Fruit Quality Response to Different Abaxial Leafy Supplemental Lighting of Greenhouse-Produced Cherry Tomato (Solanum lycopersicum var. Cerasiforme)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Supplemental Lighting Treatment
2.3. Fruit Yield and Carbon Sequestration Analyses
2.4. Fruit Appearance and Flavor Quality Analyses
2.5. Fruit Nutrient and Function Indicator Analyses
2.6. Fruit Aromatic Substances Analyses
2.7. Economic Performance Analyses
2.8. Statistical Analyses
3. Results
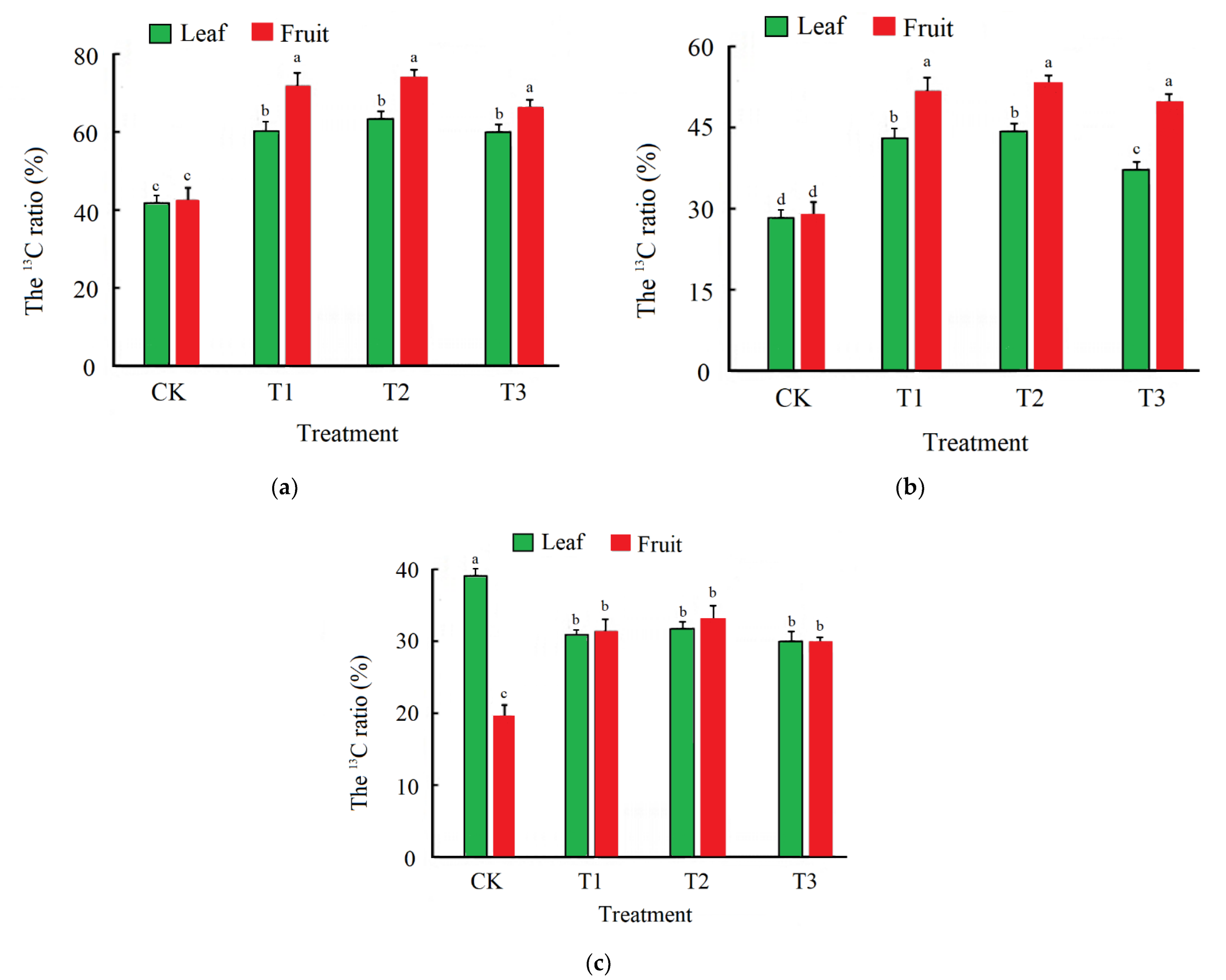
3.1. Fruit Yield and Carbon Sequestration of Function Leaves
3.2. Fruit Appearance Characters
3.3. Fruit Flavor Quality Analyses
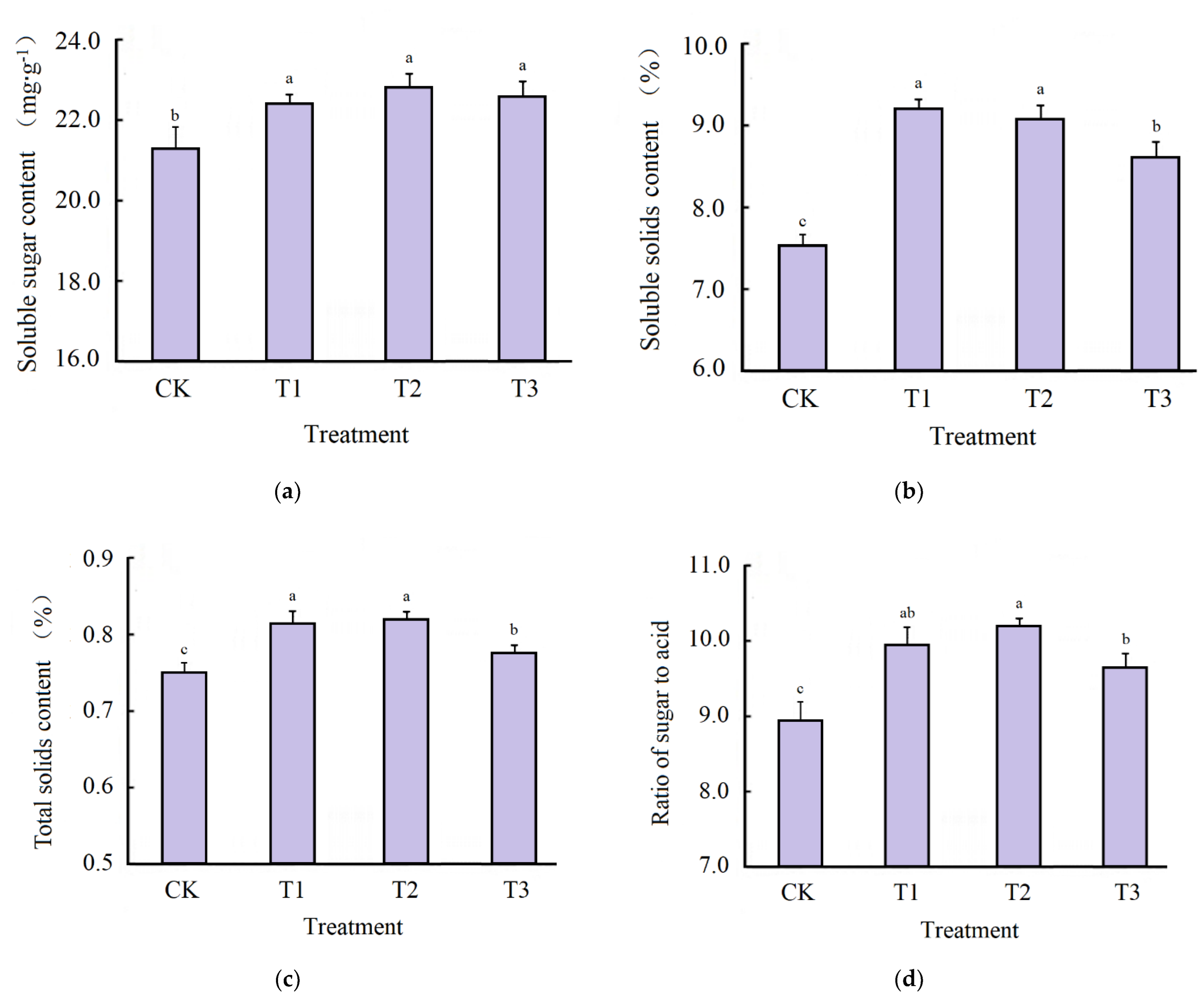
3.4. Fruit Nutrient and Function Indicator Analyses
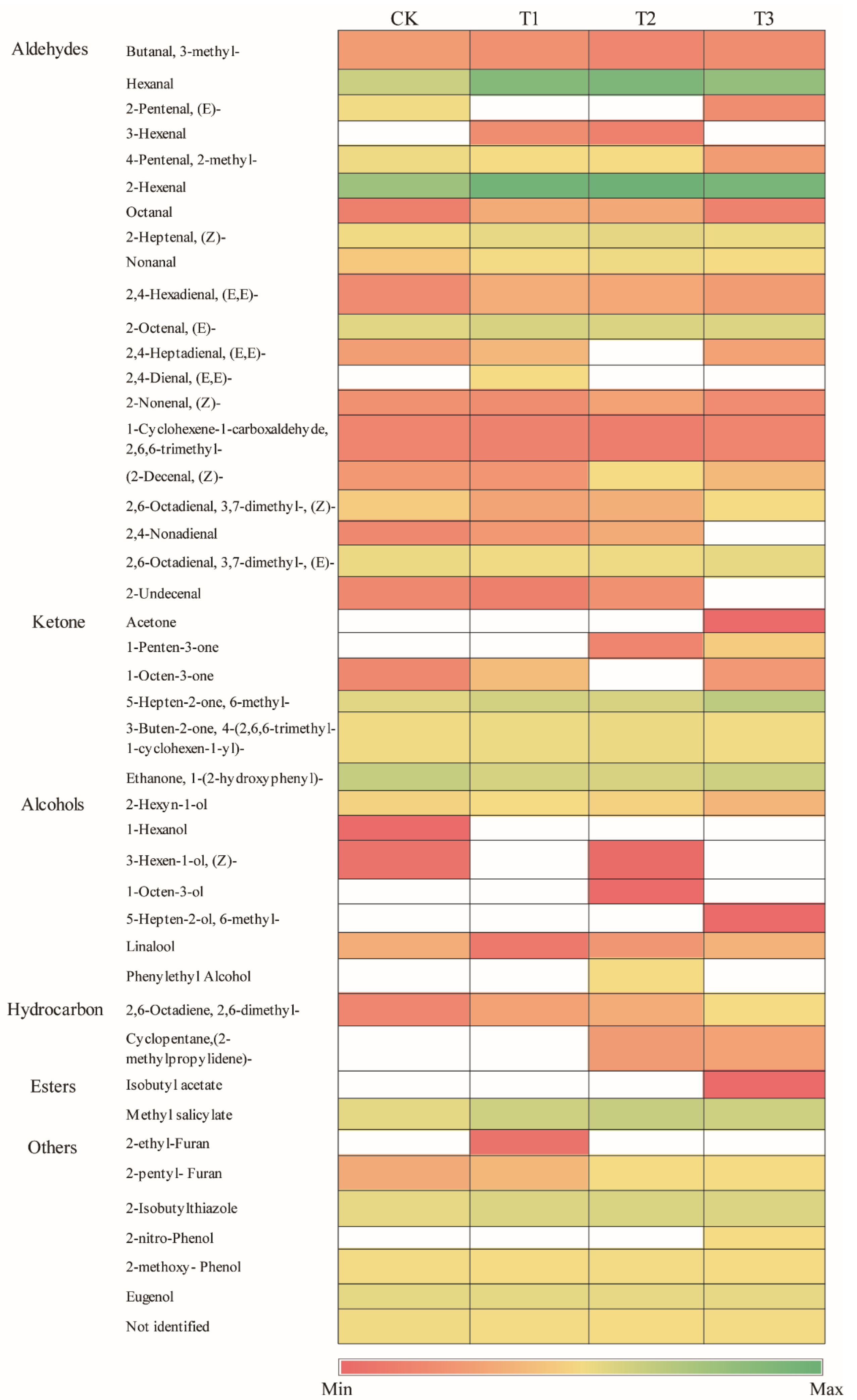
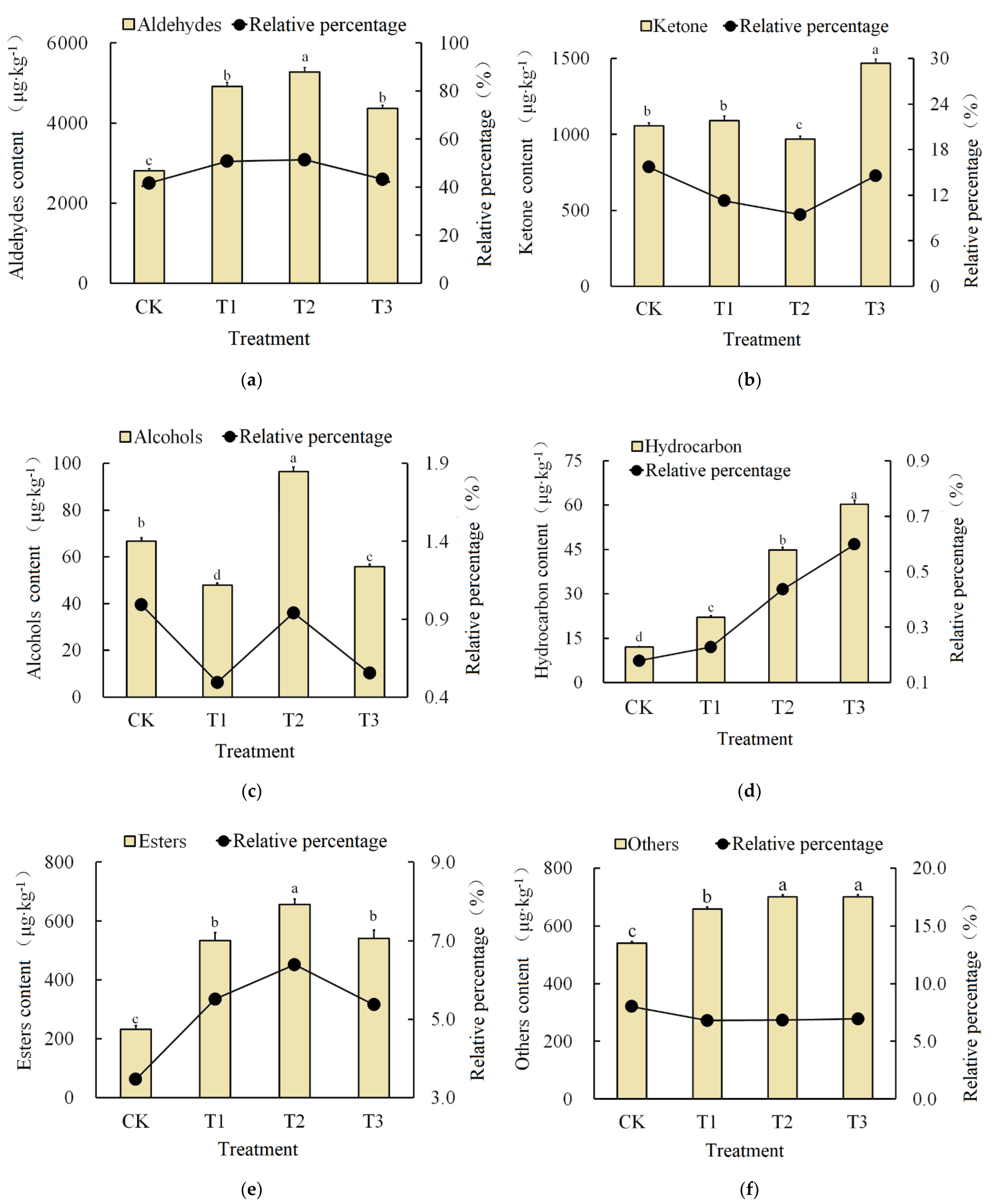
3.5. Fruit Aromatic Substances Analyses
3.6. Correlation Analysis of Fruit Yield and Quality Parameters
3.7. Economic Performance Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Villers, A.J.; van Rooyen, M.W.; Theron, G.K.; van de Venter, H.A. Germination of three Namaqualand pioneer species, as influenced by salinity, temperature and light. Seed Sci. Technol. 1994, 22, 427–433. [Google Scholar]
- Zohar, Y.; Waisel, Y.; Karschon, R. Effects of light, temperature and osmotic stress on seed germination of Eucalyptus occidentalis Endl. Aust. J. Bot. 1975, 23, 391–397. [Google Scholar] [CrossRef]
- Evans, J.; Poorter, H.R. Photosynthetic acclimation of plants to growth irradiance: The relative importance of specific leaf area and nitrogen in maximizing carbon gain. Plant Cell Environ. 2001, 24, 755–767. [Google Scholar] [CrossRef]
- Fu, Q.; Zhao, B.; Wang, X.; Wang, Y.; Ren, S.; Guo, Y. The responses of morphological trait, leaf ultrastructure, photosynthetic and biochemical performance of tomato to differential light availabilities. Agric. Sci. China 2011, 10, 1887–1897. [Google Scholar] [CrossRef]
- Cerdán, P.D.; Chory, J. Regulation of flowering time by light quality. Nature 2003, 423, 881–885. [Google Scholar] [CrossRef] [PubMed]
- Goto, N.; Kumagai, T.; Koornneef, M. Flowering responses to light-breaks in photomorphogenic mutants of Arabidopsis thaliana, a long-day plant. Physiol. Plantarum. 1991, 83, 209–215. [Google Scholar] [CrossRef]
- O’Carrigan, A.; Hinde, E.; Lu, N.; Xu, X.; Duanc, H.; Huang, G.; Mak, M.; Bellotti, B.; Chen, Z. Effects of light irradiance on stomatal regulation and growth of tomato. Environ. Exp. Bot. 2014, 98, 65–73. [Google Scholar] [CrossRef]
- Shimazaki, K.; Doi, M.; Assmann, S.M.; Kinoshita, T. Light regulation of stomatal movement. Annu. Rev. Plant Biol. 2007, 58, 219–247. [Google Scholar] [CrossRef] [Green Version]
- Mullineaux, P.; Karpinski, S. Signal transduction in response to excess light getting out of the chloroplast. Curr. Opin. Plant Biol. 2002, 5, 43–48. [Google Scholar] [CrossRef]
- Hovi, T.; Nakkila, J.; Tahvonen, R. Intra-canopy lighting improves production of year-round cucumber. Sci. Hortic. 2004, 102, 283–294. [Google Scholar] [CrossRef]
- Pettersen, R.I.; Torre, S.; Gislerod, H.R. Effects of intera-canopy lighting on photosynthesis characteristics in cucumber. Sci. Hortic. 2010, 125, 77–81. [Google Scholar] [CrossRef]
- Lu, N.; Maruo, T.; Johkan, M.; Hohjo, M.; Tsukagoshi, S.; Ito, Y.; Ichimura, T.; Shinohara, Y. Effect of supplemental lighting within the canopy at different developing stages on tomato yield and quality of single-truss tomato plant grown at high density. Environ. Control Biol. 2012, 50, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Dorais, M. The use of supplemental lighting for vegetable crop production: Light intensity, crop response, nutrition, crop management, cultural practices. In Proceedings of the Canadian Greenhouse Conference, Toronto, QN, Canada, 1 August 2003. [Google Scholar]
- Song, Y. Study on the Relationship between LED Supplementary Light and Greenhouse Tomato Light Utilization Characteristics and Growth and Development. Ph.D. Thesis, China Agricultural University, Beijing, China, 2017. (In Chinese with English Abstract). [Google Scholar]
- Tewolde, F.T.; Lu, N.; Shiina, K.; Maruo, T.; Takagaki, M.; Kozai, T.; Wataru, Y. Nighttime supplemental LED inter-lighting improves growth and yield of single-truss tomatoes by enhancing photosynthesis in both winter and summer. Front. Plant Sci. 2016, 7, 448. [Google Scholar] [CrossRef] [PubMed]
- Dou, H.; Niu, G.; Gu, M.; Masabni, J.G. Effects of Light Quality on Growth and Phytonutrient Accumulation of Herbs under Controlled Environments. Horticulturae 2017, 3, 36. [Google Scholar] [CrossRef] [Green Version]
- Rezazadeh, A.; Harkess, R.L.; Telmadarrehei, T. The Effect of Light Intensity and Temperature on Flowering and Morphology of Potted Red Firespike. Horticulturae 2018, 4, 36. [Google Scholar] [CrossRef] [Green Version]
- Zha, L.; Liu, W. Effects of light quality, light intensity, and photoperiod on growth and yield of cherry radish grown under red plus blue LEDs. Hortic. Environ. Biotechnol. 2018, 59, 511–518. [Google Scholar] [CrossRef]
- Ahmed, H.A.; Tong, Y.; Li, L.; Sahari, S.Q.; Almogahed, A.M.; Cheng, R. Integrative Effects of CO2 Concentration, Illumination Intensity and Air Speed on the Growth, Gas Exchange and Light Use Efficiency of Lettuce Plants Grown under Artificial Lighting. Horticulturae 2022, 8, 270. [Google Scholar] [CrossRef]
- Song, Y.; Jiang, C.; Gao, L. Polychromatic supplemental lighting from underneath canopy is more effective to enhance tomato plant development by improving leaf photosynthesis and stomatal regulation. Front. Plant Sci. 2016, 7, 1832. [Google Scholar] [CrossRef] [Green Version]
- Jiang, C.; Johkan, M.; Hohjo, M.; Tsukagoshi, S.; Ebihara, M.; Nakaminami, A.; Maruo, T. Photosynthesis, plant growth, and fruit production of single-truss tomato improves with supplemental lighting provided from underneath or within the inner canopy. Sci. Hortic. 2017, 222, 221–229. [Google Scholar] [CrossRef]
- Jiang, C.; Johkan, M.; Hohjo, T.; Tsukagoshi, S.; Ebihara, M.; Nakaminami, A.; Maruo, M. Supplemental lighting applied within or underneath the canopy enhances leaf photosynthesis, stomatal regulation and plant development of tomato under limiting light conditions. Acta Hortic. 2018, 1227, 645–652. [Google Scholar] [CrossRef]
- Jiang, C.; Song, Y.; Li, Y. Effects of different supplemental lightning modes on photosynthetic performance and carbon sequestration of tomato leaves in Gobi greenhouse. China Veg. 2019, 10, 32–38, (In Chinese with English Abstract). [Google Scholar]
- Song, Y.; Jiang, C.; Li, Y. Effects of different abaxial leaf supplemental lightning modes on fruit quality of tomato produced in Gobi greenhouses. Xinjiang Agric. Sci. 2021, 58, 294–303, (In Chinese with English Abstract). [Google Scholar]
- National Bureau of Statistics. China Statistical Yearbook-2021; China Statistics Press: Beijing, China, 2021.
- Aguirre, N.C.; Cabrera, F.A.V. Evaluating the fruit production and quality of cherry tomato (Solanum lycopersicum var. cerasiforme). Rev. Fac. Natl. Agric. Medellín 2012, 65, 6593–6604. [Google Scholar]
- Sun, Q.; Zhang, N.; Wang, J.; Zhang, H.; Li, D.; Shi, J.; Li, R.; Weeda, S.; Zhao, B.; Ren, S.; et al. Melatonin promotes ripening and improves quality of tomato fruit during postharvest life. J. Exp. Bot. 2015, 66, 657–668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, A.R.; Fish, W.W.; Perkins, P. A rapid spectrophotometric method for analyzing lycopene content in tomato products. Postharvest Biol. Tec. 2003, 28, 425–430. [Google Scholar] [CrossRef]
- Reich, P.B.; Walters, M.B.; Tjoelker, M.G.; Vanderklein, D.; Buschena, C. Photosynthesis and respiration rates depend on leaf and root morphology and nitrogen concentration in nine boreal tree species differing in relative growth rate. Func. Ecol. 2010, 12, 395–405. [Google Scholar] [CrossRef]
- Petrovic, I.; Savic, S.; Jovanovic, Z.; Stikić, R.; Brunel, B.; Sérino, S.; Bertin, N. Fruit quality of cherry and large fruited tomato genotypes as influenced by water deficit. Zemdirbyste 2019, 106, 123–128. [Google Scholar] [CrossRef] [Green Version]
- Dias, N.D.S.; Diniz, A.A.; de Morais, P.L.D.; Pereira, G.D.S.; Sá, F.V.D.S.; Souza, B.G.D.A.; Cavalcante, L.F.; Neto, M.F. Yield and quality of cherry tomato fruits in hydroponic cultivation. Biosci. J. 2019, 35, 1470–1477. [Google Scholar] [CrossRef] [Green Version]
- Gautier, H.; Rocci, A.; Buret, M.; Grasselly, D.; Causse, M. Fruit load or fruit position alters response to temperature and subsequently cherry tomato quality. J. Sci. Food Agric. 2005, 85, 1009–1016. [Google Scholar] [CrossRef]
- Islam, M.Z.; Lee, Y.T.; Mele, M.A.; Choi, I.L.; Kang, H.M. Effect of fruit size on fruit quality, shelf life and microbial activity in cherry tomatoes. AIMS Agric. Food 2019, 4, 340–348. [Google Scholar] [CrossRef]
- Selli, S.; Kelebek, H.; Ayseli, M.T.; Tokbas, H. Characterization of the most aroma-active compounds in cherry tomato by application of the aroma extract dilution analysis. Food Chem. 2014, 165, 540–546. [Google Scholar] [CrossRef] [PubMed]
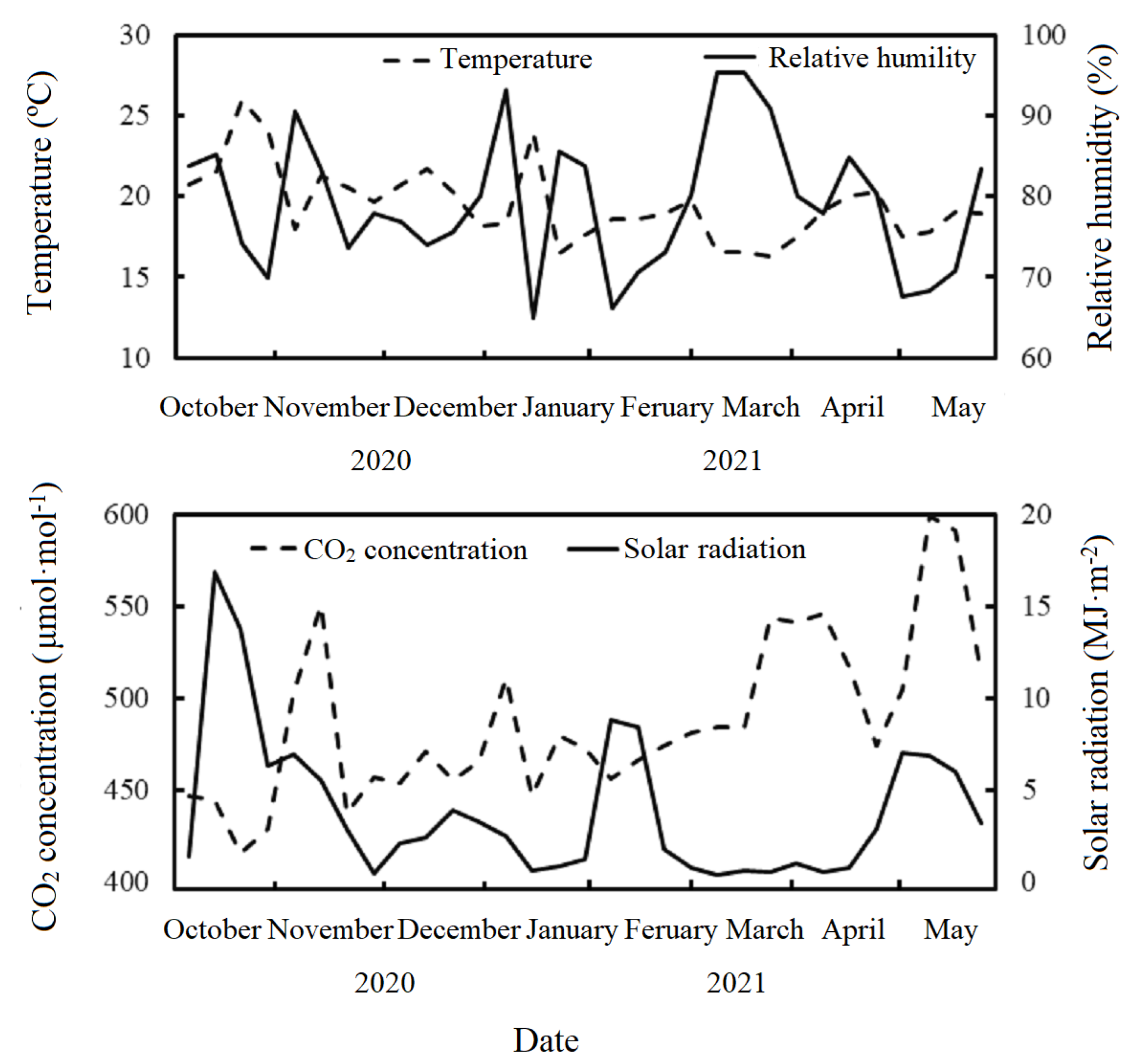


| Treatment | Supplemental Lighting Arrangement |
|---|---|
| CK | No supplement treatment, a blank control |
| T1 | Continuous supplemental lighting during 8:00–9:00 (before TIC opening), and 20:00–22:00 (after TIC closing) with PPFD of 200 μmol·m−2·s−1 |
| T2 | Dynamic altered supplemental lighting: PPFD rising from 100 μmol·m−2·s−1 to 200μmol·m−2·s−1 (8:00–9:00) and falling from 200 μmol·m−2·s−1 to 100 μmol·m−2·s−1 (20:00–22:00). |
| T3 | Intermittent supplemental lighting, which automatically used PPFD of 100 μmol·m−2·s−1 when indoor PPFD is below 150 μmol·m−2·s−1 from 8:00–22:00. |
| Treatment | Fresh Weight of Single Fruit (g) | Fresh Weight of Single Fruit Truss (g·truss−2) | Dry Weight of Single Fruit Truss (g·truss−2) | Total Yield (kg·m−2) |
|---|---|---|---|---|
| CK | 18.03 ± 0.11 c | 208.03 ± 5.11 c | 2.21 ± 0.03 c | 15.41 ± 4.61 c |
| T1 | 25.88 ± 2.14 ab | 265.18 ± 2.14 ab | 3.29 ± 0.03 a | 22.12 ± 4.14 a |
| T2 | 26.81 ± 0.87 a | 281.83 ± 4.87 a | 3.32 ± 0.02 a | 23.35 ± 5.36 a |
| T3 | 20.79 ± 1.02 b | 254.79 ± 1.02 b | 3.28 ± 0.02 a | 18.01 ± 4.61 b |
| Treatment | Transverse Diameter (mm) | Longitudinal Diameter (mm) | Fruit Shape Index |
|---|---|---|---|
| CK | 27.58 ± 0.17 c | 26.36 ± 1.21 a | 0.96 ± 0.01 a |
| T1 | 32.89 ± 1.11 a | 30.92 ± 1.25 a | 0.94 ± 0.01 b |
| T2 | 35.16 ± 3.48 a | 33.47 ± 2.98 a | 0.95 ± 0.00 a |
| T3 | 30.23 ± 2.36 a | 30.09 ± 0.56 a | 0.99 ± 0.00 a |
| Treatment | Luminosity (L*) | Pericarp Chroma | Color Saturation (C) | Hue (H) | |
|---|---|---|---|---|---|
| Red Saturation (a*) | Yellow Saturation (b*) | ||||
| CK | 45.23 ± 0.02 b | 20.13 ± 0.06 b | 12.24 ± 0.16 b | 23.56 ± 0.61 b | 31.30 ± 0.45 b |
| T1 | 52.15 ± 1.01 a | 26.18±0.13 a | 19.04 ± 0.13 a | 32.38 ± 0.03 a | 36.02 ± 0.12 a |
| T2 | 51.40 ± 0.77 a | 26.65 ± 0.16 a | 18.50 ± 0.22 a | 32.44 ± 0.15 a | 34.76 ± 0.97 ab |
| T3 | 51.67 ± 0.98 a | 26.05 ± 0.68 a | 18.41 ± 0.59 a | 31.90 ± 0.94 a | 35.25 ± 1.09 a |
| Parameter y | FWSFT | TY | FSI | L* | C | SSC | LC | AAC | ARP | KRP |
|---|---|---|---|---|---|---|---|---|---|---|
| FWSFT | 1 | |||||||||
| TY | 0.97 *z | 1 | ||||||||
| FSI | 0.64 | 0.83 | 1 | |||||||
| L* | 0.88 | 0.95 * | −0.94 * | 1 | ||||||
| C | 0.66 | −0.68 | −0.94 * | 0.90 | 1 | |||||
| SSC | 0.83 | 0.92 * | −0.85 | 0.95 * | 0.95 * | 1 | ||||
| LC | 0.87 | 0.86 | −0.87 | 0.93 * | 0.94 * | 0.92 * | 1 | |||
| AAC | 0.86 | 0.78 | −0.89 * | 0.58 | 0.62 | 0.95 * | 0.90 * | 1 | ||
| ARP | 0.91 * | 0.93 * | −0.40 | 0.84 | 0.86 | 0.91 * | 0.81 | 0.94 * | 1 | |
| KRP | −0.96 * | −0.91 * | 0.82 | −0.95 * | −0.90 | −0.88 | −0.71 | −0.68 | −0.94 * | 1 |
| Treatment | Electricity Consumption (kWh·m−2) | Yield Enhancement (kg·m−2) | Electricity Efficiency (g·kWh−1) | Cost Performance |
|---|---|---|---|---|
| T1 | 52.09 ± 2.11 a | 6.71 ± 1.12 b | 128.81 ± 3.65 b | 2.03 ± 0.62 ab |
| T2 | 45.16 ± 1.88 b | 7.94 ± 0.48 a | 175.82 ± 2.88 a | 2.64 ± 0.33 a |
| T3 | 40.99 ± 1.16 c | 2.60 ± 0.56 c | 63.43 ± 2.12 c | 1.57 ± 0.41 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, C.; Rao, J.; Rong, S.; Ding, G.; Liu, J.; Li, Y.; Song, Y. Fruit Quality Response to Different Abaxial Leafy Supplemental Lighting of Greenhouse-Produced Cherry Tomato (Solanum lycopersicum var. Cerasiforme). Horticulturae 2022, 8, 423. https://doi.org/10.3390/horticulturae8050423
Jiang C, Rao J, Rong S, Ding G, Liu J, Li Y, Song Y. Fruit Quality Response to Different Abaxial Leafy Supplemental Lighting of Greenhouse-Produced Cherry Tomato (Solanum lycopersicum var. Cerasiforme). Horticulturae. 2022; 8(5):423. https://doi.org/10.3390/horticulturae8050423
Chicago/Turabian StyleJiang, Chengyao, Jiahui Rao, Sen Rong, Guotian Ding, Jiaming Liu, Yushan Li, and Yu Song. 2022. "Fruit Quality Response to Different Abaxial Leafy Supplemental Lighting of Greenhouse-Produced Cherry Tomato (Solanum lycopersicum var. Cerasiforme)" Horticulturae 8, no. 5: 423. https://doi.org/10.3390/horticulturae8050423
APA StyleJiang, C., Rao, J., Rong, S., Ding, G., Liu, J., Li, Y., & Song, Y. (2022). Fruit Quality Response to Different Abaxial Leafy Supplemental Lighting of Greenhouse-Produced Cherry Tomato (Solanum lycopersicum var. Cerasiforme). Horticulturae, 8(5), 423. https://doi.org/10.3390/horticulturae8050423







