Anatomical and Physiological Performance of Jojoba Treated with Proline under Salinity Stress Condition
Abstract
:1. Introduction
2. Materials and Methods
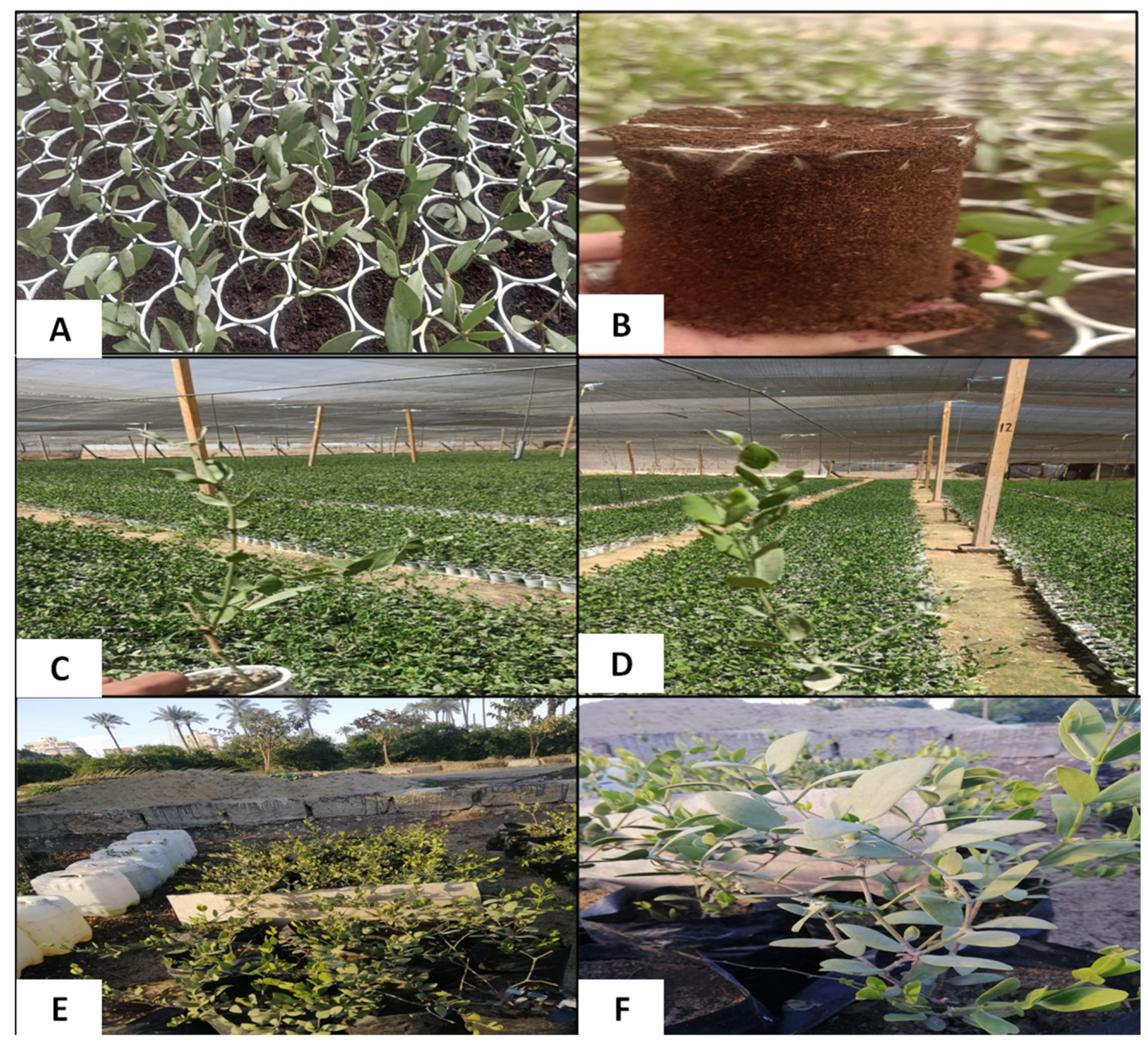
2.1. Growth Experiment and Salt–Proline Treatments
2.2. Measurement of Vegetative Growth Characteristics of Jojoba Plant
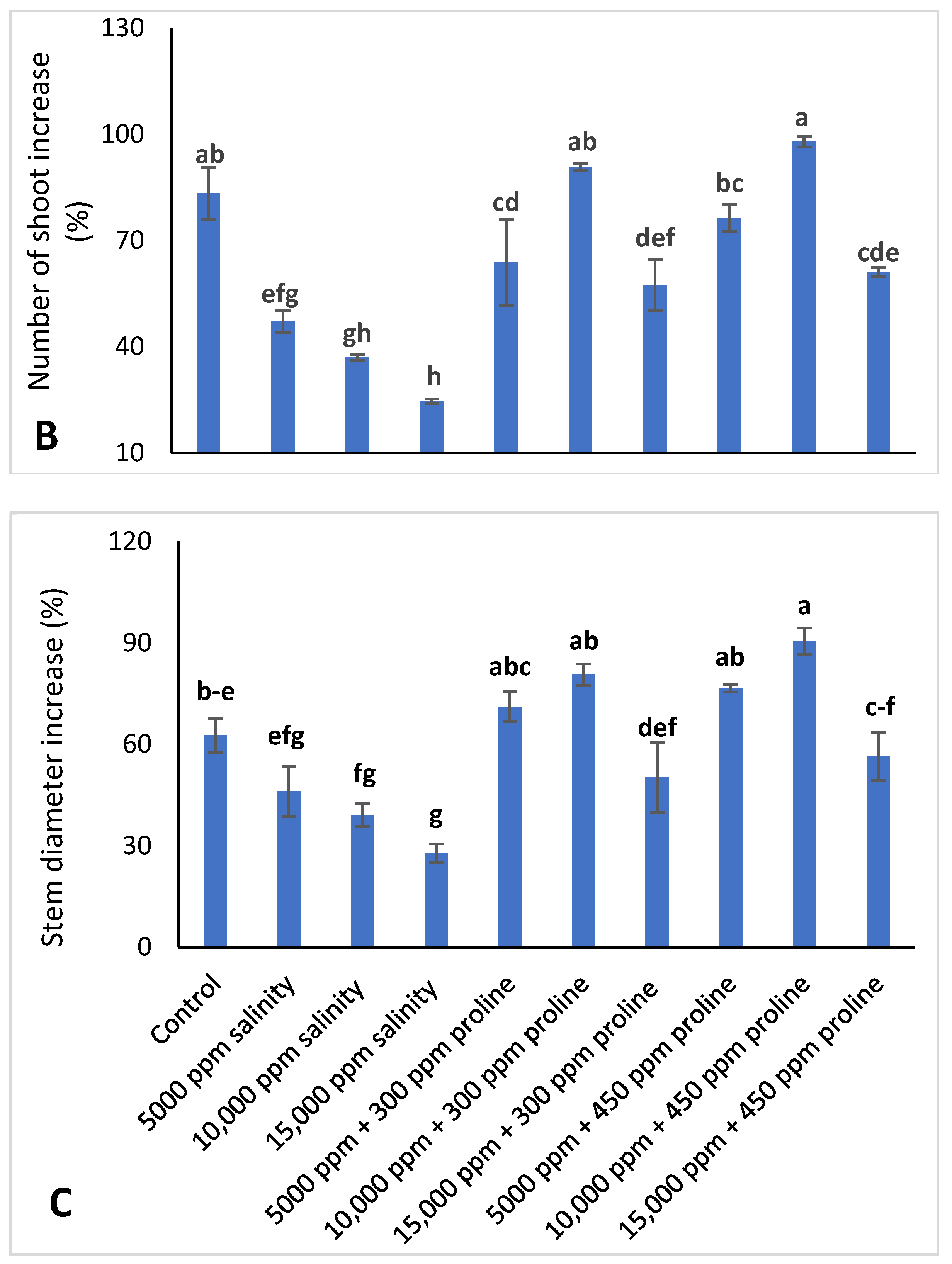
2.2.1. Shoot Number Increase Percentage (NSIP)
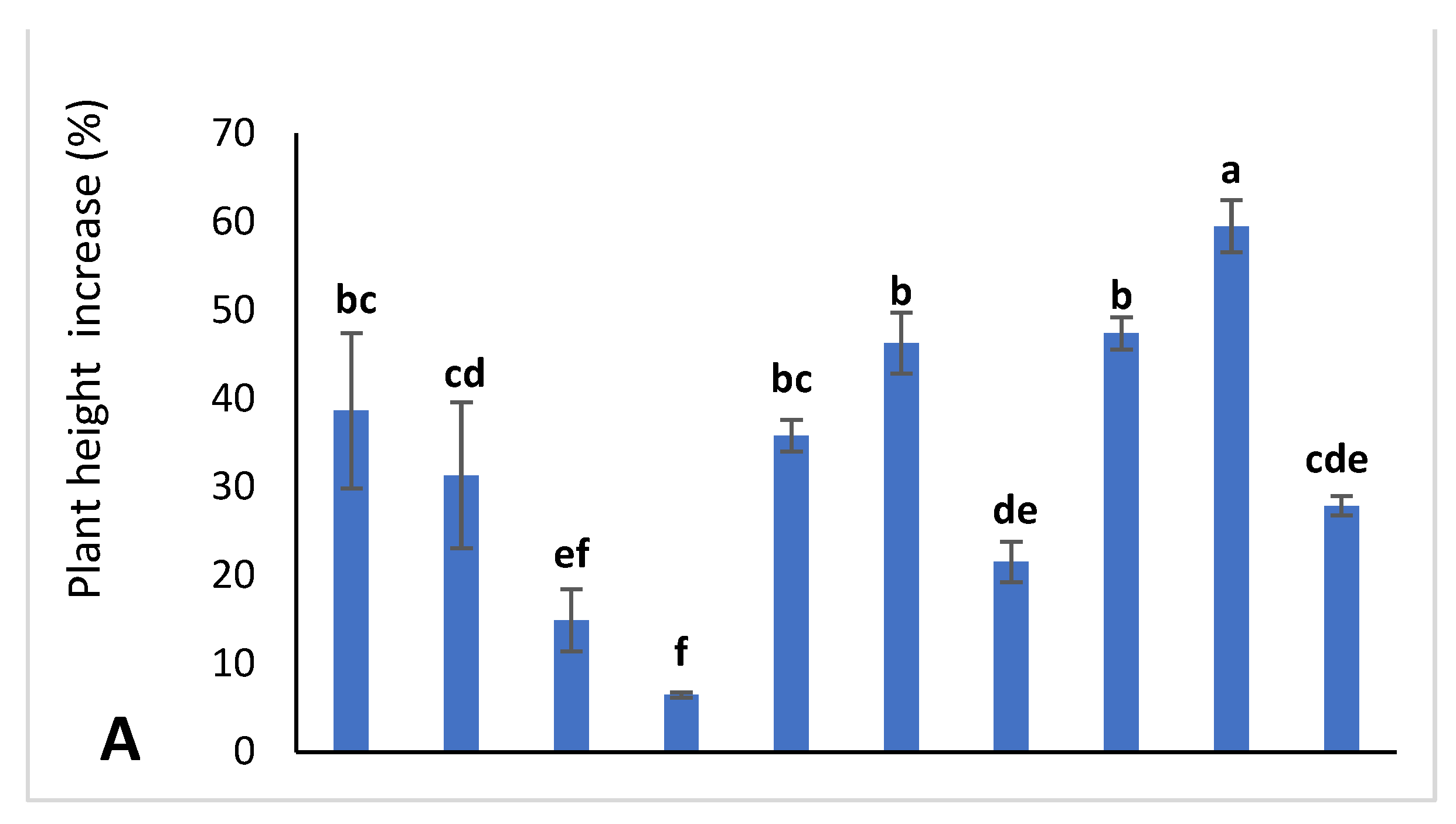
2.2.2. Plant Height Increase Percentage (PHIP)
2.2.3. Stem Diameter Increase Percentage (SDIP)
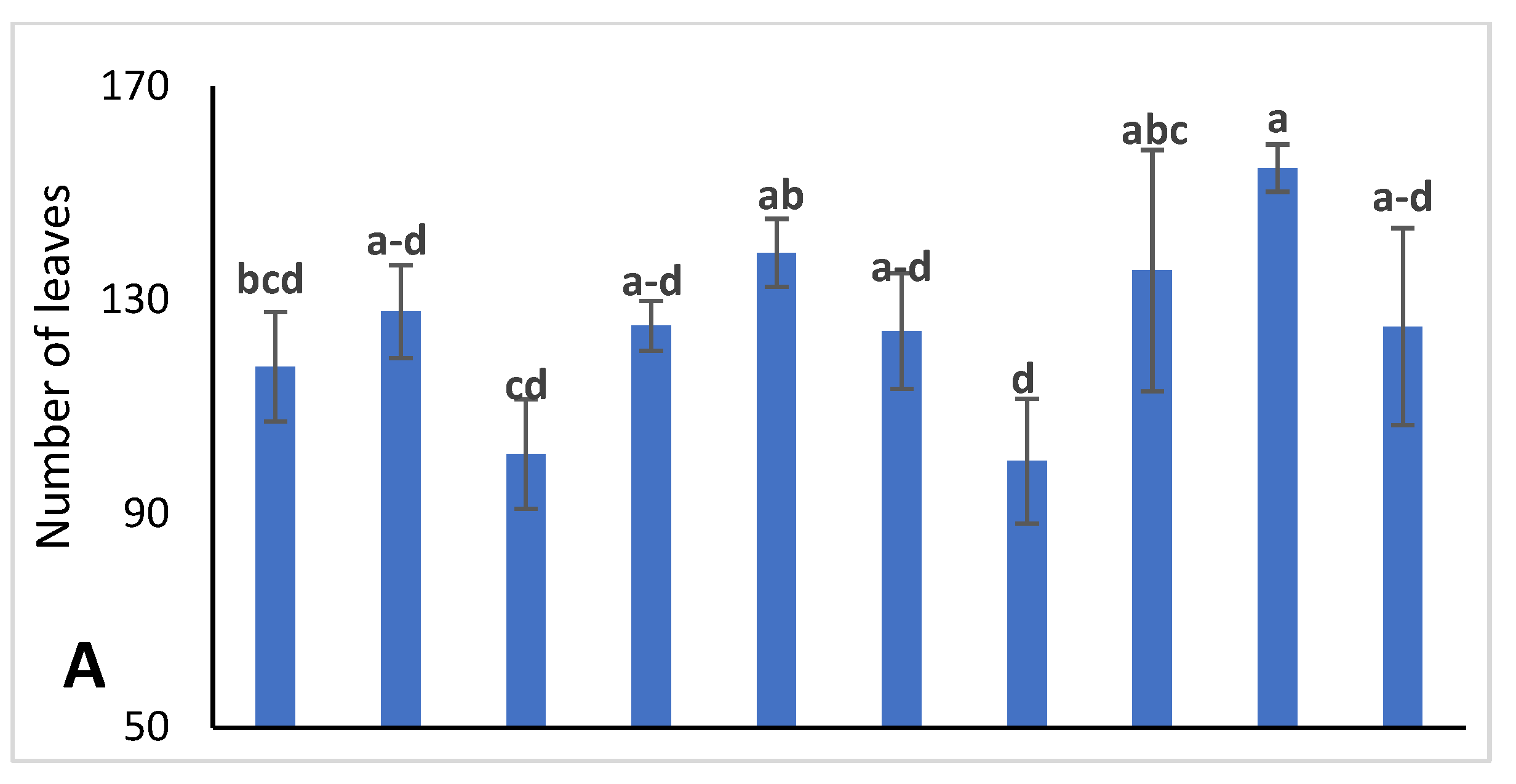
2.2.4. Number of Leaves, Leaf Thickness, and Leaf Area (LA)
2.2.5. Visible Quality
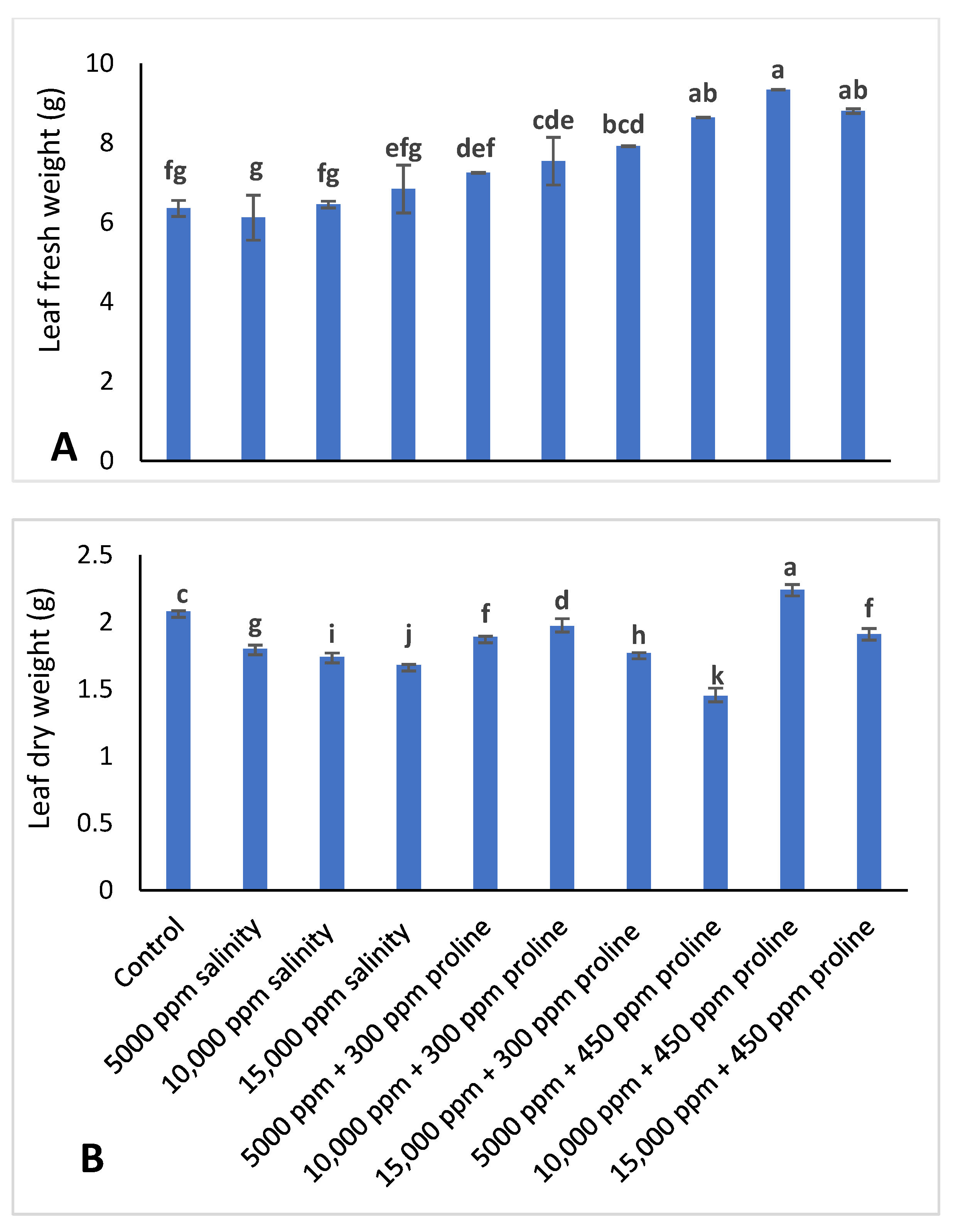
2.2.6. Leaf Fresh and Dry Weight
2.3. Chemical Characteristics of Jojoba Plant
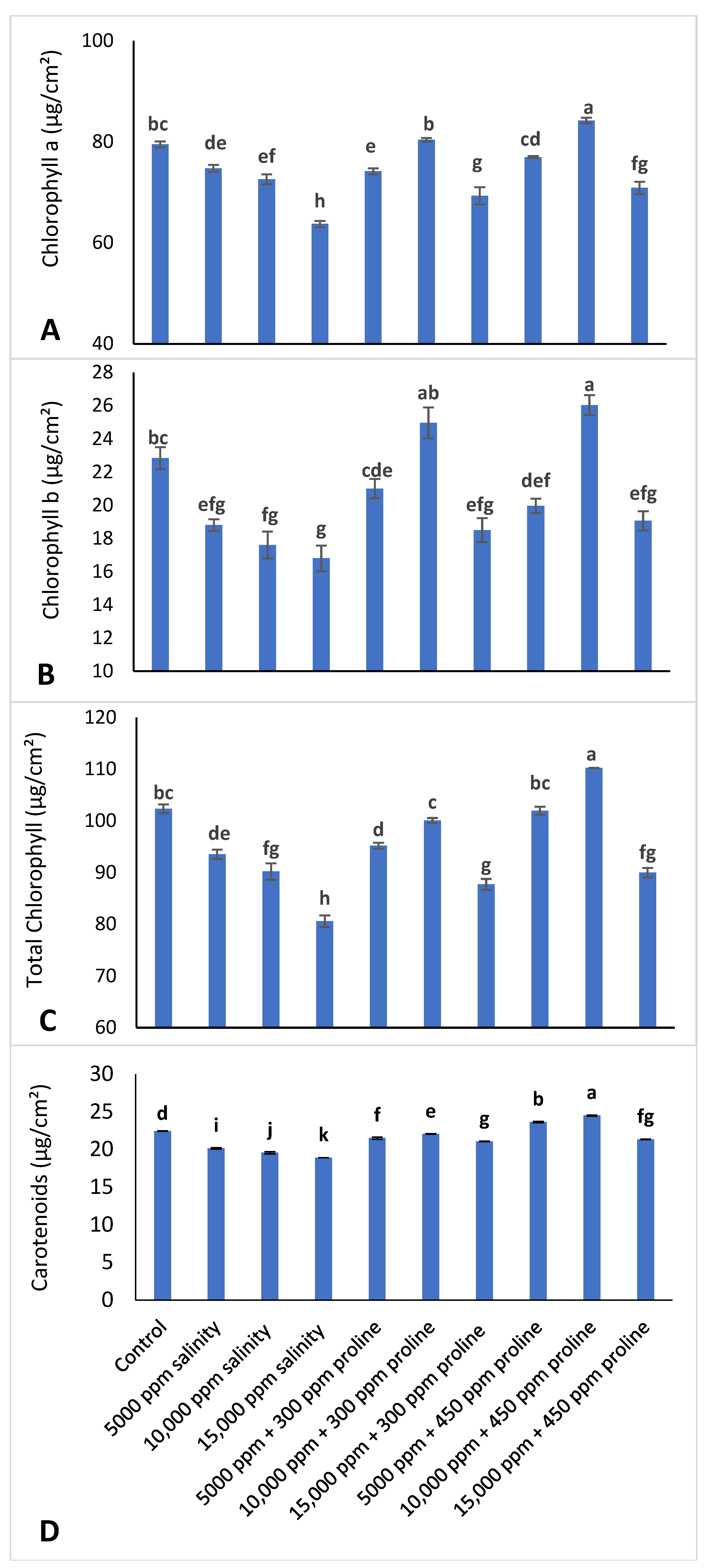
2.3.1. Pigment Measurements
- Chlorophyll a content = (12 × (E 662)) − (3.11 × (E 650))
- Chlorophyll b content = (20.78 × (E 650)) − (4.88 × (E 662))
- Total chlorophyll content = (17.67 × (E 650)) + (7.12 × (E 662))
- Carotenoids (µg/mL) = (1000 × A480 − 0.89 × chla − 52.02 × chlb)/245
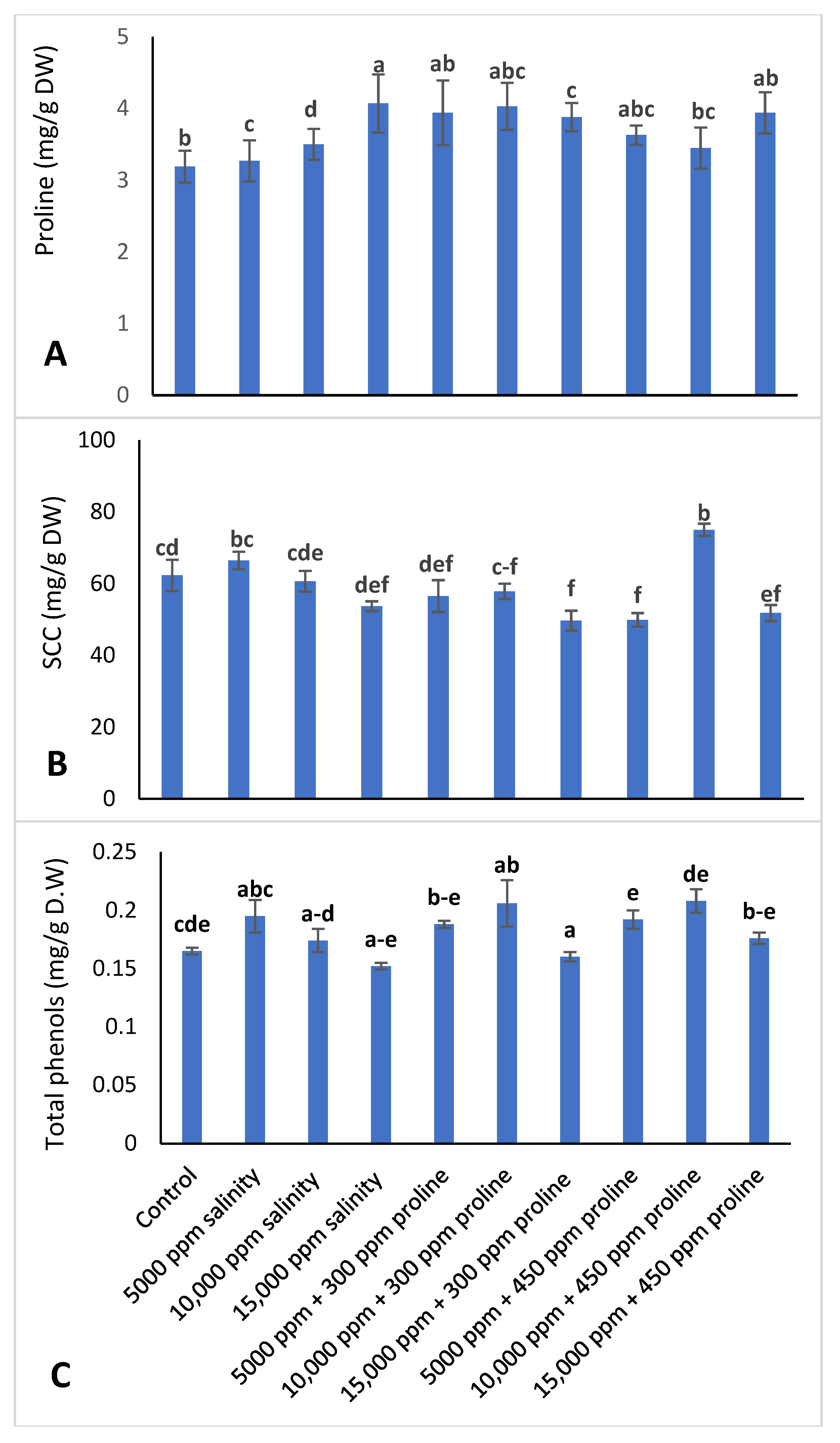
2.3.2. Proline Determination
2.3.3. Soluble Carbohydrate Content (SCC)
2.3.4. Total Phenolic Content (TPC)
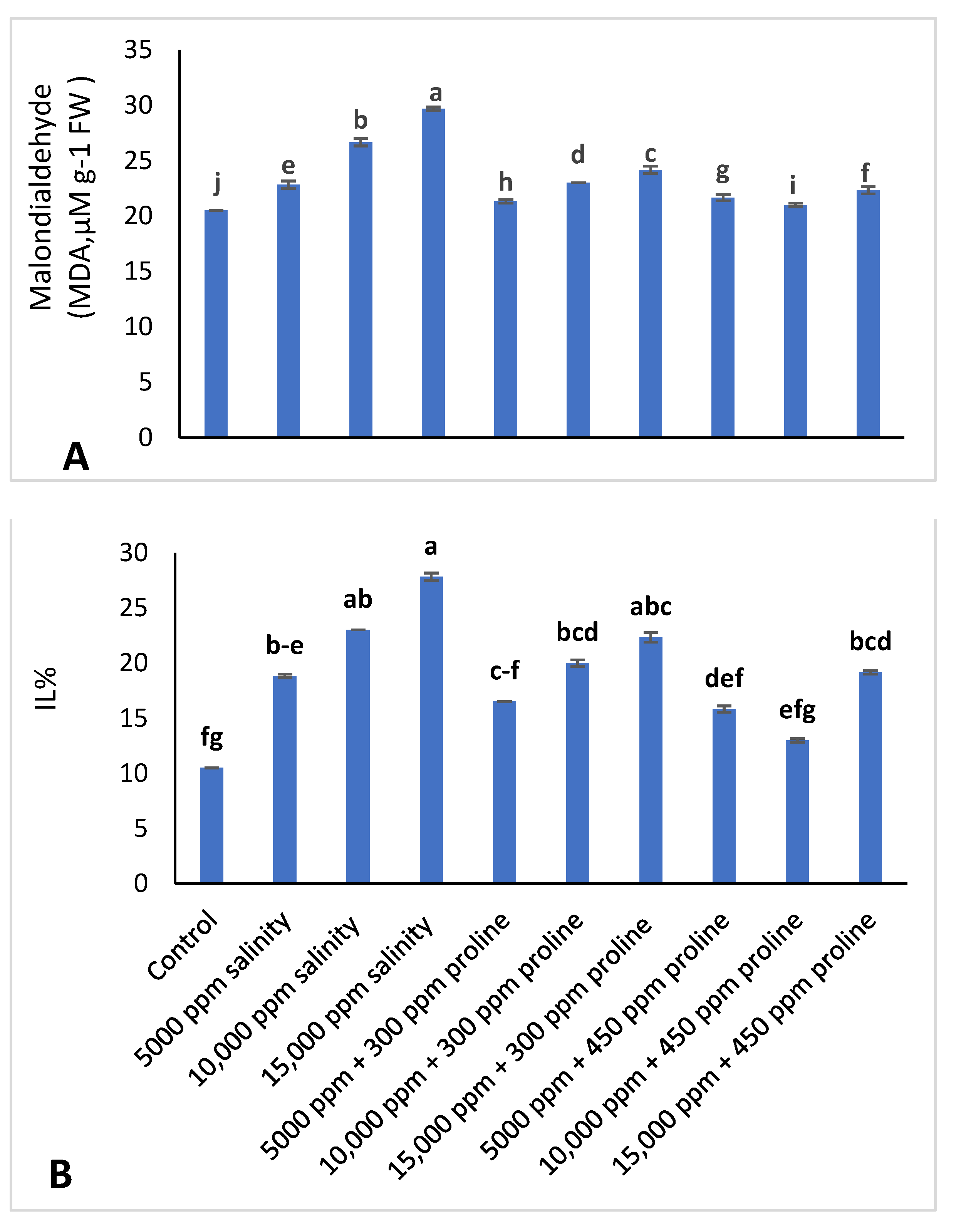
2.3.5. Ion Leakage (IL%) and Malondialdehyde (MDA) Accumulation
2.3.6. O2•− and H2O2 Production Rate
2.3.7. Leaf Mineral Content Determination
2.4. Anatomical Study
2.5. Statistical Analysis
3. Results
3.1. Vegetative Growth Characteristics of Jojoba Plant
3.2. Leaf Fresh Weight and Dry Weight
3.3. Chemical Characters of Jojoba Plant
3.3.1. Leaf Pigments
3.3.2. Ion Leakage (IL%) and Malondialdehyde (MDA)
3.3.3. O2•− and H2O2 Accumulation Rate
3.3.4. Proline Content, Soluble Carbohydrate Content (SCC), and Total Phenols
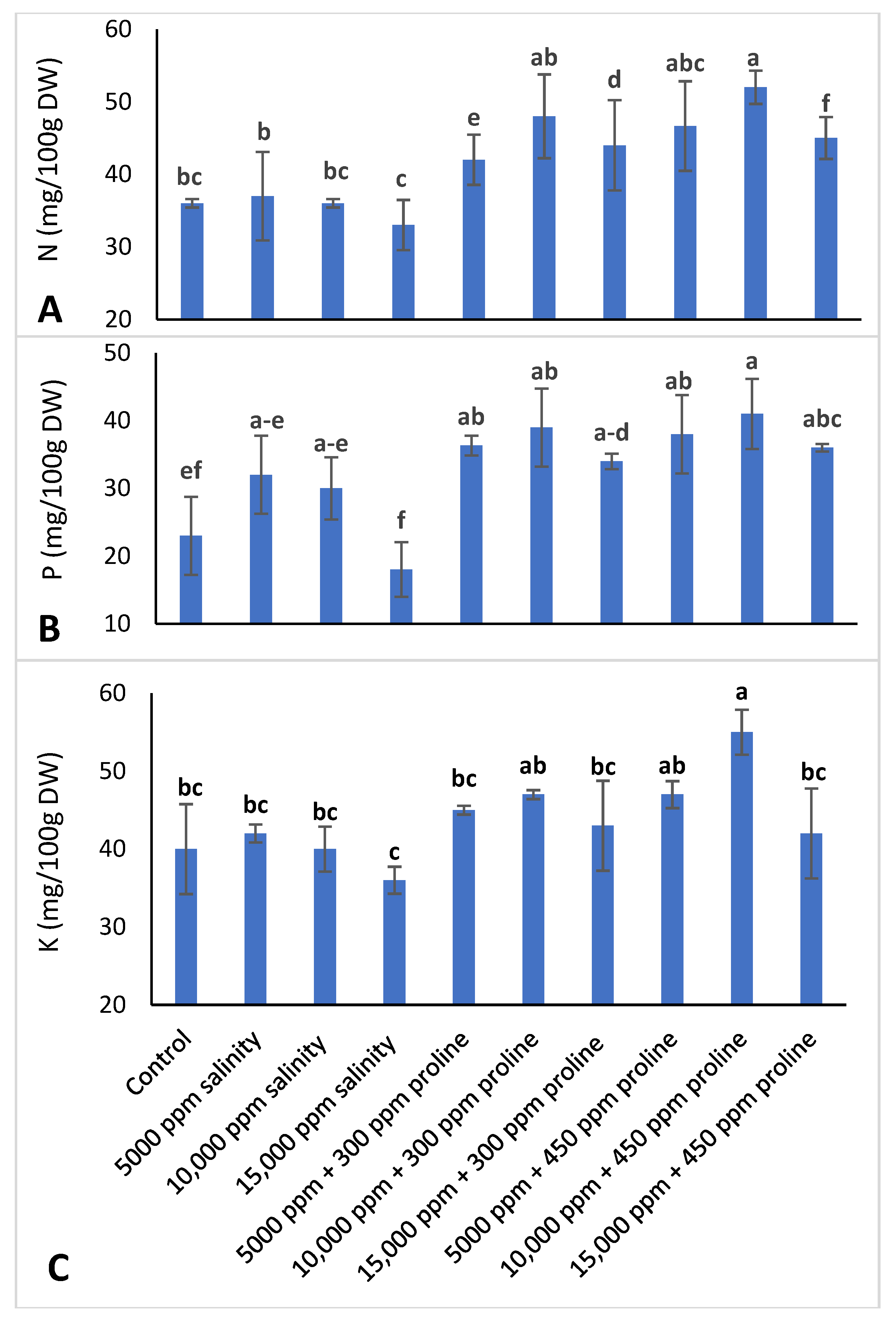
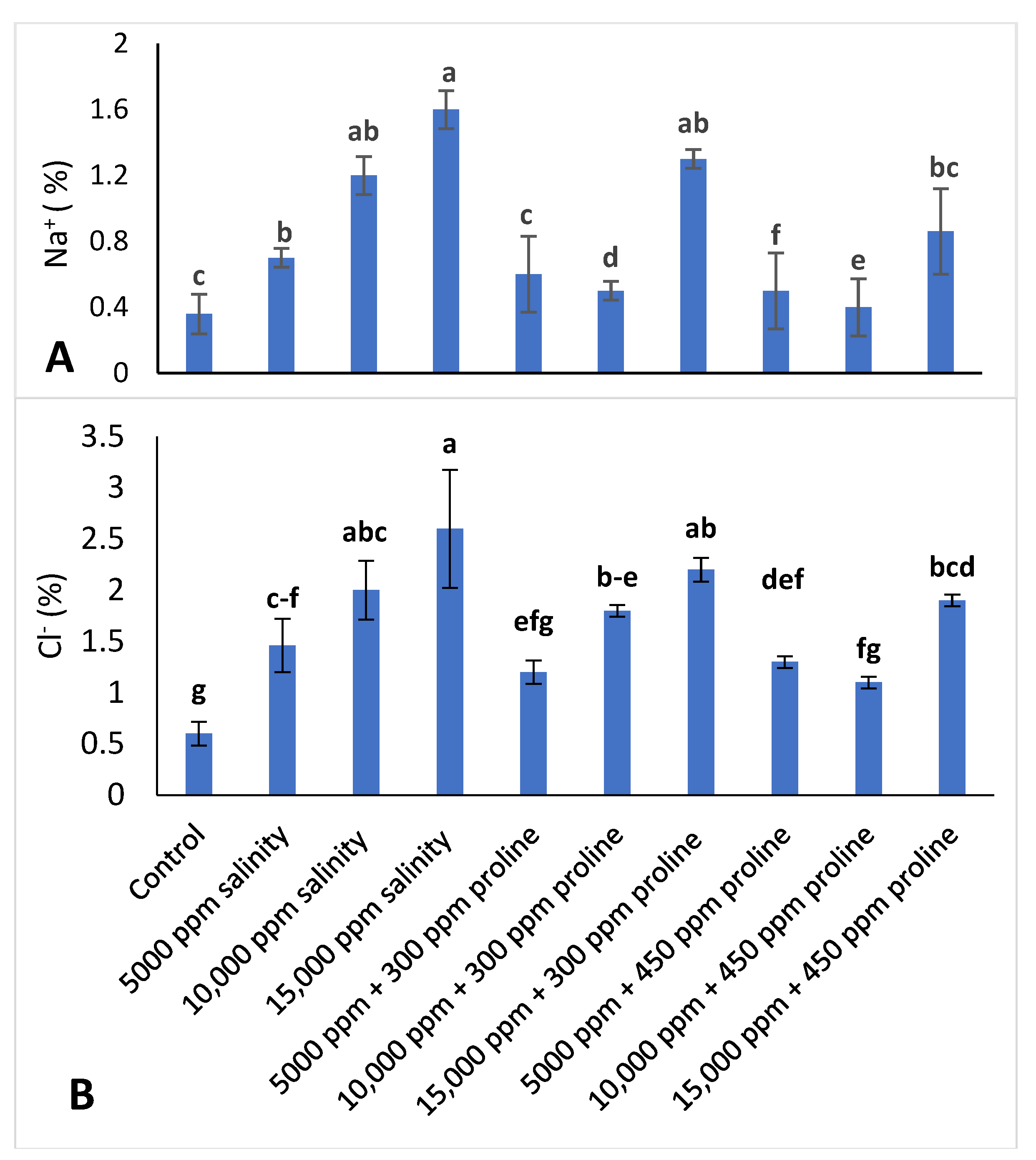
3.3.5. Leaf Mineral Concentration
3.3.6. Anatomical Characterization of the Leaf Structure
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bannari, A.; Al-Ali, Z.M. Assessing Climate Change Impact on Soil Salinity Dynamics between 1987–2017 in Arid Landscape Using Landsat TM, ETM+ and OLI Data. Remote Sens. 2020, 12, 2794. [Google Scholar] [CrossRef]
- Benzioni, A.; Nerd, A.; Rosenärtner, Y.; Mills, D. Effect of NaCl Salinity on Growth and Development of Jojoba Clones: I. Young Plants. J. Plant Physiol. 1992, 139, 731–736. [Google Scholar] [CrossRef]
- Bafeel, S.O.; Galal, H.K.; Basha, A.Z. Effect of Seawater Irrigation on Growth and Some Metabolites of Jojoba Plants. Am. J. Agric. Environ. Sci. 2016, 16, 49–59. [Google Scholar] [CrossRef]
- Botti, C.; Prat, L.; Palzkill, D.; Cánaves, L. Evaluation of jojoba clones grown under water and salinity stresses in Chile. Ind. Crops Prod. 1998, 9, 39–45. [Google Scholar] [CrossRef]
- Ahmad, P.; Azooz, M.M.; Prasad, M.N.V. Ecophysiology and responses of plants under salt stress. Ecophysiol. Responses Plants Salt Stress 2013, 9781461447474, 1–510. [Google Scholar] [CrossRef]
- Cho, U.H.; Park, J.O. Mercury-induced oxidative stress in tomato seedlings. Plant Sci. 2000, 156, 1–9. [Google Scholar] [CrossRef]
- Cabrera-Bosquet, L.; Molero, G.; Bort, J.; Nogués, S.; Araus, J.L. The combined effect of constant water deficit and nitrogen supply on WUE, NUE and Δ13C in durum wheat potted plants. Ann. Appl. Biol. 2007, 151, 277–289. [Google Scholar] [CrossRef]
- Ma, S.; Guo, S.; Chen, J.; Sun, J.; Wang, Y.; Shu, S. Enhancement of salt-stressed cucumber tolerance by application of glucose for regulating antioxidant capacity and nitrogen metabolism. Can. J. Plant Sci. 2020, 100, 253–263. [Google Scholar] [CrossRef]
- Slabbert, M.M.; Krüger, G.H.J. Antioxidant enzyme activity, proline accumulation, leaf area and cell membrane stability in water stressed Amaranthus leaves. S. Afr. J. Bot. 2014, 95, 123–128. [Google Scholar] [CrossRef] [Green Version]
- Boughalleb, F.; Denden, M.; Tiba, B. Ben Anatomical changes induced by increasing NaCl salinity in three fodder shrubs, Nitraria retusa, Atriplex halimus and Medicago arborea. Acta Physiol. Plant. 2009, 31, 947–960. [Google Scholar] [CrossRef]
- Tabot, P.T.; Adams, J.B. Salt secretion, proline accumulation and increased branching confer tolerance to drought and salinity in the endemic halophyte Limonium linifolium. S. Afr. J. Bot. 2014, 94, 64–73. [Google Scholar] [CrossRef] [Green Version]
- Abdelhamid, M.T.; Rady, M.M.; Osman, A.S.; Abdalla, M.A. Exogenous application of proline alleviates salt-induced oxidative stress in Phaseolus vulgaris L. Plants 2015, 88, 439–446. [Google Scholar] [CrossRef]
- Mansour, M.M.F. Nitrogen Containing Compounds and Adaptation of Plants to Salinity Stress. Biol. Plant. 2000, 43, 491–500. [Google Scholar] [CrossRef]
- Dawood, M.G.; Taie, H.A.A.; Nassar, R.M.A.; Abdelhamid, M.T.; Schmidhalter, U. The changes induced in the physiological, biochemical and anatomical characteristics of Vicia faba by the exogenous application of proline under seawater stress. S. Afr. J. Bot. 2014, 93, 54–63. [Google Scholar] [CrossRef] [Green Version]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments: A review. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef] [Green Version]
- Aggarwal, M.; Sharma, S.; Kaur, N.; Pathania, D.; Bhandhari, K.; Kaushal, N.; Kaur, R.; Singh, K.; Srivastava, A.; Nayyar, H. Exogenous proline application reduces phytotoxic effects of selenium by minimising oxidative stress and improves growth in bean (Phaseolus vulgaris L.) seedlings. Biol. Trace Elem. Res. 2011, 140, 354–367. [Google Scholar] [CrossRef]
- Kim, G.B.; Nam, Y.W. A novel Δ1-pyrroline-5-carboxylate synthetase gene of Medicago truncatula plays a predominant role in stress-induced proline accumulation during symbiotic nitrogen fixation. J. Plant Physiol. 2013, 170, 291–302. [Google Scholar] [CrossRef]
- Yang, S.L.; Lan, S.S.; Gong, M. Hydrogen peroxide-induced proline and metabolic pathway of its accumulation in maize seedlings. J. Plant Physiol. 2009, 166, 1694–1699. [Google Scholar] [CrossRef]
- Kaya, C.; Tuna, A.L.; Ashraf, M.; Altunlu, H. Improved salt tolerance of melon (Cucumis melo L.) by the addition of proline and potassium nitrate. Environ. Exp. Bot. 2007, 60, 397–403. [Google Scholar] [CrossRef]
- Mona, A.E.-H.; Sahar, A.E.-D.; Sohayla, M.A.; Hasan, N.A.; Ragia, H. MJFMCT_Volume 16_Issue 2_Pages 1–15.pdf. Mansoura J. Forensic Med. Clin. Toxicol. 2008, 16, 1–15. [Google Scholar]
- Ahmed, F.; Morsy, M.H. A new method for measuring leaf area in different fruit species. Minia J. Agric. Res. Dev. 1999, 19, 97–105. [Google Scholar]
- Sun, Y.; Niu, G.; Perez, C. Relative Salt Tolerance of Seven Texas Superstar® Perennials. HortScience 2015, 50, 1562–1566. [Google Scholar] [CrossRef] [Green Version]
- Porra, R.J. The chequered history of the development and use of simultaneous equations for the accurate determination of chlorophylls a and b. Photosynth. Res. 2002, 73, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Wellburn, A.R. The Spectral Determination of Chlorophylls a and b, as well as Total Carotenoids, Using Various Solvents with Spectrophotometers of Different Resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Kerepesi, I.; Tóth, M.; Boross, L. Water-Soluble Carbohydrates in Dried Plant. J. Agric. Food Chem. 1996, 44, 3235–3239. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 2002, 28, 350–356. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- Hakim, A.; Purvis, A.C.; Mullinix, B.G. Differences in chilling sensitivity of cucumber varieties depends on storage temperature and the physiological dysfunction evaluated. Postharvest Biol. Technol. 1999, 17, 97–104. [Google Scholar] [CrossRef]
- Iturbe-Ormaetxe, I.; Escuredo, P.R.; Arrese-Igor, C.; Becana, M. Oxidative Damage in Pea Plants Exposed to Water Deficit or Paraquat. Plant Physiol. 1998, 116, 173–181. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Wu, F.; Cheng, J. Reduced chilling injury in cucumber by nitric oxide and the antioxidant response. Food Chem. 2011, 127, 1237–1242. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Dong, J.; Zhang, M.; Xu, X.; Sun, L. Cold-induced endogenous nitric oxide generation plays a role in chilling tolerance of loquat fruit during postharvest storage. Postharvest Biol. Technol. 2012, 65, 5–12. [Google Scholar] [CrossRef]
- Shehata, S.A.; El-Mogy, M.M.; Mohamed, H.F.Y. Postharvest quality and nutrient contents of long sweet pepper enhanced by supplementary potassium foliar application. Int. J. Veg. Sci. 2019, 25, 196–209. [Google Scholar] [CrossRef]
- Anderson, D.L.; Henderson, L.J. Comparing Sealed Chamber Digestion with Other Digestion Methods Used for Plant Tissue Analysis. Agron. J. 1988, 80, 549–552. [Google Scholar] [CrossRef]
- Munter, R.C.; Halverson, T.L.; Anderson, R.D. Quality assurance for plant tissue analysis by ICP-AES. Commun. Soil Sci. Plant Anal. 2008, 15, 1285–1322. [Google Scholar] [CrossRef]
- Ruzin, S.E. Plant Micro Technique and Microscopy. Oxford University Press, Inc., Oxford.-References-Scientific Research Publishing. 1999. REVIEWS New Phytol. 2000, 148, 57–58. [Google Scholar]
- Qaderi, M.M.; Kurepin, L.V.; Reid, D.M. Growth and physiological responses of canola (Brassica napus) to three components of global climate change: Temperature, carbon dioxide and drought. Physiol. Plant. 2006, 128, 710–721. [Google Scholar] [CrossRef]
- Khayyat, M.; Tehranifar, A.; Davarynejad, G.H.; Sayyari-Zahan, M.H. Vegetative growth, compatible solute accumulation, ion partitioning and chlorophyll fluorescence of “Malas-e-Saveh” and “Shishe-Kab” pomegranates in response to salinity stress. Photosynthetica 2014, 52, 301–312. [Google Scholar] [CrossRef] [Green Version]
- Sadak, M.S.; Abdelhamid, M.T.; Schmidhalter, U. Effect of foliar application of aminoacids on plant yield and some physiological parameters in bean plants irrigated with seawater. Acta Biológica Colomb. 2015, 20, 141–152. [Google Scholar] [CrossRef]
- Ali, Q.; Athar, H.; Zulqurnain, M. Abiotic Stress Tolerance to Plants. Acta Physiol. Plant. 2019, 41, 1. [Google Scholar]
- El-Sherbeny, M.R.; Teixeira Da Silva, J.A. Foliar treatment with proline and tyrosine affect the growth and yield of beetroot and some pigments in beetroot leaves. J. Hortic. Res. 2014, 21, 95–99. [Google Scholar] [CrossRef] [Green Version]
- Waller, G.R.; Nowacki, E.K. Alkaloid Biology and Metabolism in Plants. Alkaloid Biol. Metab. Plants 1978, 157–189. [Google Scholar] [CrossRef]
- Lea, P.J.; Fowden, L. Asparagine Metabolism in Higher Plants. Biochem. Und Physiol. Der Pflanz. 1975, 168, 3–14. [Google Scholar] [CrossRef]
- Çiçek, N.; Çakirlar, H. The effect of salinity on some physiological parameters in two maize cultivars. Bulg. J. Plant Physiol. 2002, 28, 66–74. [Google Scholar]
- Ehsanpour, A.A.; Fatahian, N. Effects of salt and proline on Medicago sativa callus. Plant Cell Tissue Organ Cult. 2003, 73, 53–56. [Google Scholar] [CrossRef]
- Yan, Z.; Guo, S.; Shu, S.; Sun, J.; Tezuka, T. Effects of proline on photosynthesis, root reactive oxygen species (ROS) metabolism in two melon cultivars (Cucumis melo L.) under NaCl stress. Afr. J. Biotechnol. 2013, 10, 18381–18390. [Google Scholar] [CrossRef]
- Abdeldym, E.A.; El-Mogy, M.M.; Abdellateaf, H.R.L.; Atia, M.A.M. Genetic Characterization, Agro-Morphological and Physiological Evaluation of Grafted Tomato under Salinity Stress Conditions. Agronomy 2020, 10. [Google Scholar] [CrossRef]
- LI, R.-H.; Guo, P.-G.; Michael, B.; Stefania, G.; Salvatore, C. Evaluation of Chlorophyll Content and Fluorescence Parameters as Indicators of Drought Tolerance in Barley. Agric. Sci. China 2006, 5, 751–757. [Google Scholar] [CrossRef]
- Mafakheri, A.; Siosemardeh, A.; Bahramnejad, B.; Struik, P.C.; Sohrabi, E. Effect of drought stress on yield, proline and chlorophyll contents in three chickpea cultivars. Aust. J. Crop Sci. 2010, 4, 580–585. [Google Scholar]
- Jaleel, C.A.; Sankar, B.; Sridharan, R.; Panneerselvam, R. Soil salinity alters growth, chlorophyll content, and secondary metabolite accumulation in Catharanthus roseus. Turk. J. Biol. 2008, 32, 79–83. [Google Scholar]
- Banu, M.N.A.; Hoque, M.A.; Watanabe-Sugimoto, M.; Matsuoka, K.; Nakamura, Y.; Shimoishi, Y.; Murata, Y. Proline and glycinebetaine induce antioxidant defense gene expression and suppress cell death in cultured tobacco cells under salt stress. J. Plant Physiol. 2009, 166, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Ben Ahmed, C.; Ben Rouina, B.; Sensoy, S.; Boukhriss, M.; Ben Abdullah, F. Exogenous Proline Effects on Photosynthetic Performance and Antioxidant Defense System of Young Olive Tree. J. Agric. Food Chem. 2010, 58, 4216–4222. [Google Scholar] [CrossRef] [PubMed]
- Egbichi, I.; Keyster, M.; Ludidi, N. Effect of exogenous application of nitric oxide on salt stress responses of soybean. S. Afr. J. Bot. 2014, 90, 131–136. [Google Scholar] [CrossRef] [Green Version]
- Foyer, C.H.; Harbinson, J. Oxygen Metabolism and the Regulation of Photosynthetic Electron Transport. In Causes Photooxidative Stress Amelior. Def. Syst. Plants, 1st ed.; CRC Press: Boca Raton, FL, USA, 2019; p. 42. ISBN 9781351070454. [Google Scholar] [CrossRef]
- Ma, J.; Du, G.; Li, X.; Zhang, C.; Guo, J. A major locus controlling malondialdehyde content under water stress is associated with Fusarium crown rot resistance in wheat. Mol. Genet. Genom. 2015, 290, 1955–1962. [Google Scholar] [CrossRef]
- Reddy, A.R.; Chaitanya, K.V.; Vivekanandan, M. Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J. Plant Physiol. 2004, 161, 1189–1202. [Google Scholar] [CrossRef]
- Nounjan, N.; Nghia, P.T.; Theerakulpisut, P. Exogenous proline and trehalose promote recovery of rice seedlings from salt-stress and differentially modulate antioxidant enzymes and expression of related genes. J. Plant Physiol. 2012, 169, 596–604. [Google Scholar] [CrossRef]
- Verma, K.K.; Song, X.P.; Tian, D.D.; Singh, M.; Verma, C.L.; Rajput, V.D.; Singh, R.K.; Sharma, A.; Singh, P.; Malviya, M.K.; et al. Investigation of Defensive Role of Silicon during Drought Stress Induced by Irrigation Capacity in Sugarcane: Physiological and Biochemical Characteristics. ACS Omega 2021, 6, 19811–19821. [Google Scholar] [CrossRef]
- Bil Der, T.; Yildiz, M.; Terzï, H.; BİLGİSİ Araştırma Makalesi-Bitkisel Üretim Sorumlu Yazar, E. Effect of NaCl Stress on Chlorophyll Biosynthesis, Proline, Lipid Peroxidation and Antioxidative Enzymes in Leaves of Salt-Tolerant and Salt-Sensitive Barley Cultivars. J. Agric. Sci. 2013, 19, 79–88. [Google Scholar] [CrossRef]
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Szabados, L.; Savouré, A. Proline: A multifunctional amino acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef]
- Munns, R. Comparative physiology of salt and water stress. Plant. Cell Environ. 2002, 25, 239–250. [Google Scholar] [CrossRef]
- Hare, P.D.; Cress, W.A. Metabolic implications of stress-induced proline accumulation in plants. Plant Growth Regul. 1997, 21, 79–102. [Google Scholar] [CrossRef]
- Khatkar, D.; Kuhad, M.S. Short-Term Salinity Induced Changes in Two Wheat Cultivars at Different Growth Stages. Biol. Plant. 2000, 43, 629–632. [Google Scholar] [CrossRef]
- Semida, W.M.; Taha, R.S.; Abdelhamid, M.T.; Rady, M.M. Foliar-applied α-tocopherol enhances salt-tolerance in Vicia faba L. plants grown under saline conditions. S. Afr. J. Bot. 2014, 95, 24–31. [Google Scholar] [CrossRef] [Green Version]
- Gholami Zali, A.; Ehsanzadeh, P. Exogenous proline improves osmoregulation, physiological functions, essential oil, and seed yield of fennel. Ind. Crops Prod. 2018, 111, 133–140. [Google Scholar] [CrossRef]
- Farissi, M.; Aziz, F.; Bouizgaren, A.; Ghoulam, C. La symbiose Légumineuses-rhizobia sous conditions de salinité: Aspect Agro-physiologique et biochimique de la tolérance [Legume-rhizobia symbiosis under saline conditions: Agro-physiological and biochemical aspects of tolerance]. Int. J. Innov. Sci. Res. 2014, 11, 96–104. [Google Scholar]
- Freitas, P.A.F.; Carvalho, H.H.; Costa, J.H.; Miranda, R.S.; Saraiva, K.D.C.; Oliveira, F.D.B.; Coelho, D.G.; Prisco, J.T.; Gomes-Filho, E. Salt acclimation in sorghum plants by exogenous proline: Physiological and biochemical changes and regulation of proline metabolism. Plant Cell Rep. 2019, 38, 403–416. [Google Scholar] [CrossRef]
- Alam, R.; Das, D.K.; Islam, M.R.; Murata, Y.; Hoque, M.A. Exogenous proline enhances nutrient uptake and confers tolerance to salt stress in maize (Zea mays L.). Progress. Agric. 2016, 27, 409–417. [Google Scholar] [CrossRef] [Green Version]
- Khan, A.; Iqbal, I.; Ahmad, I.; Nawaz, H.; Nawaz, M. Role of proline to induce salinity tolerance in sunflower (Helianthus annus L.). Sci. Technol. Dev. 2014, 33, 88–93. [Google Scholar]
- Heuer, B. Role of Proline in Plant 9 Role Response to Drought and Salinity. In Handbook of Plant and Crop Stress; CRC Press: Boca Raton, FL, USA, 2010; pp. 213–238. [Google Scholar]
- Ben Rejeb, K.; Abdelly, C.; Savouré, A. How reactive oxygen species and proline face stress together. Plant Physiol. Biochem. 2014, 80, 278–284. [Google Scholar] [CrossRef]
- Posmyk, M.M.; Janas, K.M. Effects of seed hydropriming in presence of exogenous proline on chilling injury limitation in Vigna radiata L. seedlings. Acta Physiol. Plant. 2007, 29, 509–517. [Google Scholar] [CrossRef]
- Zhu, J.K. Regulation of ion homeostasis under salt stress. Curr. Opin. Plant Biol. 2003, 6, 441–445. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Ibrahim, M.; Farid, M.; Adrees, M.; Bharwana, S.A.; Zia-ur-Rehman, M.; Qayyum, M.F.; Abbas, F. Mechanisms of silicon-mediated alleviation of drought and salt stress in plants: A review. Environ. Sci. Pollut. Res. 2015, 22, 15416–15431. [Google Scholar] [CrossRef] [PubMed]
- Ben Ahmed, C.; Magdich, S.; Ben Rouina, B.; Sensoy, S.; Boukhris, M.; Ben Abdullah, F. Exogenous proline effects on water relations and ions contents in leaves and roots of young olive. Amino Acids 2011, 40, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Bie, Z.; Liu, Z.; Zhen, A.; Wang, W. Protective role of proline against salt stress is partially related to the improvement of water status and peroxidase enzyme activity in cucumber. Soil Sci. Plant Nutr. 2010, 55, 698–704. [Google Scholar] [CrossRef] [Green Version]
- Roussos, P.A.; Tsantili, E.; Pontikis, C.A. Responses of jojoba explants to different salinity levels during the proliferation stage in vitro. Ind. Crops Prod. 2006, 23, 65–72. [Google Scholar] [CrossRef]
- Gonzalez, A.J.; Larraburu, E.E.; Llorente, B.E. Azospirillum brasilense mitigates anatomical alterations produced by salt stress in jojoba in vitro plants. Vegetos 2021, 34, 725–737. [Google Scholar] [CrossRef]
- Akcin, T.A.; Akcin, A.; Yalcin, E. Anatomical Adaptations to Salinity in Spergularia marina (Caryophyllaceae) from Turkey. Proc. Natl. Acad. Sci. India Sect. B-Biol. Sci. 2015, 85, 625–634. [Google Scholar] [CrossRef]
- Debez, A.; Saadaoui, D.; Ramani, B.; Ouerghi, Z.; Koyro, H.W.; Huchzermeyer, B.; Abdelly, C. Leaf H+-ATPase activity and photosynthetic capacity of Cakile maritima under increasing salinity. Environ. Exp. Bot. 2006, 57, 285–295. [Google Scholar] [CrossRef]
- Bongi, G.; Loreto, F.; Nazionale, C.; Alta, V.M. Gas-Exchange Properties of Salt-Stressed Olive (Olea europea L.) Leaves. Plant Physiol. 1989, 90, 1408–1416. [Google Scholar] [CrossRef] [Green Version]
- Waisel, Y.; Eshel, A.; Agami, M. Salt balance of leaves of the mangrove Avicennia Marina. Physiol. Plant. 1986, 67, 67–72. [Google Scholar] [CrossRef]
- Debez, A.; Ben Hamed, K.; Grignon, C.; Abdelly, C. Salinity effects on germination, growth, and seed production of the halophyte Cakile maritima. Plant Soil 2004, 262, 179–189. [Google Scholar] [CrossRef]
- Shannon, M.C.; Grieve, C.M.; Francois, L.E. Whole-Plant Response to Salinity. In Plant-Environment Interactions; Wilkinson, R.E., Ed.; Marcel Dekker: New York, NY, USA, 1994; pp. 199–244. Available online: https://www.scirp.org/%28S%28vtj3fa45qm1ean45vvffcz55%29%29/ (accessed on 1 February 2021).
- Botti, C.; Palzkill, D.; Muñoz, D.; Prat, L. Morphological and anatomical characterization of six jojoba clones at saline and non-saline sites. Ind. Crops Prod. 1998, 9, 53–62. [Google Scholar] [CrossRef]
- El-Banna, M.F.; Abdelaal, K.A.A. Response of Strawberry Plants Grown in the Hydroponic System to Pretreatment with H2O2 before Exposure to Salinity Stress. J. Plant Prod. 2018, 9, 989–1001. [Google Scholar] [CrossRef]
- El-Taher, A.M.; Abd El-Raouf, H.S.; Osman, N.A.; Azoz, S.N.; Omar, M.A.; Elkelish, A.; Abd El-Hady, M.A.M. Effect of Salt Stress and Foliar Application of Salicylic Acid on Morphological, Biochemical, Anatomical, and Productivity Characteristics of Cowpea (Vigna unguiculata L.) Plants. Plants 2021, 11, 115. [Google Scholar] [CrossRef]
- El-Banna, M.F.; AL-Huqail, A.A.; Farouk, S.; Belal, B.E.A.; El-Kenawy, M.A.; El-Khalek, A.F.A. Morpho-Physiological and Anatomical Alterations of Salt-Affected Thompson Seedless Grapevine (Vitis vinifera L.) to Brassinolide Spraying. Horticulturae 2022, 8, 568. [Google Scholar] [CrossRef]
- Kanwal, S.; Ashraf, M.; Shahbaz, M.; Iqbal, M.Y. Influence of saline stress on growth, gas exchange, mineral nutrients and non-enzymatic antioxidatns in mungbean [(Vigna Radiata (L.) Wilczek]. Pak. J. Bot. 2013, 45, 763–771. [Google Scholar]
- Kozlowski, T.T. Responses of woody plants to flooding and salinity. Tree Physiol. 1997, 17, 490. [Google Scholar] [CrossRef]
- Akcin, T.A.; Akcin, A.; Yalcın, E. Anatomical changes induced by salinity stress in Salicornia freitagii (Amaranthaceae). Rev. Bras. Bot. 2017, 40, 1013–1018. [Google Scholar] [CrossRef]
- Abd Elbar, O.H.; Farag, R.E.; Shehata, S.A. Effect of putrescine application on some growth, biochemical and anatomical characteristics of Thymus vulgaris L. under drought stress. Ann. Agric. Sci. 2019, 64, 129–137. [Google Scholar] [CrossRef]
- Abdelaal, K. Effect of salicylic acid and abscisic acid on morpho-physiological and anatomical characters of faba bean plants (Vicia faba L.) under drought stress. J. Plant Prod. 2015, 6, 1771–1788. [Google Scholar] [CrossRef] [Green Version]
- Hussein, M.M.; Abo-Leila, B.H.; Metwally, S.A.; Leithy, S.Z. Anatomical structure of jatropha leaves affected by proline and salinity conditions. J. Appl. Sci. Res. 2012, 8, 491–496. [Google Scholar]



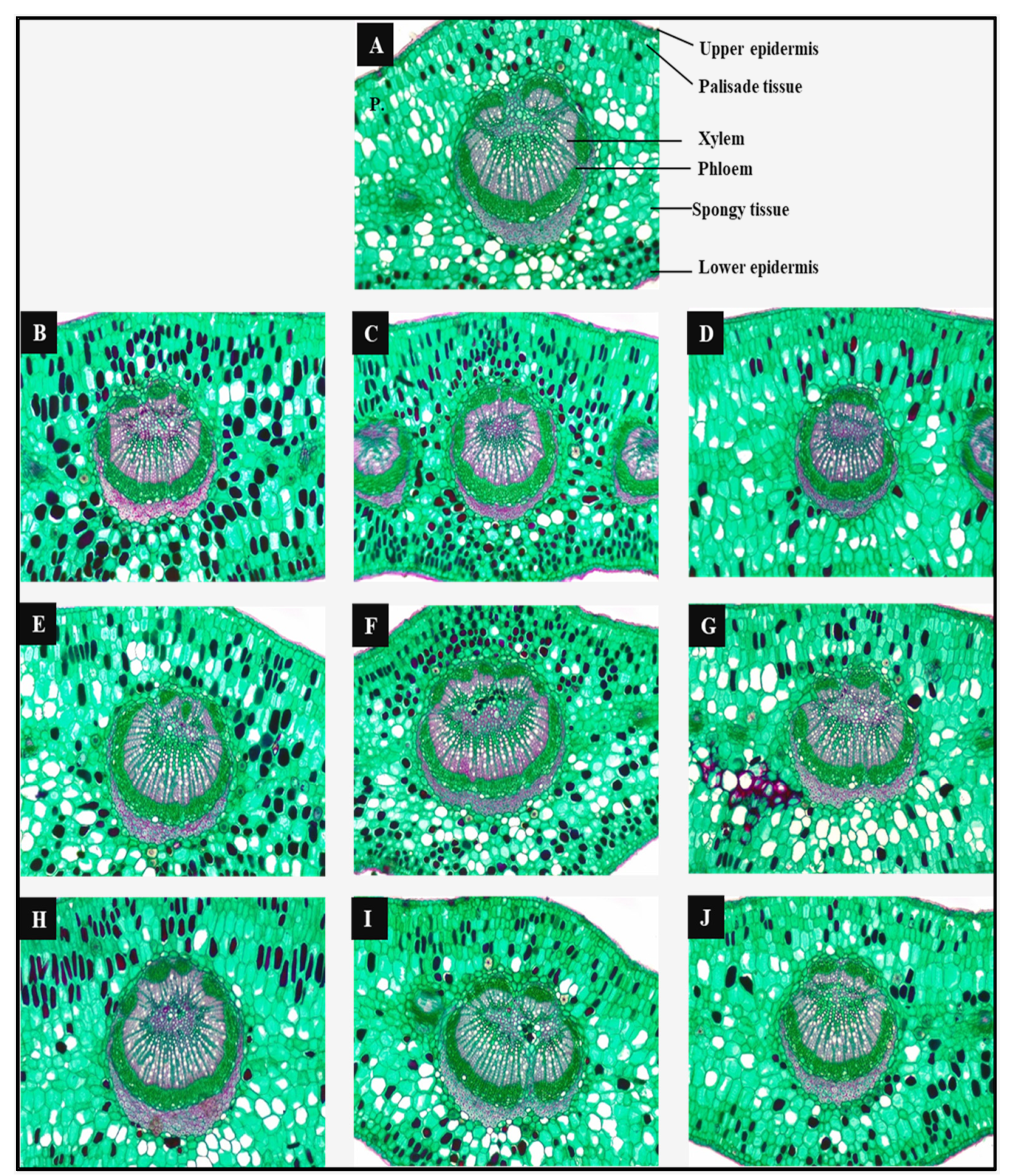

| Treatments | Lamina Thickness | Upper Epidermis Thickness | Palisade Tissue Thickness | Spongy Tissue Thickness | Lower Epidermis Thickness | Dimensions of Main Vascular Bundle | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thickness | Width | |||||||||||||
| µm | ± % to S0 * | µm | ± % to S0 | µm | ± % to S0 | µm | ± % to S0 | µm | ± % to S0 | µm | ± % to S0 | µm | ± % to S0 | |
| T1 | 144.20 ± 2.61e | 0.00 | 7.72 ± 0.37e | 0.00 | 24.50 ± 0.55cd | 0.00 | 104.65 ± 3.15d | 0.00 | 7.33 ± 0.24c | 0.00 | 98.00 ± 1.75b | 0.00 | 98.70 ± 0.42b | 0.00 |
| T2 | 172.76 ± 2.36c | +19.81 | 8.71 ± 0.37de | +12.82 | 28.00 ± 0.78b | +14.29 | 126.35 ± 1.28b | +20.74 | 9.70 ± 0.48a | +32.43 | 84.35 ± 0.65e | −13.93 | 92.05 ± 1.30c | −6.74 |
| T3 | 175.61 ± 1.45bc | +21.78 | 9.90 ± 0.31bc | +28.21 | 33.95 ± 0.89a | +38.57 | 122.85 ± 1.16b | +17.39 | 8.91 ± 0.76a-c | +21.62 | 81.20 ± 0.89f | −17.14 | 77.35 ± 0.65de | −21.63 |
| T4 | 181.80 ± 1.70ab | +26.08 | 10.30 ± 0.24ab | +33.33 | 17.85 ± 1.28e | −27.14 | 144.55 ± 1.88a | +38.13 | 9.11 ± 0.20ab | +24.32 | 78.75 ± 0.55f | −19.64 | 74.55 ± 1.18e | −24.47 |
| T5 | 159.89 ± 1.40d | +10.88 | 8.32 ± 0.24de | +7.69 | 27.65 ± 1.02bc | +12.86 | 116.20 ± 0.89c | +11.04 | 7.72 ± 0.48bc | +5.41 | 94.15 ± 0.65c | −3.93 | 98.70 ± 0.78c | 0.00 |
| T6 | 130.10 ± 1.37f | −9.78 | 10.30 ± 0.39ab | +33.33 | 18.55 ± 1.42e | −24.29 | 91.35 ± 2.02e | −12.71 | 9.90 ± 0.54a | +35.14 | 90.65 ± 0.65d | −7.50 | 89.25 ± 0.95a | −9.57 |
| T7 | 124.83 ± 4.29f | −13.43 | 9.31 ± 0.39b-d | +20.51 | 23.80 ± 1.62d | −2.86 | 84.00 ± 3.79f | −19.73 | 7.72 ± 0.37bc | +5.41 | 80.50 ± 1.66f | −17.86 | 78.05 ± 5.35de | −20.92 |
| T8 | 183.32 ± 0.96a | + 27.13 | 9.11 ± 0.19cd | +17.95 | 21.70 ± 1.80d | −11.43 | 144.20 ± 2.85a | +37.79 | 8.32 ± 0.24a–c | +13.51 | 107.80 ± 1.18a | +10.00 | 105.00 ± 2.18b | +6.38 |
| T9 | 140.82 ± 1.25e | −2.34 | 8.91 ± 0.54cd | +15.38 | 24.50 ± 0.55cd | 0.00 | 98.70 ± 1.18d | −5.69 | 8.71 ± 0.48a–c | +18.92 | 94.15 ± 1.02c | −3.93 | 102.20 ± 0.89ab | +3.55 |
| T10 | 145.65 ± 1.79e | + 1.01 | 11.09 ± 0.37a | +43.59 | 22.05 ± 0.70d | −10.00 | 103.60 ± 2.17d | −1.00 | 8.91 ± 0.70a–c | +21.62 | 86.80 ± 0.42e | −11.43 | 82.95 ± 1.18d | −15.96 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aboryia, M.S.; El-Dengawy, E.-R.F.A.; El-Banna, M.F.; El-Gobba, M.H.; Kasem, M.M.; Hegazy, A.A.; Hassan, H.M.; El-Yazied, A.A.; El-Gawad, H.G.A.; Al-Qahtani, S.M.; et al. Anatomical and Physiological Performance of Jojoba Treated with Proline under Salinity Stress Condition. Horticulturae 2022, 8, 716. https://doi.org/10.3390/horticulturae8080716
Aboryia MS, El-Dengawy E-RFA, El-Banna MF, El-Gobba MH, Kasem MM, Hegazy AA, Hassan HM, El-Yazied AA, El-Gawad HGA, Al-Qahtani SM, et al. Anatomical and Physiological Performance of Jojoba Treated with Proline under Salinity Stress Condition. Horticulturae. 2022; 8(8):716. https://doi.org/10.3390/horticulturae8080716
Chicago/Turabian StyleAboryia, M. S., El-Refaey F. A. El-Dengawy, Mostafa F. El-Banna, Mervat H. El-Gobba, Mahmoud M. Kasem, Ahmed A. Hegazy, Heba Metwally Hassan, Ahmed Abou El-Yazied, Hany G. Abd El-Gawad, Salem Mesfir Al-Qahtani, and et al. 2022. "Anatomical and Physiological Performance of Jojoba Treated with Proline under Salinity Stress Condition" Horticulturae 8, no. 8: 716. https://doi.org/10.3390/horticulturae8080716
APA StyleAboryia, M. S., El-Dengawy, E.-R. F. A., El-Banna, M. F., El-Gobba, M. H., Kasem, M. M., Hegazy, A. A., Hassan, H. M., El-Yazied, A. A., El-Gawad, H. G. A., Al-Qahtani, S. M., Al-Harbi, N. A., Dessoky, E. S., Ismail, I. A., El-Mogy, M. M., & EL-Boraie, E.-S. A. (2022). Anatomical and Physiological Performance of Jojoba Treated with Proline under Salinity Stress Condition. Horticulturae, 8(8), 716. https://doi.org/10.3390/horticulturae8080716










