Abstract
In olive (Olea europaea L.), the floral quality is a key feature affecting the final fruit crop. The aim of this study was to evaluate the inflorescence traits and the floral quality parameters of three clones of Leccino cultivar (L 1.3, L 1.4, L 1.9). To assess a possible effect of light limitations on these parameters two canopy zones, internal (IZ) and external (EZ), were considered. The inflorescences were collected over two consecutive years in order to establish: (i) the characteristics of inflorescences (length, flowers per inflorescence) and flowers (open and perfect flowers); (ii) the ovary structure by histological analysis; and (iii) the viability and germination of pollen grains by in vitro culture. The preliminary results highlighted some differences among clones. The L 1.9 was the less affected by the canopy position for inflorescence morphological traits, and the presence of ovaries with at least three fully developed ovules denoting a high female fertility. Regardless of the canopy position, L 1.4 showed the highest pollen viability, suggesting its possible use as pollinator. The lower sensitivity of female and male floral organs to partial shading of L 1.9 and L 1.4 needs further investigations aimed at evaluating their suitability in high-density olive orchards.
1. Introduction
In fruit crops, productivity is determined by the total flower number per tree and by their quality, referred to as the structural and developmental characteristics of the flower which influence its capacity for fertilization and fruit set [1]. In olive (Olea europaea L.), the period from floral induction to full blooming covers approximately 9–10 months [2,3]. In particular, the inflorescence number is determined in the previous season during the shoot growth period, whereas flower quality is mainly determined in the current season [4]. Olive produces a large number of flowers of which only a small portion eventually set fruits [5]. Thus, the flower quality is a determinant requisite to achieve economic yields. The olive inflorescences, termed panicles, differ by shape, length and number of flowers in a wide range from 10 to 40, depending on the cultivar [6]. Flowers are typically hermaphrodite (perfect flowers) but, because of pistil or anthers abortion, they can be ‘imperfect’, namely staminate or pistilate, respectively. The staminate type is the most frequent to find, especially in certain cultivars, being the phenomenon mainly under genetic control [7,8]. However, the proportion of staminate flowers depends on many other factors, such as the fruit yield in the previous year, the position of the branch and twig, the position of the inflorescences on the twig, the air temperature and the rainfall at the meiosis stage [8,9]. Thus, the number of hermaphrodite flowers is fundamental but not sufficient to determine the potential tree yield [10]. To reach this goal in a morphologically perfect flower, both the female and the male gametophyte have to be functional for fertilization. This is considered the main condition defining the ‘flower quality’ [11]. With regard to the olive female organ, the pistil has two carpels each containing two ovules of which at least one should be fertilized developing into one seed [12]. However, the appearance of abnormal ovules, namely those with a failure of embryo sac development, can hinder a regular fertilization process [13]. Concerning the male gametophyte or pollen, the viability is considered the main quality indicator because being olive an anemophilous species, the quantity of pollen grains produced per flower does not represent a limiting factor [14]. Successful fertilization requires viable pollen capable of germinating, growing and fertilizing one of the ovules [15]. It has been established that plant reproductive development is more sensitive than vegetative growth to environmental stresses [16]. The quality of both the ovule and the pollen are influenced by genetic, abiotic (i.e., heat, cold or drought) and biotic factors [17]. The majority of the studies have focused on the influence at canopy level of key parameters such as temperature and light interception on fruit quality, whereas their potential effects on flower quality have been less investigated. In particular, studies about the influence of the intercepted photosynthetically active radiation (PAR) within the canopy on fruit and oil quality have been carried out on a wide range of olive cultivars [18,19,20,21]. The evolution of planting systems has led to the adoption of training systems associated with high tree density [22,23]. The cultural intensification, requiring a modification of canopy size and shape [24,25,26], may alter the microenvironment with possible effects on the processes involved in the regulation of the floral organs’ development. In this context, studies on the impact of solar radiation interception on floral organogenesis are still scarce and have been focused on few cultivars, mainly of the Spanish germplasm [22,27].
The main objective of this study was to evaluate differences in inflorescence traits and floral quality parameters of three olive clones, which are characterized by desirable features such as constant yields and a tendency to be self-compatible (pers. comm.). Clones were selected within the Leccino cultivar, one of the most cultivated varieties which has been receiving attention because it is tolerant to the quarantine bacterium Xylella fastidiosa subsp. pauca [28], a phytopathogen associated with the “olive quick decline syndrome” (OQDS). The effect of light limitations on inflorescence traits and floral quality parameters was also evaluated in order to highlight the different suitability of these clones to high- and super-high density olive orchards.
2. Materials and Methods
2.1. Study Site, Growing Conditions and Plant Material
The study was carried out over two consecutive growing seasons (2016–2018), from October to May, at the experimental farm of the Department of Agriculture, Food and Environment (University of Pisa) located in Pisa province (Tuscany, Italy, 43°43′32.02″ N, 10°27′37.66″ E; altitude 3 m a.s.l.). Three clones (L 1.3, L 1.4, L 1.9) of the cultivar Leccino (Olea europaea L.) were evaluated. Own-rooted trees were planted in 2014 at 5 × 5 m spacing and trained with a single trunk with four scaffold branches. Trees were minimally pruned to eliminate suckers and vigorous watersprouts in the central part of the canopy to facilitate light penetration [29]. Standard agronomic practices (irrigation, fertilization, pests and diseases treatments) were applied. The experiment was carried out according to a completely randomized design with eight single-tree per each clone. The same trees were assessed in both years. For each tree, two canopy zones between 0.8 m and 1.8 m above the ground were selected: (i) the external zone (EZ), representing the external surface of the canopy where the inflorescences-bearing branches were directly exposed to the solar radiation; and (ii) the internal zone (IZ), between the EZ and the internal empty zone resulting from pruning. The photosynthetically active radiation (PAR) was measured monthly on sunny days from October to May. In both canopy zone, four measurements (one for each cardinal point) were taken between 11:00 and 12:00 a.m., at 1.5 m from the ground using a digital lux meter with a silicon sensor (T.R. Turoni Srl, Forlì, Italy). The mean PAR values (from October to May) for each zone indicated a reduction in light interception of about 30% in the inner zone (572.1 ± 19.0 µEm−2 s−1) with respect to the more illuminated external one (817.3 ± 27.2 µEm−2 s−1). Minimum and maximum daily temperatures (°C) and rainfall (mm) were retrieved from a weather station located near the experimental olive orchards.
2.2. Phenological Observations
The evolution of the phenological growth stages of the three Leccino clones were recorded for each tree according to the standardized BBCH-scale for olive tree [30]. It is widely accepted for describing the olive development stages and it is based on different codes divided into nine principal and secondary growth stages. In particular, the observations were focused from the inflorescences emergence (BBCH stage 5, code 50–59) to flowering (BBCH stage 6, code 60–69). At our study site the aforementioned stages lasted from late March to the first decade of June. The date of full bloom was determined by visual assessment when more than 50% of the flowers were open on at least 70% of the inflorescences of the selected shoots [13].
2.3. Inflorescences and Flower Observations
Inflorescences were collected from both IZ and EZ of the canopy. Since within the inflorescences the opening of the flowers occurs gradually and lasts approximately five to six days, samplings were done approximately halfway through this period. The inflorescences (n = 100 per position per clone) containing either open or closed flowers were sampled from the mid portion of one-year-old twigs. The proportion of closed (BBCH code 59, denoted by corolla changes from green to white color) and open (BBCH codes 60–65) flowers was expressed as a percentage. On each inflorescence the rachis length and the number of flowers were determined. The gender was assessed on each flower by verifying the presence of female and male organs. The entity of perfect (hermaphrodite) and imperfect (staminate) flowers, due to the absence of pistil or the presence of a tiny aborted one, was expressed as a percentage.
2.4. Ovary Histological Observations
Considering that only open flowers were considered mature and ready to be fertilized [31], pistils (n = 40 per position per clone) of perfect flowers were collected at the initial opening of petals from both EZ and IZ inflorescences. Samples were fixed in a FAA solution (10% formaldehyde, 5% glacial acetic acid, 45% ethyl alcohol; 1:1:8 v/v) until dehydration in graded ethanol series and embedding in Histoplast according to standard paraffin procedures [32]. Transverse ovary sections (10 μm) were stained with 0.05% Toluidine Blue [33] and analyzed under an optical microscope (Fluophot, Nikon Inc., Minato City, Japan) with polarized white light, equipped with a digital camera (Olympus C-2000z, Tokyo, Japan). Ovaries were scored according to the number of fully developed ovules with embryo sacs out of the four ovules [34]. The percentage of ovaries according to the rate 0/4 (zero), 1/4 (one), 2/4 (two), 3/4 (three) and 4/4 (four) fully developed ovules was then calculated.
2.5. Viability and Germination of Pollen Grains
Viability and germination rates of pollen grains were evaluated by in vitro tests using fresh samples collected at the dehiscence of anthers from flowers of EZ and IZ inflorescences (n = 20 per position per clone). Each pollen sample was obtained from at least 200 flowers.
Viable pollen grains were ascertained with a colorimetric reaction induced by the TTC (2,3,5 triphenyl tetrazolium chloride), a redox indicator of cellular respiration [35]. Pollen was sown on slides with drops of 1% TTC sucrose solution (15%) and incubated under dark conditions (37 °C overnight). The viability was established under an optical microscope (Fluophot, Nikon Inc., Minato City, Japan) by observing the pollen grains turning red in color on six selected microscopic fields. The stainability was calculated on a total number of at least 200 pollen grains. The pollen germination power was assessed on solid medium consisting of 0.65% (w/v) agar and 15% (w/v) sucrose, and then incubated in the dark at 24 °C [36]. For this purpose, plastic Petri dishes (5 cm diameter) were used. After 48 h of culture the dishes were observed under an inverted microscope (Wilovert S, Helmut Hund GmbH, Wetzlar, Germany). The germination rate was determined by counting at least 200 pollen grains per preparation. When pollen tube length was equal to or greater than the grain diameter, pollen was considered as germinated [37]. The germination ability was expressed as a percentage by the ratio between the number of germinated pollen grains and the total number of grains.
2.6. Data Analysis
Data were processed for a completely randomized design and the statistical analysis was performed using the package GraphPad Prism (version 5.00 for Windows, GraphPad Software, La Jolla, San Diego, CA, USA). Percentage data of open and perfect flowers, ovary structure, viable and germinated pollen grains were subjected to arcsine root square transformation, before tests. The standard errors (SE) of the means were calculated for each parameter measured. Values were compared by analysis of variance (ANOVA) and differences between means were assessed after the Tukey’s multiple comparison test. A two-factor ANOVA was performed to reveal if factors, year, clone and canopy zone, and their interaction had a significant influence on morphological and quality traits of inflorescences, flowers, and male and female floral organs. In all cases, the confidence level at p ≤ 0.05 was considered statistically significant. Differences in air temperature between years (from January to May) were assessed using the Kruskal-Wallys test after the analysis of the normal distribution.
3. Results
3.1. Environmental Conditions and Phenological Evolution
The daily mean temperature and rainfall recorded during the study period (2016–2018) are shown in Figure 1.
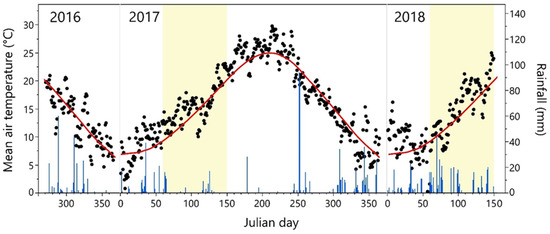
Figure 1.
Daily mean air temperatures (black dots) and rainfall (blue histograms) from October 2016 to May 2018. Yellow ranges indicate the periods of the experimental trials. The red line indicates the 20-year average (1996–2015) of the mean air temperature recorded at the experimental site.
Significant differences in air temperature between years were observed during the January–March period (Table S1), whereas the fall thermal regimes were similar. In 2017, the mean air temperature of January was 5.5 °C, which was colder (−1.5° C) than the average of the 1996–2015 time series [38], whereas February (10.7 °C) and March (13.1 °C) showed a higher mean air temperature (+2.9 and +2.3 °C, respectively) (Figure 1, Table S1). An opposite trend was observed in 2018: January was warmer (+3.6 °C), while the air temperature in February (6.8 °C) and March (9.4 °C) was colder compared with the 1996–2015 period (−1.0 and −1.4 °C, respectively) (Figure 1, Table S1). The evolution of the phenological stages, from pre- to post-flowering phases (Figure 2), fell in a timeframe which was typical of the Leccino standard [39], and it was similar among clones. The opening of the inflorescence buds (BBCH code 52) started at Julian day (JD) 95 in 2017 whereas the same phase was recorded earlier (JD 89) in 2018. The following phases ( flower clusters totally expanded and full flowering, BBCH code 55 and 65, respectively) showed a similar trend (Figure 2). The blooming time occurred around May 27, while in 2018 it was recorded around May 18.
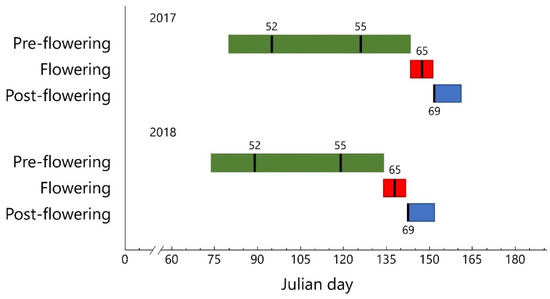
Figure 2.
Phenological stages recorded in spring 2017 and 2018 on olive trees (average of all clones) according to the BBCH scale [30]. Green, red and blue boxes indicate the pre-flowering, flowering and post-flowering period, respectively. BBCH codes: 52 (start of flower cluster development); 55 (flower cluster totally expanded); 65 (full flowering); 69 (end of flowering, fruit set).
In olive, changes in flowering time can be frequent, mainly related to weather conditions during the four weeks before flowering. A relationship between the timing of flowering and climatic factors, such as temperatures and rainfall, has been found in previous studies [8,40,41]. Concerning air temperatures, it has been observed that the thermal regime in April and May influenced the blooming time; in particular, warmer conditions had led to a flowering advance in olive [42]. A possible role of the precipitation amount on the blooming time has also been observed. Orlandi et al. [43] reported an anthesis delay in response to a reduction in precipitation, whereas abundant rainfall was associated with an earlier opening of the flowers as a response to a probable increased absorption of nutrients from the roots [44]. Accordingly, similar events occurred in our experimental trials in which the flowering advance observed in 2018 was associated with higher mean air temperatures in April (+1.6 °C) and a wetter pre-flowering period than 2017.
3.2. Inflorescence and Flower Characteristics
The main characteristics of inflorescences (length and flowers per inflorescence) and flowers (open and perfect flowers) samples collected in spring from IZ and EZ positions are shown in Table 1.

Table 1.
Characteristics of inflorescences (length and flowers per inflorescence) and flowers (open and perfect flowers) of samples collected from IZ and EZ of the canopy for three Leccino clones (L 1.3, L 1.4, L 1.9). For each spring season (2017–2018), values are means ± standard error. Different letters indicate differences at p ≤ 0.05 (Tukey test) among the Clone-Canopy Position combinations within each year. Letters are only presented when ANOVA indicate significant effect. Two-way ANOVA results; main effect: clone (C), canopy position (CP). F- and p-values are shown; ns: not significant.
In both years, significantly higher values of rachis length were measured in EZ inflorescences (112 and 118% in 2017 and 2018, respectively; average of all clones) than in IZ ones. In the EZ of clones L 1.3 and L 1.4, the inflorescence length ranged from 26.1–30.2 to 25.8–28.4 mm, whereas in the IZ values ranged from 20.0–25.1 and 23.7–24.2 mm, respectively (Table 1). These results are in accordance with those reported by Trentacoste et al. [22,45] and Moreno-Alias et al. [27], who observed significantly shorter inflorescences in the less illuminated canopy positions. Significant differences in rachis length among clones were assessed in 2018 when inflorescences sampled from L 1.9 were shorter than those from L 1.3 and L1.4. The mean number of flowers per inflorescence significantly varied among clones and with respect to the canopy position in 2017. In particular, the flower number of L 1.3 was higher in EZ (14.5) than IZ (12.0) inflorescences (Table 1). A similar trend was also observed in the following year, although a weak number decrease was recorded. Similarly, L 1.9 went from about 12 (2017) to 11 (2018) flowers per inflorescence. Instead, in L 1.4 similar values were found between positions, ranging from 12.1 to 12.9 (Table 1).
Considering that the proportion between mean rachis length and flower number indicates the type of olive inflorescence, L 1.9 was characterized by a more compact structure in comparison with the others. A certain influence of the canopy position on the flower quantity has been observed by several authors. In ornamental species, a higher light intensity positively affected the number of flowers in unshaded plants [46]. In olive, a positive, although not significant, relationship between flower number and solar radiation has been found in the cultivar Arbequina [22]. Acebedo et al. [47], in a traditional olive orchard (density of 238 trees/ha), have demonstrated that flower intensity and flower number per inflorescence increased in relation to more illuminated positions on the tree canopy. In Arbequina, the number of flowers per inflorescence was similar in trees grown under full irradiation and shaded at 50% but were significantly lower in trees shaded at 80% in three consecutive seasons [45]. The flower number per inflorescence has been related to mean daily irradiance, supporting the assumption that photosynthetic rate affects the number of flowers on each inflorescence [22]. Regarding the opening of the flowers, it was confirmed that is not uniform within inflorescences regardless of clone, similar to what was observed in other olive cultivars [6]. The first flowers to open were in terminal positions followed by those located on the laterals. The percentage of open flowers in the IZ was significantly reduced compared to the EZ. This result agrees with previous studies showing a significant influence of the position within the canopy on the blooming trend, with a flowering delay under low light intensity [6,9,46] which represent a potential drawback in assuring an adequate and uniform pollination throughout the canopy. In 2018, a further decrease was recorded for L 1.3 and L 1.4. A significant interaction ‘year × canopy position’ was found for L 1.3 (Table S2).
In both years, L 1.9 was characterized by the lowest open flower percentages, without differences between canopy positions. The perfect flower rate, considered an important parameter in determining final fruit yield, was in general high (> 93%) and similar to the standard Leccino, which generally shows a very low and negligible percentage of aborted pistils [5,39]. An exception was observed in 2018 for L 1.9, which showed the lowest values of perfect flowers (56.5–61.8% in EZ and IZ, respectively) compared to L 1.3 and L 1.4 (Table 1). A possible explanation for this occurrence could be related to a higher sensitivity of L 1.9 to the heavy rainfall that occurred in 2018 between March and May, which is a critical period during which pistil abortion has usually been observed. In our case, in 2018 precipitations were higher than in 2017, corroborating previous evidence of an inverse relationship between rainfall amount in the post dormancy phase and pistil abortion in olive [36]. Several authors have reported that, in addition to the olive genotypes, the expression of a different flower gender may be accentuated by abiotic stresses and tree physiological status [7,11,48]. The results obtained for L 1.9 suggest a greater sensitivity of this clone to environmental constraints, but further investigations may better elucidate the relationship between this genotype and environmental conditions. In this regard, a high variability within the variety-population of Leccino cultivar has been ascertained [49]. The initiation and development of pistil were similar between canopy positions, with the only exception of L 1.9 in 2018, suggesting that the approximate 30% reduction of intercepted light measured in our study is not sufficient to induce significant differences. In a previous study, Seifi et al. [50] measured a similar percentage of perfect flowers per inflorescence regardless of their location on the shoots. Trentacoste et al., [45] reported that the percentage of perfect flowers per inflorescence was a stable trait and did not show significant differences throughout three growing seasons in shaded (50 and 80%) plants in comparison to the unshaded ones. On the contrary, Dimassi et al. [51] and Trentacoste et al. [22] reported a significant increase of perfect flowers per inflorescence in more illuminated canopy positions.
3.3. Ovary Histological Observations
The number of perfect flowers constituting the inflorescences represents a key factor in determining the potential yield of the olive tree. However, it is a prerequisite which does not fully guarantee a correct fruit set which is affected by many variables such as cultivar, year, shoot position and tree water status [4,12,52]. Moreover, the occurrence of abnormal ovules characterized by a failure of embryo sac development may be the cause of an unsuccessful fertilization process [13,17]. Previous studies reported that the presence of three or four well-developed embryo-sacs is determinant for successful fertilization and fruit set [4]. In this study, the histological analysis allowed us to assess that ovaries with four fully developed ovules (4/4) were generally rare to detect in the three studied clones (Figure 3A and Figure 4). Indeed, in both years only L 1.4 showed a small percentage (20.2–15.4%) of flowers in the EZ position with four fully developed ovules (Figure 4).
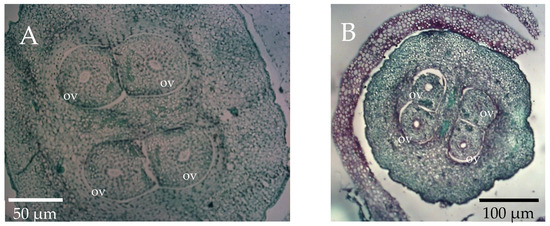
Figure 3.
Transverse sections of olive ovary containing four ovules (ov). (A) Four fully developed ovules with round open embryo sacs (4/4). (B) Three fully developed ovules and one undeveloped ovule, without embryo sac (3/4).
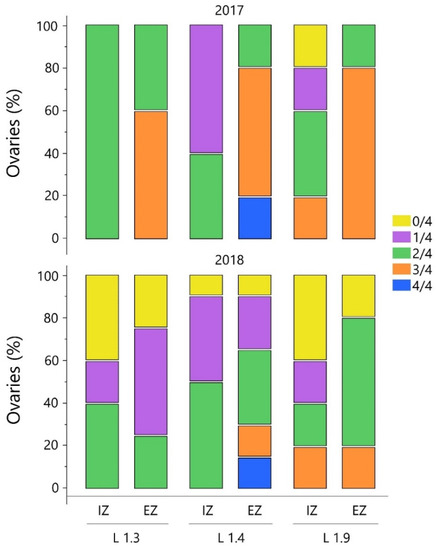
Figure 4.
Percentage distribution of ovaries with four (4/4), three (3/4), two (2/4), one (1/4) and zero (0/4) developed ovules for pistils sampled from the internal (IZ) and external zone (EZ) of the canopy. Data refer to the 2017 and 2018 spring seasons for three Leccino olive clones (L 1.3, L 1.4, L 1.9).
Ovaries with 3/4 fully developed ovules (Figure 3B) were more frequent in flowers of EZ inflorescences (between 60.4 and 80.2%) than in IZ ones (only found in 20.3% of ovaries in the L 1.9 clone). In 2018 a generalized poor ovary performance was observed, and the 3/4 proportion was only recognized in 15.2–20.3% of analyzed samples of L 1.9 and L 1.4 (Figure 4). Ovaries without functional ovules (0/4) were found in a range of 10.4–40.2%, with the highest values associated with the inner position, regardless of clone. As a general trend, pistils of perfect flowers from IZ inflorescences showed lower ovary quality rates (1/4–2/4), an occurrence that could make it difficult to achieve a regular fertilization (Figure 4). Considering that ovaries with three or four fully developed ovules have a good potential for fertilization and fruit formation, these two ovule categories were grouped for a two-way ANOVA analysis (Table 2).

Table 2.
Two-way ANOVA results relating to the quality parameters of female and male reproductive organs represented by ovaries with ≥3–4/4 ovules and pollen viability. Main effect: clone (C), canopy position (CP). F- and p-values are shown; ns: not significant. Data of pollen germination are not reported because differences between clones and canopy position were never significant.
Worthy of note is the significance of the ‘canopy position’ factor and of the ‘clone’ × ‘canopy position’ interaction in 2018. A heterogeneous light interception at internal zones of the canopy may impact the final ovule quality. Commonly, plants in low-light environments may shunt resources away from reproductive structures to parts that can increase light capture, such as leaves and stems [53]. Less illuminated canopy zones could be negatively influenced by a different nutritional status due to a direct limitation of assimilates, or indirectly through a light-induced promotion of the sink strength [54]. In apricot, low light levels have influenced the appearance of anomalous gynoecium structures, increasing the percentage of flowers with reduced or no ability to set [55,56]. On this matter, few studies have been carried out on olive. In Arbequina, a Spanish cultivar characterized by high-quality ovule development [34], the canopy position did not affect the ovule quality, so that this genotype has been suggested for hedgerows olive orchards [22,27]. The great biodiversity that characterizes the olive germplasm requires broadening investigations on the reproductive organ quality in specific genotypes. Since flower quality parameters may be strongly affected by the interaction among numerous micro- and macro-environmental conditions [47,57], the worst quality ovary found in 2018 could have been affected by the spring meteorological events which occurred during the completion of pistil development. Considering that in olive this phase starts about five to six weeks before blooming [58], under our study area it occurred in early April and lasted up to mid-May. In 2018, this period was exceptionally rainy: under our experimental conditions from post-dormancy to flowering (March–May) it rained 371 mm, in comparison to the 177 mm of rainfall during the 1996–2015 period. Such an occurrence could have determined possible hypoxia conditions for which the olive trees are particularly sensitive [59]. Considering the scarce or non-existent literature on the relationship between waterlogging stress and floral biology variables, specific studies are needed to clarify the responses of olive genotypes in view of adaptation strategies towards climate change projections as well.
3.4. Viability and Germination of Pollen Grains
The values of pollen viability and germination power measured on samples collected from the internal and external zone of the canopy are given in Figure 5.
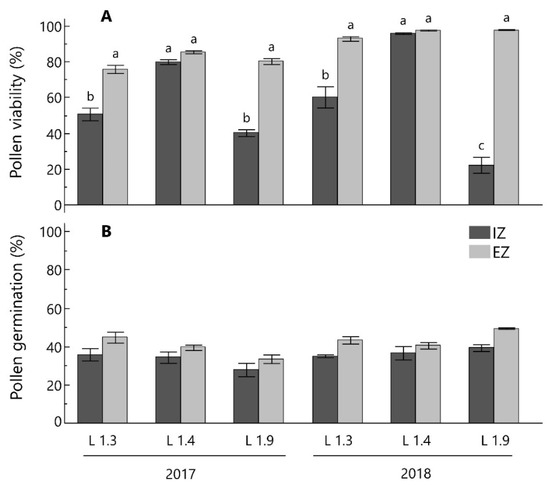
Figure 5.
Percentage of viable pollen grains (A) and pollen germination (B) from internal (IZ) and external zone (EZ) of the canopy. Data refer to the 2017 and 2018 spring seasons for three Leccino olive clones (L 1.3, L 1.4, L 1.9). Histograms are means ± standard error. Different letters indicate differences at p ≤ 0.05 (Tukey’s test) among the Clone-Canopy Position combinations within each year. Letters are only presented when ANOVA indicate significant effect.
The TTC staining test showed a wide range of pollen viability, from 22.2 to 96.2% (Figure 5A), mainly in relation to the canopy position. The highest percentages (>75%) of viable pollen grains were recorded in samples collected from flowers grown at the EZ of the canopy. At this canopy position, all of the tested clones showed high viability rates according to the ranking indicated by Rovira and Tous [60], who considered values over 50% as high. In both years, L 1.3 and L 1.9 clones showed significantly lower pollen viability in IZ flowers (50.4 and 25.3%, respectively) than in EZ ones (Figure 5). Our finding agrees with Anguilar-Garcia et al. [61] who reported that in a Cactaceae species, flowers intercepted lower PAR had worse quality pollen. Interestingly, in both years the canopy zone did not affect pollen viability in the L 1.4 clone, which showed high values, (80.3 to 97.6%) regardless of the inflorescence position and year (Table S2). In this regard, Reale et al. [48] reported that the effect of light availability on fruit development is cultivar dependent. A highly significant interaction ‘clone × canopy position’ was also observed in both years (Table 2). The rate of pollen germination power (Figure 5B) was remarkably lower than the percentage of viable pollen grains, as generally observed in several olive cultivars [62]. In only one case (L 1.9 – IZ in 2018) the germination percentagewas higher than the percentage of the viable pollen grains. As a general trend, the germination values were higher than 30%, which is considered an adequate rate for the Leccino cultivar [49]. Higher, although not significant, percentages of pollen germination were measured in EZ flowers. These results are in agreement with previous studies on wheat × maize crosses in which the pollen tube growth was positively affected by higher light intensity [63]. To the best of our knowledge, there are no similar studies in olive.
4. Conclusions
The Leccino cultivar is one of the most cultivated in Italy but also common and appreciated throughout the world because of its high yields and oil quality. The preliminary results of this study highlighted some differences in inflorescence traits and floral quality parameters in three new Leccino clones, also taking into account two canopy positions and the different meteorological climatic conditions over the two experimental years.
Among the three clones, the morphological traits of inflorescence and flower fertility of L 1.9 were the less affected by light availability. The L 1.9, characterized by shorter inflorescences with fewer flowers, showed similar inflorescence length, percentage of open flowers and flower fertility (presence of at least three fully developed ovules) between external and internal canopy zones.
In particular, the presence of ovaries with at least three fully developed ovules denoted a high flower fertility, a key condition for achieving a successful fruit set. The best male fertility by the highest pollen viability, regardless of canopy position and year, was measured on L 1.4, suggesting its possible use as a pollinator.
The lower sensitivity of female and male floral organs to partial shading needs further investigations and long term studies aimed at evaluating the suitability of the two above-mentioned clones to high- and super-high density olive orchards. Considering that the Leccino cultivar has been receiving particular attention because of its tolerance to Xylella fastidiosa subsp. pauca, these clones could represent a valid tool to counteract the bacterial phytopathogen associated with the OQDS.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae8050402/s1, Table S1: Mean air temperature; Table S2: Two-way ANOVA results of morphological and floral quality traits.
Author Contributions
Conceptualization, S.B.; methodology, S.B. and G.P.; software, G.C. and G.P.; validation, S.B., G.C. and G.P.; formal analysis, S.B., G.C. and G.P.; investigation, S.B.; resources, S.B.; data curation, S.B., G.C. and G.P.; writing—original draft preparation, S.B., G.C. and G.P.; writing—review and editing, S.B., G.C. and G.P.; funding acquisition, S.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by University funds from the Scuola Superiore Sant’Anna, Pisa, Italy.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors wish to thank Cristina Ghelardi and Fausta Rocco for their excellent technical assistance.
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Williams, R.R. The effect of summer nitrogen applications on the quality of apple blossom. J. Hortic. Sci. 1965, 40, 31–41. [Google Scholar] [CrossRef]
- Andreini, L.; Bartolini, S.; Guivarc’h, A.; Chriqui, D.; Vitagliano, C. Histological and immunohistochemical studies on flower induction in olive tree (Olea europaea L.). Plant Biol. 2008, 10, 588–595. [Google Scholar] [CrossRef]
- Fernandez-Escobar, R.; Benlloch, M.; Navarro, C.; Martin, G.C. The time of floral induction in the olive. J. Am. Soc. Hortic. Sci. 1992, 117, 304–307. [Google Scholar] [CrossRef]
- Rapoport, H.F.; Hammami, S.B.M.; Martins, P.; Pérez-Priego, O.; Orgaz, F. Influence of water deficits at different times during olive tree inflorescence and flower development. Environ. Exp. Bot. 2012, 77, 227–233. [Google Scholar] [CrossRef]
- Lavee, S. Olive. In CRC Handbook of Fruit Set and Development; Monselisa, S.P., Ed.; CRC Press: Boca Raton, FL, USA, 1986; pp. 261–276. [Google Scholar]
- Seifi, E.; Guerin, J.; Kaiser, B.; Sedgley, M. Inflorescence architecture of olive. Sci. Hortic. 2008, 116, 273–279. [Google Scholar] [CrossRef]
- Lavee, S.; Rallo, L.; Rapoport, H.F.; Troncoso, A. The floral biology of the olive: Effect of flower number, type and distribution on fruitset. Sci. Hortic. 1986, 66, 149–158. [Google Scholar] [CrossRef]
- Lavee, S.; Taryan, J.; Levin, J.; Haskal, A. The significance of cross-pollination for various cultivars under irrigated intensive growing conditions. Olivae 2002, 91, 25–36. [Google Scholar]
- Cuevas, J.; Polito, V.S. The role of staminate flowers in the breeding system of Olea europaea (Oleaceae): An andromonoecious, wind-pollinated taxon. Ann. Bot. 2004, 93, 547–553. [Google Scholar] [CrossRef]
- Rallo, L.; Fernandez-Escobar, R. Influence of cultivar and flower thinning within the inflorescences on competition among olive fruits. J. Am. Soc. Hort. Sci. 1985, 110, 303–308. [Google Scholar]
- Rapoport, H.F. The reproductive biology of the olive tree and its relationship to extreme environmental conditions. Acta Hort. 2014, 1057. [Google Scholar] [CrossRef]
- Martin, G.C.; Sibbett, G.S. Botany of the olive. In Olive Production Manual; Sibbett, G.S., Ferguson, L., Coviello, J.L., Lindstrand, M., Eds.; University of California: Oakland, CA, USA, 2005; pp. 15–19. [Google Scholar]
- Rapoport, H.F.; Rallo, L. Fruit set and enlargement in fertilized and unfertilized olive ovaries. Hortscience 1991, 26, 896–898. [Google Scholar] [CrossRef]
- Tello, J.; Montemayor, M.I.; Forneck, A.; Ibáñez, J. A new image-based tool for the high throughput phenotyping of pollen viability: Evaluation of inter- and intra-cultivar diversity in grapevine. Plant Methods 2018, 14, 3. [Google Scholar] [CrossRef]
- Perica, S.; Ban, S.G.; Bucan, L.; Poljak, M. Flower sterility and the germination ability of pollen as genetic traits of seven olive. Olea 2012, 87, 237–242. [Google Scholar]
- Higashitani, A. High temperature injury and auxin biosynthesis in microsporogenesis. Front. Plant Sci. 2013, 4, 47. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Alias, I.; Rapoport, H.F. Morphological limitations in floral development among olive cultivars. Acta Hort. 2012, 932, 23–28. [Google Scholar] [CrossRef]
- Bartolini, S.; Leccese, A.; Andreini, L. Influence of canopy fruit location on morphological, histochemical and biochemical changes in two oil olive cultivars. Plant Biosyst. 2014, 148, 1221–1230. [Google Scholar] [CrossRef]
- Caruso, G.; Gucci, R.; Sifola, M.I.; Selvaggini, R.; Urbani, S.; Esposto, S.; Taticchi, A.; Servili, M. Irrigation and fruit canopy position modify oil quality of olive trees (cv. Frantoio). J. Sci. Food. Agric. 2017, 97, 3530–3539. [Google Scholar] [CrossRef] [PubMed]
- Lémole, G.; Weibelb, A.M.; Trentacoste, E.R. Effect of shading in different periods from flowering to maturity on the fatty acid and phenolic composition of olive oil (cv. Arbequina). Sci. Hortic. 2018, 240, 162–169. [Google Scholar] [CrossRef]
- Grilo, F.; Sedaghat, S.; Di Stefano, V.; Sacchi, R.; Caruso, T.; Lo Bianco, R. Tree planting density and canopy position affect ‘Cerasuola’and ‘Koroneiki’ olive oil quality. Horticulturae 2021, 7, 11. [Google Scholar] [CrossRef]
- Trentacoste, E.R.; Moreno-Alíasa, I.; Gómez-del-Campo, M.; Beyá-Marshalla, V.; Rapoport, H.F. Olive floral development in different hedgerow positions and orientations as affected by irradiance. Sci. Hortic. 2017, 225, 226–234. [Google Scholar] [CrossRef]
- Farinelli, D.; Tombesi, S. Performance and oil quality of ‘Arbequina’ and four Italian olive cultivars under super high density hedgerow planting system cultivated in central Italy. Sci. Hortic. 2015, 192, 97–107. [Google Scholar] [CrossRef]
- De la Rosa, R.; León, L.; Guerrero, N.; Rallo, L.; Barranco, D. Preliminary results of an olive cultivar trial at high density. Aus. J. Agric. Res. 2007, 58, 392–395. [Google Scholar] [CrossRef]
- De Castro, A.; Pilar Rallo, I.; Paz Suárez, M.; Torres-Sánchez, J.; Casanova, L.; Jiménez-Brenes, F.M.; Morales-Sillero, A.; Jiménez, M.R.; López-Granados, F. High-throughput system for the early quantification of major architectural traits in olive breeding trials using UAV images and OBIA techniques. Front. Plant Sci. 2019, 10, 1472. [Google Scholar] [CrossRef] [PubMed]
- Caruso, G.; Palai, G.; Marra, F.P.; Caruso, T. High-Resolution UAV imagery for field olive (Olea europaea L.) phenotyping. Horticulturae 2021, 7, 258. [Google Scholar] [CrossRef]
- Moreno-Alías, I.; Trentacoste, E.R.; Gómez-del-Campo, M.; Beyá-Marshall, V.; Rapoport, H.F. Olive inflorescence and flower development as affected by irradiance received in different positions of an east-west hedgerow. Acta Hortic. 2018, 1199. [Google Scholar] [CrossRef]
- Giampetruzzi, A.; Morelli, M.; Saponari, M.; Loconsole, G.; Chiumenti, M.; Boscia, D.; Savino, V.N.; Martelli, G.P.; Saldarelli, P. Transcriptome profiling of two olive cultivars in response to infection by the CoDiRO strain of Xylella fastidiosa subsp. pauca. BMC Genom. 2016, 17, 475. [Google Scholar] [CrossRef]
- Gucci, R.; Cantini, C. Pruning and Training Systems for Modern Olive Growing; CSIRO Publishing: Clayton, Australia, 2000; pp. 117–121. [Google Scholar]
- Sanz-Cortés, F.; Martínez-Calvo, J.; Badenes, M.L.; Bleiholder, H.; Hack, H.; Llacer, G.; Meier, U. Phenological growth stages of olive trees (Olea europea). Ann. Appl. Biol. 2002, 140, 151–157. [Google Scholar] [CrossRef]
- Suarez, C.; Castro, A.J.; Rapoport, H.F.; Rodriguez-Garcia, M.I. Morphological, histological and ultrastructural changes in the olive pistil during flowering. Sex Plant Reprod. 2012, 25, 133–146. [Google Scholar] [CrossRef]
- Hawes, C.; Satiat-Jeunemaitre, B. Plant Cell Biology: A Practical Approach; Oxford University Press: Oxford, UK, 2001; p. 364. [Google Scholar]
- Sakai, W.S. Simple method for differential staining of paraffin embedded plant material using toluidine blue. Stain Technol. 1973, 48, 247–249. [Google Scholar] [CrossRef]
- Moreno-Alías, I.; De La Rosa, R.; Rapoport, H.F. Floral quality components of a new olive cultivar and its parents. Sci. Hortic. 2013, 154, 17–19. [Google Scholar] [CrossRef]
- Shivanna, K.R.; Rangaswamy, N.S. Pollen Biology: A Laboratory Manual; Springer: Berlin, Germany, 1992; p. 119. [Google Scholar]
- Bartolini, S.; Viti, R. Olive floral biology and climatic elements: Twenty-eight years of observations. Acta Hortic. 2018, 1229, 299–304. [Google Scholar] [CrossRef]
- Stanley, R.G.; Linskens, H.F. Pollen Biology, Biochemistry Management; Springer: Berlin, Germany, 1983; p. 310. [Google Scholar]
- SIR. Regional Hydrological and Geological Service, Tuscany. Available online: www.sir.toscana.it (accessed on 14 March 2022).
- Cimato, A.; Cantini, C.; Sani, G.; Marranci, M. Il Germoplasma Dell’olivo; CNR-ARSIA: Florence, Italy, 1997; p. 309. [Google Scholar]
- Lombardo, N.; Alessandrino, M.; Godino, G.; Madeo, A. Comparative observations regarding the floral biology of 150 Italian olive (Olea europaea L.) cultivars. Adv. Hort. Sci. 2006, 20, 246–255. [Google Scholar]
- Bartolini, S.; Viti, R. Observations on floral biology of several olive ‘Leccino’ clones. Acta Hortic. 2018, 1199, 145–152. [Google Scholar] [CrossRef]
- Orlandi, F.; Ruga, L.; Romano, B.; Fornaciari, M. Olive flowering as an indicator of local climatic changes. Theor. Appl. Climatol. 2005, 81, 169–176. [Google Scholar] [CrossRef]
- Orlandi, F.; Sgromo, C.; Bonofiglio, T.; Ruga, L.; Romano, B.; Fornaciari, M. Spring influences on olive flowering and threshold temperatures related to reproductive structure formation. Hort. Sci. 2010, 45, 1052–1057. [Google Scholar] [CrossRef]
- Lombardo, L.; Fila, G.; Lombardo, N.; Epifani, C.; Duffy, D.H.; Godino, G.; Salimonti, A.; Zelasco, S. Uncovering olive biodiversity through analysis of floral and fruiting biology and assessment of genetic diversity of 120 italian cultivars with minor or marginal diffusion. Biology 2019, 8, 62. [Google Scholar] [CrossRef] [PubMed]
- Trentacoste, E.R.; Calvo, F.E.; Sánchez, C.L.; Calderón, F.J.; Banco, A.P.; Lémole, G. Response of inflorescence structure and oil yield components to source-sink manipulation by artificial shading in olive. Theor. Exp. Plant Physiol. 2022. [Google Scholar] [CrossRef]
- Rezazadeh, A.; Harkess, R.L.; Telmadarrehei, T. The effect of light intensity and temperature on flowering and morphology of potted red firespike. Horticulturae 2018, 4, 36. [Google Scholar] [CrossRef]
- Acebedo, M.M.; Cañete, M.L.; Cuevas, J. Processes affecting fruit distribution and its quality in the canopy of olive trees. Adv. Hort. Sci. 2000, 14, 169–175. [Google Scholar]
- Reale, L.; Sgromo, C.; Bonofiglio, T.; Orlandi, F.; Fornaciari, M.; Ferranti, F.; Romano, B. Reproductive biology of Olive (Olea europaea L.) DOP Umbria cultivars. Sex Plant Reprod. 2006, 19, 151–161. [Google Scholar] [CrossRef]
- Bartolini, S.; Guerriero, R. Self-compatibility in several clones of oil olive cv. Leccino. Adv. Hort. Sci. 1995, 9, 71–74. [Google Scholar]
- Seifi, E.; Guerin, J.; Kaiser, B.; Sedgley, M. Sexual compatibility and floral biology of some olive cultivars. N. Z. J. Crop Hortic. Sci. 2011, 39, 141–151. [Google Scholar] [CrossRef]
- Dimassi, K.; Therios, I.; Balatsos, A. The blooming period and self-fruitfulness in twelve Greek and three foreign olive cultivars. Acta Hortic. 1999, 474, 275–278. [Google Scholar] [CrossRef]
- Fabbri, A.; Bartolini, G.; Lambardi, M.; Kailis, S.G. Olive Propagation Manual; CSIRO Publishing: Clayton, Australia, 2004; p. 160. [Google Scholar]
- Kilkenny, F.; Galloway, L.F. Reproductive success in varying light environments: Direct and indirect effects of light on plants and pollinators. Oecologia 2008, 155, 247–255. [Google Scholar] [CrossRef]
- Kinet, J.M.; Sachs, R.M.; Bernier, G. Photosynthesis, assimilate supply, and utilization. In The Physiology of Flowering: Volume III: The Development of Flowers; CRC Press: Boca Raton, FL, USA, 1985; pp. 179–194. [Google Scholar]
- Nuzzo, V.; Biasi, R.; Dichio, B.; Montanaro, G.; Xiloyannis, C. Influence of different seasonal light availability on flower bud quality in cv Tirynthos (Prunus armeniaca L.). Acta Hort. 1997, 488, 477–483. [Google Scholar] [CrossRef]
- Bartolini, S.; Viti, R.; Andreini, L. The effect of summer shading on flower bud morphogenesis in apricot (Prunus armeniaca L.). Cent. Eur. J. Biol. 2013, 8, 54–63. [Google Scholar] [CrossRef]
- Fernández, J.E. Understanding olive adaptation to abiotic stresses as a tool to increase crop performance. Environ. Exp. Bot. 2014, 103, 158–179. [Google Scholar] [CrossRef]
- Cuevas, J.; Pinney, K.; Polito, V.S. Flower differentiation, pistil development and pistil abortion in olive (Olea europaea L. ‘Manzanillo’). Acta Hortic. 1999, 474, 293–296. [Google Scholar] [CrossRef]
- Barranco, D.; Fernández-Escobar, R.; Rallo, L. El Cultivo del Olivo; Mundi-Prensa: Madrid, Spain, 2001; p. 724. [Google Scholar]
- Rovira, M.; Tous, J. Producción y viabilidad del polen. In Variedades de Olivo en España; Rallo, L., Ed.; Junta de Andalucía, Ministerio de Agricultura, Pesca y Alimentación y Ediciones Mundi-Prensa: Madrid, Spain, 2005; pp. 295–299. [Google Scholar]
- Aguilar-Garcıa, S.A.; Figueroa-Castro, D.M.; Valverde, P.L.; Vite, F. Effect of flower orientation on the male and female traits of Myrtillocactus geometrizans (Cactaceae). Plant. Biol. 2018, 20, 531–536. [Google Scholar] [CrossRef]
- Giordani, E.; Ferri, A.; Trentacoste, E.R.; Radice, S. Viability and in vitro germinability of pollen grains of olive cultivars grown in different environments. Acta Hort. 2014, 1057, 65–72. [Google Scholar] [CrossRef]
- Campbell, W.; Griffin, W.B.; Burritt, B.J.; Conner, J. The importance of light intensity for pollen tube growth and embryo survival in wheat x maize crosses. Ann. Bot. 2001, 87, 517–522. [Google Scholar] [CrossRef][Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).