), nickel (r = 0.883), and iron (r = 0.880); chromium and nickel (r = 0.801) and iron (r = 0.793); and the moderate negative correlation between calcium and manganese (r = −0.398).

Table 5.
Correlations between selected elements in willow bark.
3.6. Correlation Analysis between Selected Elements, Production of Photosynthetic Pigments and Phenolic Compounds, and Antioxidant Activities in Salix alba Bark Samples
The Pearson correlation matrix revealed significant positive correlations between Al, Cr, Fe, and Ni contents, as well between Cd, Fe, and Ca contents. Furthermore, Ca content was significantly positively correlated with Mg content, but negatively correlated with Mn content. With respect to the phenolic compounds, TP and TF contents were positively correlated with the CAR contents, as well as with the antioxidant activity assays (CUPRAC, FRAP, and DPPH). However, phenolic compounds—particularly TF—were significantly negatively correlated with Fe, Ni, and Mn contents. Regarding the photosynthetic pigments, CHLa content was negatively correlated with Fe and Ca contents. Antioxidant activity assays were significantly negatively correlated with the Ni and Mn contents. In addition, the FRAP assay was negatively correlated with Al, Cr, and Fe contents.
The data in Table 6 indicate significant positive correlations (Pearson coefficients) between TF and TP (r = 0.889); CHLb and CHLa (r = 0.665); CAR and TP, TF, and CHLb (r = 0.594, r = 617, and r = 0.332, respectively); CUPRAC and TP, TF, and CAR (r = 0.882, r = 0.902, and r = 0.540, respectively); FRAP and TP, TF, CAR, and CUPRAC (r = 0.854, r = 0.922, r = 0.577, and r = 0.910, respectively); and DPPH and TP, TF, CAR, CUPRAC, and FRAP (r = 0.886, r = 0.952, r = 0.568, r = 0.939, and r = 0.933, respectively).

Table 6.
Correlation analysis between selected elements, production of photosynthetic pigments and phenolic compounds, and antioxidant activities in Salix alba bark samples.
4. Discussion
High concentrations of toxic elements in surface soil and groundwater, compared to normal and permissible values, are an increasing problem, considering their threat to the environment and human health. This is primarily a result of the development of many branches of chemical-based industries and the large discharges from them. Except for two of the samples, the aluminum content in water samples was higher than the level reported by Oram B (2020) [70]. The average arsenic, chromium, and copper concentration in all samples along Sitnica river were below the permissible metal limit reported by Brent L [71]. The iron levels in several sampling sites exceeded the average values reported for the presence of iron in rivers (0.5–1 mg L−1) [72]. The obtained values for nickel were higher than those reported by Shala et al. (2015), detected at the source of Sitnica river (0.015–0.033 mg L−1) [72]. Although lead was determined in only four samples, the values indicate that, in the samples in which it was detected, the lead concentration was higher than the value allowed by the WHO (0.05 mg L−1), and the zinc content in all water samples was under the limit described by the WHO (5 mg L−1) [46]. In terms of maximum detected concentrations, most of the selected elements showed the same tendency, in that the maximum values were observed in samples taken before the river approaches the KTPPs (Al, W6; Cr, W1; Fe, W6; Ni, W10; and Zn, W2), while the maximum arsenic value was detected in a sample taken near the KTPPs (W15). Conversely, the maximum copper concentration was detected in a sample (W24) at a distance of about 10 km from the KTPPs, in the flow of the Sitnica river to the north. Of the analyzed elements, only arsenic showed a tendency toward a greater concentration in samples taken in the vicinity of (i.e., within 5 km in both directions) the source of pollution; that is, the KTPPs.
The aluminum concentrations in all soil samples collected along the Sitnica river were within the typically reported values of Lindsay (1979) and Dragun (1988) [43,44]. The arsenic concentration in most samples was above the values reported in the Anadrinia region in Kosovo [45]. Cadmium, as one of the greatest ecotoxic metals, showed content in the analyzed samples that was higher than intervention values of the Dutch list (12 mg kg−1) [50]. The chromium content in all samples was higher than results from samples around the Tunçbilek thermal power plant, Turkey [49], but presented lower concentrations when compared with data from the Anadrinia region [45]. The copper content in soil samples was above the average values of 20–30 mg kg −1 [47], and mostly above the target value presented in the Dutch list (36 mg kg−1) [50]. The iron content was within the permissible range of 7000–500,000 mg/kg [71]. Nickel content was above average values, compared with data from the area surrounding Tunçbilek thermal power plant, Turkey (25 mg kg−1) [49]. In terms of lead content, the intervention value (Dutch list; 530 mg kg) was only exceeded at sampling point S29. The zinc mean value of 226.7 mg kg−1 was higher than the natural zinc concentration in soil (10–100 mg kg−1) [73]. Compared with data in the FAO Soil Bulletins (Tietjen, 1975) for the tolerable total content of some elements in soils, cadmium and lead exceeded the tolerable values [74]. Arsenic and cadmium showed the same tendency, with maximum and minimum values at the same sampling sites: the maximum content was determined for both of these elements in sample S10 and the minimum value was in sample S18. Furthermore, the highest value of zinc was determined in sample S10 and, in nearby sample S9, the highest value of aluminum was observed. Copper, iron, and lead showed a similar tendency, in that the highest value was detected in samples from sampling sites in the north direction of the Sitnica river (S29, Cu and Pb; S28, Fe). In sample S18, in addition to arsenic and cadmium, minimum values for iron and lead were also detected, while aluminum, copper, and zinc showed the same tendency of lower values being presented in the northern part of the sampling points (S29, Al; S27, Cu and Zn). Of the analyzed elements, copper and nickel showed a tendency toward greater concentrations in samples taken in the area in close vicinity (i.e., within 5 km in both directions) to the source of pollution: KTPPs. In samples collected within 5–10 km from the source of pollution, arsenic, cadmium, chromium, and zinc presented higher concentrations; however, at a distance of more than 10 km from the pollution source on both sides, aluminum, iron, and lead tended to accumulate more.
The arsenic, lead, and molybdenum contents in Salix alba bark were below the detection limits for the methods used. The aluminum content was within the limits reported in various plants, which range up to 1000 mg kg−1. A significantly high content of calcium was detected in all willow bark samples, of which the highest content (2.2%) was within the range of acceptable values reported by the WHO (1996), and the cadmium content in all willow bark samples was higher than the value recommended for plants by the WHO (<0.02 mg kg−1) [46]. The chromium level in ten willow bark samples was higher than the acceptable level (1.30 mg kg−1) suggested by the WHO for plants, while copper in seven willow bark samples exceeded the content suggested by the WHO (10 mg kg−1). In all of the willow bark samples, the iron content exceeded the value recommended by the WHO for plants (20 mg kg−1). The magnesium content in all analyzed willow bark samples also exceeded the range suggested as acceptable by the WHO for plants (200 mg kg−1). The nickel content in willow bark was above the acceptable concentration for nickel in plant tissue (0.5–5 mg kg−1) recommended by Salt et al. (2000) [58]. Finally, the zinc content was higher than the recommended level for plants (50 mg kg−1) [46].
Considering critical heavy metal values, it was observed that levels of cadmium, iron, nickel, lead, and zinc exceeded these values in soil, while only iron exceeded the critical value reported for plants in the willow bark samples. Compared with reported average data for Salix alba, the copper and manganese mean content in our study area were higher than WHO-reported data (Cu: 9.1 mg kg−1 vs. 8.89 mg kg−1; Mn: 123.13 mg kg −1 vs. 43.94 mg kg−1), but the value was lower in the case of zinc (87.77 mg kg−1 vs. 94.10 mg/kg) [75].
In terms of the maximum reported content of selected elements in willow bark samples, with the exception of manganese (P30), in the area including samples P7–P17 the maximum content was observed for all other elements. In willow bark sample P10, the maximum contents of Ca, Cd, and Fe were observed; Ni and Zn were highest in P11; Cu and Mg were highest in sample P7; and Al and Cr were highest in sample P17. The lowest contents were mostly detected in samples P27 (Ca, Cr, Ni), P29 (Al, Fe), P12 (Cd, Cu), P13 (Mn), and P18 (Ca). Between toxic and essential elements, there was a moderate correlation between aluminum and calcium content (r = 0.316); strong correlations between aluminum and iron (r = 0.880), nickel (r = 0.883), and chromium (r = 0.893); and a moderate correlation between cadmium and iron (r = 0.392) in Salix alba bark samples. Because the metal transmission from soil to Salix alba bark is observed in the following order of significant importance, zinc ˃ copper ˃ cadmium ˃ nickel [41], the results point toward the importance of studying and applying Salix alba in the process of phytoremediation. Sustainable decision-making processes regarding ecological restoration using phytoremediation processes should find the few most favorable factors (human, earth, ethical issues, etc.) [76].
The differences in mineral elements and trace composition, the production of photosynthetic pigments and phenolic compounds, and antioxidant activities between the thirty Salix alba bark samples were analyzed using principal component analysis (PCA) and hierarchical agglomerative cluster analysis (HACA). These statistical tools were utilized to determine the variance in mineral element and heavy metal contents (Ca, Mg, Zn, Mn, Al, Cd, Cr, Cu, Fe, and Ni), the production of photosynthetic pigments (CHLa, CHLb, and CAR), the accumulation of phenolic compounds (TP) and flavonoids (TF), and antioxidant activities (CUPRAC, FRAP and DPPH) within Salix alba bark samples, as well as to cluster the samples depending on the analyzed variables (see Figure 9 and Figure 10).
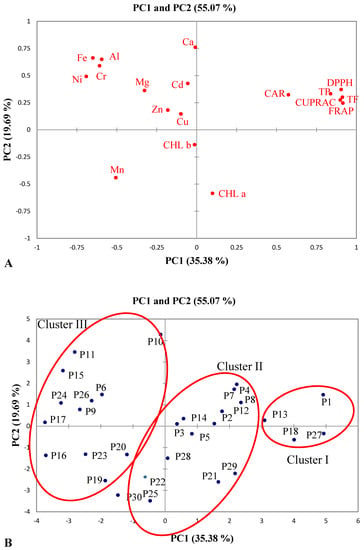
Figure 9.
Loading plot (A) and score plot (B) of principal component analysis for analyzed parameters of Salix alba bark samples.
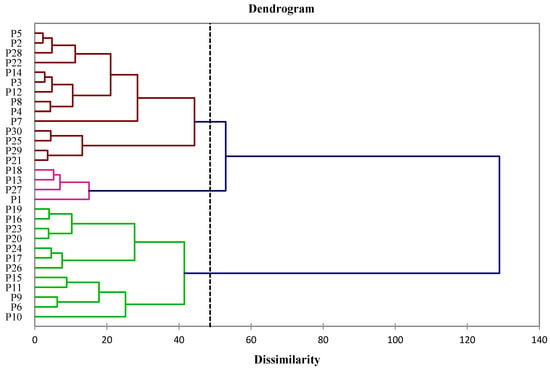
Figure 10.
Cluster analysis of analyzed parameters for Salix alba bark samples.
Two main principal components (PCs) were used to characterize the mineral element and heavy metal composition, the production of photosynthetic pigments and phenolic compounds, and the antioxidant activities of the thirty S. alba samples. The PCA data revealed that PC1 and PC2 explained 55.07% of the total variation. The PCA loading plot (Figure 9A) indicates that PC1 explained 35.38% of the variance, which was connected positively to TP, TF, CUPRAC, FRAP, and DPPH, but negatively to Ni, Fe, Cr, and Al content. Meanwhile, PC2 explained 19.69% of the variance, and was positively related to Ca content.
The results for the mineral and heavy metal composition, the production of photosynthetic pigments and phenolic compounds, and the antioxidant activities showed that samples of S. alba bark were separated with respect to PC1 and grouped into three clusters (Figure 9B and Figure 10).
Cluster I was represented by four samples (P1, P13, P18, and P27) with the largest positive scores in PC1. These samples were described as having a high production of phenolic and flavonoid compounds, as well strong antioxidant activities. In contrast, cluster III included 12 samples (P6, P9–P11, P15–P17, P19, P20, P23, P24, and P26) with the largest negative scores in PC1, which were characterized by the high accumulation of Ni, Fe, Cr, and Al. However, P10 and P11 samples were separated from this cluster, due to their high positive scores in PC2, and were therefore characterized by the increased accumulation of Ca. Cluster II was represented by 14 S. alba bark samples (P2–P5, P7, P8, P12, P14, P21, P22, P25, and P28–P30) with intermediate PC1 scores and which were characterized by average values for the production of phenolic and flavonoid compounds, antioxidant activities, and mineral element and heavy metal composition.
5. Conclusions
In this study, we showed that the group of chemical elements selected for analyses presented high contents in soil for the region around the KTPPs. As such, continuous and close monitoring—especially of arsenic, cadmium, copper, nickel, and lead—as well as their potential impacts on the environment and human health should be taken into account. Compared with WHO-recommended values, the mean cadmium content in Salix alba bark was approximately thirty times higher, while copper also exceeded the recommended values at several sampling points. Iron, magnesium, and zinc contents throughout the sampling area exceeded WHO values. The correlation between the cadmium and nickel contents in soil and their respective contents in Salix alba bark was demonstrated. Based on transfer factor data, it can be concluded that willow can be successfully used for phytoremediation, as it is able to extract and remove important quantities of heavy metals from soil, especially in terms of zinc, copper, cadmium, and nickel.
The results in this study indicate that the examined bark of Salix alba contained significant amounts of phenolic compounds and photosynthetic pigments with a strong positive correlation, proving to be sources of antioxidants with great potential. From the correlative point of view, negative moderate correlations were revealed between total phenolic compounds and iron, nickel, and manganese; photosynthetic compounds with iron and calcium; and antioxidant activity assays with nickel and manganese. In addition, the FRAP assay was negatively correlated with aluminum, chromium, and iron content in Salix alba bark. Further research is recommended, in order to analyze the impact of environmental pollution on the antioxidant capacity of plants, especially regarding Salix alba bark in regions where heavy metal/metalloid levels exceed recommended values. As already-published data have established that phenolic and flavonoid compounds are important components in drug design, isolated active components from Salix alba bark should be employed in future drug discovery research. Another aspect to be considered by future studies is the analysis of the uncertainty factors involved in making correct decisions to analyze the impact of heavy metals in Salix alba and its antioxidant potential.
Author Contributions
Conceptualization, D.B. and B.B.; methodology, D.B and L.Z.; software, D.B.; validation, D.B., B.B. and L.Z.; formal analysis, L.Z.; investigation, D.B.; resources, D.B.; data curation, D.B. and L.Z.; writing—original draft preparation, D.B.; writing—review and editing, D.B.; visualization, B.B.; supervision, B.B. and L.Z.; project administration, D.B.; funding acquisition, D.B. and L.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tawfeek, N.; Mahmoud, M.F.; Hamdan, D.I.; Sobeh, M.; Farrag, N.; Wink, M.; El-Shazly, A.M. Phytochemistry, Pharmacology and Medicinal Uses of Plants of the Genus Salix: An Updated Review. Front. Pharmacol. 2021, 12, 593856. [Google Scholar] [CrossRef] [PubMed]
- Barnes, J.; Anderson, L.A.; Phillipson, J.D. Herbal Medicines; Pharmaceutical Press: London, UK, 2007; pp. 598–600. [Google Scholar]
- Schmid, B.; Tschirdewahn, B.; Kötter, I.; Günaydin, I.; Lüdtke, R.; Selbmann, H.; Schaffner, W.; Heide, L. Analgesic effects of willow bark extract in osteoarthritis: Results of a clinical double-blind trial. Focus Altern. Complement. Ther. 2010, 3, 186. [Google Scholar] [CrossRef]
- Saller, R.; Melzer, J.; Felder, M. Pain relief with a proprietary extract of Willow bark in rheumatology. Open Trial. Schweiz. Zschr. Ganzheitsmed. Medizin. 2008, 20, 156–162. [Google Scholar] [CrossRef]
- Praciak, A.; Pasiecznik, N.M.; Sheil, D.; van Heist, M.; Sassen, M.; Correia, C.S.; Dixon Ch Fyson, G.E.; Rushforth, K.; Teeling, C. The CABI Encyclopedia of Forest Trees; CABI: Oxfordshire, UK, 2013. [Google Scholar]
- Dickmann, D.I.; Kuzovkina, J. Poplars and Willows in the World. In Poplars and Willows: Trees for Society and the Environment; Isebrands, J.G., Richardson, J., Eds.; Chapter: Poplars and Willows in the World Publisher; FAO: Rome, Italy, 2014; pp. 8–91. [Google Scholar]
- Isebrands, J.G.; Richardson, J. Poplars and Willows: Trees for Society and the Environment CABI; FAO: Rome, Italy, 2014. [Google Scholar]
- Larison, J.R.; Likens, E.; Fitzpatrick, J.W.; Crock, J.G. Cadmium toxicity among wildlife in the Colorado Rocky Mountains. Nature 2000, 406, 181–183. [Google Scholar] [CrossRef]
- Takáč, P.; Szabová, T.; Kozáková, L.; Benková, M. Heavy metals and their bioavailability from soils in the long-term polluted Central Spis Region of SR. Plant Soil Environ. 2009, 55, 167–172. [Google Scholar] [CrossRef] [Green Version]
- Casarett and Doull’s. Toxicology, the Basic Science of Poisons, 7th ed.; McGraw-Hill: New York, NY, USA, 2008; pp. 1103–1104. [Google Scholar]
- Tlustoš, P.; Szková, J.; Vysloužilová, M.; Pavlíková, D.; Weger, J.; Javorská, H. Variation in the uptake of Arsenic, Cadmium, Lead, and Zinc by different species of willows Salix spp. grown in contaminated soils. Cent. Eur. J. Biol. 2007, 2, 254–275. [Google Scholar] [CrossRef]
- Viehweger, K. How plants cope with heavy metals. Bot. Stud. 2014, 55, 35. [Google Scholar] [CrossRef] [Green Version]
- Ekmekçi, Y.; Tanyolaç, D.; Ayhan, B. Effects of cadmium on antioxidant enzyme and photosynthetic activities in leaves of two maize cultivars. J. Plant Physiol. 2008, 165, 600–611. [Google Scholar] [CrossRef]
- Zeneli, L.; Daci-Ajvazi, M.; Daci, N.M.; Hoxha, D.; Shala, A. Environmental Pollution and Relationship Between Total Antioxidant Capacity and Heavy Metals (Pb, Cd, Zn, Mn, and Fe) in Solanum tuberosum L. and Allium cepa L. Hum. Ecol. Risk Assess. Int. J. 2013, 19, 1618–1627. [Google Scholar] [CrossRef]
- Zeneli, L.; Daci, N.; Paçarazi, H.; Daci, A.M. Impact of Environmental Pollution on Human Health of Population Which Lives in Nearby Kosovo Thermopower Plants. Indoor Build Environ. 2011, 20, 479–482. [Google Scholar] [CrossRef]
- Pietarila, H.; Varjoranta, R. Dispersion of Exhaust Gases from Kosovo B Power Plant in Obilic, Kosovo; Finnish Meteorological Institute, Air Quality Research: Helsinki, Finland, 2005. [Google Scholar]
- Halliwell, B.; Gutteridge, J.M. The definition and measurement of antioxidants in biological systems. Free Radic. Biol. Med. 1995, 18, 125–126. [Google Scholar] [CrossRef]
- Halliwell, B. Biochemistry of oxidative stress. Biochem. Soc. Trans. 2007, 35, 1147–1150. [Google Scholar] [CrossRef] [PubMed]
- Justesen, U.; Knuthsen, P. Composition of flavonoids in fresh herbs and calculation of flavonoid intake by use of herbs in traditional Danish dishes. Food Chem. 2001, 73, 245–250. [Google Scholar] [CrossRef]
- Yadav, A.; Kumari, R.; Yadav, A.; Mishra, J.P.; Srivatva, S.; Prabha, S.H. Antioxidants and its functions in human body—A Review. Res. Environ. Life Sci. 2016, 9, 1328–1331. [Google Scholar]
- Anbudhasan, P.; Surendraraj, A.; Karkuzhali, S.; Sathishkumaran, S. Natural antioxidants and its benefits. Int. J. Food Nutr. Sci. 2014, 3, 225–232. [Google Scholar]
- Gupta, D. Methods for Determination of Antioxidant capacity: A Review. Int. J. Pharm. Sci. Res. 2015, 6, 546–566. [Google Scholar]
- Sin, H.P.Y.; Liu, D.T.L.; Lam, D.S.C. Life style Modification, Nutritional and Vitamins Supplements for Age-Related Macular Degeneration. Acta Ophthalmol. 2013, 91, 6–11. [Google Scholar] [CrossRef]
- Willis, L.M.; Shukitt-Hale, B.; Joseph, J.A. Recent advances in berry supplementation and age-related cognitive decline. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 91–94. [Google Scholar] [CrossRef]
- Kaneria, M.; Kanani, B.; Chanda, S. Assessment of effect of hydroalcoholic and decoction methods on extraction of antioxidants from selected Indian medicinal plants. Asian Pac. J. Trop. Biomed. 2012, 2, 195–202. [Google Scholar] [CrossRef] [Green Version]
- Huang, D.; Ou, B.; Prior, R.L. The Chemistry behind Antioxidant Capacity Assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Antolovich, M.; Prenzler, P.D.; Patsalides, E.; Mc-Donald, D.; Robards, K. Methods for testing antioxidant activity. Analyst 2002, 127, 183–198. [Google Scholar] [CrossRef] [PubMed]
- Özyürek, M.; Güçlü, K.; Tütem, E.; Başkan, K.S.; Erçağ, E.; Celik, S.E.; Baki, S.; Yıldız, L.; Karaman, S.; Apak, R. A comprehensive review of CUPRAC methodology. Anal. Methods 2011, 3, 2439–2453. [Google Scholar] [CrossRef]
- Apak, R.; Gorinstein, S.; Böhm, V.; Schaich, K.M.; Özyürek, M.; Güçlü, K. Methods of measurement and evaluation of natural antioxidant capacity/activity (IUPAC Technical Report). Pure Appl. Chem. 2013, 85, 957–998. [Google Scholar] [CrossRef] [Green Version]
- Moharram, H.A.; Youssef, M.M. Methods for Determining the Antioxidant Activity: A Review. Alex. J. Food Sci. Technol. 2014, 11, 31–42. [Google Scholar]
- Ansari, A.Q.; Ahmed, S.A.; Waheed, M.A.; Juned, A.S. Extraction and determination of antioxidant activity of Withania somnifera Dunal. Eur. J. Exp. Biol. 2013, 3, 502–507. [Google Scholar]
- Gadzovska, S.; Maury, S.; Delaunay, A.; Spasenoski, M.; Hagège, D.; Courtois, D.; Joseph, C. The influence of salicylic acid elicitation of shoots, callus, and cell suspension cultures on production of naphtodianthrones and phenylpropanoids in Hypericum perforatum L. Plant Cell Tissue Organ Cult. 2013, 113, 25–39. [Google Scholar] [CrossRef]
- Gadzovska, S.; Maury, S.; Delaunay, A.; Spasenoski, M.; Joseph, C.; Hagege, D. Jasmonic acid elicitation of Hypericum perforatum L. cell suspensions and effects on the production of phenylpropanoids and naphtodianthrones. Plant Cell Tissue Organ Cult. 2007, 89, 1–13. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef] [Green Version]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C.L.W.T. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Wang, Q.; Cui, Y.; Lin, X.M.; Dong, Y.Y.; Christie, P. Soil contamination and plant uptake of heavy metals at polluted sites in China. J. Environ. Sci. Health A Tox. Hazard. Subst. Environ. Eng. 2003, 38, 823–838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, S.-H.; Kim, D.-H.; Shin, S.-J. Bioaccumulation and Physiological, Response of Five Willows to Toxic Levels of Cadmium and Zinc. Soil Sediment Contam. 2013, 22, 241–255. [Google Scholar] [CrossRef]
- Bajraktari, D.; Bauer, B.; Kavrakovski, Z.; Zeneli, L. Correlation between Environmental Pollution and Metals Accumulation in Salix alba L. (Fam. Salicaceae). Agric. Conspec. Sci. 2019, 84, 95–101. [Google Scholar]
- Kabata-Pendias, A. Trace Elements in Soil and Plants, 4th ed.; Taylor & Francis Group: Boca Raton, FL, USA, 2011. [Google Scholar]
- Lindsay, W.L. Chemical Equilibria in Soils; John Wiley and Sons: New York, NY, USA, 1979. [Google Scholar]
- Dragun, J. Element adsorption and mobility in soil. In The Soil Chemistry of Hazardous Materials; Hazardous Materials Control Research Institute: Silver Spring, MD, USA, 1988. [Google Scholar]
- Shehu, I.; Bajraktari, N.; Demaku, S.; Bekolli, A.; Malsiu, A. The Study of Absorption of Heavy Metals from the Soil at Some Vegetables in Anadrinia Region in Kosovo. Int. J. Pharm. Sci. Rev. Res. 2016, 40, 324–329. [Google Scholar]
- WHO. Permissible Limits of Heavy Metals in Soil and Plants; WHO: Geneva, Switzerland, 1996. [Google Scholar]
- Hooda, P.S. Trace Elements in Soils; Wiley: Hoboken, NJ, USA, 2010; Volume 28, p. 443. [Google Scholar]
- Güne, A.; Alpaslan, M.; Ina, L.A. Plant Growth and Fertilizer; Pub. No: 1539; Univ. Agriculture Ankara: Ankara, Turkey, 2004. [Google Scholar]
- Cicek, A.; Koparal, A.S. Accumulation of sulfur and heavy metals in soil and tree leaves sampled from the surroundings of Tuncbilek Thermal Power Plant. Chemosphere 2004, 57, 1031–1036. [Google Scholar] [CrossRef]
- Dutch Target and Intervention Values, 2000 (the New Dutch List). Available online: https://www.esdat.net/environmental%20standards/dutch/annexs_i2000dutch%20environmental%20standards.pdf (accessed on 10 January 2022).
- Holmgren, G.G.S.; Meyer, M.W.; Chaney, R.L.; Daniels, R.B. Cadmium, lead, zinc, copper, and in agricultural soils of the United States of America. J. Environ. Qual. 1993, 22, 335–348. [Google Scholar] [CrossRef]
- Lokeshwari, H.; Chandrappa, G.T. Impact of heavy metal contamination of Bellandur Lake on soil and cultivated vegetation. Curr. Sci. 2006, 91, 622–627. [Google Scholar]
- Sparling, D.W.; Lowe, T.P. Environmental Hazards of Aluminum to Plants, Invertebrates, Fish and Wildlife. Reviews of Environmental Contamination and Toxicology; Springer: New York, NY, USA, 1996; Volume 145. [Google Scholar]
- Marschner, H. Mineral Nutrition of Higher Plants; Academic Press: London, UK, 1995. [Google Scholar]
- Bajraktari, D.; Petrovska, B.B.; Zeneli, L.; Dimitrovska, A.; Kavrakovski, Z. Soil chemical evaluation and power plant ash impact on chemical properties of Salix alba L. (Fam. Salicaceae): The impact of bioaccumulation. Toxicol. Res. Appl. 2020, 4, 1–8. [Google Scholar] [CrossRef]
- Klang-Westin, E.; Eriksson, J. Potential of Salix as phytoextractor for Cd on moderately contaminated soils. Plant Soil 2003, 249, 127–137. [Google Scholar] [CrossRef]
- Vandecasteele, B.; De Vos, B.; Tack, F.M.G. Cadmium and Zinc uptake by volunteer willow species and elder rooting in polluted dredged sediment disposal sites. Sci. Total Environ. 2002, 299, 191–205. [Google Scholar] [CrossRef]
- Benavides, M.P.; Gallego, S.M.; Tomaro, M.L. Cadmium toxicity in plants. Braz. J. Plant Physiol. 2005, 17, 21–34. [Google Scholar] [CrossRef] [Green Version]
- Morrison, R.S.; Brooks, R.D.; Reeves, R.D.; Malaise, F.; Iiorowitz, P.; Aronson, M.; Merrian, G.R. The diverse chemical forms of heavy metals in tissue extracts of metallophytes from Shaba province, Zaire. Photochem 1981, 20, 155–158. [Google Scholar] [CrossRef]
- Le Bot, J.; Goss, M.; Carvalho, M.J.G.P.R.; Van Beusichem, M.L.; Kirkby, E.A. The significance of the magnesium to manganese ratio in plant tissues for growth and alleviation of manganese toxicity in tomato (Lycopersicon esculentum) and wheat (Triticum aestivum) plants. Plant Soil 1990, 124, 205–210. [Google Scholar] [CrossRef]
- Dt, O.; Aa, A.; Oe, O. Heavy Metal Concentrations in Plants and Soil along Heavy Traffic Roads in North Central Nigeria. J. Environ. Anal. Toxicol. 2015, 5, 6. [Google Scholar] [CrossRef]
- Salt, D.E.; Kato, N.; Kramer, U.; Smith, R.D.; Raskin, I. The role of root exudates in nickel hyperaccumulation and tolerance in accumulator and nonaccumulator species of Thlaspi. In Phytoremediation of Contaminated Soil and Water; Terry, N., Banuelos, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2000; pp. 189–200. [Google Scholar]
- Herrero, E.M.; López-Gonzálvez, A.; Ruiz, M.A.; Lucas-García, J.A.; Barbas, C. Uptake and distribution of zinc, cadmium, lead and copper in Brassica napus var. oleífera and Helianthus annus grown in contaminated soils. Int. J. Phytoremediat. 2003, 3, 153–167. [Google Scholar] [CrossRef]
- Choi, J.M.; Pak, C.H.; Lee, C.W. Micronutrient toxicity in French marigold. J. Plant Nutr. 1996, 2019, 901–916. [Google Scholar] [CrossRef]
- Krishnaiah, D.; Sarbatly, R.; Bono, A. Phytochemical antioxidants for health and medicine—A move towards nature. Biotechnol. Mol. Biol. 1996, 1, 97–104. [Google Scholar]
- Tahirović, A.; Bašić, N. Determination of phenolic content and antioxidant properties of methanolic extracts from Viscum album ssp. album Beck. Bull. Chem. Technol. Bosnia Herzeg. 2017, 49, 25–30. [Google Scholar]
- Shao, Y. Phytochemischer Atlas der Schweizer Weiden. Ph.D. Thesis, University of Zürich (CH), Zürich, Switzerland, 1991. [Google Scholar]
- Apak, R.; Güçlü, K.; Demirata, B.; Özyürek, M.; Çelik, S.E.; Bektaşoğlu, B.; Berker, K.I.; Özyurt, D. Comparative Evaluation of Various Total Antioxidant Capacity Assays Applied to Phenolic Compounds with the CUPRAC Assay. Molecules 2007, 12, 1496–1547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sulaiman, G.M.; Hussien, N.N.; Marzoog, T.R.; Awad, H.A. Phenolic content, Antioxidant, Antimicrobial and Cytotoxic activities of ethanol extract of Salix alba. Am. J. Biochem. Biotechnol. 2013, 9, 41–46. [Google Scholar] [CrossRef] [Green Version]
- Oram, B. Metals in the Environment, Water Rsearch Center. 2020. Available online: https://water-research.net/index.php/metals-in-the-environment (accessed on 15 January 2022).
- Dasa, B.H.; Swarnakar, A.K. Mobility of Heavy Metals in Soils and Wastewater from Landfill, Sarona, Raipur, Chhattisgarh, India. J. Univ. Shanghai Sci. Technol. 2020, 21, 372–381. [Google Scholar]
- Shala, A.; Sallaku, F.; Shala, A.; Ukaj, S. The effects of industrial and agricultural activity on the water quality of the Sitnica River (Kosovo). Geoadria 2015, 20, 13–21. [Google Scholar] [CrossRef]
- Mertens, J.; Smolders, E. Zinc. Heavy Metals in Soils; Environmental Pollution Book Series (EPOL); Springer: Berlin/Heidelberg, Germany, 2012; Volume 22, pp. 465–493. [Google Scholar]
- FAO Soils Bulletins. Available online: https://www.fao.org/3/x5872e/x5872e0i.htm (accessed on 20 December 2021).
- Popoviciu, D.R.; Ticuţa, N. Copper, manganese and zinc bioaccumulation in three common woody species from black sea coastal area. UPB Sci. Bull. Ser. B 2018, 80, 49–56. [Google Scholar]
- Băbău, A.M.C.; Micle, V.; Damian, G.E.; Sur, I.M. Sustainable Ecological Restoration of Sterile Dumps Using Robinia pseudoacacia. Sustainability 2021, 13, 14021. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).