The Use of Opuntia ficus-indica Mucilage and Aloe arborescens as Edible Coatings to Improve the Physical, Chemical, and Microbiological Properties of ‘Hayward’ Kiwifruit Slices
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fruits and Preparation of the Edible Coating
2.2. Fruit Processing
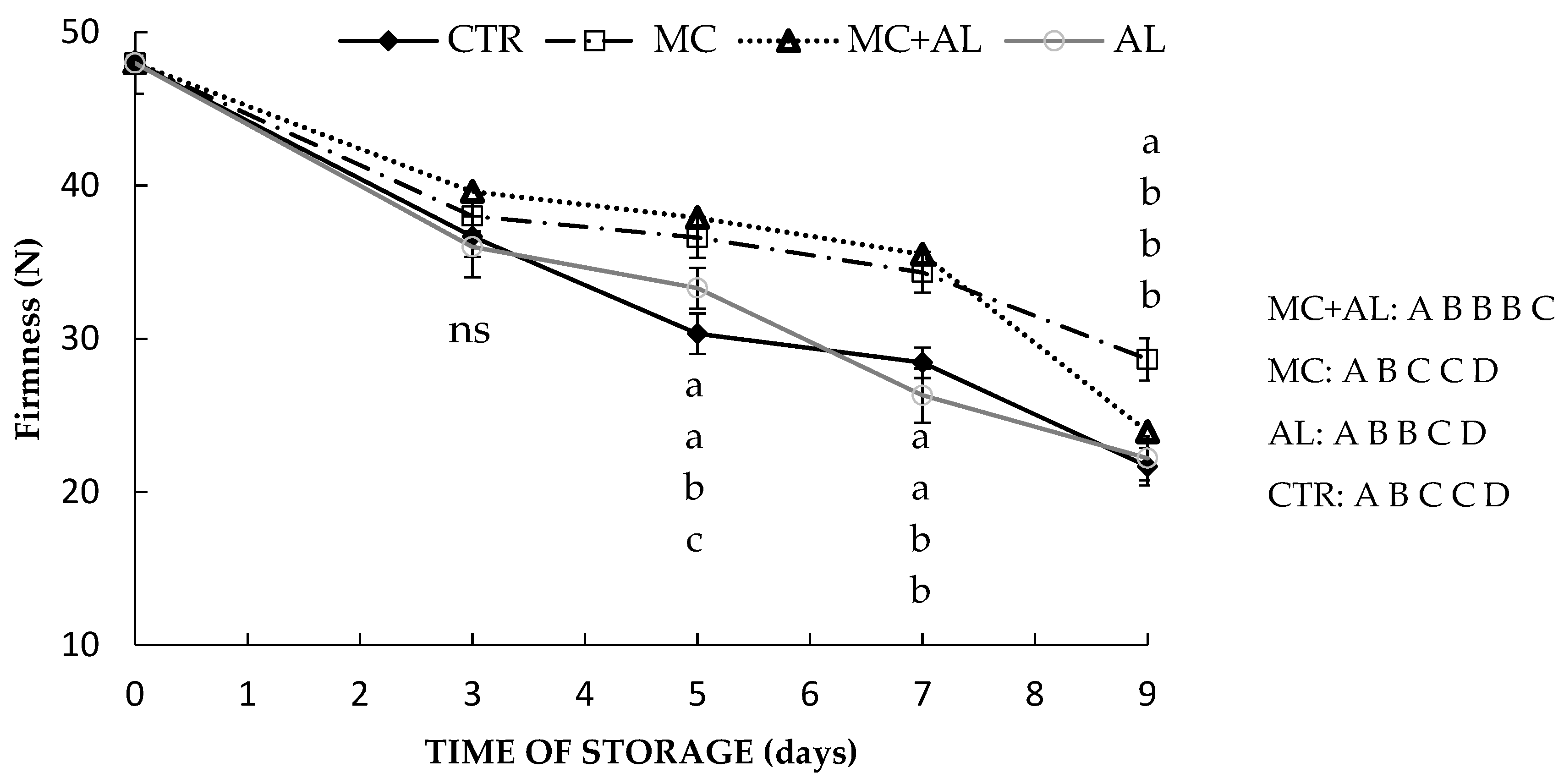
2.3. Firmness
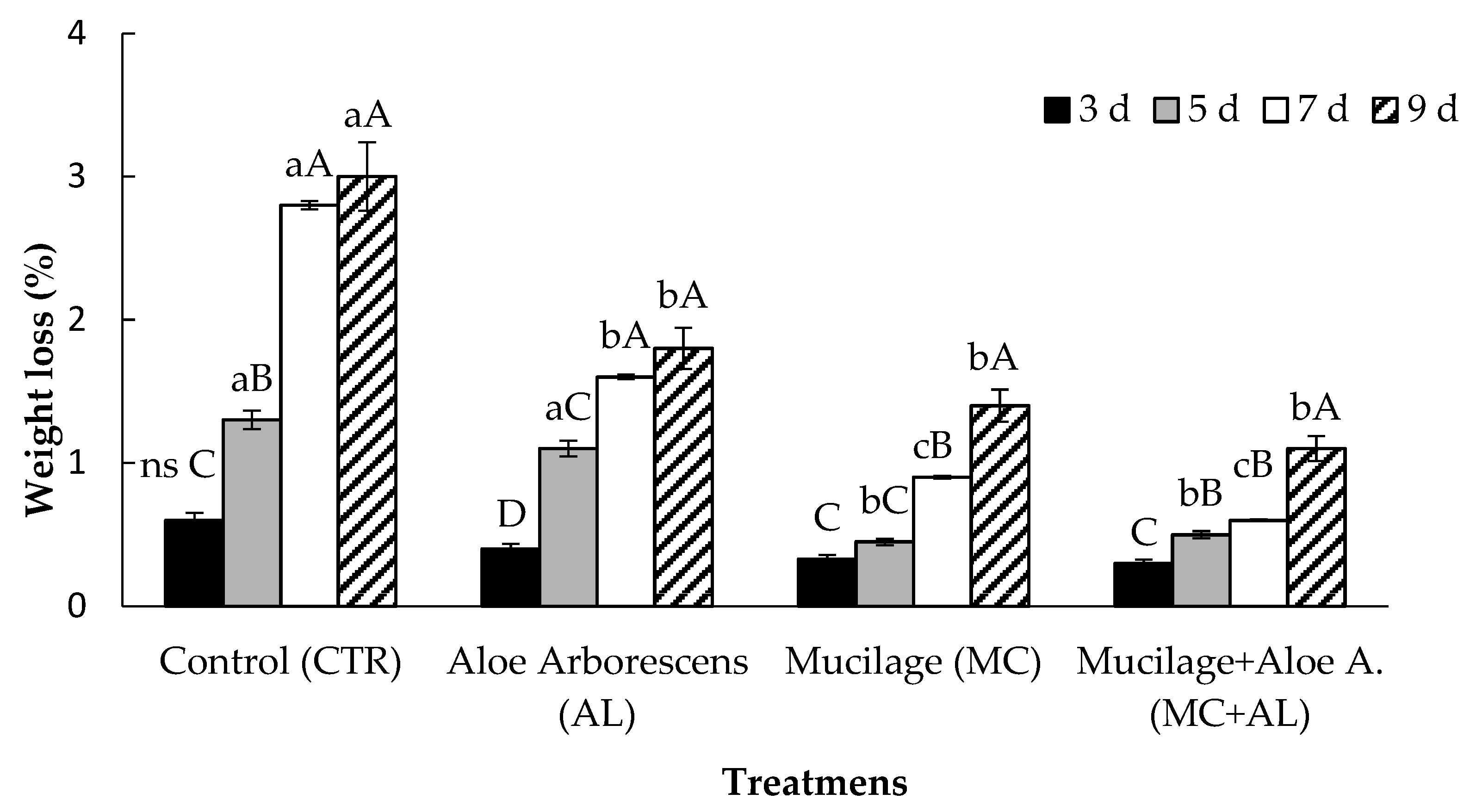
2.4. Weight Loss (%)
2.5. Total Soluble Solids Content and Titratable Acidity
2.6. Visual Appearance Score
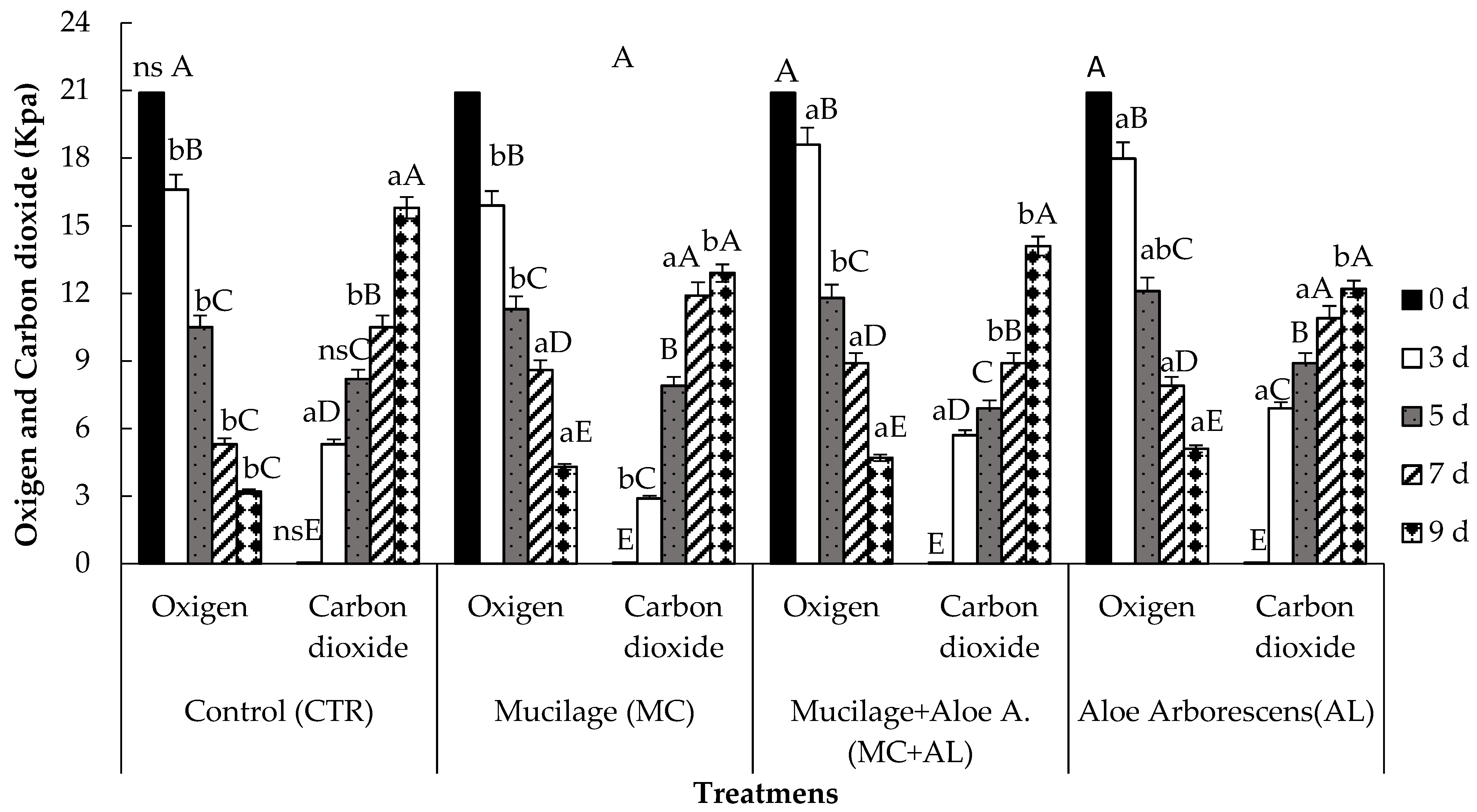
2.7. Package O2 and CO2 Analysis
2.8. Color
2.9. Microbiological Analysis
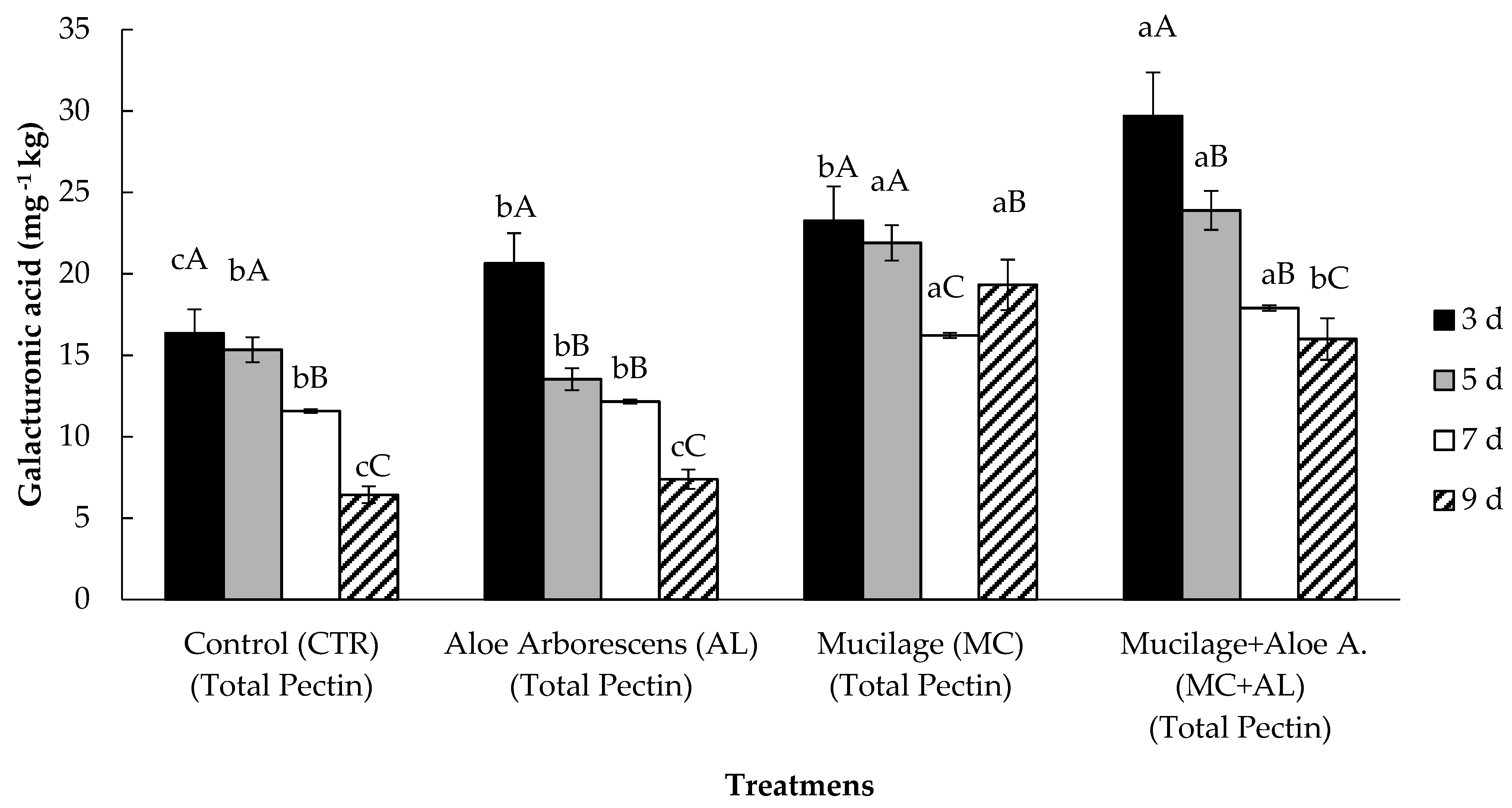
2.10. Pectin Analysis
2.10.1. Water Soluble Pectin
2.10.2. Oxalate Soluble Pectin
2.10.3. Total Pectin
2.10.4. Non-Extractable Pectin
2.10.5. Pectin Content Determination
2.11. Statistical Analysis
3. Results and Discussion
3.1. Solid Soluble Total (SST), Titratable Acidity (TA), Color and Visual Score
3.2. Firmness, Weight Loss and Pectin Content
3.3. Head Spaces Gas Composition
3.4. Microbiological Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schroder, R.; Atkinson, R.G. Kiwifruit cell walls: Towards an understanding of softening? N. Z. J. For. Sci. 2006, 36, 112–129. [Google Scholar]
- Karakurt, Y.; Huber, D.J. Activities of several membrane and cell-wall hydrolases, ethylene biosynthetic enzymes, and cell wall polyuronide degradation during low-temperature storage of intact and fresh-cut papaya (Carica papaya) fruit. Postharvest Biol. Technol. 2003, 28, 219–229. [Google Scholar] [CrossRef]
- Benitez, S.; Achaerandio, I.; Sepulcre, F.; Pujola, M.A. Aloe vera based edible coatings improve the quality of minimally processed ‘Hayward’ kiwifruit. Postharv. Biol. Technol. 2013, 81, 29–36. [Google Scholar] [CrossRef]
- Mastromatteo, M.; Mastromatteo, M.; Conte, A.; Del Nobile, M.A. Combined effect of active coating and MAP to prolong the shelf life of minimally processed kiwifruit (Actinidia deliciosa cv. Hayward). Food Res. Int. 2011, 44, 1224–1230. [Google Scholar] [CrossRef]
- Passafiume, R.; Gaglio, R.; Sortino, G.; Farina, V. Effect of Three Different A. vera Gel-Based Edible coatings on the Quality of Fresh-Cut “Hayward” Kiwifruits. Foods 2020, 9, 939. [Google Scholar] [CrossRef]
- Del Valle, V.; Hernández-Muñoz, P.; Guarda, A.; Galotto, M.J. Development of a cactus-mucilage edible coating (Opuntia ficus indica) and its application to extend strawberry (Fragaria ananassa) shelf life. Food Chem. 2005, 91, 751–756. [Google Scholar] [CrossRef]
- Passafiume, R.; Perrone, A.; Sortino, G.; Gianguzzi, G.; Saletta, F.; Gentile, C.; Farina, V. Chemical-physical characteristics, polyphenolic content and total antioxidant activity of three Italian-grown pomegranate cultivars. NFS J. 2019, 16, 9–14. [Google Scholar] [CrossRef]
- Lai, Q.; Wang, H.; Guo, X.; Abbasi, A.M.; Wang, T.; Li, T.; Liu, R.H. Comparison of phytochemical profiles, antioxidant and cellular antioxidant activities of seven cultivars of Aloe. Int. J. Food Sci. Technol. 2016, 51, 1489–1494. [Google Scholar] [CrossRef]
- Zapata, P.J.; Navarro, F.D.; Guillén, S.; Castillo, D.; Martínez-Romero, D.; Valero, D.; Serrano, M. Characterisation of gels from different Aloe spp. as antifungal treatment: Potential crops for industrial applications. Ind. Crops Prod. 2013, 42, 223–230. [Google Scholar] [CrossRef]
- Allegra, A.; Sortino, G.; Inglese, P.; Settanni, L.; Todaro, A.; Gallotta, A. The effectiveness of Opuntia ficus-indica mucilage edible coating on postharvest maintenance of ‘Dottato’ fig (Ficus carica L.) fruit. Food Packag. Shelf Life 2017, 12, 135–141. [Google Scholar] [CrossRef]
- Trachtenberg, S.; Mayer, A.M. Composition and Properties of Opuntia Ficus-indica mucilage. Phytochemistry 1981, 20, 2665–2668. [Google Scholar] [CrossRef]
- Sáenz, C.; Montoya, L.C. Nopalitos: Nueva hortaliza para Chile. El Campesino 1999, 130, 4–7. [Google Scholar]
- Medina-Torres, L.; Brito-De La Fuente, E.; Torrestiana-Sanchez, B.; Katthain, R. Rheological properties of the mucilage gum (Opuntia ficus indica). Food Hydrocoll. 2000, 14, 417–424. [Google Scholar] [CrossRef]
- Allegra, A.; Inglese, P.; Sortino, G.; Settanni, L.; Todaro, A.; Liguori, G. The influence of Opuntia ficus-indica mucilage edible coating on the quality of ‘Hayward’kiwifruit slices. Postharvest Biol. Technol. 2016, 120, 45–51. [Google Scholar] [CrossRef]
- Sáenz, C.; Vásquez, M.; Trumper, S.; Fluxá, C. Extractión y Composición Química del Mucílago de Tuna (Opuntia ficus indica). In Proceedings of the II Congreso Internacional de Tuna y Cochinilla, Santiago, Chile, 22–25 September 1992; pp. 93–96. [Google Scholar]
- Sortino, G.; Saletta, F.; Puccio, S.; Scuderi, D.; Allegra, A.; Inglese, P.; Farina, V. Extending the shelf life of white peach fruit with 1-methylcyclopropene and A. arborescens edible coating. Agriculture 2019, 10, 151. [Google Scholar] [CrossRef]
- Chironi, S.; Sortino, G.; Allegra, A.; Saletta, F.; Caviglia, V.; Ingrassia, M. Consumer assessment on sensory attributes of fresh table grapes cv ‘Italia’ and ‘Red globe’ after long cold storage treatment. Chem. Eng. Trans. 2017, 58, 421–426. [Google Scholar]
- Rouse, A.H.; Atkins, C.D. Pectinesterase and pectin in commercial citrus juices as determined by methods used at the Citrus Experiment Station. Calif. Agric. Exp. Stn. Bull. 1955, 570, 1–9. [Google Scholar]
- Rouse, A.H.; Atkins, C.D. Heat inactivation of pectinesterase in citrus juices. Food Technol. 1952, 6, 291–294. [Google Scholar]
- Ahmed, A.E.R.; Labavitch, J.M. A simplified method for accurate determination of cell wall uronide content. J. Food Biochem. 1978, 1, 361–365. [Google Scholar] [CrossRef]
- Kintner, P.K.; Buren, J.P. Carbohydrate Interference and Its Correction in Pectin Analysis Using the m-Hydroxydiphenyl Method. J. Food Sci. 1982, 47, 756–759. [Google Scholar] [CrossRef]
- Yu, L.; Reitmeier, C.A.; Love, M.H. Strawberry texture and pectin content as affected by electron beam irradiation. J. Food Sci. 1996, 61, 844–846. [Google Scholar] [CrossRef]
- Xu, F.; Liu, S.; Liu, Y.; Xu, J.; Liu, T.; Dong, S. Effectiveness of lysozyme coatings and 1-MCP treatments on storage and preservation of kiwifruit. Food Chem. 2019, 288, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Jordan, R.B.; Walton, E.F.; Klages, K.U.; Seelye, R.J. Postharvest fruit density as an indicator of dry matter and ripened soluble solids of kiwifruit. Postharvest Biol. Technol. 2000, 20, 163–173. [Google Scholar] [CrossRef]
- Sicari, V.; Loizzo, M.R.; Pellicanò, T.M.; Giuffrè, A.M.; Poiana, M. Evaluation of A. arborescens gel as new coating to maintain the organoleptic and functional properties of strawberry (Fragaria× ananassa cv. Cadonga) fruits. Int. J. Food Sci. Technol. 2019, 55, 861–870. [Google Scholar] [CrossRef]
- Famiani, F.; Battistelli, A.; Moscatello, S.; Cruz-Castillo, J.G.; Walker, R.P. The organic acids that are accumulated in the flesh of fruits: Occurrence, metabolism and factors affecting their contents—A review. Rev. Chapingo Ser. Hortic. 2015, 21, 97–128. [Google Scholar] [CrossRef]
- Allegra, A.; Gallotta, A.; Carimi, F.; Mercati, F.; Inglese, P.; Martinelli, F. Metabolic profiling and post-harvest behavior of “Dottato” fig (Ficus carica L.) fruit covered with an edible coating from O. ficus-indica. Front. Plant Sci. 2018, 9, 1321. [Google Scholar] [CrossRef]
- Soliva-Fortuny, R.; Martín-Belloso, O. Fresh-cut fruits: Apples and pears. In Controlled and Modified Atmospheres for Fresh and Fresh-Cut Produce; Gil, M.I., Beaudry, R., Eds.; Elsevier Inc.: London, UK, 2020; pp. 487–494. [Google Scholar]
- Wang, D.; Yeats, T.H.; Uluisik, S.; Rose, J.K.; Seymour, G.B. Fruit softening: Revisiting the role of pectin. Trends Plant Sci. 2018, 23, 302–310. [Google Scholar] [CrossRef]
- Brummell, D.A.; Cin, V.D.; Crisosto, C.H.; Labavitch, J.M. Cell wall metabolism during maturation, ripening and senescence of peach fruit. J. Exp. Bot. 2008, 55, 2029–2039. [Google Scholar] [CrossRef] [Green Version]
- Hamman, J.H. Composition and applications of A. vera leaf gel. Molecules 2008, 13, 1599–1616. [Google Scholar] [CrossRef] [Green Version]
- Espino-Díaz, M.; De Jesús Ornelas-Paz, J.; Martínez-Téllez, M.A.; Santillán, C.; Barbosa-Cánovas, G.V.; Zamudio-Flores, P.B.; Olivas, G.I. Development and characterization of edible films based on mucilage of Opuntia ficus-Indica (l.). J. Food Sci. 2010, 75, E347–E352. [Google Scholar] [CrossRef]
- Dave, R.K.; Ramana Rao, T.V.; Nandane, A.S. Improvement of post-harvest quality of pear fruit with optimized composite edible coating formulations. J. Food Sci. Technol. 2017, 54, 3917–3927. [Google Scholar] [CrossRef] [PubMed]
- Gol, N.B.; Patel, P.R.; Rao, T.R. Improvement of quality and shelf-life of strawberries with edible coatings enriched with chitosan. Postharvest Biol. Technol. 2013, 85, 185–195. [Google Scholar] [CrossRef]
- Pérez-Gago, M.B.; Rojas, C.; Del Río, M.A. Effect of lipid type and amount of edible hydroxypropyl methylcellulose-lipid composite coatings used to protect postharvest quality of Mandarins cv. Fortune. J. Food Sci. 2002, 67, 2903–2910. [Google Scholar] [CrossRef]
- Park, Y.B.; Cosgrove, D.J. Changes in cell wall biomechanical properties in the xyloglucan-deficient xxt1/xxt2 mutant of Arabidopsis. Plant Physiol. 2012, 158, 465–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broxterman, S.E.; Schols, H.A. Interactions between pectin and cellulose in primary plant cell walls Carbohydrate. Polymers 2018, 192, 263–272. [Google Scholar]
- Cybulska, J.; Zdunek, A.; Kozioł, A. The self-assembled network and physiological degradation of pectins in carrot cell walls. Food Hydrocoll. 2015, 43, 41–50. [Google Scholar] [CrossRef]
- Billy, L.; Mehinagic, E.; Royer, G.; Renard, C.M.; Arvisenet, G.; Prost, C. Relationship between texture and pectin composition of two apple cultivars during storage. Postharvest Biol. Technol. 2008, 47, 315–324. [Google Scholar] [CrossRef]
- Rosli, H.G.; Civello, P.M.; Martínez, G.A. Changes in cell wall composition of three Fragaria x ananassa cultivars with different softening rate during ripening. Plant Physiol. Biochem. 2004, 42, 823–831. [Google Scholar] [CrossRef]
- Banks, N.H.; Dadzie, B.K.; Cleland, D.J. Reducing gas exchange of fruits with surface coatings. Postharv. Biol. Technol. 1993, 3, 269–284. [Google Scholar] [CrossRef]
- Martinez-Romero, D.; Alburquerque, N.; Valverde, J.; Guillen, F.; Castillo, S.; Valero, D.; Serrano, M. Postharvest sweet cherry quality and safety maintenance by A. vera treatment: A new edible coating. Postharv. Biol. Technol. 2006, 39, 93–100. [Google Scholar] [CrossRef]
- Tripathi, P.; Dubey, N. Exploitation of natural products as an alternative strategy to control postharvest fungal rotting of fruit and vegetables. Postharv. Biol. Technol. 2004, 32, 235–245. [Google Scholar] [CrossRef]
- Misir, J.; Brishti, F.H.; Hoque, M.M.A. Aloe vera gel as a novel edible coating for fresh fruits: A review. Am. J. Food Sci. Technol. 2014, 2, 93–97. [Google Scholar] [CrossRef] [Green Version]


| Time of Storage (Days) | Solid Soluble Total (°Brix) | |||
|---|---|---|---|---|
| CTR | MC | MC+AL | AL | |
| 0 days | 12.9 ± 0.2 A nsA | 12.9 ± 0.2 NS | 12.9 ± 0.2 A | 12.9 ± 0.2 NS |
| 3 days | 13.2 ± 0.2 nsA | 13.1 ± 0.1 | 13.3 ± 0.2 A | 13.0 ± 0.3 |
| 5 days | 13.5 ± 0.2 aA | 13.0 ± 0.1 b | 13.5 ± 0.4 aA | 13.2 ± 0.4 a |
| 7 days | 13.7 ± 0.4 nsA | 13.3 ± 0.3 | 13.5 ± 0.2 A | 13.3 ± 0.2 |
| 9 days | 14.1 ± 0.1 nsAB | 13.9 ± 0.4 | 13.6 ± 0.5 AB | 13.5 ± 0.2 |
| Time of Storage (Days) | Titratable Acidity (g L−1) | |||
|---|---|---|---|---|
| CTR | MC | MC+AL | AL | |
| 0 days | 1.8 ± 0.2 nsA | 1.8 ± 0.2 NS | 1.8 ± 0.2 NS | 1.8 ± 0.2 A |
| 3 days | 1.5 ± 0.2 aA | 1.7 ± 0.3 a | 1.6 ± 0.2 b | 1.7 ± 0.5 bA |
| 5 days | 1.3 ± 0.1 aAB | 1.6 ± 0.2 b | 1.6 ± 0.1 b | 1.6 ± 0.3 bA |
| 7 days | 1.2 ± 0.3 aAB | 1.5 ± 0.1 b | 1.6 ± 0.4 b | 1.5 ± 0.2 bA |
| 9 days | 0.9 ± 0.6 B | 1.4 ± 0.5 b | 1.4 ± 0.2 b | 1.0 ± 0.3 aB |
| Time of Storage (Days) | L* (CieLab) | |||
|---|---|---|---|---|
| CTR | MC | MC+AL | AL | |
| 0 days | 66.6 ± 2.4 nsA | 66.6 ± 2.4 nsA | 66.6 ± 2.4 nsA | 66.6 ± 2.4 nsA |
| 3 days | 60.3 ± 1.9 cB | 67.4 ± 1.9 abA | 68.4 ± 5.3 aA | 68.2 ± 2.1 aA |
| 5 days | 58.4 ± 1.9 dB | 60.1 ± 2.9 cB | 67.4 ± 3.2 bA | 69.9 ± 2.0 aA |
| 7 days | 70.4 ± 2.8 aC | 58.9 ± 3.4 cC | 67.1 ± 1.9 bA | 51.4 ± 5.8 dB |
| 9 days | 51.5 ± 1.9 bD | 55.2 ± 5.3 aD | 45.7 ± 3.7 cB | 52.1 ± 4.7 bB |
| Time of Storage (Days) | ΔE (%) | |||
|---|---|---|---|---|
| CTR | MC | MC+AL | AL | |
| 3 days | 1.2 ± 0.3 nsA | 0.8 ± 0.2 A | 0.7 ± 0.2 A | 1.3 ± 0.5 A |
| 5 days | 3.1 ± 0.4 aB | 1.2 ± 0.4 bA | 0.9 ± 0.1 cA | 1.8 ± 0.4 bA |
| 7 days | 4.7 ± 0.8 aB | 2.2 ± 0.3 bAB | 1.1 ± 0 cA | 2.1 ± 0.4 bA |
| 9 days | 7.9 ± 1.9 aC | 5.2 ± 1.1 bB | 3.3 ± 0.9 cB | 5.1 ± 0.7 bB |
| Time of Storage (Days) | Treatments | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aerobic Mesophile Bacteria (Log CFU g−1) | Pseudomonads (Log CFU g−1) | Yeast Content (Log CFU g−1) | ||||||||||
| CTR | MC | MC+AL | AL | CTR | MC | MC+AL | AL | CTR | MC | MC+AL | AL | |
| 0 days | 0 nsD | 0 D | 0 B | 0 B | 0 nsC | 0 C | 0 C | 0 C | 0 nsC | 0 D | 0 C | 0 b |
| 3 days | 0.9 aC | 1.1 aC | 0 bB | 0 bB | 0 nsC | 0 C | 0 C | 0 C | 0 nsC | 0 D | 0 C | 0 B |
| 5 days | 1 bC | 2 aB | 0 cB | 0 cB | 0 nsC | 0 C | 0 C | 0 C | 0 cC | 2.9 aC | 0.1 bC | 0 cB |
| 7 days | 1.9 bB | 4.3 aA | 0 cB | 0 cB | 2 bB | 3.5 aB | 1 cB | 0.5 cB | 1 bB | 3.3 aB | 1.1 bB | 0 cB |
| 9 days | 3.3 bA | 5.4 aA | 1 cA | 1 cA | 4.5 aA | 5 aA | 1.5 bA | 1 bA | 2 bA | 4 aA | 1.5 bA | 1.3 cA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sortino, G.; Inglese, P.; Farina, V.; Passafiume, R.; Allegra, A. The Use of Opuntia ficus-indica Mucilage and Aloe arborescens as Edible Coatings to Improve the Physical, Chemical, and Microbiological Properties of ‘Hayward’ Kiwifruit Slices. Horticulturae 2022, 8, 219. https://doi.org/10.3390/horticulturae8030219
Sortino G, Inglese P, Farina V, Passafiume R, Allegra A. The Use of Opuntia ficus-indica Mucilage and Aloe arborescens as Edible Coatings to Improve the Physical, Chemical, and Microbiological Properties of ‘Hayward’ Kiwifruit Slices. Horticulturae. 2022; 8(3):219. https://doi.org/10.3390/horticulturae8030219
Chicago/Turabian StyleSortino, Giuseppe, Paolo Inglese, Vittorio Farina, Roberta Passafiume, and Alessio Allegra. 2022. "The Use of Opuntia ficus-indica Mucilage and Aloe arborescens as Edible Coatings to Improve the Physical, Chemical, and Microbiological Properties of ‘Hayward’ Kiwifruit Slices" Horticulturae 8, no. 3: 219. https://doi.org/10.3390/horticulturae8030219
APA StyleSortino, G., Inglese, P., Farina, V., Passafiume, R., & Allegra, A. (2022). The Use of Opuntia ficus-indica Mucilage and Aloe arborescens as Edible Coatings to Improve the Physical, Chemical, and Microbiological Properties of ‘Hayward’ Kiwifruit Slices. Horticulturae, 8(3), 219. https://doi.org/10.3390/horticulturae8030219










