Pre-Fermentative Cold Maceration and Native Non-Saccharomyces Yeasts as a Tool to Enhance Aroma and Sensory Attributes of Chardonnay Wine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Wine Production
2.2. Extraction of Volatile Compounds in Wines by Headspace-Solid Phase Microextraction (HS-SPME)
2.3. Gas Chromatography/Mass Spectrometry (GC/MS) Analysis and Gas Chromatography-Flame Ionization Detection (GC/FID) of Volatile Compounds in Wines
2.4. Quantitative HS-SPME Analysis
2.5. Sensory Analysis
2.6. Statistical Analysis
3. Results
3.1. Standard Wine Analysis
3.2. Aromatic Compounds
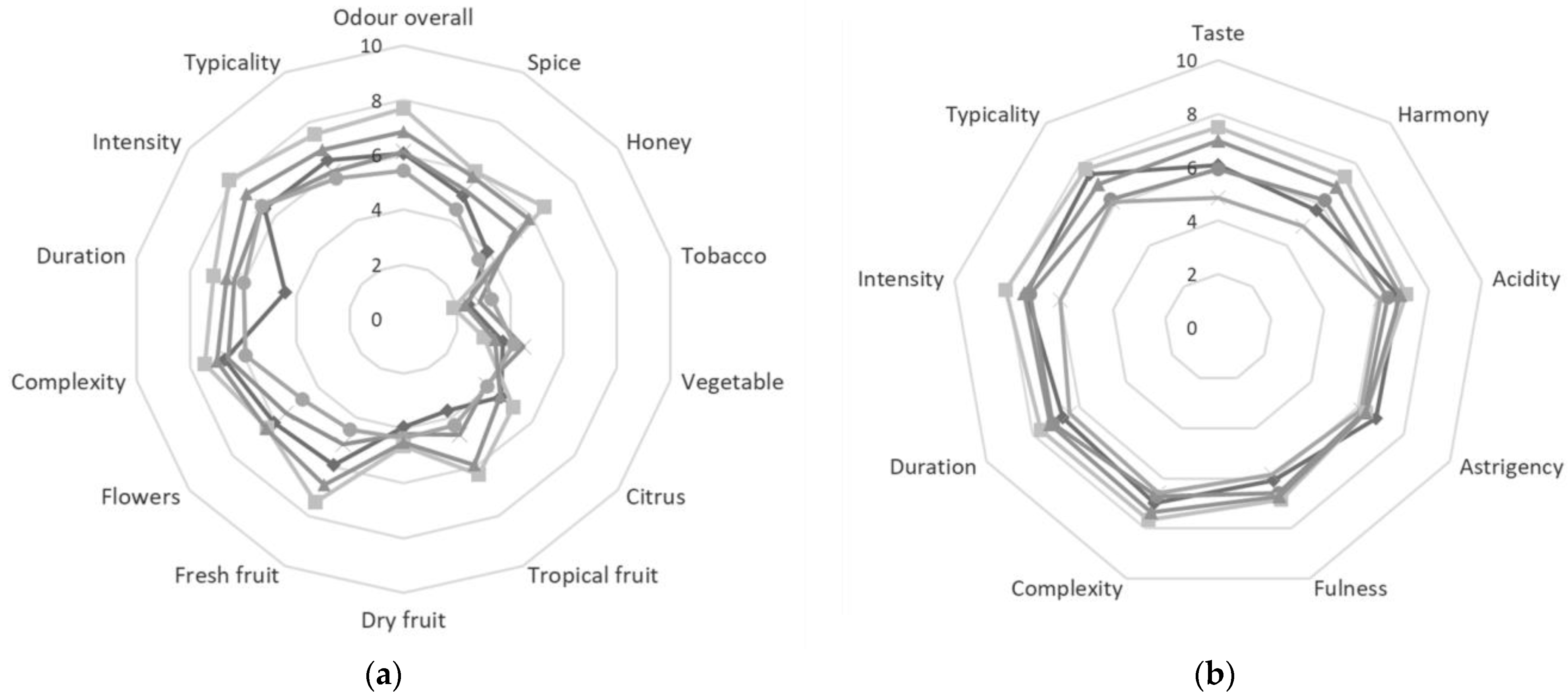
3.3. Sensory Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Samoticha, J.; Wojdyło, A.; Chmielewska, J.; Oszmiański, J. The Effects of Flash Release Conditions on the Phenolic Compounds and Antioxidant Activity of Pinot Noir Red Wine. Eur. Food Res. Technol. 2017, 243, 999–1007. [Google Scholar] [CrossRef] [Green Version]
- De Santis, D.; Frangipane, M.T. Effect of Prefermentative Cold Maceration on the Aroma and Phenolic Profiles of a Merlot Red Wine. Ital. J. Food Sci. 2010, 22, 47–53. [Google Scholar]
- Van Breda, V.; Jolly, N.; van Wyk, J. Characterisation of Commercial and Natural Torulaspora delbrueckii Wine Yeast Strains. Int. J. Food Microbiol. 2013, 163, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Van Wyk, N.; Grossmann, M.; Wendland, J.; Von Wallbrunn, C.; Pretorius, I.S. The Whiff of Wine Yeast Innovation: Strategies for Enhancing Aroma Production by Yeast during Wine Fermentation. J. Agric. Food Chem. 2019, 67, 13496–13505. [Google Scholar] [CrossRef]
- Lin, M.M.H.; Boss, P.K.; Walker, M.E.; Sumby, K.M.; Grbin, P.R.; Jiranek, V. Evaluation of Indigenous Non-Saccharomyces Yeasts Isolated from a South Australian Vineyard for Their Potential as Wine Starter Cultures. Int. J. Food Microbiol. 2020, 312, 108373. [Google Scholar] [CrossRef]
- Englezos, V.; Rantsiou, K.; Torchio, F.; Rolle, L.; Gerbi, V.; Cocolin, L. Exploitation of the Non-Saccharomyces Yeast Starmerella bacillaris (Synonym Candida zemplinina) in Wine Fermentation: Physiological and Molecular Characterizations. Int. J. Food Microbiol. 2015, 199, 12–30. [Google Scholar] [CrossRef]
- Jolly, N.P.; Varela, C.; Pretorius, I.S. Not Your Ordinary Yeast: Non-Saccharomyces Yeasts in Wine Production Uncovered. FEMS Yeast Res. 2014, 14, 215–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belda, I.; Navascués, E.; Marquina, D.; Santos, A.; Calderon, F.; Benito, S. Dynamic Analysis of Physiological Properties of Torulaspora delbrueckii in Wine Fermentations and Its Incidence on Wine Quality. Appl. Microbiol. Biotechnol. 2015, 99, 1911–1922. [Google Scholar] [CrossRef] [PubMed]
- Canonico, L.; Solomon, M.; Comitini, F.; Ciani, M.; Varela, C. Volatile Profile of Reduced Alcohol Wines Fermented with Selected Non-Saccharomyces Yeasts under Different Aeration Conditions. Food Microbiol. 2019, 84, 103247. [Google Scholar] [CrossRef]
- Contreras, A.; Hidalgo, C.; Henschke, P.A.; Chambers, P.J.; Curtin, C.; Varela, C. Evaluation of Non-Saccharomyces Yeasts for the Reduction of Alcohol Content in Wine. Appl. Environ. Microbiol. 2014, 80, 1670–1678. [Google Scholar] [CrossRef] [Green Version]
- González-Royo, E.; Pascual, O.; Kontoudakis, N.; Esteruelas, M.; Esteve-Zarzoso, B.; Mas, A.; Canals, J.M.; Zamora, F. Oenological Consequences of Sequential Inoculation with Non-Saccharomyces Yeasts (Torulaspora delbrueckii or Metschnikowia pulcherrima) and Saccharomyces cerevisiae in Base Wine for Sparkling Wine Production. Eur. Food Res. Technol. 2015, 240, 999–1012. [Google Scholar] [CrossRef]
- Jolly, N.P.; Augustyn, O.P.H.; Pretorius, I.S. The Effect of Non-Saccharomyces Yeasts on Fermentation and Wine Quality. S. Afr. J. Enol. Vitic. 2003, 24, 55–62. [Google Scholar] [CrossRef]
- Padilla, B.; Zulian, L.; Ferreres, À.; Pastor, R.; Esteve-Zarzoso, B.; Beltran, G.; Mas, A. Sequential Inoculation of Native Non-Saccharomyces and Saccharomyces cerevisiae Strains for Wine Making. Front. Microbiol. 2017, 8, 1293. [Google Scholar] [CrossRef]
- Tufariello, M.; Fragasso, M.; Pico, J.; Panighel, A.; Castellarin, S.D.; Flamini, R.; Grieco, F. Influence of Non-Saccharomyces on Wine Chemistry: A Focus on Aroma-Related Compounds. Molecules 2021, 26, 644. [Google Scholar] [CrossRef] [PubMed]
- Manzanares, P.; Vallés, S.; Viana, F. Non-Saccharomyces Yeasts in the Winemaking Process. In Molecular Wine Microbiology; Carrascosa, A.V., Muñoz, R., González, R., Eds.; Academic Press: Cambridge, MA, USA, 2011; pp. 85–110. [Google Scholar]
- Fernández-González, M.; Di Stefano, R.; Briones, A. Hydrolysis and Transformation of Terpene Glycosides from Muscat Must by Different Yeast Species. Food Microbiol. 2003, 20, 35–41. [Google Scholar] [CrossRef]
- Mančić, S.; Danilović, B.; Malićanin, M.; Stojanović, S.S.; Nikolić, N.; Lazić, M.; Karabegović, I. Fermentative Potential of Native Yeast Candida famata for Prokupac Grape Must Fermentation. Agriculture 2021, 11, 358. [Google Scholar] [CrossRef]
- Barbosa, C.; Lage, P.; Esteves, M.; Chambel, L.; Mendes-Faia, A.; Mendes-Ferreira, A. Molecular and Phenotypic Characterization of Metschnikowia pulcherrima Strains from Douro Wine Region. Fermentation 2018, 4, 8. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez, M.E.; Lopes, C.A.; Barbagelata, R.J.; Barda, N.B.; Caballero, A.C. Influence of Candida pulcherrima Patagonian Strain on Alcoholic Fermentation Behaviour and Wine Aroma. Int. J. Food Microbiol. 2010, 138, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Morata, A.; Escott, C.; Loira, I.; Manuel Del Fresno, J.; González, C.; Suárez-Lepe, J.A. Influence of Saccharomyces and Non-Saccharomyces Yeasts in the Formation of Pyranoanthocyanins and Polymeric Pigments during Red Wine Making. Molecules 2019, 24, 4490. [Google Scholar] [CrossRef] [Green Version]
- Morata, A.; Escott, C.; Bañuelos, M.A.; Loira, I.; Del Fresno, J.M.; González, C.; Suárez-lepe, J.A. Contribution of Non-Saccharomyces Yeasts to Wine Freshness. A Review. Biomolecules 2020, 10, 34. [Google Scholar] [CrossRef] [Green Version]
- Oro, L.; Ciani, M.; Comitini, F. Antimicrobial Activity of Metschnikowia Pulcherrima on Wine Yeasts. J. Appl. Microbiol. 2014, 116, 1209–1217. [Google Scholar] [CrossRef] [PubMed]
- OIV 370-2012; Guidelines for the Characterization of Wine Yeasts of the Genus Saccharomyces Isolated from Vitivinicultural Environments. International Organisation of Vine and Wine: Paris, France, 2012.
- ISO 3591; Sensory Analysis—Apparatus—Wine-Tasting Glass. International Organization for Standardization: Geneva, Switzerland, 1977.
- ISO 6658; Sensory Analysis—Methodology—General Guidance. International Organization for Standardization: Geneva, Switzerland, 2017.
- OIV. Review Document on Sensory Analysis of Wine; OIV: Paris, France, 2015. [Google Scholar]
- Jiang, B.; Zhang, Z. Volatile Compounds of Young Wines from Cabernet Sauvignon, Cabernet Gernischet and Chardonnay Varieties Grown in the Loess Plateau Region of China. Molecules 2010, 15, 9184–9196. [Google Scholar] [CrossRef] [Green Version]
- Korenika, A.M.J.; Preiner, D.; Tomaz, I.; Jeromel, A. Volatile Profile Characterization of Croatian Commercial Sparkling Wines. Molecules 2020, 25, 137–149. [Google Scholar]
- Welke, J.E.; Zanus, M.; Lazzarotto, M.; Alcaraz Zini, C. Quantitative Analysis of Headspace Volatile Compounds Using Comprehensive Two-Dimensional Gas Chromatography and Their Contribution to the Aroma of Chardonnay Wine. Food Res. Int. 2014, 59, 85–99. [Google Scholar] [CrossRef] [Green Version]
- Cameleyre, M.; Lytra, G.; Tempere, S.; Barbe, J.C. 2-Methylbutyl Acetate in Wines: Enantiomeric Distribution and Sensory Impact on Red Wine Fruity Aroma. Food. Chem. 2017, 237, 364–371. [Google Scholar] [CrossRef]
- Medina, K.; Boido, E.; Fariña, L.; Gioia, O.; Gomez, M.E.; Barquet, M.; Gaggero, C.; Dellacassa, E.; Carrau, F. Increased Flavour Diversity of Chardonnay Wines by Spontaneous Fermentation and Co-Fermentation with Hanseniaspora vineae. Food Chem. 2013, 141, 2513–2521. [Google Scholar] [CrossRef]
- Blanco, P.; Castrillo, D.; Graña, M.J.; Lorenzo, M.J.; Soto, E. Evaluation of Autochthonous Non-saccharomyces Yeasts by Sequential Fermentation for Wine Differentiation in Galicia (NW Spain). Fermentation 2021, 7, 183. [Google Scholar] [CrossRef]
- Casassa, L.F.; Bolcato, E.A.; Sari, S.E.; Barda, N. Effects of Maceration Length after Prefermentative Cold Soak: Detailed Chromatic, Phenolic and Sensory Composition of Cabernet Sauvignon, Malbec and Merlot Wines. J. Food Compos. Anal. 2021, 104, 104168. [Google Scholar] [CrossRef]
- Jordeva, S.N.; Mojsov, K.D.; Andronikov, D.; Janevski, A.; Gaber, S. The Effects of the Duration of Cold Maceration on the composition and sensory properties of Smederevka wines. Adv. Technol. 2016, 5, 60–63. [Google Scholar] [CrossRef] [Green Version]
- Zoecklein, B.W.; Fugelsang, K.C.; Gump, B.H.; Nury, F.S. Wine Analysis and Production; Springer: Boston, MA, USA, 1995; Volume 7. [Google Scholar]
- Dutraive, O.; Benito, S.; Fritsch, S.; Beisert, B.; Patz, C.D.; Rauhut, D. Effect of Sequential Inoculation with Non-Saccharomyces and Saccharomyces Yeasts on Riesling Wine Chemical Composition. Fermentation 2019, 5, 79. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Moreno, M.J.; Muñoz-Redondo, J.M.; Cuevas, F.J.; Marrufo-Curtido, A.; León, J.M.; Ramírez, P.; Moreno-Rojas, J.M. The Influence of Pre-Fermentative Maceration and Ageing Factors on Ester Profile and Marker Determination of Pedro Ximenez Sparkling Wines. Food Chem. 2017, 230, 697–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lambrechts, M.G.; Pretorius, I.S. Yeast and Its Importance to Wine Aroma—A Review. S. Afr. J. Enol. Vitic. 2000, 21, 97–129. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.; Zhang, R.; Sirisena, S.; Gan, R.; Fang, Z. Beta-Glucosidase Activity of Wine Yeasts and Its Impacts on Wine Volatiles and Phenolics: A Mini-Review. Food Microbiol. 2021, 100, 103859. [Google Scholar] [CrossRef] [PubMed]
- Olejar, K.J.; Fedrizzi, B.; Kilmartin, P.A. Influence of Harvesting Technique and Maceration Process on Aroma and Phenolic Attributes of Sauvignon Blanc Wine. Food Chem. 2015, 183, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Olejar, K.J.; Fedrizzi, B.; Kilmartin, P.A. Enhancement of Chardonnay Antioxidant Activity and Sensory Perception through Maceration Technique. LWT Food Sci. Technol. 2016, 65, 152–157. [Google Scholar] [CrossRef]
- Lappa, I.K.; Kachrimanidou, V.; Pateraki, C.; Koulougliotis, D.; Eriotou, E.; Kopsahelis, N. Indigenous Yeasts: Emerging Trends and Challenges in Winemaking. Curr. Opin. Food Sci. 2020, 32, 133–143. [Google Scholar] [CrossRef]
- Torrea, D.; Varela, C.; Ugliano, M.; Ancin-Azpilicueta, C.; Leigh Francis, I.; Henschke, P.A. Comparison of Inorganic and Organic Nitrogen Supplementation of Grape Juice—Effect on Volatile Composition and Aroma Profile of a Chardonnay Wine Fermented with Saccharomyces Cerevisiae Yeast. Food Chem. 2011, 127, 1072–1083. [Google Scholar] [CrossRef] [PubMed]
- Saberi, S.; Cliff, M.A.; van Vuuren, H.J.J. Impact of Mixed, S. cerevisiae Strains on the Production of Volatiles and Estimated Sensory Profiles of Chardonnay Wines. Food Res. Int. 2012, 48, 725–735. [Google Scholar] [CrossRef]
- Kustos, M.; Gambetta, J.M.; Jeffery, D.W.; Heymann, H.; Goodman, S.; Bastian, S.E.P. A Matter of Place: Sensory and Chemical Characterisation of Fine Australian Chardonnay and Shiraz Wines of Provenance. Food Res. Int. 2020, 130, 222–237. [Google Scholar] [CrossRef]
- Liberatore, M.T.; Pati, S.; Del Nobile, M.A.; Notte, E. La Aroma Quality Improvement of Chardonnay White Wine by Fermentation and Ageing in Barrique on Lees. Food Res. Int. 2010, 43, 996–1002. [Google Scholar] [CrossRef]
- Pietruszka, M.; Pielech-Przybylska, K.; Szopa, J.S. Synthesis of Higher Alcohols during Alcoholic Fermentation of Rye Mashes. Food Chem. Biotechnol. 2010, 74, 51–64. [Google Scholar]
- Escribano-Viana, R.; González-Arenzana, L.; Portu, J.; Garijo, P.; López-Alfaro, I.; López, R.; Santamaría, P.; Gutiérrez, A.R. Wine Aroma Evolution throughout Alcoholic Fermentation Sequentially Inoculated with Non-Saccharomyces/Saccharomyces Yeasts. Food Res. Int. 2018, 112, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Iris, L.; Antonio, M.; Antonia, B.M.; Antonio, S.L.J. Isolation, selection, and identification techniques for non-saccharomyces yeasts of oenological interest. In Biotechnological Progress and Beverage Consumption; Mihai Grumezescu, A., Holban, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2020; Volume 19, pp. 467–508. [Google Scholar]
- Ribéreau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. Handbook of Enology: The Chemistry of Wine: Stabilization and Treatments, 2nd ed.; John Wiley & Sons Ltd: Hoboken, NJ, USA, 2006; pp. 155–188. [Google Scholar]
- Puertas, B.; Jimenez-Hierro, M.J.; Cantos-Villar, E.; Marrufo-Curtido, A.; Carbú, M.; Cuevas, F.J.; Moreno-Rojas, J.M.; González-Rodríguez, V.E.; Cantoral, J.M.; Ruiz-Moreno, M.J. The Influence of Yeast on Chemical Composition and Sensory Properties of Dry White Wines. Food Chem. 2018, 253, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Pérez, A.; Vila-López, R.; Fernández-Fernández, J.I.; Martínez-Cutillas, A.; Gil-Muñoz, R. Influence of Cold Pre-Fermentation Treatments on the Major Volatile Compounds of Three Wine Varieties. Food Chem. 2013, 139, 770–776. [Google Scholar] [CrossRef] [PubMed]
- Marić, J.; Firšt-Bača, M. Sensory Evaluation and Some Acetate Esters of Bottle Aged Chardonnay Wines. Plant Soil Environ. 2003, 49, 332–336. [Google Scholar] [CrossRef] [Green Version]
- Torrens, J.; Rlu-Aumatell, M.; Vichi, S.; López-Tamames, E.; Buxaderas, S. Assessment of Volatlle and Sensory Profiles between Base and Sparkling Wines. J. Agric. Food Chem. 2010, 58, 2455–2461. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.J.; Tao, Y.S.; Wu, Y.; An, R.Y.; Yue, Z.Y. Aroma Compounds and Characteristics of Noble-Rot Wines of Chardonnay Grapes Artificially Botrytized in the Vineyard. Food Chem. 2017, 226, 41–50. [Google Scholar] [CrossRef]
- Lengyel, E.; Sikolya, L. The Influence of Aroma Compounds on Senzorial Traits of Wines from the Apold Depression. Manag. Sustain. Dev. 2015, 7, 23–28. [Google Scholar] [CrossRef]
- Delfini, C.; Gaia, P.; Mariscalco, G.; Contiero, M.; Pagliara, A. Production of Benzaldehyde, Benzyl Alcohol and Benzoic Acid by Yeasts and Botrytis Cinerea Isolated from Grape Musts and Wines. Vitis 1991, 30, 253–263. [Google Scholar]
- Zhang, X.; Kontoudakis, N.; Blackman, J.W.; Clark, A.C. Copper (II) and Sulfur Dioxide in Chardonnay Juice and Shiraz Must: Impact on Volatile Aroma Compounds and Cu Forms in Wine. Beverages 2019, 5, 70. [Google Scholar] [CrossRef] [Green Version]

| Parameter | Process | M. pulcherrima B-5 | C. famata WB-1 | S. cerevisiae QA23 | M. pulcherrima B-5 + S. cerevisiae QA23 | C. famata WB-1 + S. cerevisiae QA23 |
|---|---|---|---|---|---|---|
| Ethanol, %v/v | NM | 14.39 bA ± 0.03 | 13.58 aA ± 0.22 | 14.32 bA ± 0.16 | 14.42 bA ± 0.12 | 14.07 bA ± 0.08 |
| CM | 14.42 bcA ± 0.08 | 14.08 aB ± 0.08 | 14.48 cA ± 0.10 | 14.41 cbA ± 0.12 | 14.17 abcA ± 0.10 | |
| Total extract, g/L | NM | 21.32 aA ± 0.08 | 24.04 cA ± 0.15 | 22.22 bA ± 0.22 | 21.15 aA ± 0.15 | 26.15 dA ± 0.15 |
| CM | 25.53 aB ± 0.06 | 27.75 bB ± 0.15 | 25.34 aB ± 0.25 | 25.75 aB ± 0.15 | 29.85 cB ± 0.15 | |
| Total acids (as tartaric acid) g/L | NM | 6.20 aB ± 0.08 | 6.00 aA ± 0.15 | 6.00 abA ± 0.15 | 6.40 acB ± 0.08 | 6.40 abB ± 0.20 |
| CM | 6.00 aA ± 0.10 | 5.80 aA ± 0.20 | 5.90 aA ± 0.1 | 6.10 abA ± 0.15 | 6.20 bA ± 0.1 | |
| Volatile acids (as acetic acid) | NM | 0.39 aA ± 0.06 | 0.42 aA ± 0.03 | 0.36 abA ± 0.02 | 0.39 aA ± 0.02 | 0.48 acA ± 0.06 |
| CM | 0.38 aA ± 0.04 | 0.39 aA ± 0.04 | 0.39 aA ± 0.04 | 0.36 aA ± 0.03 | 0.39 aA ± 0.01 | |
| Reducing sugar g/L | NM | 2.45 bA ± 0.5 | 3.82 cB ± 0.10 | 2.28 bA ± 0.02 | 1.50 aA ± 0.05 | 5.44 dB ± 0.04 |
| CM | 2.45 bA ± 0.04 | 4.24 eA ± 0.04 | 2.60 cB ± 0.02 | 2.34 aB ± 0.02 | 3.44 dA ± 0.04 | |
| Total polyphenols, g/L | NM | 0.386 dA ± 0.004 | 0.359 bA ± 0.001 | 0.359 bA ± 0.002 | 0.348 cA ± 0.002 | 0.312 aA ± 0.002 |
| CM | 0.393 bA ± 0.003 | 0.370 abB ± 0.005 | 0.373 abB ± 0.006 | 0.353 aA ± 0.002 | 0.358 aB ± 0.017 | |
| Free SO2, mg/L | NM | 26.88 cB ± 0.02 | 29.44 dB ± 0.11 | 24.72 aB ± 0.02 | 25.60 bB ± 0.02 | 26.88 cB ± 0.02 |
| CM | 25.60 dA ± 0.02 | 19.20 bA ± 0.02 | 21.76 cA ± 0.06 | 21.76 cA ± 0.04 | 15.36 aA ± 0.06 | |
| Total SO2, mg/L | NM | 89.60 cB ± 0.10 | 94.72 dB ± 0.02 | 128.1 eB ± 0.21 | 81.92 bB ± 0.02 | 80.65 aB ± 0.05 |
| CM | 84.72 eA ± 0.01 | 71.44 aA ± 0.04 | 76.8 cA ± 0.02 | 79.36 dA ± 0.06 | 72.16 bA ± 0.04 |
| Compound | Aroma Descriptors | Odour Threshold Level, mg/L | Pre-Fermentative Treatment | C. famata WB-1 | C. famata WB-1 + S. cerevisiae QA23 | M. pulcherrima B-5 | M. pulcherrima B-5 + S. cerevisiae QA23 | S. cerevisiae QA23 |
|---|---|---|---|---|---|---|---|---|
| Alcohols | ||||||||
| Isobutyl alcohol | Fusel, alcohol * | 40.00 * | NM | 24.51 cA ±1.12 | 16.02 bA ± 0.68 | 21.42 cA ±1.79 | 15.20 abA ± 0.23 | 13.02 aA ± 0.19 |
| CM | 27.97 cB ±1.05 | 20.12 aB ± 0.86 | 31.56 dB ±1.64 | 25.07 bcB ± 0.58 | 21.79 abB ± 0.86 | |||
| 1-Propanol | Fresh, alcohol * | 306 * | NM | nd | nd | nd | nd | nd |
| CM | nd | nd | nd | nd | 8.16 ± 0.22 | |||
| 3-Methyl-1-butanol | Whisky, alcohol ** | 30.00 ** | NM | 145.05 bcA ±8.92 | 159.62 dA ±5.12 | 125.89 aA ±5.53 | 156.18 cdB ±1.18 | 139.68 abB ±1.44 |
| CM | 138.84 abA ±5.21 | 167.78 cA ±3.87 | 140.03 bB ±1.64 | 146.40 bA ±3.38 | 130.14 aA ±3.00 | |||
| 2-Methyl-1-butanol | Whisky, malt ** | 30.00 ** | NM | tr | tr | 21.04 aB ± 0.25 | 22.22 bB ± 0.34 | 25.96 cB ± 0.38 |
| CM | nd | nd | 10.48 aA ± 0.33 | 13.10 bA ± 0.56 | 13.25 bA ± 0.43 | |||
| 1-Pentanol | Fruity, balsamic * | 80.00 * | NM | 21.81 dA ±1.66 | 13.50 bB ± 0.41 | 24.70 eA ± 0.79 | 15.68 cB ± 0.33 | 3.53 aA ± 0.02 |
| CM | 28.46 dB ± 0.43 | 10.45 bA ± 0.34 | 25.69 cA ± 0.90 | 9.15 aA ± 0.39 | 8.31 aB ± 0.19 | |||
| (S)-(-)-2-Methyl-1-butanol | - | 30.00 * | NM | nd | tr | 9.62 aA ± 0.34 | tr | 10.00 aA ± 0.14 |
| CM | nd | nd | 10.62 aA ± 0.89 | 19.69 c ± 0.84 | 14.69 bB ± 0.61 | |||
| Isohexanol | - | - | NM | nd | nd | nd | nd | nd |
| CM | nd | tr | 3.28 ± 0.09 | tr | nd | |||
| n-Hexanol | Green grass * | 8.00 * | NM | 0.99 bA ± 0.04 | 1.15 cB ± 0.03 | nd | 1.77 d ± 0.03 | 0.70 a ± 0.01 |
| CM | 1.94 aA ± 0.04 | 0.33 bA ± 0.01 | nd | nd | nd | |||
| Phenyl ethyl alcohol | Flower, pollen * | 14.00 * | NM | 46.83 bB ±3.87 | 70.42 cB ±1.45 | 44.30 bB ±1.27 | 68.16 cB ± 0.87 | 35.85 aA ± 0.66 |
| CM | 36.61 bA ± 0.71 | 56.06 cA ±2.39 | 26.58 aA ±2.22 | 55.17 cA ±1.81 | 39.29 bB ±1.08 | |||
| S,S-2,3 butanediol | Butter ** | 668.00 ** | NM | nd | 10.27 ± 0.44 | nd | nd | nd |
| CM | nd | nd | nd | nd | nd | |||
| Total alcohols | NM | 239.19 aA ± 15.69 | 270.98 bB ± 7.97 | 246.97 aA ± 9.79 | 279.21 bA ± 3.59 | 228.47 aA ± 2.76 | ||
| CM | 233.82 aA ± 7.35 | 254.74 cA ± 7.31 | 248.24 bA ± 8.60 | 268.58 bA ± 7.47 | 235.62 aA ± 6.21 | |||
| Esters | ||||||||
| Ethyl acetate | Fruity, sweet * | 7.500 * | NM | 36.28 cA ±1.57 | 24.75 aA ± 0.81 | 37.78 cA ± 0.99 | 29.78 bA ± 0.61 | 23.73 aA ± 0.30 |
| CM | 57.62 cB ± 0.82 | 35.27 aB ±1.15 | 66.82 dB ±1.75 | 47.32 bB ± 0.97 | 32.32 aB ±1.06 | |||
| Ethyl hexanoate | Fruity, anis * | 0.005 * | NM | 0.35 eB ± 0.02 | 0.26 cB ± 0.01 | 0.33 dB ± 0.01 | 0.15 aB ± 0.00 | 0.23 bB ± 0.00 |
| CM | 0.09 cA ± 0.00 | 0.08 bA ± 0.00 | 0.11 dA ± 0.00 | 0.08 bA ± 0.00 | 0.06 aA ± 0.00 | |||
| Ethyl isohexanoate | Fruity # | - | NM | 0.17 eB ± 0.01 | 0.06 cA ± 0.00 | 0.14 dA ± 0.00 | 0.05 bA ± 0.00 | 0.02 aA ± 0.00 |
| CM | 0.13 cA ± 0.00 | 0.10 bB ± 0.00 | 0.18 fB ± 0.00 | 0.15 dB ± 0.00 | 0.03 aB ± 0.00 | |||
| Diethyl succinate | Light fruity * | 500 * | NM | nd | nd | nd | nd | nd |
| CM | 3.60 a ± 0.06 | 3.42 a ± 0.15 | tr | nd | nd | |||
| Ethyl octanoate | Pineapple, pear, floral * | 0.002 * | NM | 1.88 bB ± 0.06 | 2.52 dB ± 0.11 | 2.18 cA ± 0.03 | 2.89 eB ± 0.03 | 1.52 aA ± 0.03 |
| CM | 1.21 aA ± 0.04 | 1.80 cA ± 0.06 | 2.27 dA ± 0.06 | 2.33 dA ± 0.05 | 1.51 bA ± 0.01 | |||
| Ethyl decanoate | Fruity, pleasant * | 0.200 * | NM | 3.36 cB ± 0.10 | 2.86 bB ± 0.09 | 3.40 cB ± 0.09 | 2.81 bA ± 0.03 | 1.82 aB ± 0.03 |
| CM | 1.19 aA ± 0.04 | 1.71 bA ± 0.04 | 2.14 cA ± 0.05 | 2.74 dA ± 0.09 | 1.60 bA ± 0.05 | |||
| Ethyl dodecanoate | Floral, fruity * | 1.500 * | NM | 0.22 dB ± 0.01 | 0.20 cB ± 0.00 | 0.23 dB ± 0.01 | 0.17 bB ± 0.00 | 0.14 aA ± 0.00 |
| CM | 0.08 bA ± 0.00 | 0.07 aA ± 0.00 | 0.14 dA ± 0.00 | 0.13 cA ± 0.00 | 0.17 eB ± 0.00 | |||
| 3-Methyl butyl acetate | Banana * | 0.03 * | NM | 2.60 dB ± 0.18 | 2.40 dB ± 0.08 | 1.80 bB ± 0.06 | 2.10 cB ± 0.04 | 1.40 aB ± 0.02 |
| CM | 1.64 eA ± 0.05 | 0.90 cA ± 0.03 | 1.07 dA ± 0.02 | 0.70 bA ± 0.02 | 0.31 aA ± 0.01 | |||
| 2- Methyl butyl acetate | Blackberry, banana † | 1.083 † | NM | 11.54 eB ± 0.37 | 6.83 dB ± 0.22 | 5.31 cB ± 0.08 | 2.38 bB ± 0.05 | 2.11 aB ± 0.04 |
| CM | 0.99 dA ± 0.04 | 0.26 abA ± 0.01 | 0.34 cA ± 0.03 | 0.30 bcA ± 0.01 | 0.21 aA ± 0.00 | |||
| Methyl-2-methyl octanoate | - | - | NM | nd | nd | nd | nd | 0.14 ± 0.00 |
| CM | nd | nd | nd | nd | nd | |||
| Ethyl 3-methyl pentanoate | - | - | NM | nd | nd | nd | nd | 0.20 B ± 0.00 |
| CM | nd | nd | nd | nd | 0.01 A ± 0.00 | |||
| Ethyl-(4 E)-decenoate | - | - | NM | 0.02 bA ± 0.00 | 0.04 cA ± 0.00 | 0.02 bA ± 0.00 | 0.04 cB ± 0.00 | 0.01 aA ± 0.00 |
| CM | 0.10 dB ± 0.00 | 0.06 cB ± 0.00 | 0.02 bA ± 0.00 | 0.01 aA ± 0.00 | 0.01 aA ± 0.00 | |||
| 2-phenil-ethyl acetate | Pleasant, floral * | 0.25 * | NM | 1.62 cB ± 0.07 | 1.39 bB ± 0.04 | 1.86 dA ± 0.06 | 1.44 bA ± 0.02 | 0.97 aB ± 0.02 |
| CM | 1.10 cA ± 0.04 | 0.69 aA ± 0.05 | 1.92 dA ± 0.04 | 1.89 dB ± 0.06 | 0.81 bA ± 0.02 | |||
| Hexyl acetate | Pleasant, fruity, pear * | 0.670 * | NM | nd | nd | nd | nd | nd |
| CM | 0.03 ± 0.00 | nd | tr | nd | nd | |||
| Ethyl butanoate | Pineapple, apple, peach ** | 0.02 ** | NM | 1.13 cA ± 0.06 | 1.01 bB ± 0.02 | nd | 0.02 a ± 0.00 | nd |
| CM | 1.18 bA ± 0.03 | 0.20 aA ± 0.00 | nd | nd | nd | |||
| Total esters | NM | 59.17 dA ± 2.37 | 42.32 bA ± 1.39 | 53.05 cA ± 1.32 | 41.83 bA ± 0.82 | 32.29 aA ± 0.45 | ||
| CM | 67.96 dB ± 1.27 | 44.50 bB ± 1.50 | 75.01 eB ± 1.96 | 55.65 cB ± 1.19 | 37.04 aB ± 1.17 | |||
| Aldehydes | ||||||||
| Benzaldehyde | Almond * | 2.000 * | NM | nd | nd | nd | nd | nd |
| CM | 0.08 a ± 0.00 | 0.09 b ± 0.00 | 0.10 c ± 0.00 | 0.11 d ± 0.00 | 0.09 b ± 0.00 | |||
| Acids | ||||||||
| Hexanoic acid | Cheese aroma, fatty * | 3.000 * | NM | nd | 0.66 ± 0.01 | nd | nd | nd |
| CM | nd | nd | nd | nd | nd | |||
| Octanoic acid | Unpleasant, cheese, fatty acid * | 0.500 * | NM | 4.05 cB ± 0.15 | 4.05 cB ± 0.05 | 4.43 dB ± 0.05 | 1.57 aB ± 0.01 | 2.62 bB ± 0.03 |
| CM | 0.94 bA ± 0.01 | 0.83 aA ± 0.01 | 1.08 cA ± 0.01 | 1.0 bcA ± 0.03 | 2.19 dA ± 0.07 | |||
| Decanoic acid | Fatty, unpleasant * | 15.000 * | NM | 2.61 c ± 0.09 | 4.17 eB ± 0.14 | 3.06 dB ± 0.07 | 1.13 aA ± 0.02 | 1.46 bA ± 0.03 |
| CM | tr | 0.70 aA ± 0.02 | 0.84 bA ± 0.02 | 1.54 cB ± 0.05 | 1.77 dB ± 0.06 | |||
| Total acids | NM | 6.66 cB ± 0.23 | 8.88 eB ± 0.21 | 7.49 dB ± 0.12 | 2.70 aB ± 0.04 | 4.08 bB ± 0.05 | ||
| CM | 0.94 aA ± 0.01 | 1.53 bA ± 0.04 | 1.92 cA ± 0.03 | 2.54 dA ± 0.08 | 3.96 eA ± 0.13 | |||
| Ketones | ||||||||
| Acetoin | Floral, wet * | 150 * | NM | 0.48 cA ± 0.02 | 0.52 cB ± 0.01 | 0.22 bB ± 0.00 | 0.56 dB ± 0.00 | 0.02 aA ± 0.00 |
| CM | 0.96 bB ± 0.05 | 0.23 cA ± 0.01 | 0.11 aA ± 0.00 | 0.26 cA ± 0.01 | 0.05 aB ± 0.00 | |||
| Compound | Aroma Descriptors | Treatment | C. famata WB-1 | C. famata WB-1 + S. cerevisiae QA23 | M, pulcherrima B-5 | M. pulcherrima B-5 + S. cerevisiae QA23 | S. cerevisiae QA23 |
|---|---|---|---|---|---|---|---|
| 3-methyl-1-butanol | Whisky, alcohol | NM | 4.8 (0.4%) | 5.3 (0.4%) | 4.2 (0.3%) | 5.2 (0.3%) | 4.7 (0.5%) |
| CM | 4.6 (0.6%) | 5.6 (0.6%) | 4.7 (0.4%) | 4.9 (0.4%) | 4.3 (0.5%) | ||
| Phenyl ethyl alcohol | Flower, pollen | NM | 3.3 (0.3%) | 5.0 (0.3%) | 3.2 (0.2%) | 4.9 (0.3%) | 2.6 (0.3%) |
| CM | 2.6 (0.3%) | 4.0 (0.4%) | 1.9 (0.2%) | 3.9 (0.3%) | 2.8 (0.3%) | ||
| Ethyl acetate | Fruity, sweet | NM | 4.8 (0.4%) | 3.3 (0.2%) | 5.0 (0.4%) | 4.0 (0.2%) | 3.2 (0.3%) |
| CM | 7.7 (1%) | 4.7 (0.4%) | 8.9 (0.7%) | 6.3 (0.5%) | 4.3 (0.5%) | ||
| Ethyl hexanoate | Fruity, anis | NM | 70.0 (5.8%) | 52.0 (3.5%) | 66.0 (5.2%) | 30.0 (1.9%) | 46.0 (5.2%) |
| CM | 18.0 (2.3%) | 16.0 (1.6%) | 22.0 (1.8%) | 16.0 (1.3%) | 12.0 (1.5%) | ||
| Ethyl octanoate | Pineapple, pear, floral | NM | 940 (77.8%) | 1260 (84.5%) | 1090 (86%) | 1445 (91.2%) | 760 (86.0%) |
| CM | 605 (79.1%) | 900 (31.5%) | 1135 (92.3%) | 1165 (93.7%) | 755 (93.8%) | ||
| Ethyl decanoate | Fruity, pleasant | NM | 16.8 (1.4%) | 14.3 (1%) | 17.0 (1.3%) | 14.1 (0.9%) | 9.1 (1.3%) |
| CM | 6.0 (0.8%) | 8.6 (0.9%) | 10.7 (0.9%) | 13.7 (1.1%) | 8.0 (1%) | ||
| 3-Methyl butyl acetate | Banana | NM | 86.7 (7.2%) | 80.0 (5.4%) | 60.0 (4.7%) | 70.0 (4.4%) | 46.7 (5.3%) |
| CM | 54.7 (7.1%) | 30.0 (3.0%) | 35.7 (2.9%) | 23.3 (1.9%) | 10.3 (1.3%) | ||
| 2-Methyl butyl acetate | Blackberri, banana | NM | 10.7 (0.9%) | 6.3 (0.4%) | 4.9 (0.4%) | 2.2 (0.1%) | 1.9 (0.2%) |
| CM | 0.9 (0.1%) | 0.2 (<0.1%) | 0.3 (<0.1%) | 0.3 (<0.1%) | 0.2 (<0.1%) | ||
| 2-phenil-ethil acetate | Pleasant, floral | NM | 6.5 (0.5%) | 5.6 (0.4%) | 7.4 (0.6%) | 5.8 (0.4%) | 3.9 (0.4%) |
| CM | 4.4 (0.6%) | 2.8 (0.3%) | 7.7 (0.6%) | 7.6 (0.6%) | 3.2 (0.4%) | ||
| Ethyl butanoate | Pineapple, apple, peach | NM | 56.5 (4.7%) | 50.5 (3.4%) | 0.0 | 1.0 (<0.1%) | 0.0 |
| CM | 59.0 (7.7%) | 10.0 (1%) | 0.0 | 0.0 | 0.0 | ||
| Octanoic acid | Unpleasant, cheese aroma, fatty acid | NM | 8.1 (0.7%) | 8.1 (0.5%) | 8.9 (0.7%) | 3.1 (0.2%) | 5.2 (0.6%) |
| CM | 1.9 (0.3%) | 1.7 (0.2%) | 2.2 (0.2%) | 2.0 (0.2%) | 4.4 (0.5%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malićanin, M.; Danilović, B.; Stamenković Stojanović, S.; Cvetković, D.; Lazić, M.; Karabegović, I.; Savić, D. Pre-Fermentative Cold Maceration and Native Non-Saccharomyces Yeasts as a Tool to Enhance Aroma and Sensory Attributes of Chardonnay Wine. Horticulturae 2022, 8, 212. https://doi.org/10.3390/horticulturae8030212
Malićanin M, Danilović B, Stamenković Stojanović S, Cvetković D, Lazić M, Karabegović I, Savić D. Pre-Fermentative Cold Maceration and Native Non-Saccharomyces Yeasts as a Tool to Enhance Aroma and Sensory Attributes of Chardonnay Wine. Horticulturae. 2022; 8(3):212. https://doi.org/10.3390/horticulturae8030212
Chicago/Turabian StyleMalićanin, Marko, Bojana Danilović, Sandra Stamenković Stojanović, Dragan Cvetković, Miodrag Lazić, Ivana Karabegović, and Dragiša Savić. 2022. "Pre-Fermentative Cold Maceration and Native Non-Saccharomyces Yeasts as a Tool to Enhance Aroma and Sensory Attributes of Chardonnay Wine" Horticulturae 8, no. 3: 212. https://doi.org/10.3390/horticulturae8030212
APA StyleMalićanin, M., Danilović, B., Stamenković Stojanović, S., Cvetković, D., Lazić, M., Karabegović, I., & Savić, D. (2022). Pre-Fermentative Cold Maceration and Native Non-Saccharomyces Yeasts as a Tool to Enhance Aroma and Sensory Attributes of Chardonnay Wine. Horticulturae, 8(3), 212. https://doi.org/10.3390/horticulturae8030212








