A Tomato Pomace Enriched Gluten-Free Ready-to-Cook Snack’s Nutritional Profile, Quality, and Shelf Life Evaluation
Abstract
1. Introduction
2. Materials and Methods
2.1. Product Preparation
2.2. Physico-Chemical Analysis
2.3. Phytochemical Analysis
2.4. Antioxidant Analysis
2.5. Cooking Analysis
2.6. Sensory Evaluation
2.7. Fourier-Transform Infrared (FTIR) Spectroscopy
2.8. Storage Studies
2.9. Statistical Analysis
3. Results
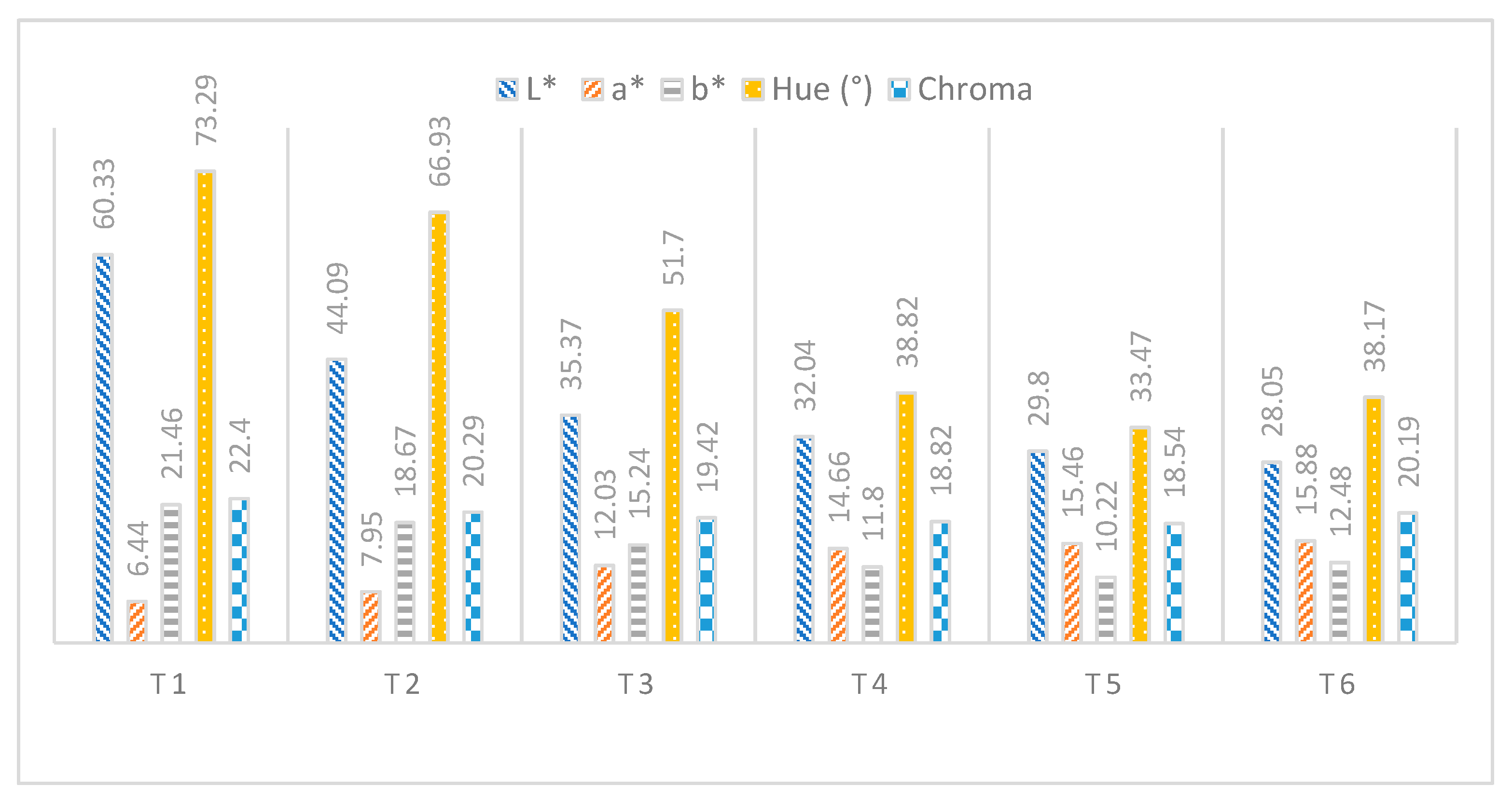
3.1. Physico-Chemical, Phytochemical, and Antioxidant Analysis
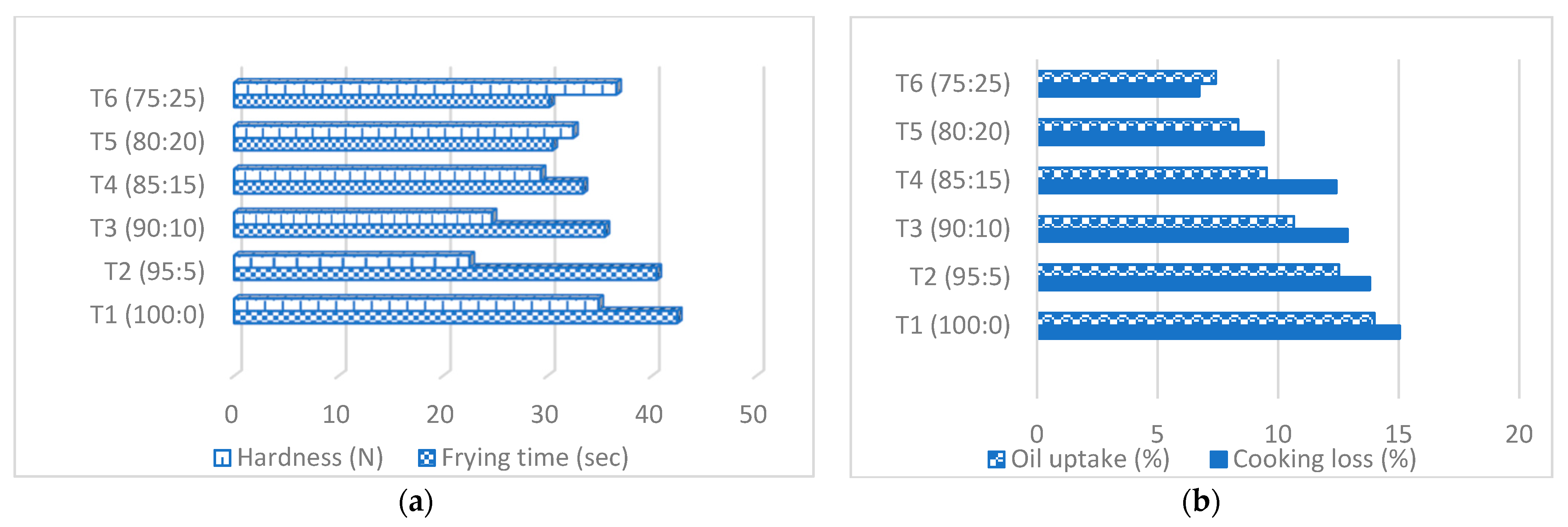
3.2. Cooking Analysis
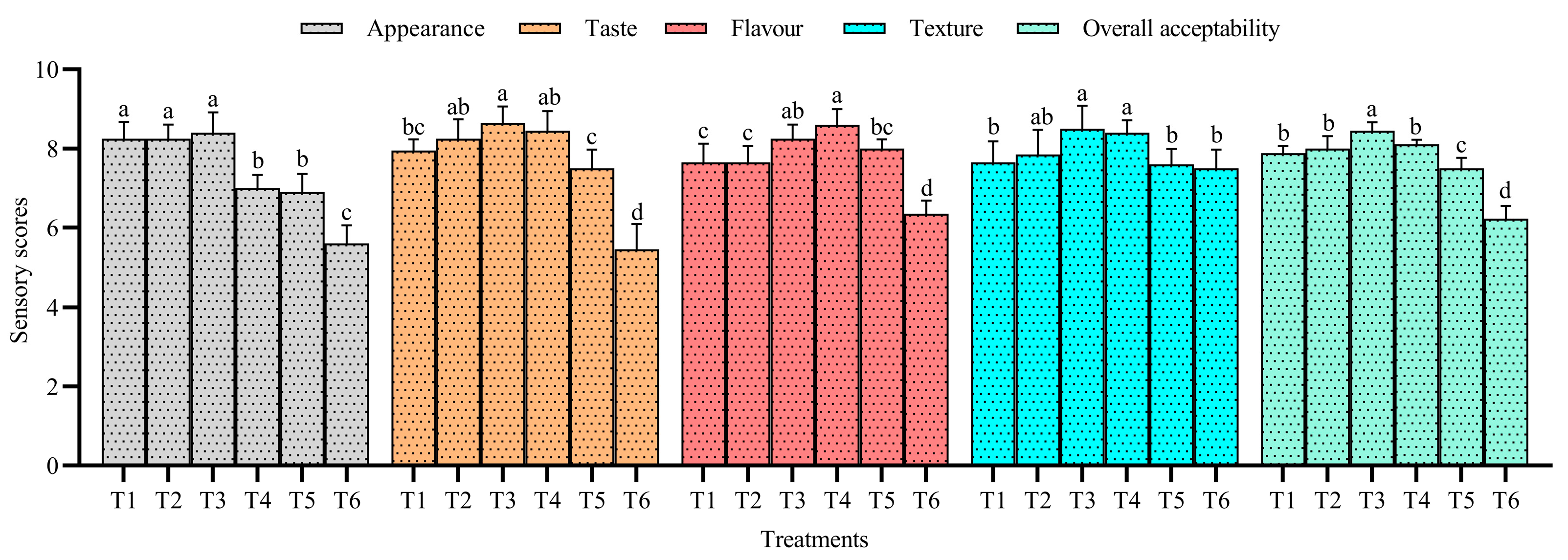
3.3. Sensory Analysis
3.4. Fourier-Transform Infrared (FTIR) Spectroscopy
3.5. Nutritional Profile of the Product
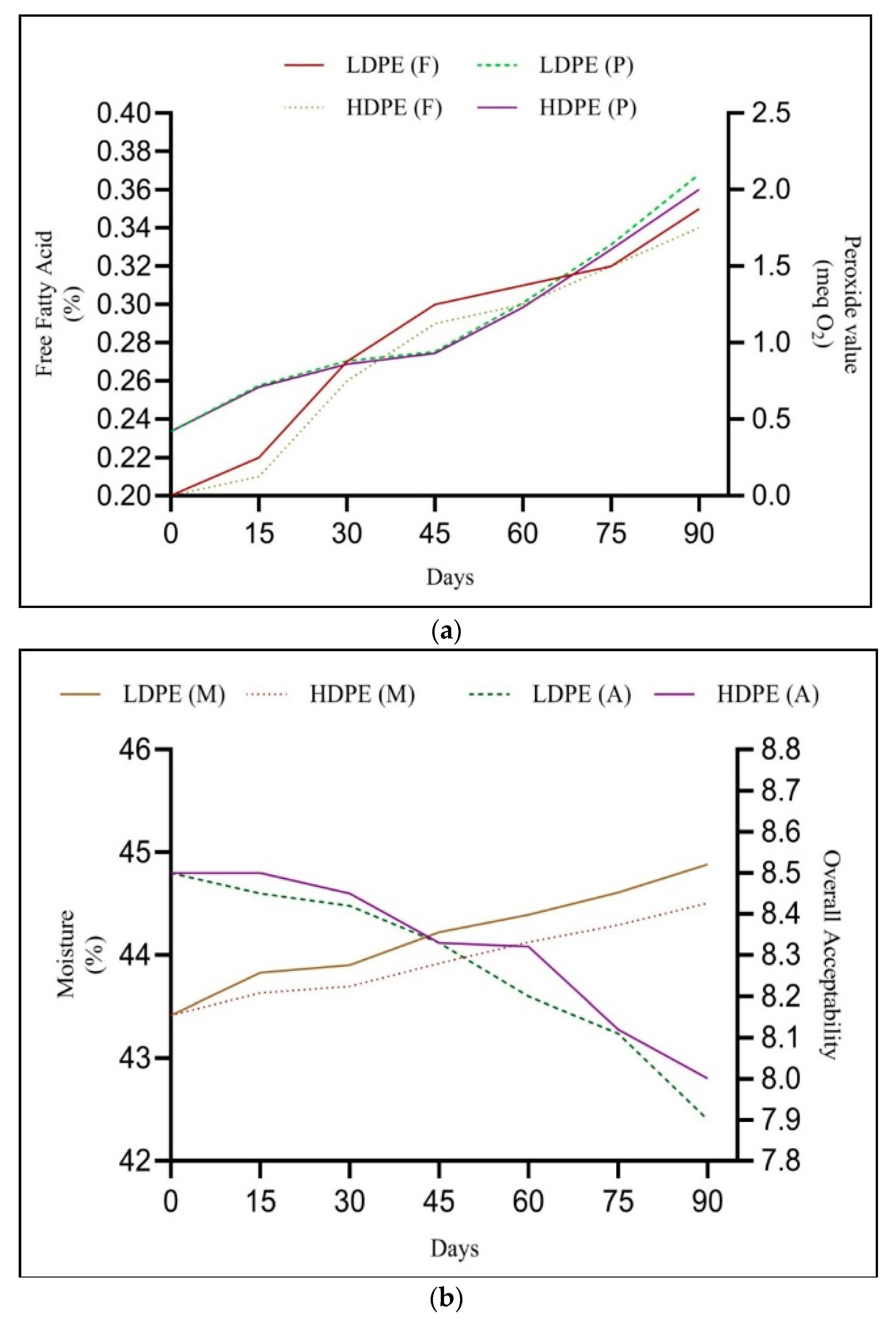
3.6. Storage Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Statista.com. India—Production Volume of Tomatoes 2015–2020. 2020. Available online: https://www.statista.com/statistics/1039712/india-production-volume-of-tomatoes/#:~:text=For%20fiscal%20year%202020%2C%20the,of%20tomato%20across%20the%20country (accessed on 1 March 2022).
- Aghajanzadeh, G.A.; Maheri, S.N.; Mirzaei, A.A.; Baradaran, H.A. Comparison of nutritional value of tomato pomace and brewer’s grain for ruminants using in vitro gas production technique. Asian J. Anim. Vet. Adv. 2010, 5, 43–51. [Google Scholar] [CrossRef][Green Version]
- Kalogeropoulos, N.; Chiou, A.; Pyriochou, V.; Peristeraki, A.; Karathanos, V.T. Bioactive phytochemicals in industrial tomatoes and their processing byproducts. LWT—Food Sci. Technol. 2012, 49, 213–216. [Google Scholar] [CrossRef]
- Ruiz Celma, A.; Cuadros, F.; López-Rodríguez, F. Characterization of pellets from industrial tomato residues. Food Bioprod. Process. 2012, 90, 700–706. [Google Scholar] [CrossRef]
- Poli, A.; Anzelmo, G.; Fiorentino, G.; Nicolaus, B.; Tommonaro, G.; Di, P. Polysaccharides from Wastes of Vegetable Industrial Processing: New Opportunities for Their Eco-Friendly Re-Use. In Biotechnology of Biopolymers; IntechOpen: London, UK, 2011; pp. 33–56. [Google Scholar] [CrossRef]
- Armstrong, M.J.; Hegade, V.S.; Robins, G. Advances in coeliac disease. Curr. Opin. Gastroenterol. 2012, 28, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Gélinas, P.; McKinnon, C. Gluten weight in ancient and modern wheat and the reactivity of epitopes towards R5 and G12 monoclonal antibodies. Int. J. Food Sci. Technol. 2016, 51, 1801–1810. [Google Scholar] [CrossRef]
- FDA. 2021. Available online: https://www.fda.gov/consumers/consumer-updates/gluten-free-means-what-it-says (accessed on 1 March 2022).
- Aziz, I.; Lewis, N.R.; Hadjivassiliou, M.; Winfield, S.N.; Rugg, N.; Kelsall, A.; Newrick, L.; Sanders, D.S. A UK study assessing the population prevalence of self-reported gluten sensitivity and referral characteristics to secondary care. Eur. J. Gastroent. Hepato. 2014, 26, 33–39. [Google Scholar] [CrossRef]
- Tanpowpong, P.; Ingham, T.; Lampshire, P.; Kirchberg, F.F.; Epton, M.J.; Crane, J.; Camargo, C.A.; Dench, C.; Duignan, M.; Fishwick, D.; et al. Coeliac disease and gluten avoidance in New Zealand children. Arch. Dis. Child. 2012, 97, 12–16. [Google Scholar] [CrossRef]
- Sehgal, H.S.; Sehgal, G.K.; Thind, S.S.; Kaur, A.; Rehal, J. Development of “fish mince pakora” from a cultured carp species, labeo rohita (HAM). J. Food Process. Preserv. 2010, 34 (Suppl. 1), 15–23. [Google Scholar] [CrossRef]
- Sen, S.; Antara, N.; Sen, S. Factors influencing consumers’ to Take Ready-made Frozen Food. Curr. Psychol. 2019, 40, 2634–2643. [Google Scholar] [CrossRef]
- Singh, J.; Whelan, K. Limited availability and higher cost of gluten-free foods. J. Hum. Nutr. Diet. 2011, 24, 479–486. [Google Scholar] [CrossRef]
- Antony, C.S.; Maharajan, T.; Ajeesh, K.T.P.; Ramakrishnan, M.; Victor, R.G.; Satish, L.; Ignacimuthu, S. Finger Millet [Eleusine coracana (L.) Gaertn.] Improvement: Current Status and Future Interventions of Whole Genome Sequence. Front. Plant Sci. 2018, 9, 1054. [Google Scholar] [CrossRef] [PubMed]
- Shobana, S.; Malleshi, N.G. Preparation and functional properties of decorticated finger millet (Eleusine coracana). J. Food Eng. 2007, 79, 529–538. [Google Scholar] [CrossRef]
- Devi, P.B.; Vijayabharathi, R.; Sathyabama, S.; Malleshi, N.G.; Priyadarisini, V.B. Health benefits of finger millet (Eleusine coracana L.) polyphenols and dietary fiber: A review. J. Food Sci. Technol. 2014, 51, 1021–1040. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Metwal, M.; Kaur, S.; Gupta, A.K.; Puranik, S.; Singh, S.; Singh, M.; Gupta, S.; Babu, B.K.; Sood, S.; et al. Nutraceutical value of finger millet [Eleusine coracana (L.) Gaertn.], and their improvement using omics approaches. Front. Plant Sci. 2016, 7, 934. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Singh, N.; Sharma, T.R.; Saxena, S.K. Physicochemical, rheological and cookie making properties of corn and potato flours. Food Chem. 2003, 83, 387–393. [Google Scholar] [CrossRef]
- Rehal, J.; Aggarwal, P.; Dhaliwal, I. Process Development of Gluten-Free Snack Utilising Finger Millets. Int. J. Curr. Microbiol. Appl. Sci. 2021, 10, 1231–1238. [Google Scholar] [CrossRef]
- AOAC. Association of Official Analytical Chemist, Official Methods of Analysis, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Yadav, D.N.; Singh, K.K.; Rehal, J. Studies on fortification of wheat flour with defatted rice bran for chapati making. J. Food Sci. Technol. 2012, 49, 96–102. [Google Scholar] [CrossRef]
- Kaur, B.; Singh, B.; Kaur, N.; Singh, D. Phytoremediation of cadmium-contaminated soil through multipurpose tree species. Agrofor. Syst. 2018, 92, 473–483. [Google Scholar] [CrossRef]
- Musaalbakri, A.M.; Noraini, S.; Madzlan, K.; Hadijah, H. Proximate and amino acids composition of Monascus fermented products with potential as functional feed ingredients. Cogent Food Agric. 2017, 3, 1295767. [Google Scholar] [CrossRef]
- Hassanien, M.M.M.; Abdel-Razek, A.G.; Rudzińska, M.; Siger, A.; Ratusz, K.; Przybylski, R. Phytochemical contents and oxidative stability of oils from non-traditional sources. Eur. J. Lipid Sci. Technol. 2014, 116, 1563–1571. [Google Scholar] [CrossRef]
- Singh, K.K.; Mridula, D.; Barnwal, P.; Rehal, J. Selected Engineering and Biochemical Properties of 11 Flaxseed Varieties. Food Bioprocess. Technol. 2013, 6, 598–605. [Google Scholar] [CrossRef]
- Ranganna, S. Handbook of Analysis and Quality Control. for Fruit and Vegetable Products; Tata McGraw-Hill Publishing Company: New Delhi, India, 1986; pp. 124–125. [Google Scholar]
- Rodriguez-Amaya, D.B.; Kimura, M. HarvestPlus Handbook for Carotenoid Analysis; International Food Policy Research Institute (IFPRI): Washington, DC, USA, 2004. [Google Scholar]
- Tapia-Salazar, M.; Arévalo-Rivera, I.G.; Maldonado-Muñiz, M.; Garcia-Amezquita, L.E.; Nieto-López, M.G.; Ricque-Marie, D.; Cruz-Suárez, L.E.; Welti-Chanes, J. The Dietary Fiber Profile, Total Polyphenol Content, Functionality of Silvetia compressa and Ecklonia arborea, and Modifications Induced by High Hydrostatic Pressure Treatments. Food Bioprocess. Technol. 2019, 12, 512–523. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Herrera-Balandrano, D.; Báez-González, J.; Carvajal-Milán, E.; Mendez-Zamora, G.; Urías-Orona, V.; Amaya-guerra, C.A.; Niño-Medina, G. Feruloylated Arabinoxylans from Nixtamalized Maize Frankfurter Sausages. Molecules 2019, 24, 2056. [Google Scholar] [CrossRef] [PubMed]
- Silva, Y.P.A.; Borba, B.C.; Pereira, V.A.; Reis, M.G.; Caliari, M.; Brooks, M.S.; Ferreira, T.A.P.C. Characterization of tomato processing by-product for use as a potential functional food ingredient: Nutritional composition, antioxidant activity and bioactive compounds. Int. J. Food Sci. Nutr. 2018, 70, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Udeh, H.O.; Duodu, K.G.; Jideani, A. Malting Period Effect on the Phenolic Composition and Antioxidant Activity of Finger Millet (Eleusine coracana L. Gaertn) Flour. Molecules 2018, 23, 2091. [Google Scholar] [CrossRef]
- Jayawardana, S.A.S.; Samarasekera, J.K.R.R.; Hettiarachchi, G.H.C.M.; Gooneratne, J.; Choudhary, M.I.; Jabeen, A. Anti-inflammatory and Antioxidant Properties of Finger Millet (Eleusine coracana (L.) Gaertn.) Varieties Cultivated in Sri Lanka. BioMed Res. Int. 2021, 2021, 7744961. [Google Scholar] [CrossRef]
- Garmakhany, A.D.; Mirzaei, H.O.; Nejad, M.K.; Maghsudlo, Y. Study of oil uptake and some quality attributes of potato chips affected by hydrocolloids. Eur. J. Lipid Sci. Technol. 2008, 110, 1045–1049. [Google Scholar] [CrossRef]
- AACC International. Approved Method of the AACC, 10th ed.; America Association of Cereal Chemists: St. Paul, MN, USA, 2000. [Google Scholar]
- Sahni, P.; Sharma, S.; Surasani, V.K.R. Influence of processing and pH on amino acid profile, morphology, electrophoretic pattern, bioactive potential and functional characteristics of alfalfa protein isolates. Food Chem. 2020, 333, 127503. [Google Scholar] [CrossRef]
- Fuentes, E.; Carle, R.; Astudillo, L.; Guzmán, L.; Gutiérrez, M.; Carrasco, G.; Palomo, I. Antioxidant and antiplatelet activities in extracts from green and fully ripe tomato fruits (Solanum lycopersicum) and pomace from industrial tomato processing. Evid.-Based Complement. Altern. Med. 2013, 2013, 867578. [Google Scholar] [CrossRef]
- Hotz, C. Dietary indicators for assessing the adequacy of population zinc intakes. Food Nutr. Bull. 2007, 28 (Suppl. 3), S430–S453. [Google Scholar] [CrossRef] [PubMed]
- Oyedeji, A.B.; Sobukola, O.P.; Henshaw, F.; Adegunwa, M.O.; Ijabadeniyi, O.A.; Sanni, L.O.; Tomlins, K.I. Effect of frying treatments on texture and colour parameters of deep fat fried yellow fleshed cassava chips. J. Food Qual. 2017, 2017, 8373801. [Google Scholar] [CrossRef]
- Primo-Martín, C.; van Deventer, H. Deep-fat fried battered snacks prepared using super heated steam (SHS): Crispness and low oil content. Food Res. Int. 2011, 44, 442–448. [Google Scholar] [CrossRef]
- Raleng, A.; Singh, A.; Chavan, P.; Attkan, A.; Singh, B. Standardization of deep-frying process and their effects on storage stability of pineapple pomace powder-incorporated rice-based extruded product. J. Food Process. Preserv. 2019, 43, e13950. [Google Scholar] [CrossRef]
- Ziaiifar, A. Oil absorption during deep-fat frying: Mechanisms and important factors. AgroParis Technol. 2008, 43, 1410–1423. [Google Scholar]
- Andrikopoulos, N.K.; Boskou, G.; Dedoussis, G.V.Z.; Chiou, A.; Tzamtzis, V.A.; Papathanasiou, A. Quality assessment of frying oils and fats from 63 restaurants in Athens, Greece. Food Serv. Technol. 2003, 3, 49–59. [Google Scholar] [CrossRef]
- Mellema, M. Mechanism and reduction of fat uptake in deep-fat fried foods. Trends Food Sci. Technol. 2003, 14, 364–373. [Google Scholar] [CrossRef]
- Califano, A.N.; Calvelo, A. Adjustment of Surface Concentration of Reducing Sugars Before Frying of Potato Strips. J. Food Process. Preserv. 1988, 12, 1–9. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, Y.; Bae, I.Y.; Lee, H.G.; Hou, G.G.; Lee, S. Utilization of preharvest-dropped apple powder as an oil barrier for instant fried noodles. LWT—Food Sci. Technol. 2013, 53, 88–93. [Google Scholar] [CrossRef]
- Moyano, P.C.; Pedreschi, F. Kinetics of oil uptake during frying of potato slices: Effect of pre- treatments. LWT—Food Sci. Technol. 2006, 39, 285–291. [Google Scholar] [CrossRef]
- Rojas-Gonzalez, J.; Avallone, S.; Brat, P.; Trystram, G.; Bohuon, P. Effect of deep-fat frying on ascorbic acid, carotenoids and potassium contents of plantain cylinders. Int. J. Food Sci. Nutr. 2006, 57, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Voong, K.Y.; Norton-Welch, A.; Mills, T.B.; Norton, I.T. Understanding and predicting sensory crispness of deep-fried battered and breaded coatings. J. Texture Stud. 2019, 50, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Vazques-Vuelvas, O.F.; Chavez-Camacho, F.A.; Meza-Velazquez, J.A.; Mendez-Merino, E.; Rios-Licea, M.M.; Contreras-Esquivel, J.C. A comparative FTIR study for supplemented agavin as functional food. Food Hydrocoll. 2020, 103, 105642. [Google Scholar] [CrossRef]
- Kamble, D.B.; Singh, R.; Rani, S.; Kaur, B.P.; Upadhyay, A.; Kumar, N. Optimization and characterization of antioxidant potential, in vitro protein digestion and structural attributes of microwave processed multigrain pasta. J. Food Process. Preserv. 2019, 43, e14125. [Google Scholar] [CrossRef]
- Jebitta, S.R.; Venkatram, S.; Aneesh, S.M.; Pasupathi, R. Functional Group Analysis of Germinated Millets and Legumes. Asian J. Dairy Food Res. 2019, 38, 134–139. [Google Scholar] [CrossRef]
- Jogihalli, P.; Singh, L.; Kumar, K.; Sharanagat, V.S. Physico-functional and antioxidant properties of sand-roasted chickpea (Cicer arietinum). Food Chem. 2017, 237, 1124–1132. [Google Scholar] [CrossRef]
- Pietrzak, L.N.; Miller, S.S. Microchemical structure of soybean seeds revealed in situ by ultraspatially resolved synchrotron Fourier transformed infrared microspectroscopy. J. Agric. Food Chem. 2005, 53, 9304–9311. [Google Scholar] [CrossRef]
- Swer, T.L.; Mukhim, C.; Bashir, K.; Chauhan, K. Optimization of enzyme aided extraction of anthocyanins from Prunus nepalensis L. LWT—Food Sci. Technol. 2018, 91, 382–390. [Google Scholar] [CrossRef]
- Gowthamraj, G.; Raasmika, M.; Sangeetha, N. Efficacy of fermentation parameters on protein quality and microstructural properties of processed finger millet flour. J. Food Sci. Technol. 2020, 58, 3223–3234. [Google Scholar] [CrossRef]
- Gull, A.; Prasad, K.; Kumar, P. Evaluation of functional, antinutritional, pasting and microstructural properties of Millet flours. J. Food Meas. Character 2016, 10, 96–102. [Google Scholar] [CrossRef]
- Bunghez, I.R.; Raduly, M.; Doncea, S.; Aksahin, I.; Ion, R.M. Lycopene determination in tomatoes by different spectral techniques (UV-VIS, FTIR and HPLC). Digest J. Nano Biostruc. 2011, 6, 1349–1356. [Google Scholar]
- Nagaprabha, P.; Devisetti, R.; Bhattacharya, S. Physicochemical and microstructural characterisation of green gram and foxtail millet starch gels. J. Food Sci. Technol. 2018, 55, 782–791. [Google Scholar] [CrossRef] [PubMed]
- Weisburger, J.H. Lycopene and tomato products in health promotion. Exp. Biol. Med. 2002, 227, 924–927. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Le Maguer, M. Lycopene in tomatoes: Chemical and physical properties affected by food processing. Cri. Rev. Food Sci. Nutr. 2000, 40, 1–42. [Google Scholar] [CrossRef] [PubMed]
- Isik, F.; Topkaya, C. Effects of tomato pomace supplementation on chemical and nutritional properties of crackers. Ital. J. Food Sci. 2016, 28, 525–535. [Google Scholar]
- Dewanto, V.; Xianzhong, W.; Adom, K.K.; Liu, R.H. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J. Agric. Food Chem. 2002, 50, 3010–3014. [Google Scholar] [CrossRef]
- Pettifor, J.M. Nutritional rickets: Deficiency of vitamin D, calcium, or both? Am. J. Clin. Nutr. 2004, 80 (Suppl. 6), 1725–1729. [Google Scholar] [CrossRef]
- He, F.J.; MacGregor, G.A. Beneficial effects of potassium on human health. Physiol. Plant. 2008, 133, 725–735. [Google Scholar] [CrossRef]
- Simopoulos, A.P. An increase in the Omega-6/Omega-3 fatty acid ratio increases the risk for obesity. Nutrients 2016, 8, 128. [Google Scholar] [CrossRef]
- Enser, M. The Role of Fats in Human Nutrition. In Oils and Fats Volume 2. Animal Carcass Fats; Rossell, B., Ed.; Leatherhead Food RA Publishing: Surrey, UK, 2001; pp. 77–122. ISBN 0905748743. [Google Scholar]
- WHO. Protein and Amino Acid Requirements in Human Nutrition: Report of a Joint FAO/WHO/UNU Expert Consultation, World Health Organisation, Technical Report Series, no. 935. 2007. Available online: http://apps.who.int/iris/bitstream/10665/43411/1/WHO_TRS_935_eng.pdf?ua=1 (accessed on 1 March 2022).
- Khidhir, Z.K.; Murad, H.O.M.; Arif, E.D. Qualitative assessment of imported frozen fish fillets in Sulaimani markets. Iraqi J. Vet. Sci. 2013, 27, 49–55. [Google Scholar] [CrossRef]
- Utrilla-Coello, R.G.; Bello-Pérez, L.A.; Vernon-Carter, E.J.; Rodriguez, E.; Alvarez-Ramirez, J. Microstructure of retrograded starch: Quantification from lacunarity analysis of SEM micrographs. J. Food Eng. 2013, 116, 775–781. [Google Scholar] [CrossRef]


| Treatments (% of TPP) | TPP(g) | FMP + PF (1:1) (g) | Spice Mixture(g) |
|---|---|---|---|
| T1 (0%) | 0 | 950 | 50 |
| T2 (5%) | 47.5 | 902.5 | 50 |
| T3 (10%) | 95.0 | 855.0 | 50 |
| T4 (15%) | 142.5 | 807.5 | 50 |
| T5 (20%) | 190.0 | 760.0 | 50 |
| T6 (25%) | 237.5 | 712.5 | 50 |
| Parameter | Finger Millet Flour | Tomato Pomace Powder | Potato Flour |
|---|---|---|---|
| Moisture content (%) | 8.56 ± 1.02 a | 5.96 ± 1.12 b | 6.08 ± 0.91 ab |
| Protein (%) | 8.10 ± 0.90 b | 14.95 ± 0.80 a | 2.84 ± 1.01 c |
| Fat (%) | 1.86 ± 0.18 b | 8.52 ± 0.32 a | 0.11 ± 0.11 c |
| Ash (%) | 2.47 ± 0.21 b | 4.27 ± 0.22 a | 1.89 ± 0.15 c |
| Total carbohydrates (%) | 79.01 ± 0.20 b | 66.3 ± 0.21 c | 89.09 ± 0.36 a |
| Crude fiber (%) | 3.61 ± 0.87 b | 39.45 ± 0.86 a | 1.80 ± 0.73 c |
| TPC (mg GAE/100 g) | 320 ± 1.10 a | 179.67 ± 0.92 b | 42.47 ± 0.87 c |
| TFC (mg QE/100 g) | 56.11 ± 0.51 b | 68.77 ± 0.57 a | 6.20 ± 0.09 c |
| DPPH radical scavenging activity (%) | 70.08 ± 0.3 a | 52.41 ± 0.40 b | 18.70 ± 0.32 c |
| FRAP (µmol FSE/100 g | 2231.19 ± 15.6 a | 1129.03 ± 7.9 b | 584.15 ± 4.2 c |
| ABTS (µmol TE/100 g | 913.01 ± 7.2 a | 609.26 ± 4.0 b | 33.19 ± 0.26 c |
| MCA (%) | 58.25 ± 0.47 a | 41.98 ± 0.36 b | 18.73 ± 0.21 c |
| Calcium (mg/100 g) | 274.3 ± 0.02 a | 76.4 ± 0.01 b | 67.2 ± 0.02 c |
| Phosphorus (mg/100 g) | 295.7 ± 0.03 a | 219.7 ± 0.02 b | 59.30 ± 0.02 c |
| Magnesium (mg/100 g) | 119.1 ± 0.01 b | 126.7 ± 0.04 a | 19.43 ± 0.01 c |
| Sodium (mg/100 g) | 35.9 ± 0.01 c | 129.1 ± 0.03 a | 40.12 ± 0.03 b |
| Potassium (mg/100 g) | 257.6 ± 0.02 c | 1011.5 ± 0.04 a | 532.6 ± 0.03 b |
| Iron (mg/100 g) | 3.61 ± 0.03 b | 9.26 ± 0.02 a | 2.79 ± 0.01 c |
| Zinc (mg/100 g) | 2.55 ± 0.04 b | 3.46 ± 0.03 a | 1.36 ± 0.02 c |
| Parameter | T1 | T3 | Parameter | T1 | T3 | |
|---|---|---|---|---|---|---|
| Moisture content (%) | 40.5 ± 0.60 b | 43.4 ± 2.6 a | Fatty acids (%) | |||
| Protein (%) | 5.01 ± 0.06 b | 6.9 ± 0.16 a | Oleic acid | 55.2 ± 0.82 a | 52.1 ± 0.69 b | |
| Fat (%) | 17.45 ± 0.26 a | 11.48 ± 0.70 b | Linoleic acid | 25.6 ± 0.39 a | 24.2 ± 0.29 b | |
| Ash (%) | 2.07 ± 0.27 b | 3.7 ± 0.22 a | Linolenic acid | 2.3 ± 0.02 b | 3.4 ± 0.38 a | |
| T. carbohydrates (%) | 32.80 ± 0.51 a | 34.52 ± 1.88 a | Palmitic acid | 25.9 ± 0.37 a | 22.8 ± 0.04 b | |
| Crude fiber (%) | 2.17 ± 0.25 b | 5.2 ± 0.11 a | Stearic acid | 6.2 ± 0.12 a | 4.1 ± 0.52 b | |
| TPC (mg GAE/100 g) | 151.2 ± 2.01 b | 186 ± 9.1 a | ω6/ω3 | 12.43: 1 | 7.1:1 | |
| TFC (mg QE/100 g) | 31.06 ± 0.27 b | 36.79 ± 0.30 a | ||||
| Lycopene (mg/100 g) | - | 5.08 ± 0.09 | ||||
| DPPH (%) | 33.7 ± 0.64 b | 46.7 ± 2.1 a | ||||
| FRAP (µmol FSE/100 g | 1427.63 ± 9.9 b | 1442.78 ± 10.11 a | ||||
| ABTS (µmol TE/100 g | 537.11 ± 4.1 b | 584.39 ± 4.4 a | ||||
| MCA (%) | 40.70 ± 0.30 b | 41.48 ± 0.34 a | Essential AAS (%) | |||
| Minerals | Valine | 5.0 ± 0.10 a | 5.2 ± 0.11 a | |||
| Calcium (mg/100 g) | 152.6 ± 2.59 b | 160 ± 1.01 a | Leucine | 3.6 ± 0.08 a | 3.5 ± 0.06 a | |
| Phosphorus (mg/100 g) | 206 ± 3.29 a | 187 ± 1.60 b | Isoleucine | 3.0 ± 0.06 b | 4.53 ± 0.07 a | |
| Magnesium (mg/100 g) | 71.3 ± 0.78 b | 89 ± 0.91 a | Threonine | 3.4 ± 0.07 b | 4.3 ± 0.06 a | |
| Sodium (mg/100 g) | 33.2 ± 0.53 b | 40 ± 0.59 a | Methionine | 2.9 ± 0.05 b | 3.1 ± 0.04 a | |
| Potassium (mg/100 g) | 356.2 ± 5.69 b | 407 ± 6.02 a | Lysine | 3.1 ± 0.08 b | 4.2 ± 0.06 a | |
| Iron (mg/100 g) | 2.79 ± 0.03 b | 3.3 ± 0.06 a | Phenyl alanine | 4.0 ± 0.12 b | 5.1 ± 0.08 a | |
| Zinc (mg/100 g) | 1.82 ± 0.02 b | 2.1 ± 0.03 a | Histidine | 2.05 ± 0.03 b | 2.2 ± 0.03 a | |
| Energy (kcal/100 g) | 308.29 ± 4.2 a | 248.2 ± 5.3 b | Tryptophan | 2.4 ± 0.04 b | 5.6 ± 0.10 a | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rehal, J.K.; Aggarwal, P.; Dhaliwal, I.; Sharma, M.; Kaushik, P. A Tomato Pomace Enriched Gluten-Free Ready-to-Cook Snack’s Nutritional Profile, Quality, and Shelf Life Evaluation. Horticulturae 2022, 8, 403. https://doi.org/10.3390/horticulturae8050403
Rehal JK, Aggarwal P, Dhaliwal I, Sharma M, Kaushik P. A Tomato Pomace Enriched Gluten-Free Ready-to-Cook Snack’s Nutritional Profile, Quality, and Shelf Life Evaluation. Horticulturae. 2022; 8(5):403. https://doi.org/10.3390/horticulturae8050403
Chicago/Turabian StyleRehal, Jagbir Kaur, Poonam Aggarwal, Inderpreet Dhaliwal, Meenakshi Sharma, and Prashant Kaushik. 2022. "A Tomato Pomace Enriched Gluten-Free Ready-to-Cook Snack’s Nutritional Profile, Quality, and Shelf Life Evaluation" Horticulturae 8, no. 5: 403. https://doi.org/10.3390/horticulturae8050403
APA StyleRehal, J. K., Aggarwal, P., Dhaliwal, I., Sharma, M., & Kaushik, P. (2022). A Tomato Pomace Enriched Gluten-Free Ready-to-Cook Snack’s Nutritional Profile, Quality, and Shelf Life Evaluation. Horticulturae, 8(5), 403. https://doi.org/10.3390/horticulturae8050403







