Iodine Enhances the Nutritional Value but Not the Tolerance of Lettuce to NaCl
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Leaf Gas Exchange and Chlorophyll a Fluorescence Measurement
2.3. Photosynthetic Pigments
2.4. Polyphenols and Soluble Proteins
2.5. Statistical Analysis
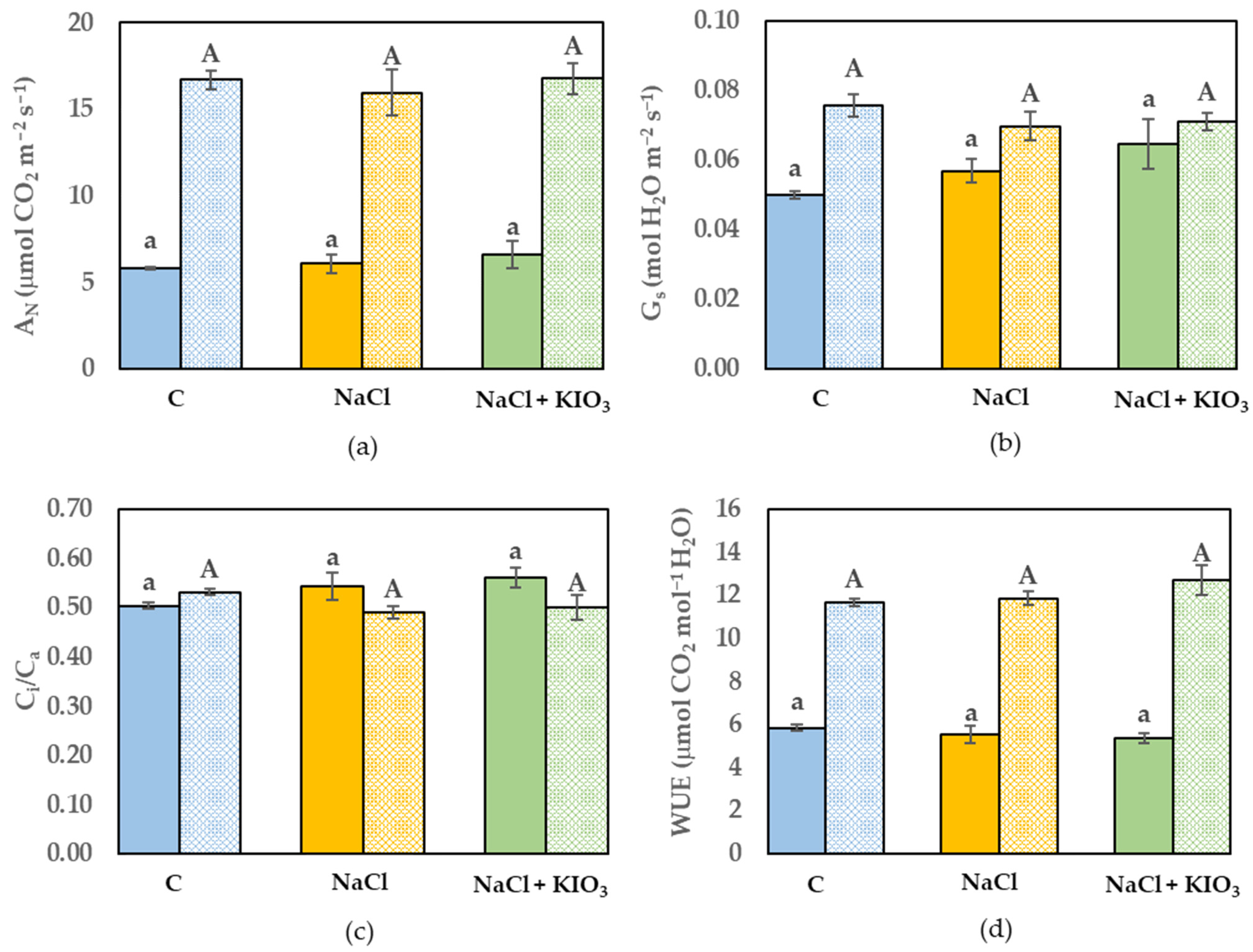
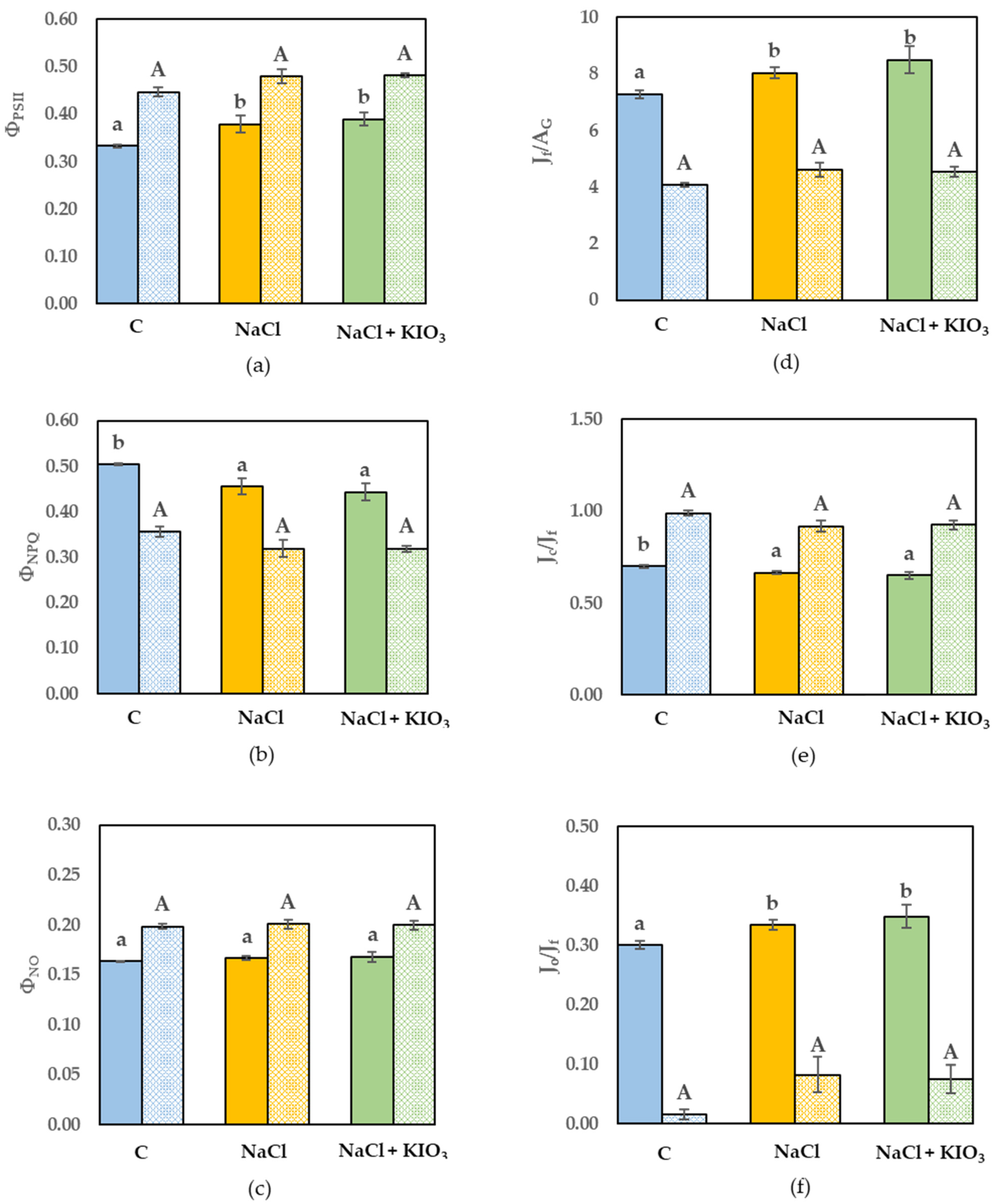
3. Results
3.1. Plant Growth
3.2. Bioactive Compounds and Soluble Proteins
3.3. Leaf Gas Exchange and Chl a Fluorescence Measurement
4. Discussion
4.1. Nutritional Value of Crops
4.2. Stress Tolerance
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Malorgio, F.; Diaz, K.E.; Ferrante, A.; Mensuali, A.; Pezzarossa, B. Effects of selenium addition on minimally processed leafy vegetables grown in floating system. J. Sci. Food Agric. 2009, 89, 2243–2251. [Google Scholar] [CrossRef]
- Montesano, F.F.; D’Imperio, M.; Parente, A.; Cardinali, A.; Renna, M.; Serio, F. Green bean biofortification for Si through soilless cultivation: Plant response and Si bioaccessibility in pods. Sci. Rep. 2016, 6, 31662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garg, M.; Sharma, N.; Sharma, S.; Kapoor, P.; Kumar, A.; Chunduri, V.; Arora, P. Biofortified crops generated by breeding, agronomy, and transgenic approaches are improving lives of millions of people around the world. Front. Nutr. 2018, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Rouphael, Y.; Kyriacou, M.C. Enhancing quality of fresh vegetables through salinity eustress and biofortification applications facilitated by soilless cultivation. Front. Plant Sci. 2018, 9, 1254. [Google Scholar] [CrossRef]
- Carillo, P.; Annunziata, M.G.; Pontecorvo, G.; Fuggi, A.; Woodrow, P. Salinity Stress and Salt Tolerance. In Abiotic Stress in Plants-Mechanisms and Adaptations; Shanker, A., Venkateswarlu, B., Eds.; Inthec Open: London, UK, 2011; Volume 1, pp. 21–38. [Google Scholar]
- Cirillo, C.; De Micco, V.; Arena, C.; Pannico, A.; De Pascale, S.; Rouphael, Y. Biochemical, Physiological and Anatomical Mechanisms of Adaptation of Callistemon citrinus and Viburnum lucidum to NaCl and CaCl2 Salinization. Front. Plant. Sci. 2019, 10, 742. [Google Scholar] [CrossRef]
- Carillo, P.; Cirillo, C.; De Micco, V.; Arena, C.; De Pascale, S.; Rouphael, Y. Morpho-anatomical, physiological and biochemical adaptive responses to saline water of Bougainvillea spectabilis Willd. trained to different canopy shapes. Agric. Water Manag. 2019, 212, 12–22. [Google Scholar] [CrossRef]
- De Micco, V.; Arena, C.; Amitrano, C.; Rouphael, Y.; De Pascale, S.; Cirillo, C. Effects of NaCl and CaCl2 Salinization on Morpho-Anatomical and Physiological Traits of Potted Callistemon citrinus Plants. Forests 2021, 12, 1666. [Google Scholar] [CrossRef]
- Maggio, A.; Hasegawa, P.M.; Bressan, R.A.; Consiglio, M.F.; Joly, R.J. Review: Unravelling the functional relationship between root anatomy and stress tolerance. Aust. J. Plant Physiol. 2001, 28, 999–1004. [Google Scholar] [CrossRef]
- Rouphael, Y.; Kyriacou, M.C.; Petropoulos, S.A.; De Pascale, S.; Colla, G. Improving vegetable quality in controlled environments. Sci. Hort. 2018, 234, 275–289. [Google Scholar] [CrossRef]
- Rouphael, Y.; Petropoulos, S.A.; Cardarelli, M.; Colla, G. Salinity as eustressor for enhancing quality of vegetables. Sci. Hort. 2018, 234, 361–369. [Google Scholar] [CrossRef]
- Santander, C.; Vidal, G.; Ruiz, A.; Vidal, C.; Cornejo, P. Salinity Eustress Increases the Biosynthesis and Accumulation of Phenolic Compounds That Improve the Functional and Antioxidant Quality of Red Lettuce. Agronomy 2022, 12, 598. [Google Scholar] [CrossRef]
- Rouphael, Y.; Kyriacou, M.C.; Carillo, P.; Pizzolongo, F.; Romano, R.; Sifola, M.I. Chemical Eustress Elicits Tailored Responses and Enhances the Functional Quality of Novel Food Perilla frutescens. Molecules 2019, 24, 185. [Google Scholar] [CrossRef] [Green Version]
- Buturi, C.V.; Mauro, R.P.; Fogliano, V.; Leonardi, C.; Giuffrida, F. Mineral Biofortification of Vegetables as a Tool to Improve Human Diet. Foods 2021, 10, 223. [Google Scholar] [CrossRef] [PubMed]
- Kiferle, C.; Martinelli, M.; Salzano, A.M.; Gonzali, S.; Beltrami, S.; Salvadori, P.A.; Hora, K.; Holwerda, H.T.; Scaloni, A.; Perata, P. Evidences for a nutritional role of iodine in plants. Front. Plant Sci. 2021, 12, 616868. [Google Scholar] [CrossRef] [PubMed]
- Caffagni, A.; Pecchioni, N.; Meriggi, P.; Bucci, V.; Sabatini, E.; Acciarri, N.; Ciriaci, T.; Pulcini, L.; Felicioni, N.; Beretta, M.; et al. Iodine uptake and distribution in horticultural and fruit tree species. Ital. J. Agron. 2012, 7, 229–236. [Google Scholar] [CrossRef]
- Medrano-Macías, J.; Leija-Martínez, P.; González-Morales, S.; Juárez-Maldonado, A.; Benavides-Mendoza, A. Use of iodine to biofortify and promote growth and stress tolerance in crops. Front. Plant Sci. 2016, 7, 1146. [Google Scholar] [CrossRef] [Green Version]
- Leyva, R.; Sánchez-Rodríguez, E.; Rubio-Wilhelmi, M.M.; Romero, L.; Ruiz, J.M.; Blasco, B. Beneficial effects of exogenous iodine in lettuce plants subjected to salinity stress. Plant Sci. 2011, 181, 195–202. [Google Scholar] [CrossRef]
- Medrano Macías, J.; López Caltzontzit, M.G.; Rivas Martínez, E.N.; Narváez Ortiz, W.A.; Benavides Mendoza, A.; Martínez Lagunes, P. Enhancement to salt stress tolerance in strawberry plants by iodine products application. Agronomy 2021, 11, 602. [Google Scholar] [CrossRef]
- Blasco, B.; Rios, J.J.; Cervilla, L.M.; Sánchez-Rodríguez, E.; Rubio-Wilhelmi, M.M.; Rosales, M.A.; Ruiz, J.M.; Romero, L. Photorespiration process and nitrogen metabolism in lettuce plants (Lactuca sativa L.): Induced changes in response to iodine biofortification. J. Plant Growth Regul. 2010, 29, 477–486. [Google Scholar] [CrossRef]
- Chiesa, A.; Frezza, D.; Fraschina, A.; Trinchero, G.; Moccia, S.; León, A. Pre-harvest factors and fresh-cut vegetables quality. Acta Hortic. 2003, 604, 153–159. [Google Scholar] [CrossRef]
- von Caemmerer, S.; Farquhar, G.D. Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 1981, 153, 376–387. [Google Scholar] [CrossRef] [PubMed]
- Genty, B.; Briantais, J.M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Kramer, D.M.; Johnson, G.; Kiirats, O.; Edwards, G.E. New fluorescence parameters for the determination of QA redox state and excitation energy fluxes. Photosynth Res. 2004, 79, 209–218. [Google Scholar] [CrossRef]
- Krall, J.P.; Edwards, G.E. Relationship between photosystem II activity and CO2 fixation in leaves. Physiol. Plant. 1992, 86, 180–187. [Google Scholar] [CrossRef]
- Epron, D.; Godard, D.; Cornic, G.; Genty, B. Limitation of net CO2 assimilation rate by internal resistances to CO2 transfer in the leaves of two tree species (Fagus sylvatica L. and Castanea sativa Mill.). Plant Cell Environ. 1995, 18, 43–51. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. In Methods in Enzymology, PlantCell Membranes; Academic Press: Cambridge, MA, USA, 1987; pp. 350–382. [Google Scholar]
- Vitale, L.; Vitale, E.; Guercia, G.; Turano, M.; Arena, C. Effects of different light quality and biofertilizers on structural and physiological traits of Spinach plants. Photosynthetica 2020, 58, 932–943. [Google Scholar] [CrossRef]
- Moulehi, I.; Bourgou, S.; Ourghemmi, I.; Tounsi, M.S. Variety and ripening impact on phenolic composition and antioxidant activity of mandarin (Citrus reticulate Blanco) and bitter orange (Citrus aurantium L.) seeds extracts. Ind. Crop. Prod. 2017, 39, 74–80. [Google Scholar] [CrossRef]
- Sun, B.; da Silva, J.M.R.; Spranger, I. Factors of Vanillin Assay for Catechins and Proanthocyanidins. J. Agric. Food Chem. 1998, 46, 4267–4274. [Google Scholar] [CrossRef]
- Mancinelli, A.L.; Huang Yang, C.P.; Lindquist, P.; Anderson, R.; Rabino, I. Photocontrol of anthocyanin synthesis. Plant Physiol. 1975, 55, 251–257. [Google Scholar] [CrossRef] [Green Version]
- Chung, Y.C.; Chen, S.J.; Hsu, C.K.; Chang, C.T.; Chou, S.T. Studies on the antioxidative activity of Graptopetalum paraguayense E. Walther. Food Chem. 2005, 91, 419–424. [Google Scholar] [CrossRef]
- George, B.; Kaur, C.; Khurdiya, D.S.; Kapoor, H.C. Antioxidants in tomato (Lycopersicum esculentum) as a function of genotype. Food Chem. 2004, 84, 45–51. [Google Scholar] [CrossRef]
- Vitale, E.; Vitale, L.; Costanzo, G.; Velikova, V.; Tsonev, T.; Simoniello, P.; De Micco, V.; Arena, C. Light spectral composition influences structural and eco-physiological traits of Solanum lycopersicum L. cv. ‘Microtom’ in response to high-LET ionizing radiation. Plants 2021, 10, 1752. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Im, J.; Ko, J.; Kim, H.Y.; Ha, B.K. Biochemical responses of Soybean (Glycine max L. Merr.) to proton beam irradiation. Plant Breed. Biotechnol. 2017, 5, 97–105. [Google Scholar] [CrossRef] [Green Version]
- Carillo, P.; Soteriou, G.A.; Kyriacou, M.C.; Giordano, M.; Raimondi, G.; Napolitano, F.; Di Stasio, E.; Mola, I.D.; Mori, M.; Rouphael, Y. Regulated Salinity Eustress in a Floating Hydroponic Module of Sequentially Harvested Lettuce Modulates Phytochemical Constitution, Plant Resilience, and Post-Harvest Nutraceutical Quality. Agronomy 2021, 11, 1040. [Google Scholar] [CrossRef]
- Nunes da Silva, M.; Machado, J.; Osorio, J.; Duarte, R.; Santos, C.S. Non-Essential Elements and Their Role in Sustainable Agriculture. Agronomy 2022, 12, 888. [Google Scholar] [CrossRef]
- Kyriacou, M.C.; Rouphael, Y. Towards a new definition of quality for fresh fruits and vegetables. Sci. Hortic. 2018, 234, 463–469. [Google Scholar] [CrossRef]
- Chutipaijit, S.; Cha-um, S.; Sompornpailin, K. High contents of proline and anthocyanin increase protective response to salinity in Oryza sativa L. spp. indica. Aust. J. Crop Sci. 2011, 5, 1191–1198. [Google Scholar]
- Blasco, B.; Rios, J.J.; Leyva, R.; Cervilla, L.M.; Sánchez-Rodríguez, E.; Rubio-Wilhelmi, M.M.; Rosales, M.Á.; Ruiz, J.M.; Romero, L. Does iodine biofortification affect oxidative metabolism in lettuce plants? Biol. Trace Elem. Res. 2010, 142, 831–842. [Google Scholar] [CrossRef]
- Blasco, B.; Rios, J.J.; Leyva, R.; Melgarejo, R.; Constan-Aguilar, C.; Sanchez-Rodrıguez, E.; Rubio-Wilhelmi, M.M.; Romero, L.; Ruiz, J.M. Photosynthesis and metabolism of sugars from lettuce plants (Lactuca sativa L. var. longifolia) subjected to biofortification with iodine. Plant Growth Regul. 2011, 65, 137–143. [Google Scholar] [CrossRef]
- Weng, H.X.; Hong, C.L.; Yan, A.L.; Pan, L.H.; Qin, Y.C.; Bao, L.T.; Xie, L.L. Mechanism of iodine uptake by cabbage: Effects of iodine species and where it is stored. Biol. Trace Elem. Res. 2008, 125, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Incrocci, L.; Carmassi, G.; Maggini, R.; Poli, C.; Saidov, D.; Tamburini, C.; Kiferle, C.; Perata, P.; Pardossi, A. Iodine Accumulation and Tolerance in Sweet Basil (Ocimum basilicum L.) With Green or Purple Leaves Grown in Floating System Technique. Front. Plant Sci. 2019, 10, 1494. [Google Scholar] [CrossRef] [PubMed]
| Parameters | Control | NaCl | NaCl + KIO3 |
|---|---|---|---|
| pHH2O | 7.28 ± 0.03 a | 7.16 ± 0.03 a | 7.18 ± 0.01 a |
| EC (dS m−1) | 1.80 ± 0.00 a | 6.70 ± 0.05 b | 6.55 ± 0.13 b |
| Parameters | Control | NaCl | NaCl + KIO3 |
|---|---|---|---|
| Shoot (g FW p−1) | 36.60 ± 1.40 a | 36.00 ± 1.05 a | 34.20 ± 1.39 a |
| N° leaves | 35.80 ± 1.11 a | 34.20 ± 0.58 a | 34.40 ± 0.51 a |
| Parameters | Control | NaCl | NaCl + KIO3 |
|---|---|---|---|
| Chlorophylls (a + b) (μg cm−2) | 49.78 ± 0.92 a | 53.02 ± 2.52 a | 64.68 ± 2.76 b |
| Carotenoids (x + c) (μg cm−2) | 10.03 ± 0.13 a | 10.97 ± 0.46 a | 12.99 ± 0.40 b |
| Total polyphenols (mg GAE g−1 FW) | 0.42 ± 0.08 c | 0.11 ± 0.02 a | 0.18 ± 0.02 b |
| Anthocyanins (μmol g−1 FW) | 0.021 ± 0.006 a | 0.041 ± 0.003 b | 0.119 ± 0.007 c |
| Flavonoids (mg CE g−1 FW) | 6.78± 0.42 a | 6.31± 0.34 a | 6.16± 0.20 a |
| Antioxidant capacity (μmol TE g−1 FW) | 0.43 ± 0.01 a | 0.52 ± 0.02 b | 0.25 ± 0.01 c |
| Soluble proteins (mg BSA eq g−1 FW) | 1.61± 0.07 a | 1.47± 0.05 a | 1.56± 0.03 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maglione, G.; Vitale, E.; Costanzo, G.; Polimeno, F.; Arena, C.; Vitale, L. Iodine Enhances the Nutritional Value but Not the Tolerance of Lettuce to NaCl. Horticulturae 2022, 8, 662. https://doi.org/10.3390/horticulturae8070662
Maglione G, Vitale E, Costanzo G, Polimeno F, Arena C, Vitale L. Iodine Enhances the Nutritional Value but Not the Tolerance of Lettuce to NaCl. Horticulturae. 2022; 8(7):662. https://doi.org/10.3390/horticulturae8070662
Chicago/Turabian StyleMaglione, Giuseppe, Ermenegilda Vitale, Giulia Costanzo, Franca Polimeno, Carmen Arena, and Luca Vitale. 2022. "Iodine Enhances the Nutritional Value but Not the Tolerance of Lettuce to NaCl" Horticulturae 8, no. 7: 662. https://doi.org/10.3390/horticulturae8070662
APA StyleMaglione, G., Vitale, E., Costanzo, G., Polimeno, F., Arena, C., & Vitale, L. (2022). Iodine Enhances the Nutritional Value but Not the Tolerance of Lettuce to NaCl. Horticulturae, 8(7), 662. https://doi.org/10.3390/horticulturae8070662









