Control of Seed-Borne Fungi by Selected Essential Oils
Abstract
1. Introduction
2. Materials and Methods
2.1. Seed Samples
2.2. Determination of Predominant Fungi
2.3. Essential Oils Efficacy Assay
2.4. Statistics
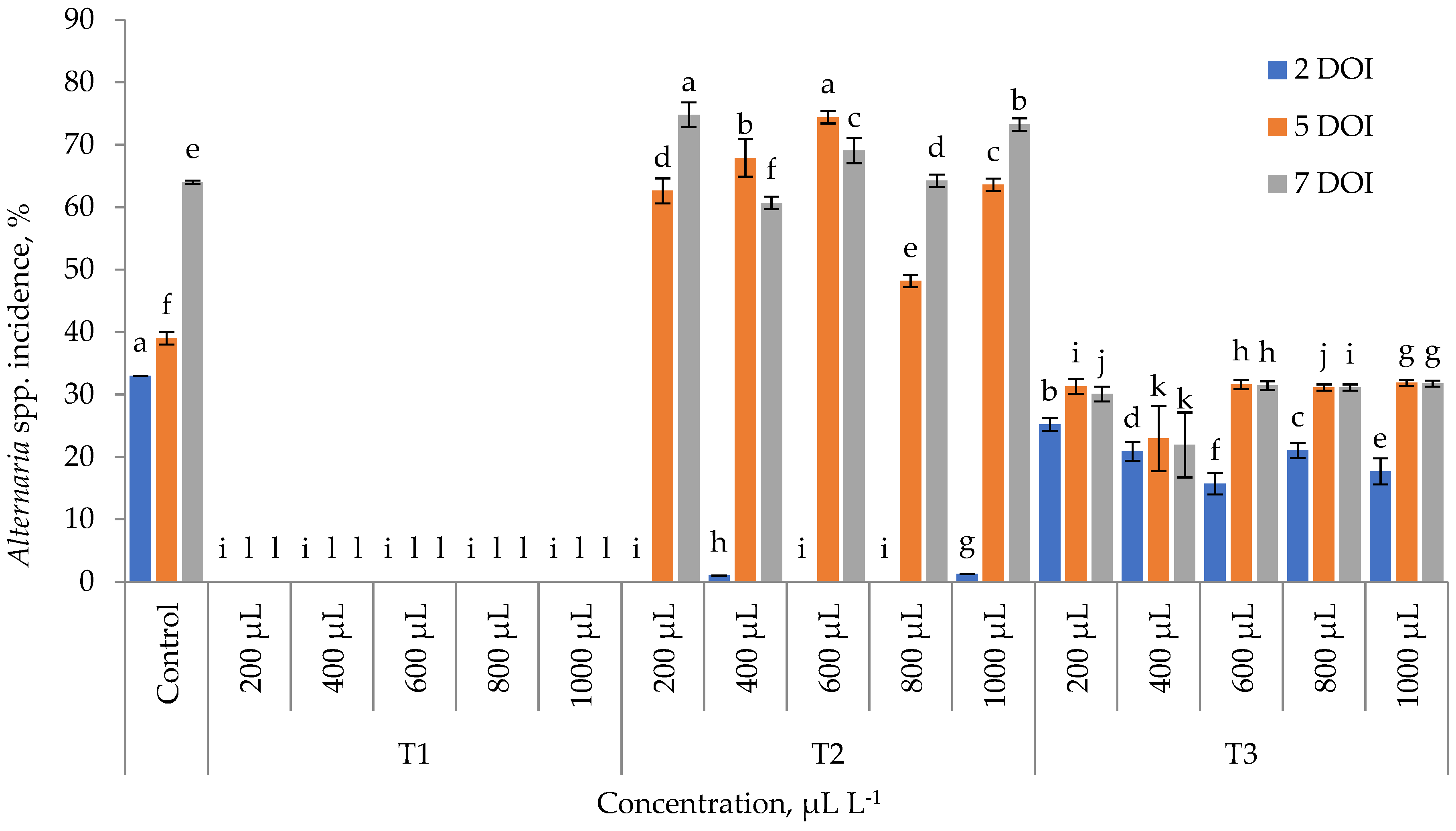
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Tournas, V.H. Spoilage of vegetable crops by bacteria and fungi and related health hazards. Crit. Rev. Microbiol. 2005, 31, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Nowicki, M.; Nowakowska, M.; Niezgoda, A.; Kozik, E. Alternaria Black Spot of Crucifers: Symptoms, Importance of Disease, and Perspectives of Resistance Breeding. J. Fruit Ornam. Plant Res. 2012, 76, 5–19. [Google Scholar] [CrossRef]
- Gaur, A.; Kumar, A.; Kiran, R.; Kumari, P. Importance of seed-borne diseases of agricultural crops: Economic losses and impact on society. In Seed-Borne Diseases of Agricultural Crops: Detection, Diagnosis & Management; Springer: Singapore, 2020; pp. 3–23. [Google Scholar] [CrossRef]
- EPPO Standard. PP 2/1 (1) Guideline on good plant protection practice: Umbelliferous crops. Bull. OEPP/EPPO Bull. 1994, 24, 233–240. [Google Scholar]
- Farrar, J.J.; Pryor, B.M.; Davis, R.M. Alternaria diseases of carrot. Plant Dis. 2004, 88, 776–784. [Google Scholar] [CrossRef] [PubMed]
- Chaerani, R.; Voorrips, R.E. Tomato early blight (Alternaria solani): The pathogen, genetics, and breeding for resistance. J. Gen. Plant Pathol. 2006, 72, 335–347. [Google Scholar] [CrossRef]
- Singh, V. Alternaria diseases of vegetable crops and its management control to reduce the low production. Int. J. Agric. Sci. 2015, 7, 834–840. [Google Scholar]
- Mohsin, S.M.; Islam, M.R.; Ahmmed, A.N.F.; Nisha, H.A.C.; Hasanuzzaman, M. Cultural, morphological and pathogenic characterization of Alternaria porri causing purple blotch of onion. Not. Bot. Horti Agrobot. Cluj-Napoca 2016, 44, 222–227. [Google Scholar] [CrossRef]
- Solfrizzo, M.; Girolamo, A.D.; Vitti, C.; Tylkowska, K.; Grabarkiewicz-Szczęsna, J.; Szopińska, D.; Dorna, H. Toxigenic profile of Alternaria alternata and Alternaria radicina occurring on umbelliferous plants. Food Addit. Contam. 2005, 22, 302–308. [Google Scholar] [CrossRef]
- Addrah, M.E.; Zhang, Y.; Zhang, J.; Liu, L.; Zhou, H.; Chen, W.; Zhao, J. Fungicide treatments to control seed-borne fungi of sunflower seeds. Pathogens 2020, 9, 29. [Google Scholar] [CrossRef]
- Siciliano, I.; Gilardi, G.; Ortu, G.; Gisi, U.; Gullino, M.L.; Garibaldi, A. Identification and characterization of Alternaria species causing leaf spot on cabbage, cauliflower, wild and cultivated rocket by using molecular and morphological features and mycotoxin production. Eur. J. Plant Pathol. 2017, 149, 401–413. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, R.; Ning, H.; Li, W.; Bai, Y.; Li, Y. Evaluation and management of fungal-infected carrot seeds. Sci. Rep. 2020, 10, 10808. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.M. Carrot diseases and their management. In Diseases of Fruits and Vegetables; Naqvi, S.A.M.H., Ed.; Springer: Dordrecht, The Netherlands, 2004; Volume I, pp. 397–439. [Google Scholar]
- González, M.; Caetano, P.; Sánchez, M.E. Testing systemic fungicides for control of Phytophthora oak root disease. For. Pathol. 2017, 47, e12343. [Google Scholar] [CrossRef]
- Lamichhane, J.R.; You, M.P.; Laudinot, V.; Barbetti, M.J.; Aubertot, J.N. Revisiting sustainability of fungicide seed treatments for field crops. Plant Dis. 2020, 104, 610–623. [Google Scholar] [CrossRef] [PubMed]
- Singh, U.B.; Chaurasia, R.; Manzar, N.; Kashyap, A.S.; Malviya, D.; Singh, S.; Kannojia, P.; Sharma, P.K.; Imran, M.; Sharma, A.K. Chemical Management of Seed-Borne Diseases: Achievements and Future Challenges. In Seed-Borne Diseases of Agricultural Crops: Detection, Diagnosis & Management; Kumar, R., Gupta, A., Eds.; Springer: Singapore, 2020; pp. 665–682. [Google Scholar] [CrossRef]
- Töfoli, J.G.; Domingues, R.J.; Tortolo, M.P.L. Effect of various fungicides in the control of Alternaria Leaf Blight in carrot crops. Biol. São Paulo 2019, 81, 1–30. [Google Scholar] [CrossRef]
- Sharma, K.K.; Singh, U.S.; Sharma, P.; Kumar, A.; Sharma, L. Seed treatments for sustainable agriculture-A review. J. Appl. Nat. Sci. 2015, 7, 521–539. [Google Scholar] [CrossRef]
- Mancini, V.; Romanazzi, G. Seed treatments to control seedborne fungal pathogens of vegetable crops. Pest Manag. Sci. 2014, 70, 860–868. [Google Scholar] [CrossRef]
- The European Commission Regulation (EC) No. 1452/2003. Official Journal of the European Community. 2003. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2003:206:0017:0021:EN:PDF (accessed on 9 March 2021).
- Silva, V.; Mol, H.G.; Zomer, P.; Tienstra, M.; Ritsema, C.J.; Geissen, V. Pesticide residues in European agricultural soils–A hidden reality unfolded. Sci. Total Env. 2019, 653, 1532–1545. [Google Scholar] [CrossRef]
- Cozma, P.; Apostol, L.C.; Hlihor, R.M.; Simion, I.M.; Gavrilescu, M. Overview of human health hazards posed by pesticides in plant products. In Proceedings of the 2017 E-Health and Bioengineering Conference (EHB), Sinaia, Romania, 22–24 June 2017; IEEE: Piscataway, NJ, USA, 2017; Volume 17066084, pp. 293–296. [Google Scholar] [CrossRef]
- Ložienė, K.; Venskutonis, P.R. Juniper (Juniperus communis L.) oils. In Essential Oils in Food Preservation, Flavor and Safety; Academic Press: Cambridge, MA, USA, 2016; pp. 495–500. [Google Scholar] [CrossRef]
- Hanif, M.A.; Nisar, S.; Khan, G.S.; Mushtaq, Z.; Zubair, M. Essential oils. In Essential Oil Research; Springer: Cham, Switzerland, 2019; pp. 3–17. [Google Scholar] [CrossRef]
- Valdivieso-Ugarte, M.; Gomez-Llorente, C.; Plaza-Díaz, J.; Gil, Á. Antimicrobial, antioxidant, and immunomodulatory properties of essential oils: A systematic review. Nutrients 2019, 11, 2786. [Google Scholar] [CrossRef]
- Moulodi, F.; Khezerlou, A.; Zolfaghari, H.; Mohamadzadeh, A.; Alimoradi, F. Chemical Composition and Antioxidant and Antimicrobial Properties of the Essential Oil of Hyssopus officinalis L. J. Kermanshah Univ. Med. Sci. 2018, 22, e85256. [Google Scholar] [CrossRef]
- Dauqan, E.M.; Abdullah, A. Medicinal and functional values of thyme (Thymus vulgaris L.) herb. J. Appl. Biol. Biotechnol. 2017, 5, 17–22. [Google Scholar] [CrossRef]
- Karaca, G.; Bilginturan, M.; Olgunsoy, P. Effects of some plant essential oils against fungi on wheat seeds. Indian J. Pharm. Educ. Res 2017, 51, S385–S388. [Google Scholar] [CrossRef]
- Muthukumar, A.; Sangeetha, G.; Naveenkumar, R. Antimicrobial activity of essential oils against seed borne fungi of rice (Oryza sativa L.). J. Environ. Biol. 2016, 37, 1429–1436. [Google Scholar]
- Dorna, H.; Qi, Y.; Szopińska, D. The effect of acetic acid, grapefruit extract and selected essential oils on germination, vigour and health of carrot (Daucus carota L.) seeds. Acta Sci. Pol. Hortorum Cultus 2018, 17, 27–38. [Google Scholar] [CrossRef]
- Koch, E.; Schmitt, A.; Stephan, D.; Kromphardt, C.; Jahn, M.; Krauthausen, H.J.; Forsberg, G.; Werner, S.; Amein, T.; Wright, S.A.I.; et al. Evaluation of non-chemical seed treatment methods for the control of Alternaria dauci and A. radicina on carrot seeds. Eur. J. Plant Pathol. 2010, 127, 99–112. [Google Scholar] [CrossRef]
- Morkeliūnė, A.; Rasiukevičiūtė, N.; Šernaitė, L.; Valiuškaitė, A. The Use of Essential Oils from Thyme, Sage and Peppermint against Colletotrichum acutatum. Plants 2021, 10, 114. [Google Scholar] [CrossRef]
- Soković, M.D.; Vukojević, J.; Marin, P.D.; Brkić, D.D.; Vajs, V.; van Griensven, L.J.L.D. Chemical composition of essential oils of Thymus and Mentha species and their antifungal activities. Molecules 2009, 14, 238–249. [Google Scholar] [CrossRef]
- Graj, W.; Lisiecki, P.; Szulc, A.; Chrzanowski, Ł.; Wojtera-Kwiczor, J. Bioaugmentation with petroleum-degrading consortia has a selective growth-promoting impact on crop plants germinated in diesel oil-contaminated soil. Water Air Soil Pollut. 2013, 224, 1676. [Google Scholar] [CrossRef]
- Mathur, S.B.; Kongsdal, O. Common Laboratory Seed Health Testing Methods for Detecting Fungi, 1st ed.; International Seed Testing Association: Bassersdorf, Switzerland, 2003; 425p, ISBN 3906549356. [Google Scholar]
- Acumedia. A Subsidiary of NEOGEN Corporation. Neogen Food Safety. Potato Dextrose Agar (7149)-Protocol. PI7149 Rev 4. 2011. Available online: http://biotrading.com/assets/productinformatie/acumedia/tds/7149.pdf (accessed on 10 March 2021).
- Thobunluepop, P. The inhibitory effect of the various seed coating substances against rice seed borne fungi and their shelf-life during storage. Pak. J. Biol. Sci. 2009, 12, 1102. [Google Scholar] [CrossRef][Green Version]
- Mancini, V.; Murolo, S.; Romanazzi, G. Diagnostic methods for detecting fungal pathogens on vegetable seeds. Plant Pathol. 2016, 65, 691–703. [Google Scholar] [CrossRef]
- Mamgain, A.; Roychowdhury, R.; Tah, J. Alternaria pathogenicity and its strategic controls. Res. J. Biol. 2013, 1, 1–9. [Google Scholar]
- Stankevičienė, A.; Lugauskas, A. Mikromicetų įvairovė augalų rizosferoje skirtingose oranžerijos sekcijose. Vytauto Didžiojo Univ. Bot. Sodo Raštai = Scr. Horti Bot. Univ. 2008, 12, 84–93. [Google Scholar]
- AOAC. Volatile oil in spices. In Official Methods of Analysis, 15th ed.; Helrich, K., Ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1990; Volume 1. [Google Scholar]
- Šernaitė, L.; Rasiukevičiūtė, N.; Valiuškaitė, A. The extracts of cinnamon and clove as potential biofungicides against strawberry grey mould. Plants 2020, 9, 613. [Google Scholar] [CrossRef] [PubMed]
- Judžentienė, A. Juniperus communis L.: A review of volatile organic compounds of wild and cultivated common juniper in Lithuania. Chemija 2019, 30, 184–193. [Google Scholar] [CrossRef]
- Morkeliūnė, A.; Rasiukevičiūtė, N.; Valiuškaitė, A. Pathogenicity of Colletotrichum acutatum to different strawberry cultivars and anthracnose control with essential oils. Zemdirb. Agric. 2021, 10, 173–180. [Google Scholar] [CrossRef]
- Alexa, E.; Sumalan, R.M.; Danciu, C.; Obistioiu, D.; Negrea, M.; Poiana, M.A.; Dehelean, C. Synergistic antifungal, allelopatic and anti-proliferative potential of Salvia officinalis L., and Thymus vulgaris L. essential oils. Molecules 2018, 23, 185. [Google Scholar] [CrossRef]
- Riccioni, L.; Orzali, L. Activity of tea tree (Melaleuca alternifolia, Cheel) and thyme (Thymus vulgaris, Linnaeus.) essential oils against some pathogenic seed borne fungi. J. Essent. Oil Res. 2011, 23, 43–47. [Google Scholar] [CrossRef]
- Fraternale, D.; Ricci, D.; Epifano, F.; Curini, M. Composition and antifungal activity of two essential oils of hyssop (Hyssopus officinalis L.). J. Essent. Oil Res. 2004, 16, 617–622. [Google Scholar] [CrossRef]
- Judžentienė, A. Hyssop (Hyssopus officinalis L.) Oils. In Essential Oils in Food Preservation, Flavor and Safety; Academic Press: Cambridge, MA, USA, 2016; pp. 471–479. [Google Scholar] [CrossRef]
- Zabka, M.; Pavela, R.; Slezakova, L. Antifungal effect of Pimenta dioica essential oil against dangerous pathogenic and toxinogenic fungi. Ind. Crops Prod. 2009, 30, 250–253. [Google Scholar] [CrossRef]
- Menghani, E.; Sharma, S.K. Antimicrobial activity of Juniperus communis and Solanum xanthocarpum. Int. J. Pharm. Sci. Res. 2012, 3, 2815. [Google Scholar] [CrossRef]



| Common Name | Botanical Name | Cultivar |
|---|---|---|
| Carrot | Daucus carota sativus L. | Svalia |
| Tomato | Solanum lycopersicum L. | Rutuliai |
| Onion | Allium cepa L. | Babtų didieji |
| Seeds | Total Seeds Infected, % | Fungal Contamination, % | |||||
|---|---|---|---|---|---|---|---|
| Alternaria spp. | Fusarium spp. | Aspergillus spp. | Mucor spp. | Penicillium spp. | Mycelia sterilia | ||
| Carrot | 100 | 93.4 | 3.77 | 0 | 0 | 2.83 | 0 |
| Tomato | 20 | 50 | 0 | 15 | 25 | 0 | 10 |
| Onion | 15 | 20 | 0 | 0 | 40 | 40 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chrapačienė, S.; Rasiukevičiūtė, N.; Valiuškaitė, A. Control of Seed-Borne Fungi by Selected Essential Oils. Horticulturae 2022, 8, 220. https://doi.org/10.3390/horticulturae8030220
Chrapačienė S, Rasiukevičiūtė N, Valiuškaitė A. Control of Seed-Borne Fungi by Selected Essential Oils. Horticulturae. 2022; 8(3):220. https://doi.org/10.3390/horticulturae8030220
Chicago/Turabian StyleChrapačienė, Simona, Neringa Rasiukevičiūtė, and Alma Valiuškaitė. 2022. "Control of Seed-Borne Fungi by Selected Essential Oils" Horticulturae 8, no. 3: 220. https://doi.org/10.3390/horticulturae8030220
APA StyleChrapačienė, S., Rasiukevičiūtė, N., & Valiuškaitė, A. (2022). Control of Seed-Borne Fungi by Selected Essential Oils. Horticulturae, 8(3), 220. https://doi.org/10.3390/horticulturae8030220







