Abstract
Drought affects plant growth and yield in many agricultural areas worldwide by producing negative water potentials in the root zone that reduce water availability, affecting plant development and metabolism. This study investigated the effect of varying moisture regimes (100% field capacity (FC), well-watered plants, 50% FC (moderate water stress), and 25% FC (severe water stress)) on growth parameters, chlorophyll content, and bioactive molecule patterns, and the impact on antioxidant, lipoxygenase (LOX), and acetylcholinesterase (AChE) activities in Rosa damascena. The water deficit treatments reduced biomass production for both treatments (−29 and −33%, respectively, for MWS and SWS) and total chlorophyll (−18 and −38% respectively for MWS and SWS), relative to the control. The 50% FC treatment had the greatest effect on the phenolic profiles and their respective functionalities, with significant increases in the levels of total phenolic, benzoic (gallic, p-coumaric, and syringic acids) (+32%), and cinnamic (caffeic and trans-cinnamic acid) acids (+19%) and flavonoids (epicatechin-3-O-gallate) (+15%) compared to well-watered leaves (control leaves). The 50% FC treatment also exhibited the highest potential antioxidant activities (apart from NO-quenching activity), evidenced by the lowest IC50 and EC50 values. The inhibitory LOX and AChE capacities varied depending on the severity of stress, with superior activity in the 50% FC treatment. Overall, the drought tolerance in rose was associated mainly with its suitable manipulation of antioxidant production and orderly regulation of LOX and AChE activities.
1. Introduction
The severity and incidence of drought are expected to increase with the predicted change in typical precipitation patterns associated with climate change [1]. Water deficits are anticipated to reduce world crop production by up to 30% by 2025 compared to current yields [2]. In arid and semi-arid zones, the potential of water resources to expand landscapes and grow ornamental plants is threatened. Water distribution to the floral industry is in strong competition with other demands, such as agriculture, urban management, and human consumption [3], and should be used optimally and with high efficiency [4].
Limited water supply to plants incites a chemical signal in the aerial system through xylem sap, eliciting partial stomatal closure to avert water loss by evaporation. As a result, plants shift to a water-saving strategy that decreases intracellular CO2, reducing the amount of NADPH+, H+, and ATP available for CO2 fixation within the Calvin cycle, thus decreasing NADP+ regeneration and affecting the photosynthetic electron transport chain [5,6]. Such effects promote the production of free radicals and reactive oxygen species (ROS), generating oxidative stress [7].
Alternatively, to manage water status, a non-antioxidant system (phenolic compounds) can be synthesized in different plant parts to keep ROS production below toxic levels during abiotic/biotic stresses. Depending on the stress intensity and plant efficiency to trigger these mechanisms, the production of such metabolites can increase or decrease under drought conditions [8].
To confront drought constraints, dehydrated plants could normalize a latent source of phenols valuable for economic exploitation. Nonetheless, abiotic constraints have the opposite effect on polyphenol yields, i.e., the increment of polyphenol quantity in tissues is negatively correlated with plant biomass production [9]. Furthermore, water scarcity may be related to increases in phenolic pools by reallocating the incorporated C as plant growth progressively declines [10]; thus, optimal polyphenol yields would be required with respect to stress-tolerant species [11].
Saudi Arabia is rich in flora, including a multiplicity of aromatic and ornamental species such as Damask rose (Rosa damascena Mill. var. trigentipetala) that belongs to the Rosaceae family, a perennial bushy shrub renowned in the perfumery, cosmetic trade, and food industries [12].
The major bioactive molecules isolated from different organs of Rosa damascena are flavonoids, glycosides (kaempferol, cyanidin 3,5, d-glycoside, and quercetin), gallic acid, terpenes, and anthocyanins. The leaves are noteworthy sources of vitamins C, A, B, and K, pectin, tannins, and carotenoids [13].
The flowers of R. damascena have analgesic, anti-inflammatory, astringent, antibacterial, antidepressant, and antiviral activity, as well as diuretic effects, and they are used in popular medicine as a sedative [13]. A leaf methanol extract of Damask rose with high amounts of (+)-catechin and (+)-epi-catechin had higher antioxidant activities than butylated hydroxytoluene (BHT), Trolox, and butylated hydroxyanisole (BHA) standards.
Agricultural practices, genetic makeup, and environmental factors affect the quality of plant products [11]. In semi-arid and rainfed areas where water is scant, incorporating different shade net houses and mulch types over the soil surface are key to meeting the increasing demand for herbs [3,4].
Despite the economic importance of Rosa damascena Mill. var. trigentipetala for the livelihood for Saudi smallholder farmers, it is cultivated in a traditional and primitive manner [14]. Yet, no published information is available on (i) its limits of tolerance, or (ii) the underlying mechanisms implicated in its tolerance to water deficit. Therefore, an investigation of its responses to drought and the mechanisms involved may support to know how to improve drought tolerance in Rosa species. This study aimed to (i) determine the limits of tolerance to water deficit of Damask rose, and (ii) identify the main physiological and biochemical mechanisms that are linked to drought resistance. Such information will be crucial in defining culture conditions that optimize biomass, biomolecules production and anti-oxidation efficiency, along with a better valorization of this underused species in water management to improve secondary metabolites production with a global goal of new water-efficient genotype screening programs.
2. Materials and Methods
2.1. Experimental Design and Irrigation Treatment
The experiment was undertaken from January to May 2020 in a greenhouse at the Biology Department, Faculty of Science, Taïf University, Saudi Arabia (21°26′02.4′′ N, 40°29′36.9′′ E), with a natural photoperiod (approximately 14 h light), temperature (day/night) of 32/22 °C, and relative humidity of 70%.
Two-year-old rooted cuttings of Rosa damascena Mill. var. trigentipetala were transplanted to Wagner pots (height: 30 cm; diameter: 20 cm) filled with sandy soil and watered daily with half-strength nutrient solution [15]. Uniform cuttings were selected, grouped into ten replicates (main factor), and exposed to one of three irrigation regimes—25%, 50%, or 100% field capacity (FC) as non-stress (WW), moderate water stress (MWS), and severe water stress (SWS) treatments, respectively—for 90 days. The pots were watered to their corresponding weights every two days with quarter-, half-, and full-strength Hewitt nutrient solution, respectively. Soil FC (%) for the 100%, 50%, and 25% FC treatments were 11.5%, 5.75%, and 2.9%, respectively. Evaporation from the soil surface was prevented by enclosing the pots in plastic bags. Ten pots without plants were used to monitor soil evaporation.
2.2. Growth Parameters and Leaf Water Potential (Ψw) Measurement
Leaf water potential (Ψleaf) was recorded on five mature and fully spread leaves using a Scholander pressure chamber (Soil Moisture Equipment Corp., Santa Barbara, CA, USA) at first light (ΨPD, 07:00 h) and midday (ΨMD, 12:00 h).
Fresh material of each plant was then placed in clean paper bags, labeled, and oven-dried at 60 °C for 48 h to determine the respective dry weight (DW) following the protocol of Wasli et al. [8].
2.3. Relative Chlorophyll Content (RCC)
The pigment concentrations in rose leaves were determined by measuring the absorption spectra of frond extracts using a UV spectrophotometer (Spectro UV-VIS Dual Beam 8 auto cell UUS-2700). Two hundred mg of leaf plant material frozen in liquid N2 was ground to a fine powder (on ice) and immediately immersed in 5 mL acetone (80/20 v/v) solution. The total extraction took place after 72 h in darkness at 4 °C, with the absorbance of the extracts measured at 663, 645 and 470 nm for Chl a and Chl b and carotenoids, respectively [16]. Varian 220Z, Mulgrave, Victoria, Australia
2.4. Characterization and Quantification of Phenolic Pools by Colorimetric and Chromatographic Analysis
Contents of total phenolic compounds and flavonoids (obtained with 3 g dry powder in 30 mL methanol 80%) were determined according to the method of Wasli et al. [17], and the results were expressed as mg of gallic acid or mg catechin per gram of dried residue, respectively using a UV-spectrometer (Varian 220Z, Mulgrave, Victoria, Australia).
In turn, to characterize and quantify individual phenolics dried samples (evaporated with in a rotavap at 40 °C) were hydrolyzed according to the method of Proestos et al. [18] with some modifications. Next, 10 mL of MeOH (80:20 v/v) containing butylated hydroxytoluene (0.5 g/L) was added to 250 µg of the dried sample. Then, 5 mL of 1 M HCl was added. The mixture was stirred carefully and sonicated for 15 min and refluxed in a water bath at 90 °C for 2 h. The obtained mixture was injected to RP-HPLC using a system model Agilent 1200 with the UV spectra of standards measured from 200–400 nm. The column was a reversed phase Zorbax SB-C18 of 4.6 mm× 250 mm and 3.5 μm particle size. The column temperature was thermostated at 25 °C. The injected sample volume was 20 μL, and peaks were monitored at 280 nm. The mobile phase comprised acetonitrile (solvent A) and water/sulfuric acid (98:2) (solvent B). The optimized gradient elution occurred as at a flow rate of 0.6 mL/min: 0–5 min, 10–20% A; 5–10 min, 20–30% A; 10–15 min, 30–50% A; 15–20 min, 50–70% A; 20–25 min, 70–90% A; 25–30 min, 90–50% A; 30–35 min, return to initial conditions.
The compounds were identified by comparing the retention times and peak area with pure standards and reported as mg per g sample dry weight. For quantitative analysis, the limits of detection and quantification were calculated from calibration curve parameters obtained by injecting known concentrations of a different standard. The results were expressed in mg per g of dry weight.
Standards with high purities were purchased from Sigma–Aldrich, including catechin hydrate (≥96% purity), chlorogenic acid (≥96% purity), caffeic acid (≥97% purity), p-coumaric acid (98% purity), ellagic acid (≥95% purity), epicatechin-3-O-gallate (≥96% purity), ferulic acid (≥95% purity), gallic acid (≥95% purity), rosmarinic acid (95% purity), luteolin-7-O-glucoside (≥95% purity), kaempferol (≥97% purity), sinapic acid (98% purity), syringic acid (≥96% purity), and trans-cinnamic acid (≥95% purity).
2.5. Biological Activities
The antiradical capacity of rose extract (RE) against the 2,2′-azino-bis (3-ethlybenzothiazoline-6-sulfonic acid) (ABTS) radical was assessed according to Wasli et al. [8]. Briefly, 250 µL of stable radical ABTS (prepared by reacting ABTS stock solution (7 mM) with potassium persulfate (2.45 mM) in a 1:1 ratio) was added to 50 µL of increasing RE concentrations ranging from 25 to 100 µg/mL. After 6 min of incubation at room temperature, the absorbance was read against a blank at 734 nm using an ELX800 microplate reader (Bio-Tek Instruments, lnc.; Winooski, VT, USA). The ABTS scavenging ability was expressed as IC50 (mg/mL), the inhibiting concentration of 50% of the synthetic radical.
The inhibition percentage (IP%) of ABTS radical was calculated as follows:
IP (%) = [(Acontrol − Asample)/Acontrol] × 100
The NO scavenging assay followed the protocol of Wasli et al. [16]. Briefly, 200 μL of different rose extracts (25–150 µg/mL) were mixed with 200 µL sodium nitroprusside (3.33 mM) in 100 mM PBS (pH 7.4). The reaction was initiated by adding Griess reagent, and the absorbance was measured at 562 nm. The results were expressed as CI50 (µg/mL).
For ORAC assessment, AAPH (240 mM), fluorescein (70.30 nM), and Trolox (3.24–130.88 µM) were prepared in 75 mM of phosphate buffer with a pH of 7.4. Fluorescence intensity (an excitation wavelength of 485 nm and emission wavelength of 520 nm) was applied every 90 s over a total measurement period of 120 min. The results were expressed in micromoles of Trolox equivalents (TE) per gram (mmol TE/g).
The FRAP assay (reaction of reductants) was traduced by the altering of the test solution from yellow to green.
One milliliter of RE from different treatments at different concentrations ranging from 50 to 500 µg/mL was mixed with 2.5 mL of Na3PO4 buffer (0.2 M, pH 6.6) and 2.5 mL potassium ferricyanide (K3Fe (CN)6; 1% w/v). The mixtures were incubated in a water bath at 50 °C for 20 min. Then 2.5 mL of TCA (10%, w/v) were inserted followed by a vigorous centrifugation for 10 min at 650 g. At the final step, 2.5 mL of the supernatant was blended with 2.5 mL of deionized water and 0.5 mL of FeCl3 solution (0.1%, w/v). The absorbance was assessed at 700 nm against a blank sample and ascorbic acid was used as a positive control. The EC50 value (µg/mL) expressed the RP [17].
For the β-carotene test, 20 mg of β-carotene was suspended in 10 mL of chloroform; linoleic acid (50 mg) and Tween 80 (1 g) were then added to 1 mL of this solution [19]. Chloroform was removed using a vacuum at 40 °C, before adding 100 mL of oxygenated water. The resulting β-carotene/linoleic acid emulsion was vigorously shaken before 250 µL was added to each well of 96-well microliter plates along with 50 µL of test samples. The initial absorbance at 470 nm was recorded.
The emulsion system with two controls (one containing BHA as a positive control (Figure 1) and the other with the same volume of distilled water instead of the extracts) was incubated at 50 °C for 2 h, and the absorbance at 470 nm was read using a model ELX800 microplate reader (Bio-Tek Instruments, nc; Winooski, VT, USA).
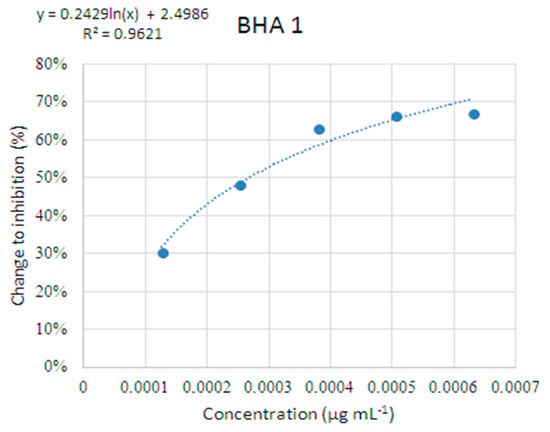
Figure 1.
Standard curve of butylated hydroxyanisol (BHA) as a positive control in the β-carotene linoleic acid model system.
Readings for all samples were taken immediately and after 2 h incubation. Blanching inhibition of the β-carotene was determined as follows:
2.6. Lipoxygenase (LOX) Inhibitory Activity
LOX activity was assessed according to Wasli et al. [17]. In a 96-well quartz plate, 10 μL R. damascena leaf extract (10–100 µg/mL) was added to 5 μL enzyme solution (0.054 g/mL), 50 μL linoleic acid (0.001 M), and borate buffer 937 μL (0.1 M, pH 9), and the absorbance measured at 234 nm. Enzymatic activity was approximated as (A0 − Ae/A0) × 100, where A0 is the absorbance of the control reaction, and Ae is the absorbance of the extract. The results were expressed as IC50 values.
2.7. Acetylcholinesterase (AChE) Inhibitory Activity
Sixty microliters of R. damascena sample at different concentrations (50–100 µg/mL) was mixed with 425 μL Tris-HCl buffer (0.1 M, pH 8) and 25 μL enzyme (0.28 µM/L), incubated at 37 °C for 15 min, before adding 75 μL substrate (0.005 g iodine acetylcholine in 10 mL buffer) and 475 μL 5,5′-dithiobis (2-nitrobenzoic acid) (DTNB) (0.059 g in 50 mL buffer) to finish the reaction. The absorbance was read at 405 nm, and the results were traduced to IC50 values [20].
2.8. Data Analysis
Data were analyzed using one-way ANOVA followed by Tukey’s post hoc test. The statistical tests were applied using Graph Pad Prism, version 6, at a p < 0.05 significance level. Multivariate data analysis was carried out using Pearson’s correlation in XLSTAT, considering variables centered on their means.
3. Results and Discussion
3.1. Effect of Moderate and Severe Drought Stress on Growth Activity and Chlorophyll Content
Biomass production and water status are considered to be the most-used criteria for assessing plant behavior to osmotic stress [14,21]. Both water deficit treatments decreased whole-plant FW and DW, declining by approximately 29% under moderate (50% FC) water stress and 48% and 33% under severe (25% FC) water stress, respectively, relative to the well-watered plants. In turn, water stress increased the root-to-shoot dry weight ratio, relative to the control plants (Table 1, p ≤ 0.05).

Table 1.
Changes in physiological parameters of Rosa damascena Mill. var. trigentipetala plants under three watering regimes: well-watered (100% field capacity (FC), WW), moderate water stress (50% FC, MWS), or severe water stress (25% FC, SWS).
Water stress reduced leaf water potential (Ψw) from –1.6 MPa (for control plants) to −2 and −2.4 MPa, respectively, for MWS and SWS (Table 1, p ≤ 0.05). Leaf water content also declined in response to water stress, more so in the SWS treatment (Table 1, p ≤ 0.05).
Reduced biomass accumulation in response to drought is mainly due to the reduction in leaf biomass explained by the reduction in leaf area, leaf number, and leaf size [22]. The greater inhibition in shoot growth than root growth (as indicated by the lower shoot-to-root ratio) is explained by the preferential allocation of dry matter to roots, representing a criterion for drought adaptation [23,24]. Changes in root architecture in cereals due to the fact of enhanced cytokinin degradation are a promising strategy for enhancing nutrient uptake, biofortification, and drought tolerance [25].
In the same line, total chlorophyll (T Chl), Chl a, and Chl b concentrations decreased with water stress (Table 1). Chl b was the most affected pigment, declining by 37% in the MWS treatment and 54% in the SWS treatment, relative to well-watered plants. The Chl a/b ratio increased with water stress by 29% and 46% in the MWS and SWS treatments, respectively (Table 1).
A chlorophyll reduction might be considered an adjustment mechanism to ROS generation from energy absorption by photosynthetic apparatus [7], owing to the presence of antioxidant leaf pigment betalain (betacyanin and betaxanthin), which absorbed a significant amount of radiation and protected drought-stressed chloroplasts from harmful excessive light.
In addition, the increased Chl a/b ratio could be correlated with the reduced size of the PSII light-harvesting antenna, ensuring the supply of electrons from PSII to keep pace with the excitation rate of PSI [26].
3.2. Variation in Phenolic Pools under Moderate and Severe Drought Stress
Table 2 showed the TPh and TF values based on the absorbance results of the FC reagent–reactive extract solutions and aluminum chloride method compared with the gallic acid and catechin equivalent standard solutions. Under the control condition (WW; 100% FC), R. damascena leaves had 45.63 mg GAE/g DW and 13.44 mg CE/g DW for TPh and TF contents, respectively. At 50% and 25% FC, the levels of TPC significantly increased in leaves by about 31% and 13% respectively for MWS and SWS, relative to the control.

Table 2.
Total polyphenol and flavonoid contents in Rosa damascena Mill. var. trigentipetala plants under three watering regimes: well-watered (100% field capacity (FC), WW), moderate water stress (50% FC, MWS), or severe water stress (25% FC, SWS).
Correspondingly, a higher concentration and stimulation of flavonoids was detected, with TFC values changing from 13.44 to 16.97 mg CE g−1 DW (under MWS) and from 13.44 to 15.74 mg CEg−1 DW (under SWS) in control and dehydrated leaves, respectively (Table 2).
Phenolic characterization by the RP-HPLC method, showed ten phenolic acids (caffeic chlorogenic, p-coumaric, ellagic, ferulic, gallic, syringic, sinapic, rosmarinic, and trans-hydroxy-cinnamic acids) with four flavonols/flavonones (catechin hydrate, epicatechin-3-O-gallate, luteolin-7-O-glucoside, and kaempferol-3-O-rutinoside) (Figure 2). Rosmarinic acid was the major detected phenolic acid, with an amount of 33.94 ± 0.05 mg/g DW, followed by syringic (6.05 ± 0.33 mg/g DW) and trans-cinnamic acids (6.55 ± 0.45 mg/g DW). Moderate water stress enhanced cinnamic and benzoic forms by approximately 1.5-fold compared to well-watered plants (Figure 2). It was observed that severe water dehydration induced a significant increase in the biosynthesis of phenolics, in particularly with respect to ferulic acid and its derivatives (Figure 2).
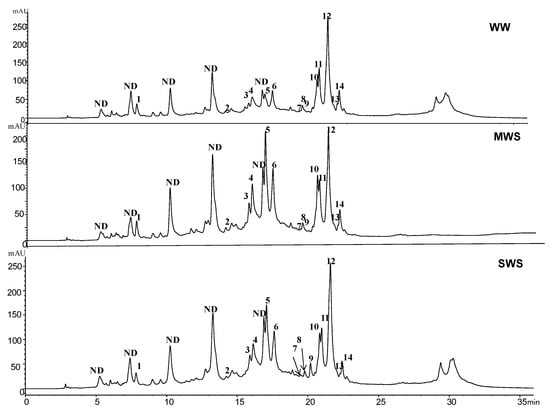
Figure 2.
RP-HPLC chromatograms of Rosa damascena plants. The signal was monitored at 280 nm. The peak numbers corresponded to (1) gallic acid; (2) catechin hydrate; (3) chlorogenic acid; (4) epicatechin3-O-gallate; (5) caffeic acid; (6) syringic acid; (7) p-coumaric acid; (8) sinapic acid; (9) ferulic acid; (10) luteolin 7-O-glucoside; (11) trans-hydroxycinnamic acid; (12) rosmarinic acid; (13) ellagic acid; (14) kaempferol-3-O-rutinoside. Well-watered (100% field capacity (FC), WW), moderate water stress (50% FC, MWS), and severe water stress (25% FC, SWS).
The flavonol and flavanone groups accounted for approximately 32% of the total phenolic constituents in control leaves, which were mostly represented by luteolin-7-O-glucoside, epicatechin-3-O-gallate, and kaempferol-3-O-rutinoside, with minor amounts of catechin hydrate (Table 2). Moderate water stress raised flavonoid levels by 1.2-fold despite the decline in luteolin-7-O-glucoside (–12%), and kaempferol (–23%). Although severe water stress increased flavonoid content by about 8.48%.
Higher phenolic contents suggest that rose can efficiently accumulate secondary metabolites in order to adapt to water deficiency [27]. Phenols play a key role in cell protection and osmotic adjustment, either directly by inducing ROS detoxification processes or indirectly by stimulating the antioxidative defense system [28]. Phenols can function as a filter to absorb radiation by limiting chlorophyll excitation in the photosynthetic apparatus during unfavorable conditions [29].
The shielding contribution of flavonoids is ascribed to their OH groups, the omnipresence of double bonds, and their predilection to glycosylation and methylation [30]. Flavonoids with an ortho-dihydroxy pattern in the B-ring in skeleton verge are thought to preserve in higher amounts than mono-hydroxylated in the B-ring [31]. Such biomolecules can also uphold the integrity of the envelope membrane through lipid adjusting during cellular dehydration [32].
Many studies have reported a variation in phenolic composition under abiotic stress. For example, Meot-Duros and Magné [33] reported an accumulation of caffeic acid in the leaves of Crithmum maritimum exposed to water stress (due to the nature of sandy substrate) and ionic stress (due to the sea sprays). In Prunella vulgaris, moderate drought stress enhanced the production of ursolic, rosmarinic, and oleanolic acids [34]. As recorded by Bettaieb-Rebey et al. [28], ferulic acid was involved in the adaptation of S. officinalis to drought stress through the assimilation of UV light and its transformation into blue fluorescence, which sheltered the plants from the destructive effects of UV light. Cinnamic, vanillin, p-hydroxybenzoic, and vanillic acids were showed to be involved in drought tolerance of Q8 rice cultivar [35], which could be related to cell wall lignification correlated with the implication of specific amino acids for osmotic adjustment [36]. Al Yasi et al. [21] suggested that the cell wall rigidity of Damask rose exposed to drought allows it to cope with toxic molecules and ROS [37,38].
Changes in flavonoid groups have occurred in diverse plants under water limitation; for instance, kaempferol and quercetin increased in dehydrated tomato plants [39]. Ding et al. [40] suggested that characteristic catechins (a subgroup of flavan-3-ols) play essential key roles in the stress response of tea plants by minimizing excess ROS production.
Luteolin is an inhibitor of α-amylase activity in plants. Thus, drought-stressed Achillea pachycephala species reduced their photosynthetic rate and soluble sugars, producing signals for discriminate gene expression patterns [31].
The metabolic investigation of flavonoid alternation by LC-QTOF-MS, during drought stress in Arabidopsis thaliana (wild type, Col-0); proved that glycosides flavonoid forms (kaempferol, quercetin, and cyanidin) were assigned in the mitigation of oxidative and drought stress.
3.3. Moderate and Severe Drought Stress Effects on Antioxidant Activities
Rosa damascena Mill. var. trigentipetala extracts were explored for their antioxidant potentialities using distinct in vitro methods—ABTS●+, NO●, ORAC, FRAP, and β-carotene–linoleic acid model systems—to estimate their quenching ability for distinct radicals and their aptitude for reducing trivalent iron +(Fe III) to its bivalent form (Fe II) and inhibiting the bleaching of the antioxidant pigment β-carotene [41].
The results confirmed an increment in antioxidant activities in both drought stress treatments (Table 3). Indeed, quenching activities against ABTS●+ and peroxyl radicals were found to be increased, as shown by their superior ORAC values and decreased IC50 values. A similar trend occurred for RPA, with EC50 values decreasing from 356 to 189 µg/mL (for MWS) and from 356 to 234 µg/mL (for SWS), respectively. The MWS samples (IC50 = 0.42 mg/mL) could also inhibit β-carotene bleaching more than the control (IC50 = 0.67 mg/mL) and SWS (IC50 = 0.55 mg/mL) samples.

Table 3.
Antioxidant activities in Rosa damascena Mill. var. trigentipetala plants under three watering regimes: well-watered (100% field capacity (FC), WW), moderate water stress (50% FC, MWS), or severe water stress (25% FC, SWS).
Water limitation also affected NO-quenching activity, despite not being completely aligned with the previous tests. The inhibited activity of NO radicals raised by approximately two-fold in both treatments, suggesting that, despite the overall increase in antioxidant activity in water-stressed plants, the effects on leaves vary depending on the specific reaction and/or mechanism involved. Our results showed that Rosa damascena Mill. var. trigentipetala extracts were more active against NO than the reference compound, ascorbic acid (IC50 = 213 µg/mL).
The dependence on antioxidant activity, obtained from various assays, in relation to TPh and TF had a linear correlation between the IC50 values for the ABTS●+ (r = −0.87 and −0.76) scavenging activity, ORAC quantity (r = −0.75 and −0.69), EC50 values of FRAP (r = −0.99 and −0.86) and β-carotene linoleic acid (r = −0.90 and −0.84) model systems, respectively (Table 4).

Table 4.
Correlation coefficients between total phenolic content (TPC)/total flavonoid content (TFC), individual phenolic compounds, and the IC50/EC50 values for ABTS●+, NO, ORAC, FRAP, and β-carotene activities.
Indeed, the antioxidant capacity and extract phenolic content are always positively correlated, owing to the direct contribution of phenolic compounds in antioxidant activities [42]. Nevertheless, a moderate correlation between TPh (or TFC) and antioxidant activity was observed for the NO-quenching potential with r values ranging from 0.5 to 0.6. Likewise, a high linear correlation occurred between the ABTS●+ and NO● scavenging activities; FRAP and β-carotene assays and caffeic acid; and syringic acid, trans-cinnamic acid, and luteolin-7-O-glucoside, indicating the potential role of cinnamic and benzoic acids with flavonol groups in offsetting oxidative stress under water-limited conditions.
3.4. Moderate and Severe Drought Stress Effects on LOX and AChE Inhibitory Enzyme Activities
LOX isoforms play a pivotal role in the mobilization of storage lipids during the germination process [43], and play a critical role in the generation of protective components, such as jasmonates, divinyl ethers, and leaf aldehydes, which assist plants to recover from biotic (insects and pathogens) and abiotic stress [44,45]. In turn, LOX reactions with unsaturated fatty acids can produce off-flavors/off-odors and cause food spoilage [41].
The inhibitory potential for LOX activity varied depending on the severity of stress. Moderate water stress had higher anti-LOX activity (27 µg/mL) compared to SWS (48 µg/mL), as reflected in the lower IC50 values (Table 5). Increased lipid peroxidation under stress conditions is principally induced by higher lipolytic activity in the membrane, stimulating LOX activity [42,45,46]. The ability to reduce LOX activity (either directly or by down-regulating its expression) is considered beneficial for plants, as LOX are oxidative enzymes that can set radicals and ROS free [47,48]. The stimulation of PgLOX3 (LOX3 isoform) gene expression is a possible adaptative strategy under water deficit [48].

Table 5.
Inhibitory activities of lipoxygenase (LOX) and acetylcholinesterase (AChE) in hydromethanolic extracts in leaves of rose plants under three watering regimes: well-watered (100% field capacity (FC), WW), moderate water stress (50% FC, MWS), or prolonged water stress (25% FC, SWS).
The genomic DNA structure of PgLOX3 disclosed a nucleotide sequence with high identity with EST in severely drought-stressed Populus encoding the PgPsad2 protein, which suggests the involvement of PgLOX3 genes in drought stress tolerance [48].
The recognition of new AChE inhibitors derived from natural sources with few side effects is required [41]. Our research showed that moderately dehydrated rose leaves had a higher inhibitory AChE capacity (CI50 = 205 µg/mL) than severely dehydrated leaves (CI50 = 281 µg/mL).
The correlation analysis (Table 5) showed strong correlation coefficients between TPC/TFC and the IC50 values of LOX (r = −0.74; r = −0.85) and AChE (r = −0.99; r = −0.96), indicating the potential role of non-antioxidant compounds in hampering LOX and AChE enzymes under drought stress. The inhibition of AChE activity was considered a tolerance mechanism in Pimpinella anisum leaves exposed to Zn excess, which could be linked to the omnipresence of bioactive molecules [41].
4. Conclusions
In summary, an integrated approach combining biochemical and physiological studies revealed new insights into the mechanisms and processes involved in Rosa damascena drought adaptation. When cultivated under water-limiting conditions, R. damascena shoot and whole plant biomass production significantly declined, whereas that of shoot/root was not affected. In addition, such constraints resulted in a significant reduction of chlorophyll linked with an alteration in water potential, probably to support its nutrient use efficiency associated with the preservation of an adequate level of chlorophyll in leaves. In turn, the contents of biomolecules under water deficit were positively correlated with antioxidant and inhibitory enzyme activities (LOX, AChE), as evaluated using different test systems, suggesting an adequate protection against oxidative damage, and thus adaptation to water limitation. Moisture deficit can successfully enhance health-promoting phytochemicals in rose, which could be manipulated through agricultural techniques and screening programs to develop drought-tolerant genotypes.
Further omics technologies such as transcriptomics and proteomics could help us to identify pathways/cycles involved in the establishment of enhanced drought tolerance.
Author Contributions
K.H.: performed the experimental work, the analysis and data interpretation, statistical analysis, writing- original draft preparation. H.W.: contributed in data analysis and co-wrote the manuscript. H.M.A.-Y., E.F.A., A.A.I. and F.A.S.H.: helped in experimental work and paper revision. K.H.M.S.: revised the paper. Conceptualization, K.H. and H.W.; methodology, K.H., E.F.A. and H.M.A.-Y.; software, K.H., F.A.S.H. and A.A.I.; validation, K.H., H.W. and H.M.A.-Y.; formal analysis, E.F.A., F.A.S.H., A.A.I., H.W.; data curation, K.H. and H.W.; writing—original draft preparation, H.W. and K.H.; writing—review and editing, K.H. and K.H.M.S., visualization, E.F.A., H.M.A.-Y., A.A.I. and F.A.S.H.; supervision, K.H. and K.H.M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the deputyship for research & Innovation, Ministry of Education in Saudi Arabia: 1-441-131.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors extend their appreciation to the Deputyship for Research and Innovation, Ministry of Education in Saudi Arabia for funding this research work through the project number 1-441-131.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Klein, R.J.T.; Midgley, G.F.; Preston, B.L.; Alam, M.; Berkhout, F.G.H.; Dow, K.; Shaw, M.R. Adaptation opportunities, constraints, and limits. In Climate Change 2014—Impacts, Adaptation and Vulnerability: Part A: Global and Sectoral Aspects: Working Group II Contribution to the IPCC Fifth Assessment; Field, C.B., Barros, V.R., Dokken, D.J., Mach, K.J., Mastrandrea, M.D., Bilir, T.E., Chatterjee, M., Ebi, K.L., Estrada, Y.O., Genova, R.C., et al., Eds.; Cambridge University Press: Cambridge, UK, 2014; pp. 899–943. [Google Scholar]
- Grafton, R.Q.; Daugbjerg, C.; Qureshi, M.E. Towards food security by 2050. Food Secur. 2015, 7, 179–183. [Google Scholar] [CrossRef]
- Marín-de la Rosa, N.; Lin, C.W.; Kang, Y.J.; Dhondt, S.; Gonzalez, N.; Inzé, D.; FalterBraun, P. Drought resistance is mediated by divergent strategies in closely related Brassicaceae. New Phytol. 2019, 223, 783–797. [Google Scholar] [CrossRef] [PubMed]
- Jafari, S.; Garmdareh, S.E.H.; Azadegan, B. Effects of drought stress on morphological, physiological, and biochemical characteristics of stock plant (Matthiola incana L.). Sci. Hortic. 2019, 253, 128–133. [Google Scholar] [CrossRef]
- Caser, M.; D’Angiolillo, F.; Chitarra, W.; Lovisolo, C.; Ruffoni, B.; Pistelli, L.; Pistelli, L.; Scariot, V. Ecophysiological and phytochemical responses of Salvia sinaloensis Fern. to drought stress. Plant Growth Regul. 2018, 84, 383–394. [Google Scholar] [CrossRef]
- Mandoulakani, B.A.; Eyvazpour, E.; Ghadimzadeh, M. The effect of drought stress on the expression of key genes involved in the biosynthesis of phenylpropanoids and essential oil components in basil (Ocimum basilicum L.). Phytochemistry 2017, 139, 1–7. [Google Scholar] [CrossRef]
- Maleki, M.; Shojaeiyan, A.; Mokhtassi-Bidgoli, A. Genotypic variation in biochemical and physiological responses of fenugreek (Trigonellafoenum-graecum L.) landraces to prolonged drought stress and subsequent rewatering. Sci. Hortic. 2021, 287, 110224. [Google Scholar] [CrossRef]
- Wasli, H.; Jelali, N.; Silva, A.M.S.; Ksouri, R.; Cardoso, S.M. Variation of polyphenolic composition, antioxidants and physiological characteristics of dill (Anethum graveolens L.) as affected by bicarbonate-induced iron deficiency conditions. Ind. Crops Prod. 2018, 126, 466–476. [Google Scholar] [CrossRef]
- Sreenivasulu, N.; Grimm, B.; Wobus, U.; Weschke, W. Differential response of antioxidant compounds to salinity stress in salt-tolerant and salt-sensitive seedlings of foxtail millet (Setaria italica). Physiol. Plant. 2002, 109, 435–442. [Google Scholar] [CrossRef]
- Abreu, I.N.; Mazzafera, P. Effect of water and temperature stress on the content of active constituents of Hypericum Brasiliense Choisy. Plant Physiol. Biochem. 2005, 43, 241–248. [Google Scholar] [CrossRef]
- Bettaieb-Rebey, I.; Jabri-Karoui, I.; Hamrouni-Sellami, I.; Bourgou, S.; Limam, F.; Marzouk, B. Effect of drought on the biochemical composition and antioxidant activities of cumin (Cuminum cyminum L.) seeds. Ind. Crops Prod. 2012, 36, 238–245. [Google Scholar] [CrossRef]
- Naquvi, K.J.; Ansari, S.H.; Ali, M.; Najmi, A.K. Volatile oil composition of Rosa damascena Mill. (Rosaceae). J. Pharmacogn. Phytochem. 2014, 2, 130–134. [Google Scholar]
- Baydar, N.G.; Baydar, H. Phenolic compounds, antiradical activity and antioxidant capacity of oil-bearing rose (Rosa damascena Mill.) extracts. Ind. Crops Prod. 2014, 41, 375–380. [Google Scholar] [CrossRef]
- Al-Yasi, H.; Attia, H.; Alamer, K.; Hassan, F.; Ali, E.; Elshazly, S.; Siddique, K.H.; Hessini, K. Impact of drought on growth, photosynthesis, osmotic adjustment, and cell wall elasticity in Damask rose. Plant Physiol. Biochem. 2020, 150, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, E.J. Sand and Water Culture Methods Used in the Study of Plant Nutrition, 2nd ed.; Commonwealth Agric. Bureaux, East Mailing: Kent, WA, USA, 1966; pp. 431–446. [Google Scholar]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Wasli, H.; Jelali, N.; Ksouri, R.; Cardoso, S.M. Insights on the adaptation of Foeniculum vulgare Mill to iron deficiency. Appl. Sci. 2021, 11, 7072. [Google Scholar] [CrossRef]
- Proestos, C.; Boziaris, I.S.; Nychas, G.J.E.; Komaitis, M. Analysis of flavonoids and phenolic acids in Greek aromatic plants: Investigation of their antioxidant capacity and antimicrobial activity. Food Chem. 2006, 95, 664–671. [Google Scholar] [CrossRef]
- Koleva, I.I.; Van Beek, T.A.; Linssen, J.P.H.; de Groot, A.; Evstatieva, L.N. Screening of plant extracts for antioxidant activity: A comparative study on three testing methods. Phytochem. Anal. 2002, 13, 8–17. [Google Scholar] [CrossRef]
- Majdoub, N.; El-Guendouz, S.; Rezgui, M.; Carlier, J.; Costa, C.; Bettaieb-Ben Kaaba, L.; Miguel, M.G. Growth, photosynthetic pigments, phenolic content and biological activities of Foeniculum vulgare Mill., Anethum graveolens L. and Pimpinella anisum L. (Apiaceae) in response to zinc. Ind. Crops Prod. 2017, 109, 627–636. [Google Scholar] [CrossRef]
- Negrão, S.; Schmöckel, S.M.; Tester, M. Evaluating physiological responses of plants to salinity stress. Ann. Bot 2017, 119, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Hessini, K.; Issaoui, K.; Ferchichi, S.; Saif, T.; Abdelly, C.; Siddique, K.H.; Cruz, C. Interactive effects of salinity and nitrogen forms on plant growth, photosynthesis and osmotic adjustment in maize. Plant Physiol. Biochem. 2019, 139, 171–178. [Google Scholar] [CrossRef]
- Mejri, M.; Siddique, K.H.; Saif, T.; Abdelly, C.; Hessini, K. Comparative effect of drought duration on growth, photosynthesis, water relations, and solute accumulation in wild and cultivated barley species. J. Plant Nutr. Soil Sci. 2016, 179, 327–335. [Google Scholar] [CrossRef]
- Farhat, N.; Belghith, I.; Senkler, J.; Hichri, S.; Abdelly, C.; Braun, H.P.; Debez, A. Recovery aptitude of the halophyte Cakilemaritima upon water deficit stress release is sustained by extensive modulation of the leaf proteome. Ecotoxicol. Environ. Saf. 2019, 179, 198–211. [Google Scholar] [CrossRef] [PubMed]
- Ramireddy, E.; Hosseini, S.A.; Eggert, K.; Gillandt, S.; Gnad, H.; Von Wirén, N.; Schmülling, T. Root engineering in barley: Increasing cytokinin degradation produces a larger root system, mineral enrichment in the shoot and improved drought tolerance. Plant Physiol. 2018, 177, 1078–1095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitajima, K.; Hogan, K.P. Increases of chlorophyll a/b ratios during acclimation of tropical woody seedlings to nitrogen limitation and high light. Plant Cell Environ. 2003, 26, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Caser, M.; Chitarra, W.; D’Angiolillo, F.; Perrone, I.; Demasi, S.; Lovisolo, C.; Pistelli, L.; Pistelli, L.; Scariot, V. Drought stress adaptation modulates plant secondary metabolite production in Salvia dolomitica Codd. Ind. Crops Prod. 2019, 129, 85–96. [Google Scholar] [CrossRef]
- Bettaieb-Rebey, I.; Jabri-Karoui, I.; Hamrouni-Sellami, I.; Bourgou, S.; Limam, F.; Marzouk, B. Drought effects on polyphenol composition and antioxidant activities in aerial parts of Salvia officinalis L. Acta Physiol. Plant. 2011, 33, 1103–1111. [Google Scholar] [CrossRef]
- Nogués, S.; Baker, N.R. Effects of drought on photosynthesis in Mediterranean plants grown under enhanced UV-B radiation. J. Exp. Bot. 2000, 51, 1309–1317. [Google Scholar] [CrossRef]
- Hodaei, M.; Rahimmalek, M.; Arzani, A.; Talebi, M. The effect of water stress on phytochemical accumulation, bioactive compounds and expression of key genes involved in flavonoid biosynthesis in Chrysanthemum morifolium L. Ind. Crops Prod. 2018, 120, 295–304. [Google Scholar] [CrossRef]
- Gharibia, S.; Tabatabaei, B.E.S.; Saeidia, G.; Majid Talebi, M.; Matkowsk, A. The effect of drought stress on polyphenolic compounds and expression of flavonoid biosynthesis related genes in Achillea pachycephala Rech. f. Phytochemistry 2019, 162, 90–98. [Google Scholar] [CrossRef]
- Jorge, T.F.; Tohge, T.; Wendenburg, R.; Ramalho, J.C.; Lidon, F.C.; Ribeiro-Barros, A.I.; Fernie, A.R.; António, C. Salt-stress secondary metabolite signatures involved in the ability of Casuarina glauca to mitigate oxidative stress. Environ. Exp. Bot. 2019, 166, 103808. [Google Scholar] [CrossRef]
- Meot-Duros, L.; Magné, C. Antioxidant activity and phenol content of Crithmum maritimum L. leaves. Plant Physiol. Biochem. 2009, 47, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Guo, Q.; Liu, L.; Liao, L.; Zhu, Z. Influence of fertilization and drought stress on the growth and production of secondary metabolites in Prunella vulgaris L. J. Med. Plants Res. 2011, 5, 1749–1755. [Google Scholar]
- Quan, N.T.; Anh, L.H.; Khang, D.T.; Tuyen, P.T.; Toan, N.P.; Minh, T.N.; Xuan, T.D. Involvement of secondary metabolites in response to drought stress of rice (Oryza sativa L.). Agriculture 2016, 6, 23. [Google Scholar] [CrossRef] [Green Version]
- Ayaz, F.A.; Kadioglu, A.R.; Turgut, R. Water stress effects on the content of low molecular weight carbohydrates and phenolic acids in Ctenanthesetosa (Rosc.) Eichler. Can. J. Plant Sci. 2000, 80, 373–378. [Google Scholar] [CrossRef]
- Salem, N.; Msaada, K.; Dhifi, W.; Sriti, J.; Mejri, H.; Limam, F.; Marzouk, B. Effect of drought on safflower natural dyes and their biological activities. Excli J. 2014, 13, 1–18. [Google Scholar] [PubMed]
- Okunlola, G.O.; Olatunji, O.A.; Akinwale, R.O.; Tariq, A.; Adelusi, A.A. Physiological response of the three most cultivated pepper species (Capsicum spp.) in Africa to drought stress imposed at three stages of growth and development. Sci. Hortic. 2017, 224, 198–205. [Google Scholar] [CrossRef]
- Sanchez-Rodriguez, E.; Moreno, D.A.; Ferreres, F.; Rubio-WilhelmiMdel, M.; Ruiz, J.M. Differential responses of five cherry tomato varieties to water stress: Changes on phenolic metabolites and related enzymes. Phytochemistry 2011, 72, 723–729. [Google Scholar] [CrossRef]
- Ding, C.; Lei, L.; Yao, L.; Wang, L.; Hao, X.; Li, N.; Wang, Y.; Yin, P.; Guo, G.; Yang, Y.; et al. The involvements of calcium-dependent protein kinases and catechins in tea plant [Camellia sinensis (L.) O. Kuntze] cold responses. Plant Physiol. Biochem. 2019, 143, 190–202. [Google Scholar] [CrossRef]
- Saada, M.; Wasli, H.; Jallali, I.; Kbouki, R.; Girard-Lalancette, K.; Mshvildadzer, V.; Ksouri, R.; Legault, J.; Cardoso, S.M. Bio-Guided Fractionation of Retama raetam (Forssk.) Webb & Berthel Polar Extracts. Molecules 2021, 26, 5800. [Google Scholar]
- New comer, M.E.; Brash, A.R. The structural basis for specificity in lipoxygenase catalysis. Protein Sci. 2015, 24, 298–309. [Google Scholar] [CrossRef] [Green Version]
- Pallavi, P.C.; Singh, A.K.; Singh, S.; Singh, N.K. In silico structural and functional insights into the lipoxygenase enzyme of legume Cajanus cajan. Int. J. Recent Innov. Trends Comput. Commun. 2014, 5, 87–91. [Google Scholar]
- Porta, H.; Rocha-Sosa, M. Plant lipoxygenases. Physiological and molecular features. Plant Physiol. 2002, 130, 15–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahman, U.; Uddin, T.; Choudhary, G.; Iqbal, M. Discovery and molecular docking simulation of 7-hydroxy-6-methoxy-2H-chromen-2-one as a LOX Inhibitor. Pak. J. Pharm. Sci. 2019, 32, 217–220. [Google Scholar] [PubMed]
- Bae, K.S.; Rahimi, S.; Kim, Y.J.; Renuka Devi, B.S.; Khorolragchaa, A.; Sukweenadhi, J.; Silva, J.; Myagmarjav, D.; Yang, D.C. Molecular characterization of lipoxygenase genes and their expression analysis against biotic and abiotic stresses in Panax ginseng. Eur. J. Plant Pathol. 2016, 145, 331–343. [Google Scholar] [CrossRef]
- Siedow, J.N. Plant lipoxygenase: Structure and function. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1991, 42, 145–188. [Google Scholar] [CrossRef]
- Maccarrone, M.; Veldink, G.A.; Agro, A.F.; Vliegenthart, J.F. Modulation of soybean lipoxygenase expression and membrane oxidation by water deficit. FEBS Lett. 1995, 371, 223–226. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).