Abstract
Mungbean is a nutritionally and economically important pulse crop cultivated around Asia, mainly in India. The crop is sensitive to drought at various developmental stages of its growing period. However, there is limited or almost no research on a comparative evaluation of mung-bean plants at the flowering stage under drought conditions. Hence, the aim of this research was to impose the drought stress on two mungbean cultivars VRM (Gg) 1 and CO6 at the flowering stage and assess the physio-biochemical and transcriptional changes. After imposing the drought stress, we found that VRM (Gg) 1 exhibited a low reduction in physiological traits (Chlorophyll, relative water content, and plant dry mass) and high proline content than CO6. Additionally, VRM (Gg) 1 has a low level of H2O2 and MDA contents and higher antioxidant enzymes (SOD, POD, and CAT) activity than CO6 during drought stress. The transcriptional analysis of photosynthesis (PS II-PsbP, PS II-LHC, PS I-PsaG/PsaK, and PEPC 3), antioxidant (SOD 2, POD, CAT 2), and drought-responsive genes (HSP-90, DREB2C, NAC 3 and AREB 2) show that VRM (Gg) 1 had increased transcripts more than CO6 under drought stress. Taken together, VRM (Gg) 1 had a better photosynthetic performance which resulted in fewer reductions in chlorophyll, relative water content, and plant dry mass during drought stress. In addition, higher antioxidative enzyme activities led to lower H2O2 and MDA levels, limiting oxidative damage in VRM (Gg) 1. This was positively correlated with increased transcripts of photosynthesis and antioxidant-related genes in VRM (Gg) 1. Further, the increased transcripts of drought-responsive genes indicate that VRM (Gg) 1 has a better genetic basis against drought stress than CO6. These findings help to understand the mungbean response to drought stress and will aid in the development of genotypes with greater drought tolerance by utilizing natural genetic variants.
1. Introduction
With the effects of global warming and drastic climate changes, drought is the major abiotic stress that affects crop production in arid and semi-arid regions of the world. It is brought about by a scarcity of rain or a vast difference in rainfall quantity [1,2]. Drought impairs plant growth and development and accounts for over 70% of agriculture yield losses worldwide. However, it relies on the drought intensity, duration, phenophases of the crop, and environmental stress factors. An increasingly warming climate and decreased water availability are likely to upsurge the occurrence and severity of drought in the near future. Therefore, boosting the tolerance to drought is a major aim of crop improvement programs. Much progress has been made in understanding the effect of drought stress on plants. Decoding the molecular mechanisms underpinning plant response during drought is not easy due to the intricacy of drought behavior, environmental factors, and their interactions [3]. When subject to drought, plants undergo a series of morphological, physiological, and biochemical changes that seriously reduce plant growth and development [4,5] Physiological responses include (i) a reduction in the content of chlorophyll, rate of photosynthesis, and transpiration, (ii) stomatal closure, (iii) dehydration of cells [6,7,8]. Drought stress causes increased peroxidation of lipid membranes and mass accumulation of reactive oxygen species (ROS) [9,10,11]. The augmented ROS accumulation causes damage to proteins, lipids, cell membranes, carbohydrates, and nucleic acids and leads to disruption of cellular homeostasis and subsequently cellular death [12]. Both enzymatic and non-enzymatic antioxidant systems are fundamental to protecting the cells against toxic ROS and minimizing the oxidative stress effects [13,14,15,16,17]. Previously, it was shown that ROS production under drought stress can be minimized by increasing the antioxidant enzymatic activities in mungbean [18]. Masoumi et al. [19] reported that tolerant soybean plants enhanced their antioxidant enzyme activities and antioxidant contents in response to drought stress, whereas drought-sensitive plants were unable to do so. The lower level of MDA along with enhanced activities of SOD and CAT in black gram plants can be linked to its ability to cope up with water scarcity by limiting the damaging effects of drought through up-regulation of antioxidant enzymes [20].
Physiological and transcriptome responses of soybean to drought stress were investigated by Xu et al. [21]. Physiological traits such as photosynthetic rate, stomatal conductance, transpiration, and water potential were reduced, while SOD and CAT activities were enhanced, and POD activity remained unchanged. Furthermore, in drought-stressed plants, a total of 2771 differentially expressed genes were identified, and they were involved in different biochemical and molecular pathways, including ABA biogenesis, compatible compound accumulation, secondary metabolite synthesis, fatty acid desaturation, and transcription factors. In another study, Mahdavi Mashaki, et al. [22] employed RNA-Seq to investigate transcriptome profiles in drought-responsive contrasting genotypes of Iranian kabuli chickpea under drought stress in root and shoot tissues at the early flowering stage. Of these, 261 and 169 drought stress-responsive genes were identified in the shoots and the roots, respectively, and 17 genes were common in the shoots and the roots. Several molecular mechanisms are involved in the stress response and their corresponding drought-related pathway, (i.e., ABA, proline, and flavonoid biosynthesis). Lopez et al. [23] showed the importance of phosphorous homeostasis, as well as several other key factors, in response to drought stress in the common bean. Upregulation of several key transcription factors, remodeling of cell walls, synthesis of osmoprotectant oligosaccharides, protection of the photosynthetic apparatus, and downregulation of genes involved in cell expansion were all revealed by RNA-seq analysis of the drought-tolerant landrace PHA-683 in response to drought, but there was a significant proportion of DEGs related to phosphate starvation response.
Mungbean (Vigna radiata), native to India, is a short-duration grain legume and is extensively cultivated in Asia. This crop mainly features high protein (25%) and nutrient (carbohydrates, lipids, minerals, and vitamins) contents. It also improves soil fertility by fixing atmospheric nitrogen [24]. India is the world’s leading mungbean grower, with 2.17 million tonnes of grains from a 4.32 m ha area. Mungbean yield in India is still low (502 kg/ha), considerably lower than the productivity of other main pulse crops [25]. In India, mungbean is mainly grown under rainfed conditions at high temperatures, with low humidity and rainfall. Thus, the mungbean is exposed to drought at various developmental stages of its growing period [26,27]. This crop is comparatively surviving under drought conditions. However, in comparison to other developmental stages, mungbean growth is sensitive to drought during flowering and post-flowering. Drought stress during these stages can decrease the grain yield ranging from 30% to 80%. Regardless of their importance, the studies that investigated the impacts of drought-influenced mungbean are limited [28,29]. Under drought, comparative evaluation of physio-biochemical and transcriptional changes between mungbean cultivars at the flowering stage lacks existing information in the literature. Taking into account the above, in this research, we aimed at revealing the physio-biochemical and transcriptional alterations in two mungbean cultivars (VRM (Gg) 1 and CO6) at the flowering stage under drought conditions.
2. Materials and Methods
2.1. Plant Materials and Drought Treatment
Seeds of mungbean varieties CO6 and VRM (Gg) 1 were provided by Agricultural Research Station, Tamil Nadu Agricultural University, Virinjipuram, Tamil Nadu, India. The seeds for two varieties were sown in pots (15 L, 30 cm height, 33.0 cm diameter at top and 25.5 cm bottom diameter) containing 3:1 ratio of soil and compost in a greenhouse at Agricultural College and Research Station, Madurai, Tamil Nadu, India. The experimental design was a completely randomized design (CRD) with three replications of 15 plants (5 plants per pot). During the experiment period, the temperature was maintained at 28 °C, and relative humidity was 65%. Three different sets of plants were maintained. Watering was done regularly until the flowering started. When the flowering was observed, drought stress for 6 days (soil moisture, 50%) and 12 days (soil moisture, 25%) were imposed in two sets; the third set was kept as the control 0 days (soil moisture, >80%). Soil moisture was measured using Lutron PMS-714 soil moisture meter.
2.2. Plant Sampling
The fully expanded leaves from three to five plants were sampled following the 0, 6, and 12 days of drought stress and immediately frozen into liquid nitrogen and then stored at −80 °C. Leaf relative water content (RWC), chlorophyll content, leaf gas exchange parameters, and plant dry mass were used to estimate at 0 and 12 days of drought stress plants. Proline, protein, hydrogen peroxide (H2O2), malondialdehyde (MDA), enzymatic (SOD, POD, and CAT), and non-enzymatic (Ascorbic acid) antioxidants assays were conducted at 0, 6, and 12 days of drought stress plants. Quantitative real-time polymerase chain reaction (qRT-PCR) analysis was conducted at 0 and 12 days.
2.3. Physiological Traits
Total chlorophyll content was measured according to Arnon [30]. Plant dry mass was measured after drying the plant at 80 °C to a constant weight. RWC was measured as the standard method described by Barrs and Weatherly [31]. RWC = (FW − DW)/(TW − DW) × 100, where FW is the fresh leaf weight, DW is the dry leaf weight, and TW is the turgid weight of the leaves. Photosynthetic gas exchange parameters were measured by portable photosynthesis system Li-6400 (LiCor, Lincoln, NE, USA).
2.4. Proline Estimation
Proline content was estimated according to the methodology described by Bates et al. [32]. Leaf samples (1 g) were taken and homogenized in 3% aqueous sulphosalicylic acid and centrifuged at 10,000 rpm for 15 min at 4 °C. The supernatant was carefully taken, and then the acid-ninhydrin solution (1.25 g of ninhydrin in 30 mL glacial acetic acid) was added. The mixture was then incubated for an hour at 100 °C and cooled in ice to stop the reaction. For extracting the reaction mixture 4 mL of toluene was added and vortexed thoroughly for 2 min. The solute is then measured for absorbance at 520 nm. Toluene was considered as a blank, and the content of proline was calculated giving to the formula: [(µg proline/mL × mL toluene)/115.5 µg/µmole]/[(g sample)/5] = µg proline/g FW.
2.5. Damage Index; Determination of MDA and H2O2 Contents
MDA content estimation was done following the Stewart and Bewly method [33] to determine lipid peroxidation. Leaf samples (1 g) were homogenized in 0.1% of trichloroacetic acid (TCA) and then centrifuged at 12,000 rpm for 15 min at 4 °C. After centrifugation, 0.5 mL of the supernatant was collected and mixed with a 1 mL volume of 20% TCA containing a 0.5% thiobarbituric acid (TBA) solution. The sample was incubated for another 30 min at 95 °C and placed in ice bath to stop the reaction followed by centrifuging at 12,000 rpm for 10 min. The resulting solute was measured for absorbance at 532 nm. MDA content was estimated by the 155 mM−1 cm−1 extinction coefficient. Results were stated as μmol/g fresh weight. The H2O2 content was determined following the method previously elucidated by Loreto and Velikova [34]. Leaf samples (1 g) were homogenized in ice-cold 0.1% TCA and centrifuged at 12,000 rpm for 15 min at 4 °C. After that, 0.5 mL of 10 mM potassium phosphate buffer and 0.75 mL of 1 M KI were added to 0.5 mL of the supernatant. The absorbance was measured at 390 nm against a blank, and the H2O2 content was inferred by a standard calibration curve, previously made solutions with known H2O2 concentration. H2O2 concentration was expressed as µmol/g fresh weight.
2.6. Assay of Antioxidant Enzymes
Approximately 1 g of sampled leaves were weighed and finely chopped to powder with liquid nitrogen. About 10 mL of ice-cold 50 mM potassium phosphate buffer with 0.1-mM Na2 EDTA and 1% (w/v) polyvinylpyrrolidone (PVP) was used to homogenize the powder. The pH of the buffer should be 7.8 for SOD and POD assays whereas it is 7.0 for CAT assay. After filtering the homogenate with a 4-layered muslin cloth, it was centrifuged at 12,000 rpm at 4 °C for 15 min. The aliquot part in the supernatant was gathered for enzyme activity assays. For recording the SOD activity, the protocol of Madamanchi and Alscher [35] was followed. The amount of enzyme that reduces nitroblue tetrazolium (NBT) to half was referred to be one unit of SOD. The solute was read at an absorbance of 560 nm. As guaiacol was the electron donor, the POD activity was recorded at 470 nm as proposed by Chance and Maehly [36] in which one unit POD function was referred to as one unit change in absorbance of 0.01 unit in a minute. As described by Aebi [37], the activity of CAT was found. In an interval of 2 min, a reduction in the absorbance at 240 nm was recorded after the digestion of H2O2. It is found that one unit of CAT produces 1 mM of H2O2 in a minute for which the results are expressed in units/mg of protein.
2.7. Ascorbic Acid Content
Ascorbic acid (referred to as vitamin C) was measured following the method previously reported by Arakawa et al. [38] with minor modifications. Leaf samples and 5 mL of 5% trichloroacetic acid (TCA) were homogenized in the mortar and centrifuged at 10,000 rpm for 10 min at 4 °C. Then, 1 mL of clear supernatant, 1 mL of 5% TC, 1 mL alcohol, 0.5 mL 0.4% phosphoric acid (H3PO4)-alcohol, 1 mL of 0.5% 4,7-diphenyl-1,10-phenanthroline (BP)-alcohol, and 0.5 mL 0.03% ferric trichloride (FeCl3)-alcohol were added into to a tube and incubated at 40 °C for 1 h. The reaction was ended at room temperature, and absorbance was measured at 534 nm with a spectrophotometer (Unicam UV-330, Cambridge, UK). Results were expressed as the unit’s μmol/g fresh weight.
2.8. RNA Isolation, cDNA Synthesis, and qRT-PCR Analysis
According to the manufacturer’s guidelines, total RNA was isolated using an RNeasy plant mini kit (Qiagen, Hilden, Germany) and treated with RNase-free DNAseI (Promega, Madison, WI, USA). The RNA quantity was assessed using the bio spectrometer (Eppendorf, Hamburg, Germany) based on the absorbance ratio at 280 nm. Further, the quality of RNA was tested on 1% agarose gel via electrophoresis. The first-strand cDNA was done by transcriptor First Strand cDNA Synthesis Kit (Roche Applied Science, Penzberg, Germany) following the manufacturer’s guidelines. Sequence information of the following genes, photosystem II oxygen-evolving complex protein (PS II-PsbP), photosystem II chlorophyll A/B binding protein (PS II-LHC), photosystem I PsaG/PsaK (PS I-PsaG/PsaK), phosphoenolpyruvate carboxylase 3 (PEPC 3), superoxide dismutase 2 (SOD 2), peroxidase (POD), catalase-2 (CAT 2), heat shock protein-90 (HSP-90), dehydration responsive element-binding transcription factor (DREB2C), NAC transcription factor 3 (NAC 3), and abscisic acid-responsive elements-binding factor 2 (ABF 2) were obtained from NCBI database [https://www.ncbi.nlm.nih.gov/ (accessed on 29 May 2019); Vigna radiata var. radiata (Mungbean)]. Primer 5.0 software was used to design the corresponding primer pairs of the concerned gene sequences for qRT-PCR reaction (Table S1) and were verified to produce a single peak in the melting curve by using a Light Cycler 480® Real-Time PCR System (Roche Applied Science, Penzberg, Germany). Aliquots for qRT-PCR reactions included 10 μL of final volumes containing 1 μL of cDNA, 0.5 μL each primer (10 μM), and 5 μL (2×) of FastStart Essential DNA Green Master mix (Roche Applied Science, Penzberg, Germany) and 3 μL of ddH2O. All reactions were performed in 96-well plates using a Light Cycler 480® Real-Time PCR System with three technical replicates. The thermal conditions are as follows: 95 °C for 5 min, followed by 40 cycles of 95 °C for 10 s, 60 °C for 30 s, and then 72 °C for 30 s. Actin gene (internal control) from mungbean was used to normalize, and transcripts change was calculated using the 2−ΔΔCT method.
2.9. Statistical Analysis
The experimental data are presented as the mean and standard error of the mean. All statistical analyses were performed using SPSS statistical package (SPSS Inc., Chicago, IL, USA). In order to find out the differences among the groups, Duncan’s multiple range test for one-way ANOVA was performed at a p-value < 0.05 statistical significance.
3. Results
3.1. The Effect of Drought Stress on Physiological Traits
Chlorophyll content, plant dry mass, and RWC were observed in two mungbean cultivars following the 12 days of drought stress and compared with the control (Figure 1). After 12 days under drought stress, CO6 plants showed severe wilting, whereas a few leaves of the VRM (Gg) 1 plants had slowly begun to curl. Notably, considerable reductions in chlorophyll content and plant dry mass were observed in the CO6 compared to the respective control. Next, we determined the RWC at the control and drought stress conditions. Mungbean cultivar VRM (Gg) 1 did not show any considerable changes in RWC when subjected to drought stress. In contrast, RWC had a considerable decrease in the CO6 after 12 days of drought stress.
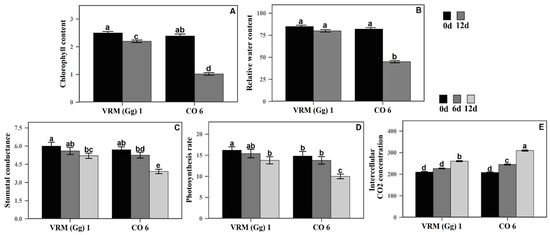
Figure 1.
(A) Chlorophyll content (mg g−1 FW), (B) relative water content (%), (C) stomatal conductance (mol H2O m−2 s−1), (D) photosynthesis rate (µmol CO2 m−2 s−1), and (E) intercellular CO2 concentration (ppm) of two mungbean cultivars (VRM (Gg) 1 and CO6) grown under control and drought-stressed conditions. Values followed by the same letter are not significantly different (p ≤ 0.05) according to duncan’s multiple range test. Bars present means ± SE (n = 3).
During the 12 days of drought stress, photosynthetic gas exchange parameters (leaf net photosynthesis rate, stomatal conductance, and intercellular CO2 concentration) were determined in both cultivars (Figure 1). After 6 days of the drought stress, no major difference in the leaf net photosynthetic rate among VRM (Gg) 1 and CO6 was observed compared to their respective control. However, after 12 days of drought stress, the reduction in CO6 was higher than observed in VRM (Gg) 1. The differences observed in stomatal conductance after drought stress were also similar to the differences seen in leaf net photosynthetic rate, with the same trend as in leaf net photosynthetic rate but to a smaller extent. However, after drought stress, CO6 plants showed an increased intercellular CO2 concentration than in VRM (Gg) 1 revealing the opposite relationship between stomatal conductance and leaf net photosynthetic rate.
3.2. Proline Content
Proline accumulation is an eminent metabolic response of plants to drought and is also used as an indicator to determine drought tolerance. The difference in proline content during the 12 days of drought stress was estimated in both cultivars. Under the control conditions, a slight difference was seen in the proline content of both cultivars. VRM (Gg) 1 showed a considerably high amount of proline than CO6 after 6 and 12 days of drought stress. The proline content of VRM (Gg) 1 and CO6 under drought stress are presented in Figure 2.
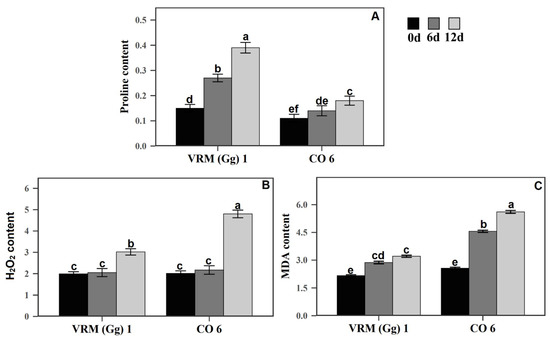
Figure 2.
(A) Effect of drought stress on proline (µg g−1 FW), (B) H2O2 (µmol g−1 FW), and (C) MDA contents (μmol g−1 FW) in two mungbean cultivars VRM (Gg) 1 and CO6. Values followed by the same letter are not significantly different (p ≤ 0.05) according to duncan’s multiple range test. Bars present means ± SE (n = 3).
3.3. Changes in MDA and H2O2 Accumulation
During the 12 days of drought stress, MDA, a product of lipid peroxidation was detected among two cultivars. Compared to their respective controls, the amount of MDA content was increased in both cultivars under stress. However, the amounts of upsurge were the degree of difference. After 12 days of stress, the content of MDA was considerably increased in CO6 (119%) than in VRM (Gg) 1 (49%), further revealing that the VRM (Gg) 1 plants cope with a smaller amount of membrane damage compared to CO6 (Figure 2). Likewise, the accumulation level of H2O2 in plants as a response to drought stress imposed on VRM (Gg) 1 and CO6 was also estimated (Figure 2). Six days after drought stress, no major changes in H2O2 content were obtained in both cultivars under stress and control conditions. However, after 12 days, H2O2 levels exhibited a significant increase between both types of plants compared with respective controls. Notably, the magnitudes of increase were different in CO6 (139%) and VRM (Gg) 1 (51.75%). Under control conditions, both cultivars showed no major difference in MDA and H2O2 (Figure 2).
3.4. Activity of Enzymatic and Non-Enzymatic Antioxidants
The effect of drought on enzymatic antioxidants viz., SOD, POD, and CAT were evaluated on the mungbean cultivars (Figure 3). After 12 days of drought stress, the three antioxidant enzyme activities were higher in both cultivars. No major changes in SOD activity were found 6 days after drought stress in the VRM (Gg) 1 and CO6 compared to their respective control. However, a significant increase was found in 12 days after drought stress in both cultivars. The increase in VRM (Gg) 1 was high compared to CO6. Unlike SOD, POD and CAT activities in the VRM (Gg) 1 and CO6 significantly increased 6 days after drought stress and maintained a higher level after 12 days of drought stress compared to their respective controls. However, the increase in VRM (Gg) 1 was high compared to CO6. Ascorbic acid is one of the most abundant water-soluble antioxidant compounds in plants. The response of ascorbic acid content for drought stress in VRM (Gg) 1 and CO6 was estimated and is presented in Figure 3. The ascorbic acid content was higher in the VRM (Gg) 1 than the CO6. After 12 days of drought stress, the ascorbic acid content was slightly increased in the VRM (Gg) 1. No significant changes were seen in the CO6 than their respective controls.
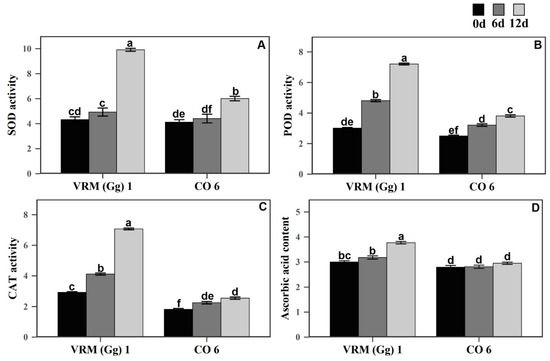
Figure 3.
Activities of enzymatic and non-enzymatic antioxidants in two mungbean cultivars VRM (Gg) 1 and CO6. (A) SOD (Umg−1 Protein), (B) POD (Umg−1 Protein), (C) CAT (Umg−1 Protein), and (D) ascorbic acid (μmolg−1 FW). Values followed by the same letter are not significantly different (p ≤ 0.05) according to duncan’s multiple range test. Bars present means ± SE (n = 3).
3.5. Transcriptional Profiling of Photosynthesis, Antioxidant, and Stress-Responsive Genes
After 12 days of drought stress, four photosynthesis-related genes (PS II-PsbP, PS II-LHCB, PS I-PsaG/PsaK, and PEPC 3) transcripts levels were analyzed between VRM (Gg) 1 and CO6 by qRT-PCR analysis (Figure 4). Under control conditions, the transcripts of photosynthesis-related genes in VRM (Gg) 1 and CO6 showed no major differences. However, after 12 days of drought stress, transcripts level increased in both cultivars compared with respective controls. Noticeably, the transcripts level was higher in VRM (Gg) 1 than CO6. Further, we analyzed the three antioxidants (SOD 2, POD, and CAT 2) and four drought stress-responsive genes (HSP-90, DREB2C, NAC 3, and AREB 2) in both cultivars. Like photosynthesis genes, transcripts of antioxidant and drought stress-responsive genes also did not exhibit major differences in VRM (Gg) 1 and CO6 under controlled conditions. However, the transcripts levels were increased in VRM (Gg) 1 than CO6 after 12 days of drought stress. Together, these results suggest that VRM (Gg) 1 exhibited better performance under drought stress compared to CO6, owing to the transcripts differences of these genes under drought stress.
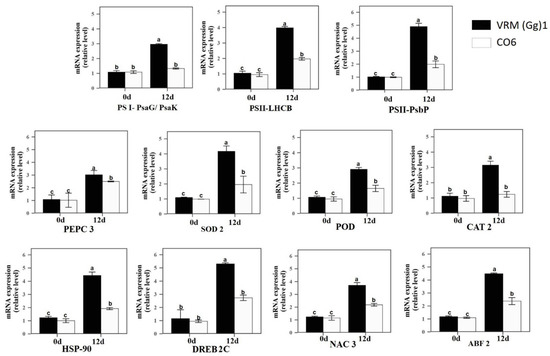
Figure 4.
Effect of drought stress on the relative expression level of photosynthesis (PS II-PsbP, PS II-LHCB, PS I-PsaG/PsaK, and PEPC 3), antioxidants (SOD 2, POD, and CAT 2), and stress (HSP-90, DREB2C, NAC 3 and AREB 2) related genes in two mungbean cultivars VRM (Gg) 1 and CO6. Values followed by the same letter are not significantly different (p ≤ 0.05) according to duncan’s multiple range test. Bars present means ± SE (n = 3).
4. Discussion
In the present study, the response of mungbean cultivars to drought stress was investigated in terms of analyzing the physio-biochemical and transcriptional changes. We found that chlorophyll content and plant dry mass were decreased during drought stress, and the cultivar VRM (Gg) 1 showed a lower decrease compared to CO6, indicating improved photosynthesis and plant growth development. Additionally, VRM (Gg) 1 subjected to drought stress did not show any major changes in RWC. However, the RWC had a significant decrease in CO6, suggesting better water maintaining capacity in VRM (Gg) 1. We also found that when VRM (Gg) 1 and CO6 were exposed to drought stress, the photosynthetic gas exchange parameters (leaf net photosynthetic rate and stomatal conductance) were decreased. Notably, the stomatal conductance in VRM (Gg) 1 plants showed lower decreases than that in CO6 under drought stress. This changing pattern in stomatal conductance is comparable to that in leaf net photosynthetic rate among the plants of VRM (Gg) 1 and CO6, showing that the better leaf net photosynthetic rate in VRM (Gg) 1 was related to the regulation of stomatal conductance. In contrast, the intercellular CO2 concentration of VRM (Gg) 1 was lower than CO6 plants. This fact was because of the varied reduction of leaf net photosynthetic rate in VRM (Gg) 1 and CO6. It might be the reason for increased CO2 assimilation and decreased intercellular CO2 concentration in the VRM (Gg) 1 plants compared to CO6. Therefore, photosynthesis and growth in the VRM (Gg) 1 were better when imposed the drought stress. Moreover, we investigated the transcripts level differences of photosynthesis-related genes under drought stress. PS II-PsbP, PS II-LHCB, PS I-PsaG/PsaK, and PEPC 3 are major genes related to photosynthesis. In our study, following 12 days of drought stress, the transcripts levels of all the genes excluding PS I-PsaG/PsaK considerably increased in both cultivars compared to their control. Notably, the transcripts level in VRM (Gg) 1 was high compared to CO6, suggesting that VRM (Gg) 1 had better photosynthetic capacity than CO6 under drought stress. Proline accumulation is an important metabolic response to drought in plants and it is also employed as an indicator to regulate the drought tolerance. After 6 and 12 days of drought stress, VRM (Gg) 1 had a much higher level of proline than CO6. Collectively, these results are in line with the reports of Li et al. [39]. Ansari et al. [40] Favero Peixoto-Junior et al. [41], who described that the genotype is referred to as tolerant to drought stress if it keeps better photosynthetic performance, chlorophyll content, RWC, plant dry mass, and proline under stress conditions.
Many studies showed that drought stress causes oxidative damage, characterized as an accumulation of H2O2 and MDA [42,43]. Our results showed that, after the drought stress, the accumulation of H2O2 and MDA was low in VRM (Gg) 1 whereas high in CO6. A lower H2O2 and MDA content in VRM (Gg) 1 specified that it has stable ROS scavenging and better protective mechanism. In several crops, including mungbean, wheat, and muskmelon [28,40,44] under drought stress, the genotypes with contrasting drought tolerance showed differences in H2O2 and MDA content. Additionally, a higher accumulation of proline in VRM (Gg) 1 was vital, and it acts as a compatible solute that prevents the protein and membrane structure while also scavenging ROS to maintain the cellular redox level under drought stress and agrees with the statement of Yamada et al. [45]. Plants with tolerance to abiotic stress possess a robust antioxidant system to defend them from oxidative stress by keeping increased antioxidant enzymes and antioxidant molecule activity and contents under stress conditions [46]. SOD, POD, and CAT are major enzymes protecting the plants against ROS-induced oxidative damage [14,47]. Many research reports detailed that the up-regulated expression of SOD, POD, and CAT leads to decreased ROS production under stress conditions [44,48]. In our study, SOD, POD, and CAT activities were heightened over time in VRM (Gg) 1 compared with the CO6 under drought stress and corroborate with the low ROS production observed in VRM (Gg) 1. Abid et al. [44] and Ali et al. [28] showed SOD, POD, and CAT activities were higher in VRM (Gg) 1 than CO6 under drought stress which was in concurrence with our findings. Likewise, the accumulation of non-enzymatic antioxidant ascorbic acid was higher in VRM (Gg) 1 than in CO6. However, the increase was non-significant, and only a marginal increase was observed. Taking together, we conclude that VRM (Gg) 1 has a stronger antioxidant system than CO6.
Drought stress regulates the expression of genes in plants at both transcriptional and post-transcriptional levels. Drought tolerance in plants is thought to be mediated by many genes and biological pathways. Heat-shock proteins serve as molecular chaperones for various client proteins in abiotic stress response and play a significant role in preventing the plants against abiotic stresses. The plant’s HSP90 genes had a major role in response to abiotic stresses, including drought [49,50]. Song et al. [51] reported that the overexpression of Hsp90 in Arabidopsis thaliana improved the plant’s sensitivity to drought stresses. VRM (Gg) 1 exhibited higher expression of Hsp90 in leaves than CO6 during drought stress, suggesting the possible role of preventing the cells from oxidative damage in VRM (Gg) 1 plants. DREB is the key transcription factor playing a pivotal role in drought stress response and tolerance to drought [52,53,54,55]. In the present study, the DREB2C transcription factor was examined in VRM (Gg) 1 and CO6 under drought stress. After ten days of drought stress, the expression level of DREB2C in leaves was expressed considerably higher in VRM (Gg) 1 than in CO6. Similarly, NAC 3 and AREB 2, which play critical roles during various abiotic stresses, also showed higher expression in VRM (Gg) 1 compared to CO6. Previously, several studies demonstrated the possible involvement of NAC 3 and AREB 2 in drought tolerance [56,57]. From these outcomes, we inferred that it might be possible that the higher expression of Hsp90, DREB1, NAC 3, and AREB 2 is likely to contribute to the better performance of VRM (Gg) 1 during drought stress.
VRM (Gg) 1 had better photosynthetic activity during drought stress, resulting in fewer losses in chlorophyll, relative water content, and plant dry mass. Furthermore, enhanced antioxidative enzyme activities resulted in decreased H2O2 and MDA levels in VRM (Gg) 1, limiting oxidative damage. These physio-biochemical alterations positively correlated with increased transcripts of photosynthesis and antioxidant-related genes in VRM (Gg) 1 and were consistent with the earlier studies on, mungbean, faba bean, and alfalfa responses to drought stress [58,59,60]. The increased transcripts of drought-responsive genes suggest that VRM (Gg) 1 has a stronger genetic base for drought tolerance than CO6. However, supplement research is needed to understand the exact genetic and molecular mechanism underlying drought tolerance.
5. Conclusions
In this study, we found that the mungbean cultivar VRM (Gg) 1 performed well and exhibited tolerance to drought stress compared to CO6, as supported by the physio-biochemical and gene transcriptional changes. In the future, VRM (Gg) 1 will need to be tested under field conditions before being employed in mungbean drought-tolerant breeding programs. VRM (Gg) 1 is a potential source to detect the quantitative trait locus (QTL)/gene (s) associated with drought tolerance. Collectively, the obtained results from our study could be used in the future search for drought-tolerant genotypes or in breeding programs with an aim to obtain tolerant mungbean genotypes.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae8050424/s1. Table S1: Details of primers used for quantitative real-time PCR (qRT-PCR) analysis. Reference [61] is cited in the supplementary materials.
Author Contributions
Conceptualization, A.K., N.S. and M.P.; methodology, A.K., V.G.R. and G.A.; formal analysis, S.P., V.M. and M.D.; investigation, G.A., A.K., A.A. and I.M.; resources, M.P. and N.S.; data curation, G.A., M.A. and A.K.; writing—review and editing, G.A. and A.K.; supervision, M.P. and N.S.; funding acquisition, A.K. and N.S. All authors have read and agreed to the published version of the manuscript.
Funding
A.K. and N.S. acknowledged the support of the Science and Engineering Research Board (SERB), Department of Science and Technology (DST), Government of India for the DST-SERB NPDF fellowship program (PDF/2016/003676).
Acknowledgments
All the authors wish to acknowledge NationalAgricultural Development Programme (NADP)/Rashtriya KrishiVikas Yojana (RKVY)—Government of Tamil Nadu, and Centre of Innovation (CI), Agricultural Collegeand Research Institute, Tamil Nadu Agricultural University, Madurai, for providing instrumentation facilities.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflicts of interest.
References
- Saini, H.S.; Westgate, M.E. Reproductive Development in Grain Crops during Drought. Adv. Agron. 1999, 68, 59–96. [Google Scholar] [CrossRef]
- Zarei, A.R.; Moghimi, M.M.; Mahmoudi, M.R. Parametric and non-parametric trend of drought in arid and semi-arid regions using RDI index. Water Resour. Manag. 2016, 30, 5479–5500. [Google Scholar] [CrossRef]
- Varshney, R.K.; Tuberosa, R.; Tardieu, F. Progress in understanding drought tolerance: From alleles to cropping systems. J. Exp. Bot. 2018, 69, 3175–3179. [Google Scholar] [CrossRef]
- Shinozaki, K.; Yamaguchi-Shinozaki, K. Gene networks involved in drought stress response and tolerance. J. Exp. Bot. 2007, 58, 221–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toscano, S.; Farieri, E.; Ferrante, A.; Romano, D. Physiological and biochemical responses in two ornamental shrubs to drought stress. Front. Plant Sci. 2016, 7, 645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoshika, Y.; Omasa, K.; Paoletti, E. Both ozone exposure and soil water stress are able to induce stomatal sluggishness. Environ. Exp. Bot. 2013, 88, 19–23. [Google Scholar] [CrossRef]
- Hu, L.; Wang, Z.; Huang, B. Diffusion limitations and metabolic factors associated with inhibition and recovery of photosynthesis from drought stress in a C3 perennial grass species. Physiol. Plant. 2010, 139, 93–106. [Google Scholar] [CrossRef]
- Manes, F.; Vitale, M.; Donato, E.; Giannini, M.; Puppi, G. Different ability of three Mediterranean oak species to tolerate progressive water stress. Photosynthetica 2006, 44, 387–393. [Google Scholar] [CrossRef]
- Carvalho, M.; Castro, I.; Moutinho-Pereira, J.; Correia, C.; Egea-Cortines, M.; Matos, M.; Rosa, E.; Carnide, V.; Lino-Neto, T. Evaluating stress responses in cowpea under drought stress. J. Plant Physiol. 2019, 241, 153001. [Google Scholar] [CrossRef]
- Dhanda, S.; Sethi, G.; Behl, R. Indices of drought tolerance in wheat genotypes at early stages of plant growth. J. Agron. Crop Sci. 2004, 190, 6–12. [Google Scholar] [CrossRef]
- Miller, G.; Suzuki, N.; Ciftci-Yilmaz, S.; Mittler, R. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ. 2010, 33, 453–467. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, W.; Zhu, Y.M.; Chen, Y.; Qiu, C.W.; Zhu, S.; Wu, F. Genotypic differences in leaf secondary metabolism, plant hormones and yield under alone and combined stress of drought and salinity in cotton genotypes. Physiol. Plant. 2019, 165, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Al Hassan, M.; Chaura, J.; Donat-Torres, M.P.; Boscaiu, M.; Vicente, O. Antioxidant responses under salinity and drought in three closely related wild monocots with different ecological optima. AoB Plants 2017, 9, plx009. [Google Scholar] [CrossRef] [PubMed]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [Green Version]
- Dudziak, K.; Zapalska, M.; Börner, A.; Szczerba, H.; Kowalczyk, K.; Nowak, M. Analysis of wheat gene expression related to the oxidative stress response and signal transduction under short-term osmotic stress. Sci. Rep. 2019, 9, 2743. [Google Scholar] [CrossRef] [Green Version]
- Manavalan, L.P.; Nguyen, H.T. Drought tolerance in crops: Physiology to genomics. In Plant Stress Physiology; Shabala, S., Ed.; CABI: Wallingford, UK, 2017; pp. 1–23. [Google Scholar]
- Wu, Q.S.; Zou, Y.N.; Xia, R.X. Effects of water stress and arbuscular mycorrhizal fungi on reactive oxygen metabolism and antioxidant production by citrus (Citrus tangerine) roots. Eur. J. Soil Biol. 2006, 42, 166–172. [Google Scholar] [CrossRef]
- Alderfasi, A.A.; Alzarqaa, A.A.; AL-Yahya, F.A.; Roushdy, S.S.; Dawabah, A.A.; Alhammad, B.A. Effect of combined biotic and abiotic stress on some physiological aspects and antioxidant enzymatic activity in mungbean (Vigna radiate L.). Afr. J. Agric. Res. 2017, 12, 700–705. [Google Scholar]
- Masoumi, H.; Darvish, F.; Daneshian, J.; Normohammadi, G.; Habibi, D. Effects of water deficit stress on seed yield and antioxidants content in soybean (Glycine max L.) cultivars. Afr. J. Agric. Res. 2011, 6, 1209–1218. [Google Scholar]
- Baroowa, B.; Gogoi, N. The effect of osmotic stress on anti-oxidative capacity of black gram (Vigna mungo L.). Exp. Agric. 2017, 53, 84–99. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Xia, C.; Xia, Z.; Zhou, X.; Huang, J.; Huang, Z.; Liu, Y.; Jiang, Y.; Casteel, S.; Zhang, C. Physiological and transcriptomic responses of reproductive stage soybean to drought stress. Plant Cell Rep. 2018, 37, 1611–1624. [Google Scholar] [CrossRef]
- Mahdavi Mashaki, K.; Garg, V.; Nasrollahnezhad Ghomi, A.A.; Kudapa, H.; Chitikineni, A.; Zaynali Nezhad, K.; Yamchi, A.; Soltanloo, H.; Varshney, R.K.; Thudi, M. RNA-Seq analysis revealed genes associated with drought stress response in kabuli chickpea (Cicer arietinum L.). PLoS ONE 2018, 13, e0199774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López, C.M.; Pineda, M.; Alamillo, J.M. Transcriptomic response to water deficit reveals a crucial role of phosphate acquisition in a drought-tolerant common bean landrace. Plants 2020, 9, 445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karthikeyan, A.; Shobhana, V.; Sudha, M.; Raveendran, M.; Senthil, N.; Pandiyan, M.; Nagarajan, P. Mungbean yellow mosaic virus (MYMV): A threat to green gram (Vigna radiata) production in Asia. Int. J. Pest Manag. 2014, 60, 314–324. [Google Scholar] [CrossRef]
- Project Coordinators Report. All India Coordinated Research Project on MULLaRP (Mungbean, Urdbean, Lentil, Lathyrus, Rajmash, Fieldpea); ICAR-Indian Institute of Pulses Research: Kanpur, India, 2018; pp. 1–46.
- Ahmad, A.; Selim, M.M.; Alderfasi, A.; Afzal, M. Effect of drought stress on mung bean (Vigna radiata L.) under arid climatic conditions of Saudi Arabia. In Ecosystems and Sustainable Development; Garcia, M., Brebbia, C.A., Eds.; WIT Press: Southampton, UK, 2015; Volume 192, pp. 185–193. [Google Scholar]
- Dutta, P.; Bera, A. Screening of mungbean genotypes for drought tolerance. Legume Res. 2008, 31, 145–148. [Google Scholar]
- Ali, Q.; Javed, M.T.; Noman, A.; Haider, M.Z.; Waseem, M.; Iqbal, N.; Waseem, M.; Shah, M.S.; Shahzad, F.; Perveen, R. Assessment of drought tolerance in mung bean cultivars/lines as depicted by the activities of germination enzymes, seedling’s antioxidative potential and nutrient acquisition. Arch. Agron. Soil Sci. 2018, 64, 84–102. [Google Scholar] [CrossRef]
- Bangar, P.; Chaudhury, A.; Tiwari, B.; Kumar, S.; Kumari, R.; Bhat, K.V. Morphophysiological and biochemical response of mungbean [Vigna radiata (L.) Wilczek] varieties at different developmental stages under drought stress. Turk. J. Biol. 2019, 43, 58–69. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Barrs, H.; Weatherley, P. A re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust. J. Biol. Sci. 1962, 15, 413–428. [Google Scholar] [CrossRef] [Green Version]
- Bates, L.S.; Waldren, R.P.; Teare, I. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Stewart, R.R.; Bewley, J.D. Lipid peroxidation associated with accelerated aging of soybean axes. Plant Physiol. 1980, 65, 245–248. [Google Scholar] [CrossRef] [Green Version]
- Loreto, F.; Velikova, V. Isoprene produced by leaves protects the photosynthetic apparatus against ozone damage, quenches ozone products, and reduces lipid peroxidation of cellular membranes. Plant Physiol. 2001, 127, 1781–1787. [Google Scholar] [CrossRef] [PubMed]
- Madamanchi, N.R.; Alscher, R.G. Metabolic bases for differences in sensitivity of two pea cultivars to sulfur dioxide. Plant Physiol. 1991, 97, 88–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chance, B.; Maehly, A. Assay of catalases and peroxidases. Methods Enzymol. 1955, 2, 764–775. [Google Scholar]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar]
- Arakawa, N.; Otsuka, M.; Kurata, T.; Inagaki, C. Separative determination of ascorbic acid and erythorbic acid by high-performance liquid chromatography. J. Nutr. Sci. Vitaminol. 1981, 27, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Li, R.-H.; Guo, P.-G.; Michael, B.; Stefania, G.; Salvatore, C. Evaluation of chlorophyll content and fluorescence parameters as indicators of drought tolerance in barley. Agric. Sci. Chin. 2006, 5, 751–757. [Google Scholar] [CrossRef]
- Ansari, W.; Atri, N.; Singh, B.; Pandey, S. Changes in antioxidant enzyme activities and gene expression in two muskmelon genotypes under progressive water stress. Biol. Plant. 2017, 61, 333–341. [Google Scholar] [CrossRef]
- Fávero Peixoto-Junior, R.; Mara de Andrade, L.; dos Santos Brito, M.; Macedo Nobile, P.; Palma Boer Martins, A.; Domingues Carlin, S.; Vasconcelos Ribeiro, R.; de Souza Goldman, M.H.; Nebo Carlos de Oliveira, J.F.; Vargas de Oliveira Figueira, A.; et al. Overexpression of ScMYBAS1 alternative splicing transcripts differentially impacts biomass accumulation and drought tolerance in rice transgenic plants. PLoS ONE 2018, 13, e0207534. [Google Scholar] [CrossRef] [Green Version]
- Amoah, J.N.; Ko, C.S.; Yoon, J.S.; Weon, S.Y. Effect of drought acclimation on oxidative stress and transcript expression in wheat (Triticum aestivum L.). J. Plant Interact. 2019, 14, 492–505. [Google Scholar] [CrossRef] [Green Version]
- Nxele, X.; Klein, A.; Ndimba, B. Drought and salinity stress alters ROS accumulation, water retention, and osmolyte content in sorghum plants. S. Afr. J. Bot. 2017, 108, 261–266. [Google Scholar] [CrossRef]
- Abid, M.; Ali, S.; Qi, L.K.; Zahoor, R.; Tian, Z.; Jiang, D.; Snider, J.L.; Dai, T. Physiological and biochemical changes during drought and recovery periods at tillering and jointing stages in wheat (Triticum aestivum L.). Sci. Rep. 2018, 8, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Morishita, H.; Urano, K.; Shiozaki, N.; Yamaguchi-Shinozaki, K.; Shinozaki, K.; Yoshiba, Y. Effects of free proline accumulation in petunias under drought stress. J. Exp. Bot. 2005, 56, 1975–1981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang-Quan, W.; Rui-Chang, L. Enhancement of superoxide dismutase activity in the leaves of white clover (Trifolium repens L.) in response to polyethylene glycol-induced water stress. Acta Physiol. Plant. 2008, 30, 841–847. [Google Scholar] [CrossRef]
- Wang, S.Y.; Chen, H.; Ehlenfeldt, M.K. Variation in antioxidant enzyme activities and nonenzyme components among cultivars of rabbiteye blueberries (Vaccinium ashei Reade) and V. ashei derivatives. Food Chem. 2011, 129, 13–20. [Google Scholar] [CrossRef]
- Huo, Y.; Wang, M.; Wei, Y.; Xia, Z. Overexpression of the maize psbA gene enhances drought tolerance through regulating antioxidant system, photosynthetic capability, and stress defense gene expression in tobacco. Front. Plant Sci. 2016, 6, 1223. [Google Scholar] [CrossRef] [Green Version]
- Song, H.; Wang, H.; Xu, X. Overexpression of AtHsp90. 3 in Arabidopsis thaliana impairs plant tolerance to heavy metal stress. Biol. Plant. 2012, 56, 197–199. [Google Scholar] [CrossRef]
- Xu, J.; Xue, C.; Xue, D.; Zhao, J.; Gai, J.; Guo, N.; Xing, H. Overexpression of GmHsp90s, a heat shock protein 90 (Hsp90) gene family cloning from soybean, decrease damage of abiotic stresses in Arabidopsis thaliana. PLoS ONE 2013, 8, e69810. [Google Scholar] [CrossRef]
- Song, H.; Zhao, R.; Fan, P.; Wang, X.; Chen, X.; Li, Y. Overexpression of AtHsp90. 2, AtHsp90. 5 and AtHsp90. 7 in Arabidopsis thaliana enhances plant sensitivity to salt and drought stresses. Planta 2009, 229, 955–964. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, L.; Yan, H.; Zhang, X.; Xu, B.; Ma, X. Cloning and characterization of an ABA-independent DREB transcription factor gene, HcDREB2, in Hemarthria compressa. Hereditas 2016, 153, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Nakashima, K.; Shinwari, Z.K.; Sakuma, Y.; Seki, M.; Miura, S.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Organization and expression of two Arabidopsis DREB2 genes encoding DRE-binding proteins involved in dehydration-and high-salinity-responsive gene expression. Plant Mol. Biol. 2000, 42, 657–665. [Google Scholar] [CrossRef]
- Qin, Q.-L.; Liu, J.-G.; Zhang, Z.; Peng, R.-H.; Xiong, A.-S.; Yao, Q.-H.; Chen, J.-M. Isolation, optimization, and functional analysis of the cDNA encoding transcription factor OsDREB1B in Oryza Sativa L. Mol. Breed. 2007, 19, 329–340. [Google Scholar] [CrossRef]
- Sakuma, Y.; Maruyama, K.; Osakabe, Y.; Qin, F.; Seki, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Functional analysis of an Arabidopsis transcription factor, DREB2A, involved in drought-responsive gene expression. Plant Cell 2006, 18, 1292–1309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, Y.; Liao, K.; Du, H.; Xu, Y.; Song, H.; Li, X.; Xiong, L. A stress-responsive NAC transcription factor SNAC3 confers heat and drought tolerance through modulation of reactive oxygen species in rice. J. Exp. Bot. 2015, 66, 6803–6817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, T.; Fujita, Y.; Sayama, H.; Kidokoro, S.; Maruyama, K.; Mizoi, J.; Shinozaki, K.; Yamaguchi-Shinozaki, K. AREB1, AREB2, and ABF3 are master transcription factors that cooperatively regulate ABRE-dependent ABA signaling involved in drought stress tolerance and require ABA for full activation. Plant J. 2010, 61, 672–685. [Google Scholar] [CrossRef]
- Abid, G.; M’hamdi, M.; Mingeot, D.; Aouida, M.; Aroua, I.; Muhovski, Y.; Sassi, K.; Souissi, F.; Mannai, K.; Jebara, M. Effect of drought stress on chlorophyll fluorescence, antioxidant enzyme activities and gene expression patterns in faba bean (Vicia faba L.). Arch. Agron. Soil Sci. 2017, 63, 536–552. [Google Scholar] [CrossRef]
- Filippou, P.; Antoniou, C.; Fotopoulos, V. Effect of drought and rewatering on the cellular status and antioxidant response of Medicago truncatula plants. Plant Signal. Behav. 2011, 6, 270–277. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Ayachit, G.; Sahoo, L. Screening of mungbean for drought tolerance and transcriptome profiling between drought-tolerant and susceptible genotype in response to drought stress. Plant Physiol. Biochem. 2020, 157, 229–238. [Google Scholar] [CrossRef]
- Li, S.; Wang, R.; Jin, H.; Ding, Y.; Cai, C. Molecular Characterization and Expression Profile Analysis of Heat Shock Transcription Factors in Mungbean. Front. Genet. 2019, 9, 736. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).