Relationships between Soil Tillage Systems, Nematode Communities and Weed Seed Predation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Trials
2.2. Nematode Community Analysis
2.3. Weed Data Collection
2.4. Weed Seed Predation
2.5. Pepper Yield
2.6. Statistical Analysis
3. Results
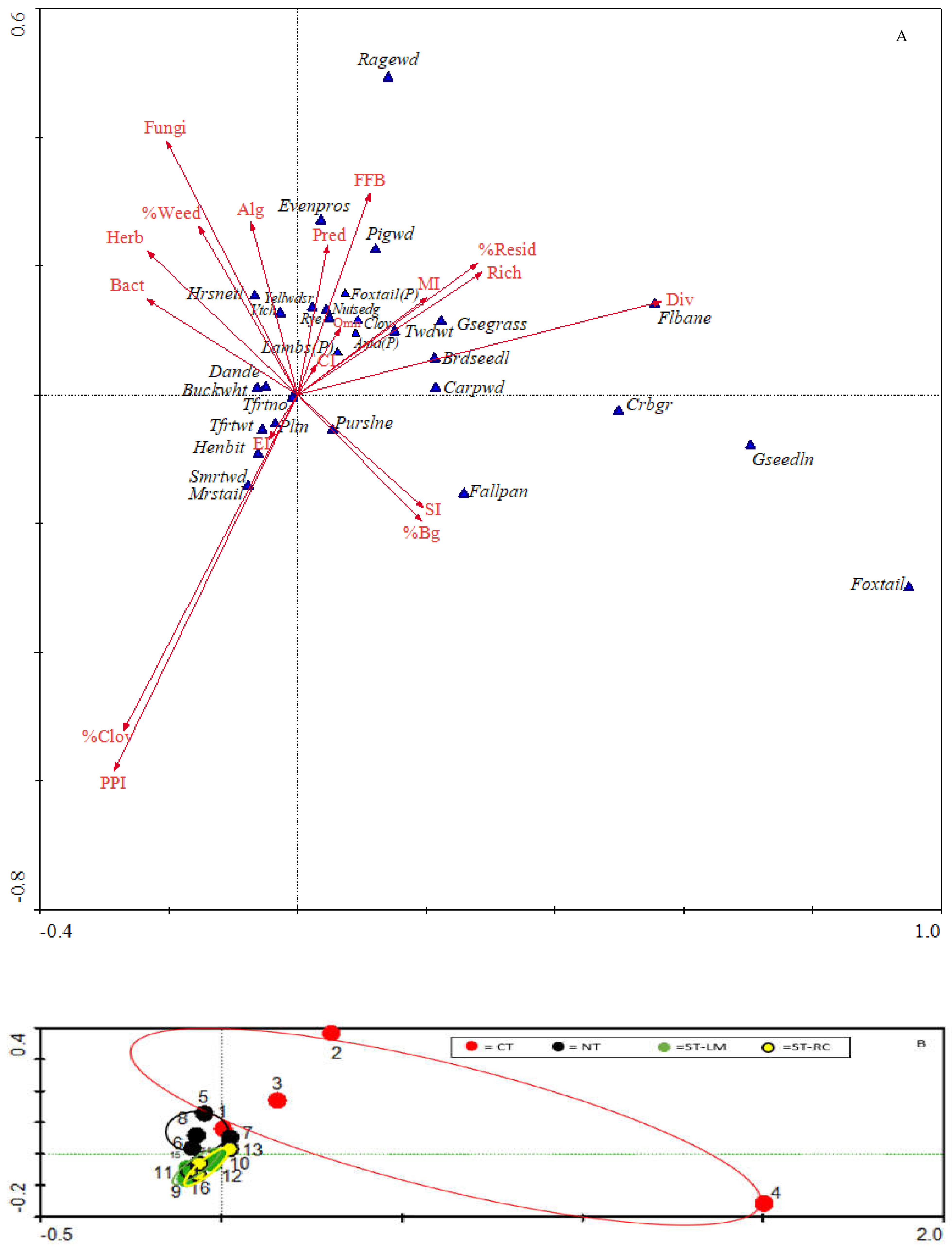
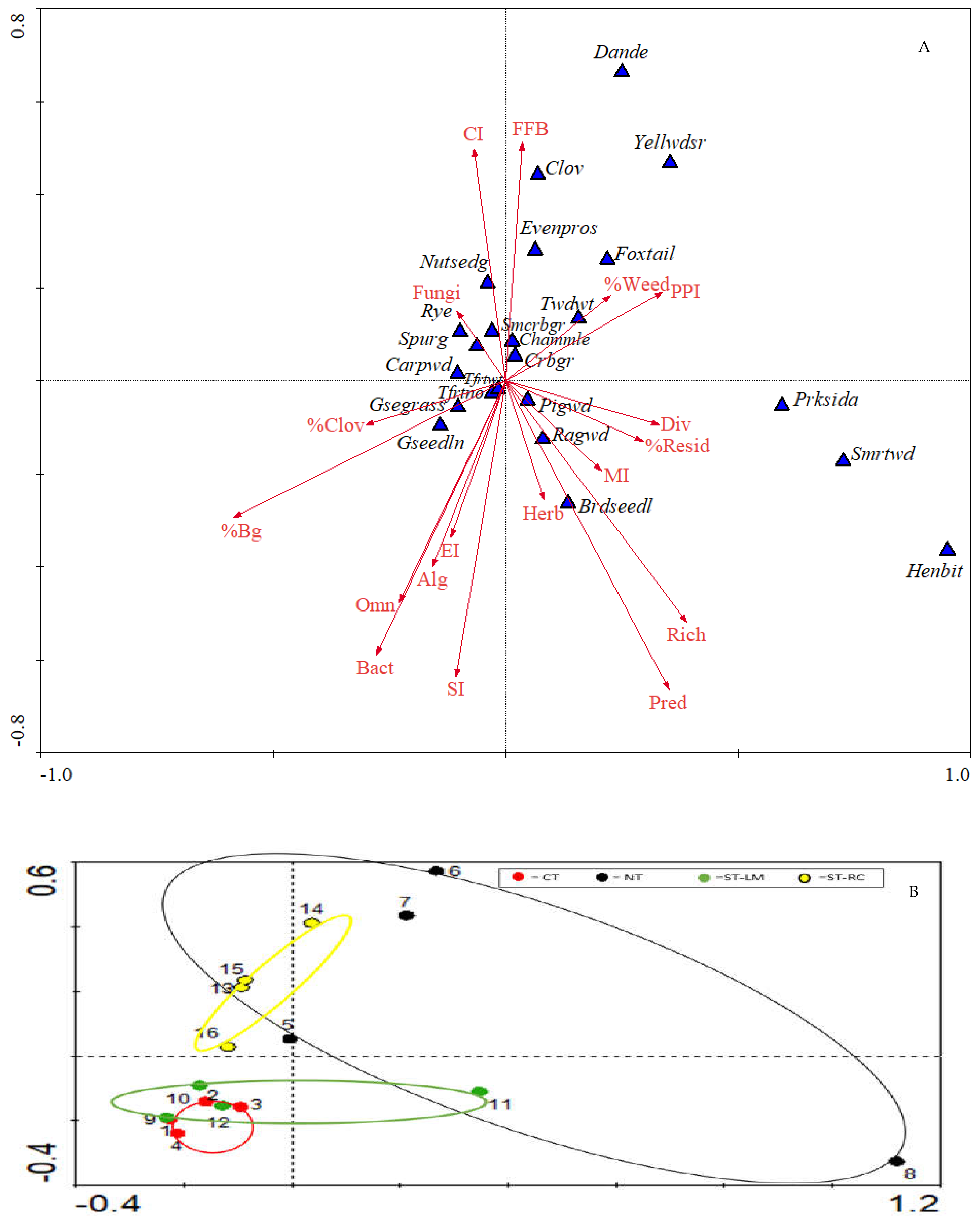
3.1. Nematode Community Analysis as Soil Health Indicators
3.2. Relationships of Soil Health with Weed Suppression and Pepper Yield
4. Discussion
4.1. Effects of Tillage Treatment on Nematode Community Assemblage
4.2. Relationships of Weed Suppression to Cover Crop Residues, Soil Health, and Pepper Yield
4.3. Relationships between Weed Seed Predation with Soil Health Indicators
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, K.H.; Hooks, C.R.R.; Marahatta, S.P. Can using a strip-tilled cover cropping system followed by surface mulch practice enhance organisms higher up in the soil food web hierarchy? Appl. Soil Ecol. 2011, 49, 107–117. [Google Scholar] [CrossRef]
- Reeves, D.W. The role of soil organic matter in maintaining soil quality in continuous cropping systems. Soil Tillage Res. 1997, 43, 131–167. [Google Scholar] [CrossRef]
- Liebman, M.; Davis, A.S. Integration of soil, crop and weed management in low-external-input farming systems. Weed Res. 2000, 40, 27–47. [Google Scholar] [CrossRef] [Green Version]
- Dabney, S.M.; Delgado, J.A.; Reeves, D.W. Using winter cover crops to improve soil and water quality. Commun. Soil Sci. Plant Anal. 2001, 32, 1221–1250. [Google Scholar] [CrossRef]
- McIntyre, D.S. Permeability measurements of soil crusts formed by raindrop impact. Soil Sci. 1958, 85, 185–189. [Google Scholar] [CrossRef]
- Dabney, S.M. Cover crop impacts on watershed hydrology. J. Soil Water Conserv. 1998, 53, 207–213. [Google Scholar]
- Sexton, A.M.; Shirmohammadi, A.; Montas, H.; Gish, T.J. Pesticide loss in surface runoff under various tillage and pesticide formulation conditions. In Proceedings of the 2000 ASAE Annual International Meeting, Milwaukee, WI, USA, 9–12 July 2000. [Google Scholar]
- Neher, D.A.; Nishanthan, T.; Grabau, Z.J.; Chen, S.Y. Crop rotation and tillage affect nematode communities more than biocides in monoculture soybean. Appl. Soil Ecol. 2019, 140, 89–97. [Google Scholar] [CrossRef]
- Altieri, M.A. The ecological role of biodiversity in agroecosystems. Agric. Ecosyst. Environ. 1999, 74, 19–31. [Google Scholar] [CrossRef] [Green Version]
- Creamer, N.G.; Bennett, M.A.; Stinner, B.R.; Cardina, J.; Regnier, E.E. Mechanisms of weed suppression in cover crop-based production systems. HortScience 1996, 31, 410–413. [Google Scholar] [CrossRef]
- Gonzalez-Martin, C.; Teigell-Perez, N.; Valladares, B.; Griffin, D.W. The global dispersion of pathogenic microorganisms by dust storms and its relevance to agriculture. Adv. Agron. 2014, 127, 1–41. [Google Scholar] [CrossRef]
- Davis, A.S. Cover-Crop Roller-crimper contributes to weed management in no-till soybean. Weed Sci. 2010, 58, 300–309. [Google Scholar] [CrossRef]
- Creamer, N.G.; Dabney, S.M. Killing cover crops mechanically: Review of recent literature and assessment of new research results. Am. J. Altern. Agric. 2002, 17, 32–40. [Google Scholar] [CrossRef]
- Osterholz, W.R.; Culman, S.W.; Herms, C.; de Oliveira, F.J.; Robinson, A.; Doohan, D. Knowledge gaps in organic research: Understanding interactions of cover crops and tillage for weed control and soil health. Org. Agric. 2021, 11, 13–25. [Google Scholar] [CrossRef]
- Bensch, C.N.; Horak, M.J.; Peterson, D. Interference of redroot pigweed (Amaranthus retroflexus), Palmer amaranth (A. palmeri), and common waterhemp (A. rudis) in soybean. Weed Sci. 2003, 51, 37–43. [Google Scholar] [CrossRef]
- Menalled, F.D.; Smith, R.G.; Dauer, J.T.; Fox, T.B. Impact of agricultural management on carabid communities and weed seed predation. Agric. Ecosyst. Environ. 2007, 118, 49–54. [Google Scholar] [CrossRef]
- Shearin, A.F.; Chris Reberg-Horton, S.; Gallandt, E.R. Cover Crop Effects on the Activity-Density of the Weed Seed Predator Harpalus rufipes (Coleoptera: Carabidae). Weed Sci. 2008, 56, 442–450. [Google Scholar] [CrossRef]
- Chauhan, B.S.; Migo, T.; Westerman, P.R.; Johnson, D.E. Post-dispersal predation of weed seeds in rice fields. Weed Res. 2010, 50, 553–560. [Google Scholar] [CrossRef]
- Brust, G.E.; House, G.J. Weed seed destruction by arthropods and rodents in low-input soybean agroecosystems. Am. J. Altern. Agric. 1988, 3, 19–25. [Google Scholar] [CrossRef]
- Harrison, S.K.; Regnier, E.E.; Schmoll, J.T. Postdispersal predation of giant ragweed (Ambrosia trifida) seed in no-tillage corn. Weed Sci. 2003, 51, 955–964. [Google Scholar] [CrossRef]
- Magdoff, F. Concept, components, and strategies of soil health in agroecosystems. J. Nematol. 2001, 33, 169–172. [Google Scholar]
- Roger-Estrade, J.; Anger, C.; Bertrand, M.; Richard, G. Tillage and soil ecology: Partners for sustainable agriculture. Soil Tillage Res. 2010, 111, 33–40. [Google Scholar] [CrossRef]
- Ferris, H.; Griffiths, B.S.; Porazinska, D.L.; Powers, T.O.; Wang, K.H.; Tenuta, M. Reflections on plant and soil nematode ecology: Past, present and future. J. Nematol. 2012, 44, 115–126. [Google Scholar]
- Bongers, T. The Maturity Index: An ecological measure of environmental disturbance based on nematode species composition. Oecologia 1990, 83, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Hoyt, G.D. Tillage and cover residue effects on Vegetable Yields. Horttechnology 1999, 9, 351–358. [Google Scholar] [CrossRef]
- DuPont, S.T.; Ferris, H.; Van Horn, M. Effects of cover crop quality and quantity on nematode-based soil food webs and nutrient cycling. Appl. Soil Ecol. 2009, 41, 157–167. [Google Scholar] [CrossRef]
- NCSS. Anapolis Series; National Cooperative Soil Survey: National Academic Soil Survey, Lincoln, NE, USA. 2015. Available online: https://soilseries.sc.egov.usda.gov/OSD_Docs/A/ANNAPOLIS.html (accessed on 28 March 2022).
- Jenkins, W.R. A rapid centrifugal-flotation technique for separating nematodes from soil. Plant Dis. Rep. 1964, 48, 692. [Google Scholar]
- Yeates, G.W.; Bongers, T.; De Goede, R.G.M.; Freckman, D.W.; Georgieva, S.S. Feeding habits in soil nematode families and genera-an outline for soil ecologists. J. Nematol. 1993, 25, 315–331. [Google Scholar]
- Simpson, E.H. Measurement of diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Freckman, D.W.; Ettema, C.H. Assessing nematode communities in agroecosystems of varying human intervention. Agric. Ecosyst. Environ. 1993, 45, 239–261. [Google Scholar] [CrossRef]
- Bongers, T.; Bongers, M. Functional diversity of nematodes. Appl. Soil Ecol. 1998, 10, 239–251. [Google Scholar] [CrossRef]
- Yeates, G.W. Modification and qualification of the nematode maturity index. Pedobiologia 1994, 38, 97–101. [Google Scholar]
- Ferris, H.; Bongers, T.; De Goede, R.G.M. A framework for soil food web diagnostics: Extension of the nematode faunal analysis concept. Appl. Soil Ecol. 2001, 18, 13–29. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture, NASS. United States Standards for Grades of Sweet Peppers; USDA AMS. 2005. Available online: https://www.ams.usda.gov/about-ams/contact-us (accessed on 28 March 2022).
- Ferris, H. NEMAPLEX: The Nematode-Plant Expert Information System. 2001. Available online: http://nemaplex.ucdavis.edu/ (accessed on 28 March 2022).
- Mendoza, R.B.; Franti, T.G.; Doran, J.W.; Powers, T.O.; Zanner, C.W. Tillage Effects on Soil Quality Indicators and Nematode Abundance in Loessial Soil under Long-Term No-till Production. Commun. Soil Sci. Plant Anal. 2008, 39, 2169–2190. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Moreno, S.; Ferris, H. Suppressive Service of the Soil Food Web: Effects of Environmental Management. Agric. Ecosyst. Environ. 2007, 119, 75–87. [Google Scholar] [CrossRef]
- Quintanilla-Tornel, M.A.; Wang, K.H.; Tavares, J.; Hooks, C.R.R. Effects of mulching on above and below ground pests and beneficials in a green onion agroecosystem. Agric. Ecosyst. Environ. 2016, 224, 75–85. [Google Scholar] [CrossRef] [Green Version]
- Anderson, W.P. Weed Science: Principles and Applications; West Publishing Co.: St. Paul, MN, USA, 1996. [Google Scholar]
- Mirsky, S.B.; Ryan, M.R.; Teasdale, J.R.; Curran, W.S.; Reberg-Horton, C.S.; Spargo, J.T.; Wells, M.S.; Keene, C.L.; Moyer, J.W. Overcoming Weed Management Challenges in Cover Crop–Based Organic Rotational No-Till Soybean Production in the Eastern United States. Weed Technol. 2013, 27, 193–203. [Google Scholar] [CrossRef]
- Liebman, M.; Sundberg, D.N. Seed mass affects the susceptibility of weed and crop species to phytotoxins extracted from red clover shoots. Weed Sci. 2006, 54, 340–345. [Google Scholar] [CrossRef]
- Davis, A.S.; Cardina, J.; Forcella, F.; Johnson, G.A.; Kegode, G.; Lindquist, J.L.; Luschei, E.C.; Renner, K.A.; Sprague, C.L.; Williams, M.M. Environmental factors affecting seed persistence of annual weeds across the U.S. corn belt. Weed Sci. 2005, 53, 860–868. [Google Scholar] [CrossRef] [Green Version]
- Kremer, R.J.; Li, J. Developing weed-suppressive soils through improved soil quality management. Soil Tillage Res. 2003, 72, 193–202. [Google Scholar] [CrossRef]
- Thorbek, P.; Bilde, T.; Bilde, T. Reduced numbers of generalist arthropod predators after crop management. J. Appl. Ecol. 2004, 41, 526–538. [Google Scholar] [CrossRef]
- Gallandt, E.R.; Weiner, J. Crop–Weed Competition. In Els; John Wiley & Sons, Ltd.: Chichester, UK, 2015; pp. 1–9. ISBN 9780470015902. [Google Scholar]
| Nematode | Guild | Nematode | Guild | Nematode | Guild |
|---|---|---|---|---|---|
| Achromadora | A-3 | Aphelenchus | F-2 | Ecumenicus | O-4 |
| Paracyatholaimus | A-3 | Aphelenchoides | F-2 | Enchodelus | O-4 |
| Alirhabditis | B-1 | Ecphyadophora | F-2 | Epidorylaimus | O-4 |
| Anguilluloides | B-1 | Neotylenchus | F-2 | Eudorylaimus | O-4 |
| Diploscapter | B-1 | Filenchus | F-2 | Labronema | O-4 |
| Panagrellus | B-1 | Psilenchus | F-2 | Mesodorylaimus | O-4 |
| Panagrolaimus | B-1 | Nothotylenchus | F-2 | Miodorylaimus | O-4 |
| Rhabditidae | B-1 | Diptherophora | F-3 | Pungentus | O-4 |
| Acrobeles | B-2 | Dorylaimoides | F-4 | Timmus | O-4 |
| Acrobeloides | B-2 | Leptonchus | F-4 | Aporcelaimellus | O-5 |
| Cephalobus | B-2 | Tylencholaimus | F-4 | Aporcelaimus | O-5 |
| Cervidillus | B-2 | Tylencholaimellus | F-4 | Laimydorus | O-5 |
| Drilocephalobus | B-2 | ||||
| Eucephalobus | B-2 | Tylenchus | H-2 | Tripyla | P-3 |
| Heterocephalobus | B-2 | Hoplolaimus | H-3 | Trobilus | P-3 |
| Monhystera | B-2 | Helicotylenchus | H-3 | Clarkus | P-4 |
| Plectus | B-2 | Ditylenchus | H-3 | Ironus | P-4 |
| Wilsonema | B-2 | Pratylenchus | H-3 | Mononchus | P-4 |
| Zeldia | B-2 | Tylenchorhynchus | H-3 | Mylonchulus | P-4 |
| Chronogaster | B-3 | Meloidogyne | H-3 | Aetholaimus | P-5 |
| Prismatolaimus | B-3 | Paratrichodorus | H-4 | Discolaimium | P-5 |
| Teratocephalus | B-3 | Nygolaimus | P-5 | ||
| Alaimus | B-4 | Paravulvus | P-5 |
| Nematode z | CT y | NT | ST-LM | ST-RC |
|---|---|---|---|---|
| 17 June 2017 | ||||
| Abundance | -------------------------------nematodes/250 cm3 soil ----------------------- | |||
| Algivores | 3 ± 2 a | 0 ± 0 a | 1 ± 1 a | 0 ± 0 a |
| Bacterivores | 356 ± 74 ab | 258 ± 47 ab | 667 ± 229 a | 150 ± 36 b |
| Fungivores | 338 ± 86 a | 339 ± 39 a | 372 ± 65 a | 384 ± 66 a |
| Herbivores | 36 ± 10 a | 166 ± 75 a | 53 ± 14 a | 66 ± 21 a |
| Omnivores | 28 ± 7 a | 36 ± 7 a | 30 ± 6 a | 32 ± 19 a |
| Predators | 10 ± 7 a | 24 ± 10 a | 24 ± 6 a | 21 ± 6 a |
| Indices | ||||
| F/(F + B) x | 0.52 ± 0.03 bc | 0.62 ± 0.03 ab | 0.40 ± 0.10 c | 0.73 ± 0.03 a |
| Diversity | 6.36 ± 0.61 a | 5.52 ± 0.61 ab | 4.49 ± 0.86 ab | 3.98 ± 0.42 b |
| Maturity index (1–5) | 1.81 ± 0.05 ab | 2.11 ± 0.08 a | 1.74 ± 0.15 b | 2.16 ± 0.07 a |
| Enrichment index (%) | 74.61 ± 1.48 a | 62.87 ± 5.88 ab | 77.36 ± 6.93 a | 56.43 ± 1.96 b |
| Structure index (%) | 29.38 ± 5.45 a | 43.49 ± 4.47 a | 36.26 ± 2.18 a | 35.96 ± 5.30 a |
| Channel index (%) | 27.21 ± 2.11 b | 47.76 ± 9.72 ab | 23.64 ± 10.60 b | 64.13 ± 5.46 a |
| 2 August 2017 | ||||
| Abundance | ---------------------------nematodes/250 cm3 soil -------------------------- | |||
| Algivores | 22 ± 4 a | 53 ± 28 a | 2 ± 2 b | 5 ± 2 ab |
| Bacterivores | 494 ± 121 c | 7555 ± 1901 a | 3019 ± 728 b | 841 ± 158 c |
| Fungivores | 282 ± 57 b | 1315 ± 426 a | 969 ± 266 a | 203 ± 31 b |
| Herbivores | 49 ± 15 b | 375 ± 87 a | 207 ± 79 a | 39 ± 12 b |
| Omnivores | 32 ± 8 b | 193 ± 44 a | 144 ± 47 ab | 29 ± 7 b |
| Predators | 18 ± 3 b | 115 ± 44 a | 54 ± 16 ab | 22 ± 6 b |
| Indices | ||||
| F/(F + B) | 0.37 ± 0.03 a | 0.17 ± 0.05 b | 0.25 ± 0.02 b | 0.20 ± 0.02 b |
| Diversity | 8.67 ± 0.54 a | 3.54 ± 0.65 c | 4.58 ± 0.52 bc | 5.68 ± 0.71 b |
| Maturity index (1–5) | 1.85 ± 0.03 a | 1.89 ± 0.07 a | 1.90 ± 0.12 a | 1.56 ± 0.10 a |
| Enrichment index (%) | 74.87 ± 1.83 ab | 56.49 ± 7.18 b | 56.60 ± 10.13 b | 89.08 ± 1.87 a |
| Structure index (%) | 38.89 ± 3.49 b | 22.86 ± 3.87 c | 27.59 ± 1.13 c | 48.31 ± 5.00 a |
| Channel index (%) | 19.57 ± 1.16 ab | 15.52 ± 5.43 ab | 26.16 ± 5.44 a | 7.32 ± 1.36 b |
| 23 October 2017 | ||||
| Abundance | -------------------------nematodes/250 cm3 soil ---------------------------- | |||
| Algivores | 160 ± 91 a | 60 ± 28 a | 78 ± 6 a | 88 ± 13 a |
| Bacterivores | 2298 ± 594 a | 1495 ± 256 a | 2079 ± 161 a | 2498 ± 442 a |
| Fungivores | 1853 ± 364 a | 1553 ± 287 a | 1555 ± 152 a | 1653 ± 299 a |
| Herbivores | 358 ± 117 a | 303 ± 36 a | 383 ± 68 a | 395 ± 144 a |
| Omnivores | 420 ± 24 a | 258 ± 36 a | 410 ± 32 a | 285 ± 63 a |
| Predators | 170 ± 34 a | 85 ± 15 a | 160 ± 17 a | 135 ± 25 a |
| Indices | ||||
| F/(F + B) | 0.46 ± 0.04 a | 0.50 ± 0.06 a | 0.43 ± 0.04 a | 0.40 ± 0.06 a |
| Diversity | 7.87 ± 0.45 ab | 7.21 ± 0.65 b | 10.04 ± 0.68 a | 7.45 ± 1.36 b |
| Maturity index (1–5) | 2.25 ± 0.08 a | 2.15 ± 0.03 a | 2.36 ± 0.05 a | 2.10 ± 0.12 a |
| Enrichment index (%) | 64.37 ± 5.08 a | 69.80 ± 3.35 a | 59.01 ± 4.40 a | 72.43 ± 6.46 a |
| Structure index (%) | 60.40 ± 1.53 a | 58.14 ± 2.23 a | 63.46 ± 2.81 a | 61.80 ± 4.93 a |
| Channel index (%) | 33.39 ± 6.44 a | 30.86 ± 6.18 a | 33.97 ± 3.88 a | 24.58 ± 7.48 a |
| Nematode z | CT y | NT | ST-LM | ST-RC |
|---|---|---|---|---|
| Richness | 30 ± 2 a | 28 ± 1 a | 28 ± 2 a | 28 ± 2 a |
| Plant-parasitic index (3–5) | 2.60 ± 0.08 b | 2.73 ± 0.08 ab | 2.84 ± 0.06 a | 2.84 ± 0.06 a |
| Nematode z | CT y | NT | ST-LM | ST-RC |
|---|---|---|---|---|
| ----------------------------------nematodes/250 cm3 soil --------------------------------- | ||||
| Abundance | ||||
| Algivores | 18 ± 4 ab | 2 ± 1 c | 34 ± 14 a | 5 ± 2 bc |
| Fungivores | 173 ± 28 ab | 142 ± 25 b | 160 ± 35 ab | 211 ± 31 a |
| Herbivores | 100 ± 65 b | 123 ± 42 ab | 297 ± 88 a | 166 ± 77 b |
| Omnivores | 32 ± 6 a | 13 ± 3 b | 28 ± 5 a | 23 ± 5 ab |
| Predators | 23 ± 6 a | 16 ± 5 a | 18 ± 4 a | 13 ± 3 a |
| Indices | ||||
| Richness | 22 ± 1 a | 21 ± 1 a | 22 ± 1 a | 20 ± 1 a |
| F/(F + B) x | 0.44 ± 0.06 b | 0.45 ± 0.05 b | 0.39 ± 0.06 b | 0.54 ± 0.06 a |
| Diversity | 6.88 ± 0.81 a | 7.69 ± 1.05 a | 6.83 ± 1.43 a | 5.32 ± 0.78 a |
| Maturity index (1–5) | 2.15 ± 0.05 a | 2.05 ± 0.08 a | 2.14 ± 0.09 a | 2.07 ± 0.05 a |
| Plant-parasitic index (3–5) | 2.78 ± 0.10 a | 2.94 ± 0.04 a | 2.94 ± 0.03 a | 2.84 ± 0.05 a |
| Enrichment index (%) | 71.46 ± 3.07 a | 70.54 ± 3.31 a | 68.64 ± 4.81 a | 66.39 ± 3.41 a |
| Structure index (%) | 59.84 ± 4.72 a | 51.63 ± 4.39 ab | 61.45 ± 5.50 a | 46.44 ± 5.02 b |
| Channel index (%) | 30.78 ± 6.61 a | 30.07 ± 6.09 a | 30.49 ± 6.90 a | 42.70 ± 7.90 a |
| Bacterivores z | CT y | NT | ST-LM | ST-RC |
|---|---|---|---|---|
| ---------------------------nematodes/250 cm3 soil ------------------------------ | ||||
| 21 June 2018 | 112 ± 27 a | 121 ± 16 a | 211 ± 80 a | 76 ± 20 a |
| 14 August 2018 | 345 ± 40 a | 285 ± 72 a | 347 ± 82 a | 214 ± 24 a |
| 4 October 2018 | 190 ± 33 ab | 117 ± 9 b | 136 ± 29 b | 245 ± 52 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.-H.; Waisen, P.; Leslie, A.W.; Paudel, R.; Meyer, S.L.F.; Hooks, C.R.R. Relationships between Soil Tillage Systems, Nematode Communities and Weed Seed Predation. Horticulturae 2022, 8, 425. https://doi.org/10.3390/horticulturae8050425
Wang K-H, Waisen P, Leslie AW, Paudel R, Meyer SLF, Hooks CRR. Relationships between Soil Tillage Systems, Nematode Communities and Weed Seed Predation. Horticulturae. 2022; 8(5):425. https://doi.org/10.3390/horticulturae8050425
Chicago/Turabian StyleWang, Koon-Hui, Philip Waisen, Alan W. Leslie, Roshan Paudel, Susan L. F. Meyer, and Cerruti R. R. Hooks. 2022. "Relationships between Soil Tillage Systems, Nematode Communities and Weed Seed Predation" Horticulturae 8, no. 5: 425. https://doi.org/10.3390/horticulturae8050425
APA StyleWang, K.-H., Waisen, P., Leslie, A. W., Paudel, R., Meyer, S. L. F., & Hooks, C. R. R. (2022). Relationships between Soil Tillage Systems, Nematode Communities and Weed Seed Predation. Horticulturae, 8(5), 425. https://doi.org/10.3390/horticulturae8050425









