Top Ten Most Important U.S.-Regulated and Emerging Plant-Parasitic Nematodes
Abstract
:1. Introduction
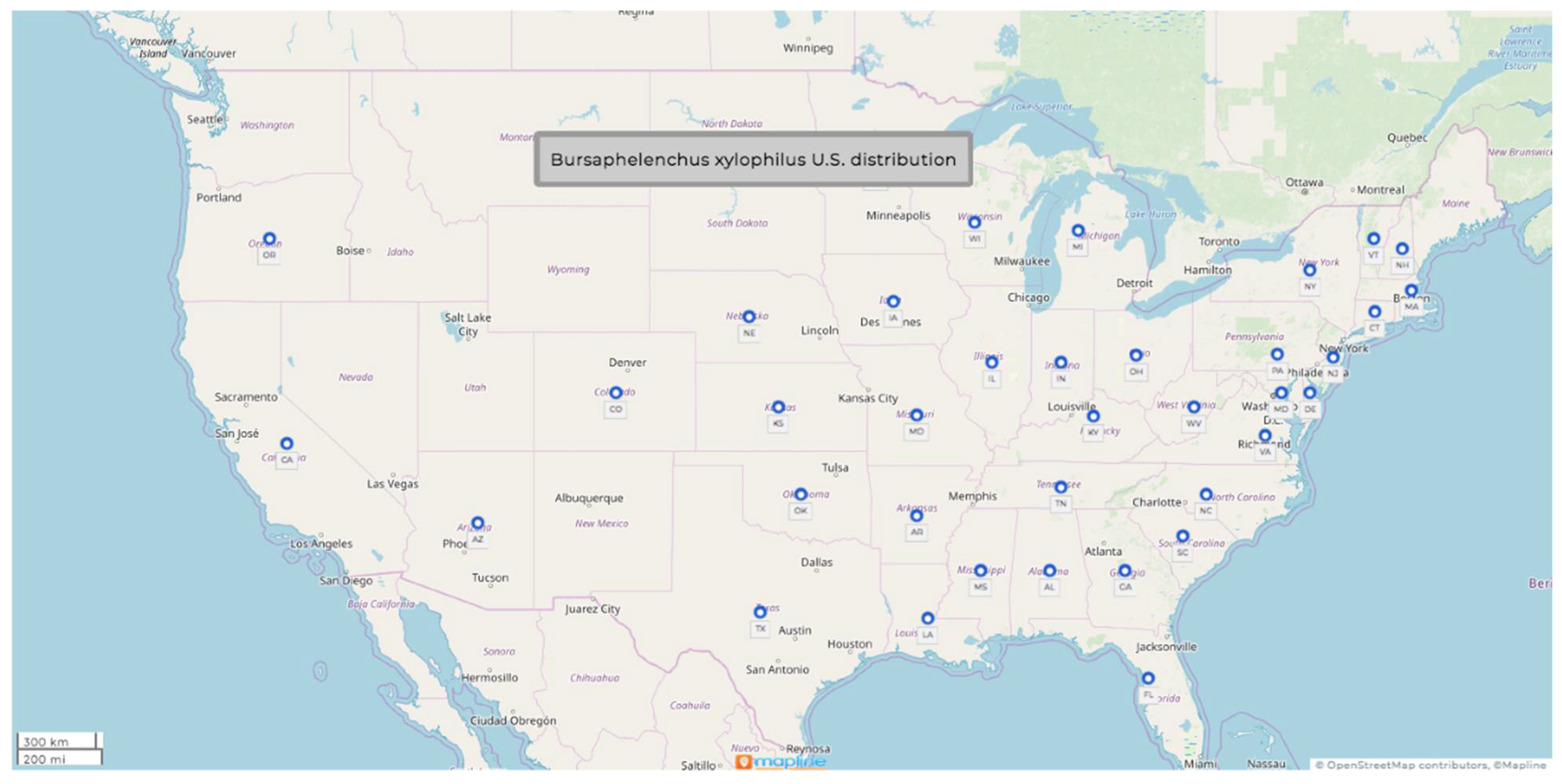
1.1. Bursaphelenchus xylophilus (Steiner & Buhrer, 1934) Nickle, 1970
- Common name: pine-wood nematode
- Type plant host: Pinus palustris Mill. or longleaf Louisiana pine
- Type locality: Bogalusa, LA, USA
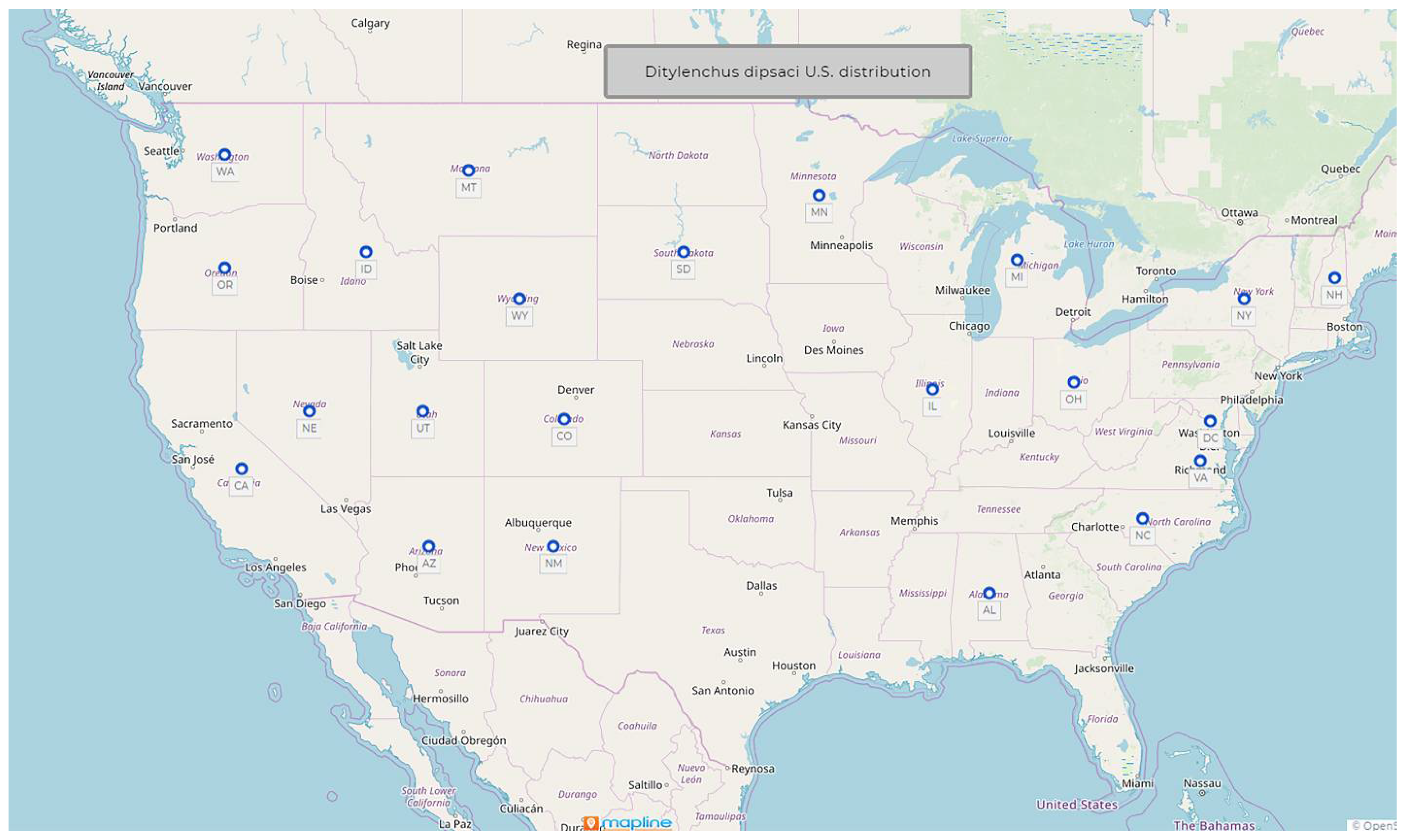
1.2. Ditylenchus dipsaci (Kuhn, 1857) Filipjev, 1936
- Common name: Stem and bulb nematode
- Type plant host: Dipsacus fullonum L., fuller’s teasel
- Type locality: Poppelsdorf near Bonn, Germany
- First U.S. report: 1914, Edgerton, KS
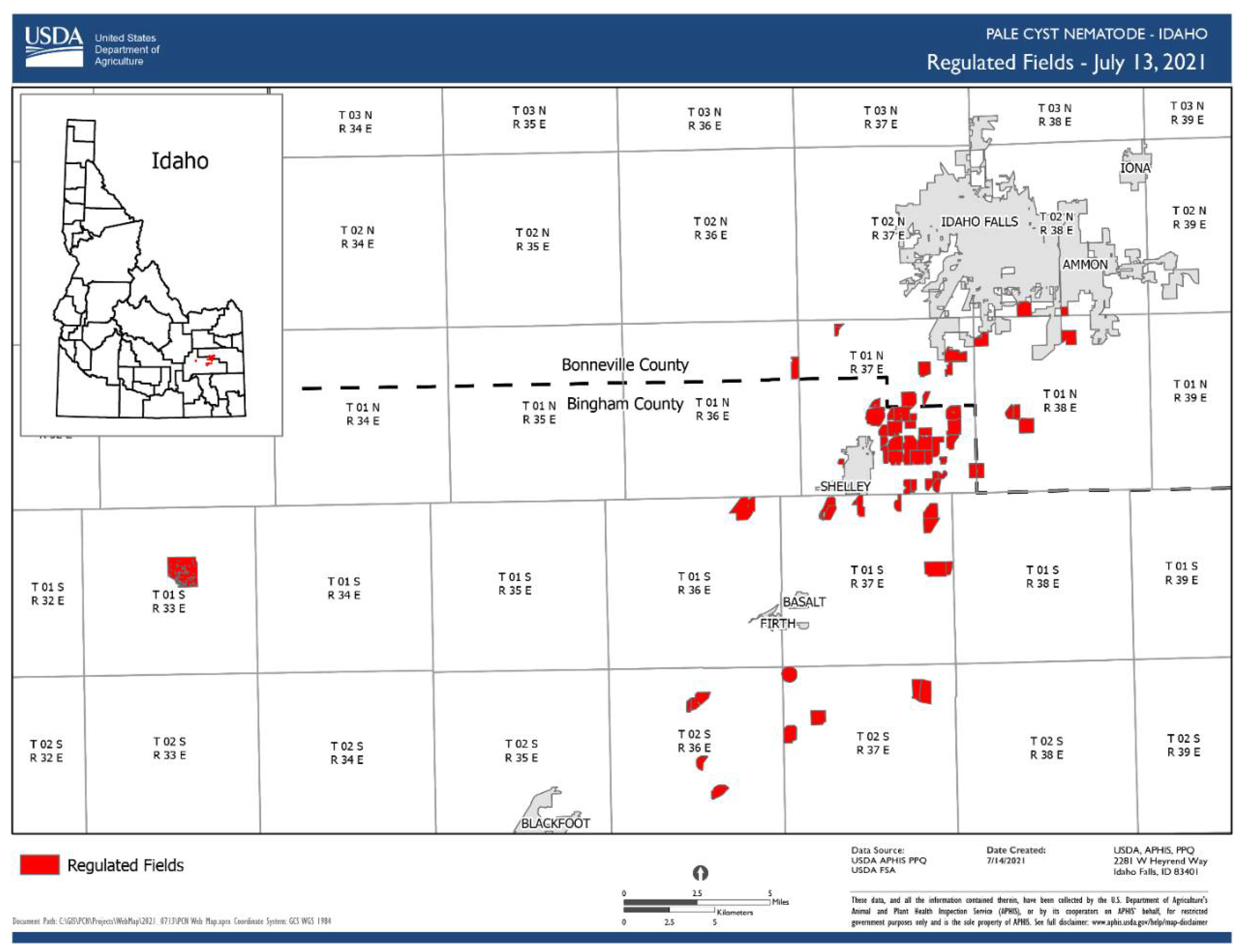
1.3. Globodera pallida (Stone, 1973) Behrens, 1975
- Common name: Pale (white) potato cyst nematode
- Type plant host: Solanum tuberosum L.
- Type locality: Epworth, Lincolnshire, Eastern England
- First U.S. report: 2006, ID
- Cyst: The morphological characters of cysts of this population agree with those of G. pallida except for the slightly higher mean in the distance from the anus to the nearest edge of fenestra 53.5 (30–80 μm). Both cyst, and J2 morphometrics and J2 tail morphology of the Idaho specimens fit well within the ranges observed for G. pallida, indicating that the Idaho specimens must represent G. pallida.
- Juvenile: The second-stage juveniles morphological characters of the Idaho population are similar to those of G. pallida but have a slightly shorter mean body length (452 ± 36 μm) than the lengths reported by Stone (1972) for the Epworth, Lincolnshire, England, population (486 ± 23 μm) and for the Duddingston, Scotland, population (482 ± 18 μm).
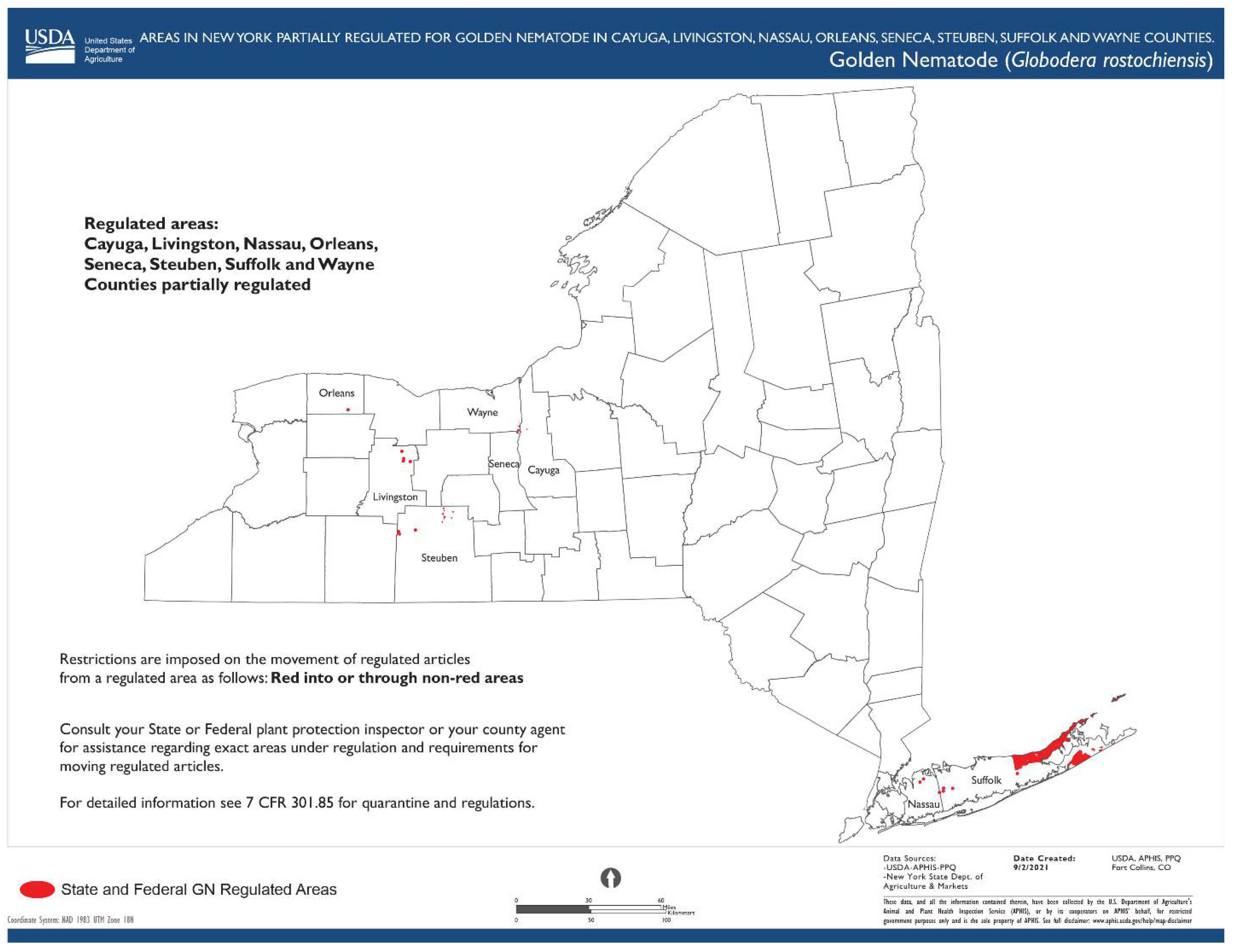
1.4. Globodera rostochiensis (Wollenweber, 1923) Behrens, 1975
- Common name: Golden (yellow) potato cyst nematode
- Type plant host: Solanum tuberosum L.
- Type locality: Epworth, Lincolnshire, Eastern England
- First U.S. report: 1941, Hicksville, NY
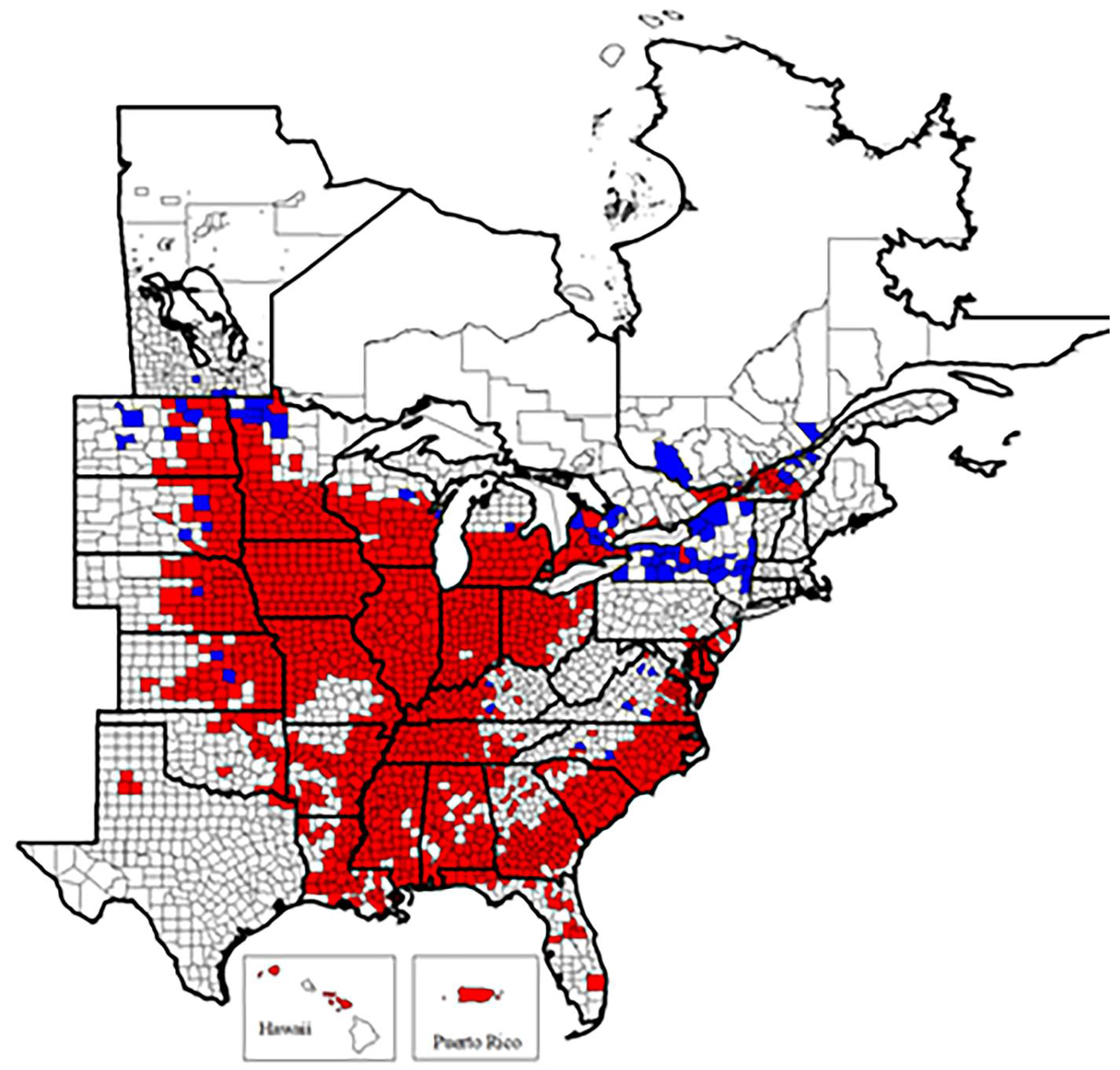
1.5. Heterodera glycines Ichinobe, 1952
- Common name: Soybean cyst nematode
- Type plant host: Glycine max, soybean
- Type locality: Obihiro-shi, Hokkaido, Japan
- First U.S. report: 1955, NC
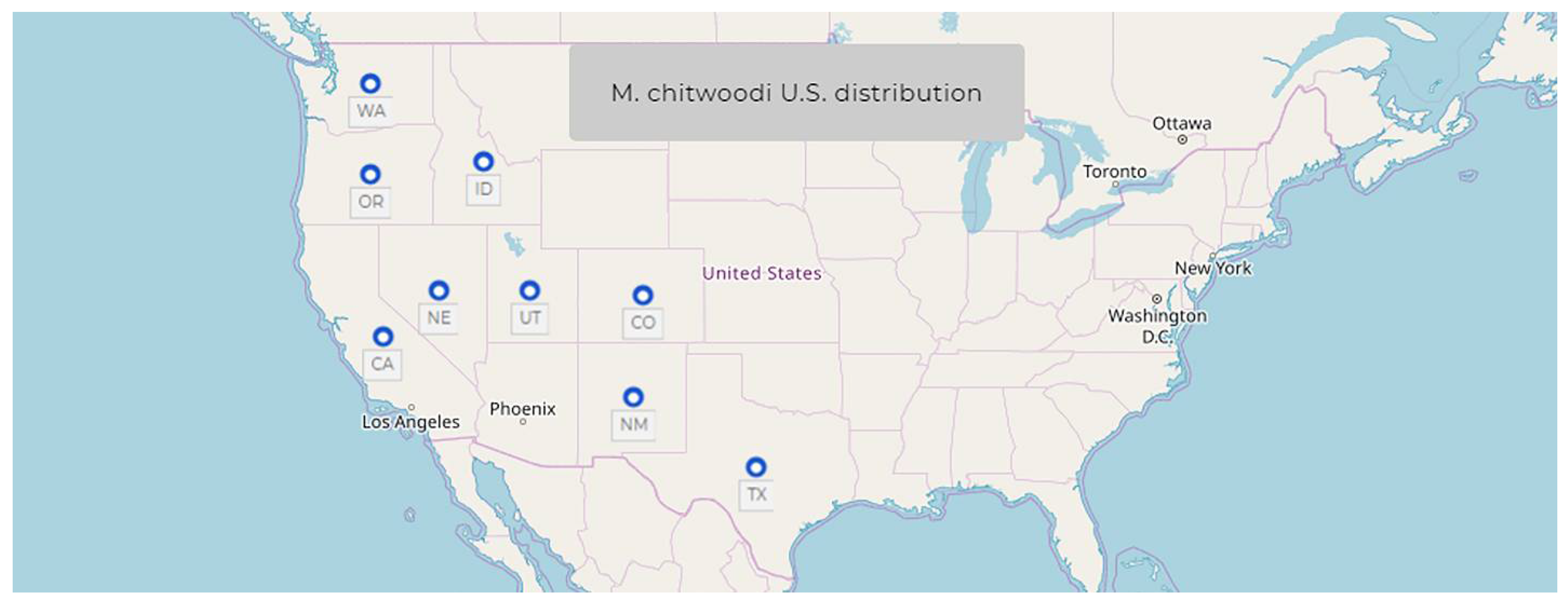
1.6. Meloidogyne chitwoodi Golden, O’Bannon, Santo & Finley, 1980
- Common name: Columbia root-knot nematode
- Type plant host: Solanum tuberosum L. potato
- Type locality: Quincy, Washington, USA
1.7. Meloidogyne enterolobii Yang & Eisenback, 1983
- Common name: Guava root-knot nematode
- Type plant host: Enterolobium contortisiliquum (Vell.) Morong, pacara earpod tree.
- Type locality: Hainan Island, Hainan Province, China.
- First U.S. report: 2001, FL.
1.8. Meloidogyne fallax Karssen, 1996
- Common name: False Columbia root-knot nematode
- Type plant host: Solanum lycopersicum L., tomato
- Type locality: Limburg, Netherlands
- First U.S. report: San Francisco County, CA
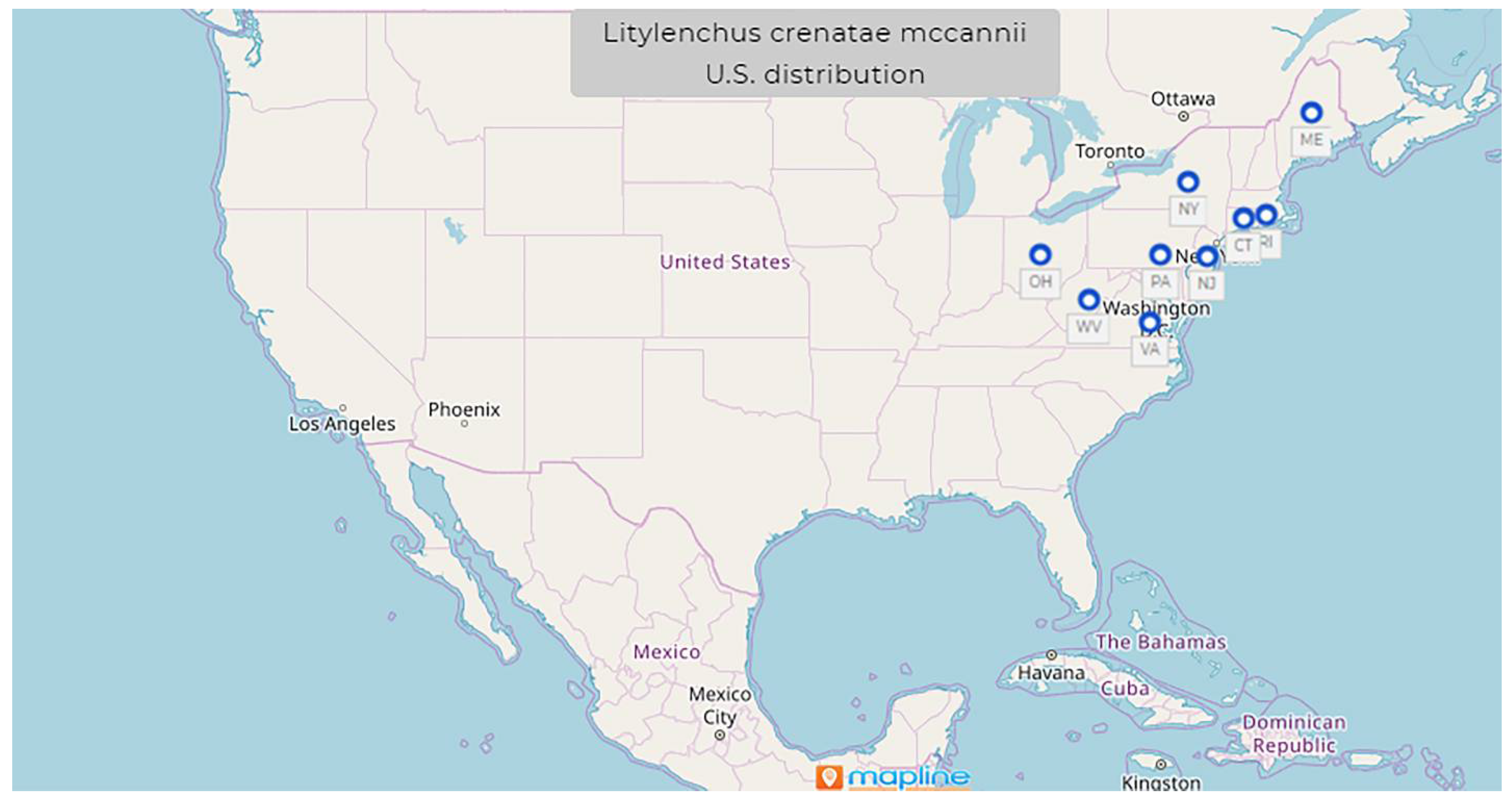
1.9. Litylenchus crenatae Kanzaki et al., 2019 mccannii ssp. Carta, Handoo, Li, Kantor, Bauchan, McCann, Gabriel, Yu, Reed, Koch, Martin, Burke 2020
- Common name: North American beech leaf nematode
- Type plant host: Fagus grandifolia, American beech
- Type locality: Perry, OH, U.S.
- First U.S. report: 2012, Perry, OH
1.10. Pratylenchus fallax Seinforst, 1968
- Common name: lesion nematode
- Type plant host: Malus spp., apple orchard with grass
- Type locality: Doornenburg, Netherlands
- First U.S. report: 1977, ND
2. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Animal and Plant Health Inspection Service (APHIS). Available online: https://www.aphis.usda.gov/aphis/ourfocus/planthealth/import-information/rppl/rppl-table (accessed on 30 November 2021).
- National Invasive Species Information Center. Available online: https://www.invasivespeciesinfo.gov/terrestrial/invertebrates (accessed on 30 November 2021).
- Golden, A.M.; Ellington, D.M. Redescription of Heterodera rostochiensis (Nematoda: Heteroderidae) with a key and notes on closely related species. Proc. Helminthol. Soc. Wash. 1972, 39, 64–78. [Google Scholar]
- Gamalero, E.; Glick, B.R. The use of plant growth-promoting bacteria to prevent nematode damage to plants. Biology 2020, 9, 381. [Google Scholar] [CrossRef] [PubMed]
- Gartner, U.; Hein, I.; Brown, L.H.; Chen, X.; Mantelin, S.; Sharma, S.K.; Dandurand, L.M.; Kuhl, J.C.; Jones, J.T.; Bryan, G.J.; et al. Resisting potato cyst nematodes with resistance. Front. Plant Sci. 2021, 12, 483. [Google Scholar] [CrossRef] [PubMed]
- Haroon, S.A.; Handoo, Z.; Kantor, M.; Skantar, A.; Hult, M. Molecular and morphological characterization of Globodera rostochiensis (Wollenweber, 1923) Skarbilovich, 1959 from Egypt. Not. Sci. Biol. 2021, 13, 11083. [Google Scholar] [CrossRef]
- Turner, S.J.; Subbotin, S.A. Cyst Nematodes. In Plant Nematology; Perry, R.N., Moens, M., Eds.; CAB International: Wallingford, UK, 2013; pp. 109–143. [Google Scholar]
- Animal and Plant Health Inspection Service (APHIS). Available online: https://www.aphis.usda.gov/aphis/ourfocus/planthealth/plant-pest-and-disease-programs/pests-and-diseases/nematode/pcn/ct_pcn_programupdates (accessed on 2 December 2021).
- Tylka, G.L.; Marett, C.C. Known distribution of the soybean cyst nematode, Heterodera glycines, in the United States and Canada in 2020. Plant Health Progress 2021, 22, 72–74. [Google Scholar] [CrossRef]
- Davis, E.L.; Tylka, G.L. Soybean cyst nematode disease. Plant Health Instr. 2000, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Ye, W.; Giblin-Davis, R.M. Molecular Characterization and Development of Real-Time PCR Assay for Pine-Wood Nematode Bursaphelenchus xylophilus (Nematoda: Parasitaphelenchidae). PLoS ONE 2013, 8, e78804. [Google Scholar]
- European and Mediterranean Plant Protection Organization. PM 3/69 (1) Meloidogyne chitwoodi and M. fallax: Sampling potato tubers for detection. Bull. OEPP EPPO Bull. 2006, 36, 421–422. [Google Scholar] [CrossRef]
- Subbotin, S.A.; Rius, J.E.P.; Castillo, P. Systematics of Root-Knot Nematodes (Nematoda: Meloidogynidae); Brill: Leiden, The Netherlands, 2021. [Google Scholar]
- Nischwitz, C.; Skantar, A.; Handoo, Z.A.; Hult, M.N.; Schmitt, M.E.; McClure, M.A. Occurrence of Meloidogyne fallax in North America, and molecular characterization of M. fallax and M. minor from U.S. golf course greens. Plant Dis. 2013, 97, 1424–1430. [Google Scholar] [CrossRef] [Green Version]
- Anonymous. Exotic Nematode Survey; 2012 Final Accomplishment Report; California Department of Food and Agriculture: Sacramento, CA, USA, 2013; p. 7. [Google Scholar]
- Haque, Z.; Khan, M.R. Handbook of Invasive Plant-Parasitic Nematodes: Novel Ingredients for Use in Pet, Aquaculture and Livestock Diets; Center for Agriculture and Bioscience International: Wallingford, UK, 2021. [Google Scholar]
- Castagnone-Sereno, P. Meloidogyne enterolobii (= M. mayaguensis): Profile of an emerging, highly pathogenic, root-knot nematode species. Nematology 2012, 14, 133–138. [Google Scholar] [CrossRef]
- Brito, J.; Powers, T.O.; Mullin, P.G.; Inserra, R.N.; Dickson, D.W. Morphological and molecular characterization of meloidogyne mayaguensis isolates from Florida. J. Nematol. 2004, 36, 232. [Google Scholar] [PubMed]
- Carta, L.K.; Handoo, Z.A.; Li, S.; Kantor, M.; Bauchan, G.; McCann, D.; Gabriel, C.K.; Yu, Q.; Reed, S.; Koch, J.; et al. Beech leaf disease symptoms caused by newly recognized nematode subspecies Litylenchus crenatae mccannii (Anguinata) described from Fagus grandifolia in North America. For. Pathol. 2020, 50, e12580. [Google Scholar] [CrossRef]
- Handoo, Z.; Kantor, M.; Carta, L. Taxonomy and Identification of Principal Foliar Nematode Species (Aphelenchoides and Litylenchus). Plants 2020, 9, 1490. [Google Scholar] [CrossRef] [PubMed]
- Kantor, M.R.; Handoo, Z.A.; Carta, L.; Li, S. First report of beech leaf disease, caused by Litylenchus crenatae mccannii, on american beech (Fagus grandifolia) in Virginia. Plant Dis. 2021, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Donald, P.A.; Hosford, R.M., Jr. Plant parasitic nematodes of North Dakota. Plant Dis. 1980, 64, 45–47. [Google Scholar] [CrossRef]
- Saikai, K.; Handoo, Z.A.; MacGuidwin, A.E. First report of the root-Lesion nematode, Pratylenchus fallax, on Soybean in Wisconsin, U.S.A. Plant Dis. 2019, 103, 2141. [Google Scholar] [CrossRef]
- Yu, Q.; Potter, J.W.; Gilby, G.A. First report of Pratylenchus fallax on turfgrass in Ontario. Plant Dis. 1997, 81, 1331. [Google Scholar] [CrossRef]
- Jeszke, A.; Budziszewska, M.; Dobosz, R.; Stachowiak, A.; Protasewicz, D.; Wieczorek, P.; Obrępalska-Stęplowska, A. A Comparative and Phylogenetic Study of the Ditylenchus dipsaci, Ditylenchus destructor and Ditylenchus gigas Populations Occurring in Poland. J. Phytopathol. 2014, 162, 61–67. [Google Scholar] [CrossRef]
- CABI-ISC. Invasive Species Compedium; Center for Agriculture and Bioscience International: Wallingford, UK, 2021; Available online: https://www.cabi.org/isc/datasheet/19287 (accessed on 15 December 2021).
- Nickle, W.R.; Golden, A.M.; Mamiya, Y.; Wergin, W. On the Taxonomy and Morphology of the Pine Wood Nematode, Bursaphelenchus xylophilus (Steiner & Buhrer 1934) Nickle 1970. J. Nematol. 1981, 13, 385–392. [Google Scholar]
- Donald, P.; Stamps, W.T.; Linit, M.J.; Todd, T.C. Pine wilt disease. Plant Health Instr. 2003. [Google Scholar] [CrossRef]
- Kikuchi, T.; Aikawa, T.; Oeda, Y.; Karim, N.; Kanzaki, N. A Rapid and Precise Diagnostic Method for Detecting the Pinewood Nematode Bursaphelenchus xylophilus by Loop-Mediated Isothermal Amplification. Phytopathology 2009, 99, 1365–1369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thorne, G. Principles of Nematology; Mc Graw-Hill: New York, NY, USA, 1961; p. 553. [Google Scholar]
- Hooper, D.J. Ditylenchus dipsaci. In C.I.H. Descriptions of Plant-Parasitic Nematodes; Set 1:14; Center for Agriculture and Bioscience International: Wallingford, UK, 1972. [Google Scholar]
- Mollov, D.S.; Subbotin, S.A.; Rosen, C. First Report of Ditylenchus dipsaci on garlic in Minnesota. Plant Dis. 2012, 96, 1707. [Google Scholar] [CrossRef] [PubMed]
- Testen, A.; Walsh, E.K.; Taylor, C.G.; Miller, S.A.; Lopez-Nicora, H.D. First report of bloat nematode (Ditylenchus dipsaci) infecting garlic in Ohio. Plant Dis. 2014, 98, 859. [Google Scholar] [CrossRef] [PubMed]
- Loughlin, D. Pests of Ornamental Trees, Shrubs and Flowers. Int. Pest Control 2013, 55, 220. [Google Scholar]
- Sturhan, D.; Brzeski, M.W. Stem and Bulb Nematodes, Ditylenchus spp. In Manual of Agricultural Nematology; Nickle, W.R., Ed.; Marcel Dekker: New York, NY, USA, 1991. [Google Scholar]
- Subbotin, S.A.; Madani, M.; Krall, E.; Sturhan, D.; Moens, M. Molecular diagnostics, taxonomy, and phylogeny of the stem nematode Ditylenchus dipsaci species complex based on the sequences of the internal transcribed spacer-rDNA. Phytopathology 2005, 95, 1308–1315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrássy, I. Free-living nematodes of Hungary (Nematoda Errantia) II. In Pedazoologica Hungarica; No. 4; Systematic Zoology Research Group of the Hungarian Academy of Sciences: Budapest, Hungary, 2007; p. 496. [Google Scholar]
- Vovlas, N.; Troccoli, A.; Palomares-Rius, J.E.; De Luca, F.; Liébanas, G.; Landa, B.B.; Subbotin, S.A.; Castillo, P. Ditylenchus gigas n. sp. parasitizing broad bean: A new stem nematode singled out from the Ditylenchus dipsaci species complex using a polyphasic approach with molecular phylogeny. Plant Pathol. 2011, 60, 762–775. [Google Scholar] [CrossRef]
- Karssen, G.; Willemsen, N.M. The spiculum: An additional useful character for the identification of Ditylenchus dipsaci and D. destructor (Nematoda: Anguinidae). EPPO Bull. 2010, 40, 211–212. [Google Scholar] [CrossRef]
- Skantar, A.M.; Handoo, Z.A.; Carta, L.K.; Chitwood, D.J. Morphological and molecular identification of globodera pallida associated with potato in Idaho. J. Nematol. 2007, 39, 133. [Google Scholar]
- Subbotin, S.A.; Franco, J.; Knoetze, R.; Roubtsova, T.V.; Bostock, R.M.; Del Prado Vera, I.C. DNA barcoding, phylogeny and phylogeography of the cyst nematode species from the genus Globodera (Tylenchida: Heteroderidae). Nematology 2020, 22, 269–297. [Google Scholar] [CrossRef]
- Camacho, M.J.; Inácio, M.L.; Mota, M.; de Andrade, E. Development and Validation of a Loop-Mediated Isothermal Amplification Diagnostic Method to Detect the Quarantine Potato Pale Cyst Nematode, Globodera pallida. Pathogens 2021, 10, 744. [Google Scholar] [CrossRef]
- Wasala, S.K.; Howe, D.K.; Dandurand, L.M.; Zasada, I.A.; Denver, D.R. Genomic analyses of Globodera pallida, a quarantine agricultural pathogen in Idaho. Pathogens 2021, 10, 363. [Google Scholar] [CrossRef]
- Wollenweber, H.W. Krankheiten und Beschadigungen der Kartoffel. Arb. Forsch. Inst. Kartoff. 1923, 7, 1–56. [Google Scholar]
- Manduric, S.; Olsson, E.; Englund, J.-E.; Andersson, S. Separation of Globodera rostochiensis and G. pallida (Tylenchida: Heteroderidae) using morphology and morphometrics. Nematology 2004, 6, 171–181. [Google Scholar] [CrossRef]
- Stone, A. Heterodera pallida N. Sp. (Nematoda: Heteroderidae), a second species of potato cyst nematode. Nematology 1972, 18, 591–606. [Google Scholar] [CrossRef]
- Lownsbery, B.F.; Lownsbery, J.W. Heterodera tabacurn new species, a parasite of solanaceous plants in Connecticut. Proc. Helminthol. Soc. Wash. 1954, 21, 42–47. [Google Scholar]
- Miller, L.I.; Gray, B.J. Horsenettle cyst nematode, Heterodera virginiae n. sp., a parasite of solanaceous plants. Nematology 1968, 14, 535–543. [Google Scholar] [CrossRef]
- Miller, L.I.; Gray, B.J. Heterodera solanacearurn n. sp., a parasite of solanaceous plants. Nematologica 1972, 18, 404–413. [Google Scholar] [CrossRef]
- Golden, A.M.; Klindic, O. Heterodera achilleae n. sp. (Nematoda: Heterocleridae) from yarrow in Yugoslavia. J. Nematol. 1973, 5, 196–201. [Google Scholar]
- Brzeski, M.W. Nematodes of Tylenchina in Poland and Temperate Europe; Muzeum I lnstytut Zoologii Polska Akademia Nauk: Warsaw, Poland, 1998; p. 396. [Google Scholar]
- Eroshenko, A.S.; Kasachenko, I.P. Heterodera arternisiae sp. n. (Nematoda: Heteroderidae)-a new cyst-forming nematode species from the Primorsk territory. Parazitologiya 1972, 6, 166–170. (In Russian) [Google Scholar]
- Subbotin, S.A.; Mundo-Ocampo, M.; Baldwin, J.G. Systematics of Cyst Nematodes (Nematoda: Heteroderinae); Part A; Brill: Leiden, The Netherlands, 2010; Volume 8. [Google Scholar]
- Cannon, O.S. Heterodera schachtii found in a Long Island potato field. Plant Dis. Rep. 1941, 25, 408. [Google Scholar]
- Spears, J.F. The golden nematode handbook. In Agriculture Handbook No. 353; U. S. Department of Agriculture: Washington, DC, USA, 1968; pp. 1–81. [Google Scholar]
- Animal and Plant Health Inspection Service (APHIS). Available online: https://www.aphis.usda.gov/aphis/ourfocus/planthealth/plant-pest-and-disease-programs/pests-and-diseases/golden-nematode/nematodes (accessed on 23 December 2021).
- Skantar, A.M.; Handoo, Z.A.; Zasada, I.A.; Ingham, R.E.; Carta, L.K.; Chitwood, D.J. Morphological and Molecular Characterization of Globodera Populations from Oregon and Idaho. Phytopathology 2011, 101, 480–491. [Google Scholar] [CrossRef] [Green Version]
- Wainer, J.; Dinh, Q. Taxonomy, Morphological and Molecular Identification of the Potato Cyst Nematodes, Globodera pallida and G. rostochiensis. Plants 2021, 10, 184. [Google Scholar] [CrossRef]
- Bulman, S.R.; Marshall, J.W. Differentiation of Australasian potato cyst nematode (PCN) populations using the polymerase chain reaction (PCR). N. Z. J. Crop Hortic. Sci. 1997, 25, 123–129. [Google Scholar] [CrossRef]
- Peng, D.; Shiqi, P.H. Globodera Rostochiensis SCAR Mark as Well as LAMP Fast Detection Method and Application of Method. CN 104059909A, 24 September 2014. [Google Scholar]
- Peng, D.; Shiqi, P.H. Globodera rostochiensis SCARMark and LAMPMethod for Quick and Application. CN 104059909B, 4 May 2016. [Google Scholar]
- Brodie, B.B.; Mai, W.F. Control of the Golden Nematode in the United States. Annu. Rev. Phytopathol. 1989, 27, 443–461. [Google Scholar] [CrossRef]
- Hirschmann, H. Comparative morphological studies on the soybean cyst nematode, Heterodera glycines, and the clover cyst nematode, H. trifolii (Nematoda:Heteroderidae). Proc. Helminthol. Soc. Wash. 1956, 23, 140–151. [Google Scholar]
- Burrows, P.R.; Stone, A.R. Heterodera glycines. In CIH Description of Plant-Parasitic Nematodes; Set 8 (No. 118); Center for Agriculture and Bioscience International: Wallingford, UK, 1985. [Google Scholar]
- Wang, X.; Bergstrom, G.C.; Chen, S.; Thurston, D.M.; Cummings, J.; Handoo, Z.A.; Hult, M.N.; Skantar, A.M. First Report of the Soybean Cyst Nematode, Heterodera glycines, in New York. Plant Dis. 2017, 101, 1957. [Google Scholar] [CrossRef]
- Subbotin, S.A.; Mundo-Ocampo, M.L.; Baldwin, J.G. Systematics of Cyst Nematodes (Nematoda: Heteroderinae); Part B; Brill: Leiden, The Netherlands, 2010. [Google Scholar]
- Handoo, Z.A.; Subbotin, S.A. Taxonomy, Identification and Principal Species. In Cyst Nematodes; Center for Agriculture and Bioscience International: Wallingford, UK, 2018; pp. 365–398. [Google Scholar]
- Ko, H.R.; Kang, H.; Park, E.H.; Kim, E.H.; Lee, J.K. Identification of Heterodera glycines (Tylenchida; Heteroderidae) Using qPCR. Plant Pathol. J. 2019, 35, 654–661. [Google Scholar] [CrossRef]
- Allen, T.W.; Bradley, C.A.; Sisson, A.J.; Byamukama, E.; Chilvers, M.I.; Coker, C.M.; Collins, A.A.; Damicone, J.P.; Dorrance, A.E.; Dufault, N.S.; et al. Soybean yield loss estimates due to diseases in the United States and Ontario, Canada, from 2010 to 2014. Plant Health Prog. 2017, 18, 19–27. [Google Scholar] [CrossRef] [Green Version]
- Koenning, S.R.; Wrather, J.A. Suppression of soybean yield potential in the continental United States by plant diseases from 2006 to 2009. Plant Health Prog. 2010, 11, 11. [Google Scholar] [CrossRef] [Green Version]
- Bandara, A.Y.; Weerasooriya, D.K.; Bradley, C.A.; Allen, T.W.; Esker, P.D. Dissecting the economic impact of soybean diseases in the United States over two decades. PLoS ONE 2020, 15, e0231141. [Google Scholar]
- Niblack, T.L.; Colgrove, A.L.; Colgrove, K.; Bond, J.P. Shift in virulence of soybean cyst nematode is associated with use of resistance from PI 88788. Plant Health Prog. 2008, 9, 29. [Google Scholar] [CrossRef] [Green Version]
- Golden, A.M.; O’Bannon, J.H.; Santo, G.S.; Finley, A.M. Description and SEM Observations of Meloidogyne chitwoodi n. sp. (Meloidogynidae), a Root-knot Nematode on Potato in the Pacific Northwest. J. Nematol. 1980, 12, 319. [Google Scholar]
- Eisenback, J.D.; Triantaphyllou, H.H. Root-knot Nematodes: Meloidogyne species and races. In Manual of Agricultural Nematology; Nickle, W.R., Ed.; Marcel Dekker: New York, NY, USA, 1991. [Google Scholar]
- Zijlstra, C.; Lever, A.E.M.; Uenk, B.J.; Van Silfhout, C.H. Differences between ITS regions of isolates of root-knot nematodes Meloidogyne hapla and M. chitwoodi. Phytopathology 1995, 85, 1231–1237. [Google Scholar] [CrossRef]
- Zijlstra, C.; Uenk, B.J.; Van Silfhout, C.H. A reliable, precise method to differentiate species of root-knot nematodes in mixtures on the basis of ITS-RFLPs. Fundam. Appl. Nematol. 1997, 20, 59–63. [Google Scholar]
- Zijlstra, C.; Donkers-Venne, D.T.; Fargette, M. Identification of Meloidogyne incognita, M. javanica and M. arenaria using sequence characterised amplified region (SCAR) based PCR assays. Nematology 2000, 2, 847–853. [Google Scholar]
- Wishart, J.; Phillips, M.S.; Blok, V.C. Ribosomal Intergenic Spacer: A Polymerase Chain Reaction Diagnostic for Meloidogyne chitwoodi, M. fallax, and M. hapla. Phytopathology 2002, 92, 884–892. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Gleason, C. Loop-Mediated Isothermal Amplification for the Diagnostic Detection of Meloidogyne chitwoodi and M. fallax. Plant Dis. 2019, 103, 12–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ingham, R.E.; Hamm, P.B.; Ocamb, C.M. Potato (Solanum tuberosum)–Nematode, Root-Knot. In Pacific Northwest Plant Disease Management Handbook; Oregon State University: Corvallis, OR, USA, 2018. [Google Scholar]
- Yang, B.; Eisenback, J.D. Meloidogyne enterolobii n. sp.(Meloidogynidae), a root-knot nematode parasitizing pacara earpod tree in China. J. Nematol. 1983, 15, 381. [Google Scholar]
- FINDMe.org. Available online: https://www.findmenematode.org/about-findme (accessed on 28 December 2021).
- Blok, V.C.; Phillips, M.S.; Fargette, M. Comparison of Sequences from the Ribosomal DNA Intergenic Region of Meloidogyne mayaguensis and Other Major Tropical Root-Knot Nematodes. J. Nematol. 1997, 29, 16. [Google Scholar]
- Blok, V.C.; Wishart, J.; Fargette, M.; Phillips, M. Mitochondrial DNA differences distinguishing Meloidogyne mayaguensis from the major species of tropical root-knot nematodes. Nematology 2002, 4, 773–781. [Google Scholar] [CrossRef]
- Xu, J.; Liu, P.; Meng, Q.; Long, H. Characterisation of Meloidogyne species from China using Isozyme Phenotypes and Amplified Mitochondrial DNA Restriction Fragment Length Polymorphism. Eur. J. Plant Pathol. 2004, 110, 309–315. [Google Scholar] [CrossRef]
- Kiewnick, S.; Holterman, M.; van den Elsen, S.; van Megen, H.; Frey, J.E.; Helder, J. Comparison of two short DNA barcoding loci (COI and COII) and two longer ribosomal DNA genes (SSU & LSU rRNA) for specimen identification among quarantine root-knot nematodes (Meloidogyne spp.) and their close relatives. Eur. J. Plant Pathol. 2014, 140, 97–110. [Google Scholar]
- Long, H.; Wang, X.; Xu, J. Molecular cloning and life-stage expression pattern of a new chorismate mutase gene from the root-Knot nematode Meloidogyne arenaria. Plant Pathol. 2006, 55, 559–563. [Google Scholar] [CrossRef]
- Tigano, M.; De Siqueira, K.; Castagnone-Sereno, P.; Mulet, K.; Queiroz, P.; Dos Santos, M.; Teixeira, C.; Almeida, M.; Silva, J.; Carneiro, R.M.D.G. Genetic diversity of the root-knot nematode Meloidogyne enterolobii and development of a SCAR marker for this guava-damaging species. Plant Pathol. 2010, 59, 1054–1061. [Google Scholar] [CrossRef] [Green Version]
- Niu, J.H.; Jian, H.; Guo, Q.X.; Chen, C.L.; Wang, X.Y.; Liu, Q.; Guo, Y.D. Evaluation of loop-mediated isothermal amplification (LAMP) assays based on 5S rDNA-IGS2 regions for detecting Meloidogyne enterolobii. Plant Pathol. 2012, 61, 809–819. [Google Scholar] [CrossRef]
- He, X.F.; Peng, H.; Ding, Z.; He, W.T.; Huang, W.K.; Peng, D.L. Loop-mediated isothermal amplification assay for rapid diagnosis of Meloidogyne enterolobii directly from infected plants. Sci. Agric. Sin. 2013, 46, 534–544. [Google Scholar]
- Kiewnick, S.; Frey, J.E.; Braun-Kiewnick, A. Development and Validation of LNA-Based Quantitative Real-Time PCR Assays for Detection and Identification of the Root-Knot Nematode Meloidogyne enterolobii in Complex DNA Backgrounds. Phytopathology. 2015, 105, 1245–1249. [Google Scholar] [CrossRef] [Green Version]
- Braun-Kiewnick, A.; Viaene, N.; Folcher, L.; Ollivier, F.; Anthoine, G.; Niere, B.; Sapp, M.; van de Vossenberg, B.; Toktay, H.; Kiewnick, S. Assessment of a new qPCR tool for the detection and identification of the root-knot nematode Meloidogyne enterolobii by an international test performance study. Eur. J. Plant Pathol. 2016, 144, 97–108. [Google Scholar] [CrossRef]
- Subbotin, S.A. Recombinase polymerase amplification assay for rapid detection of the root-knot nematode Meloidogyne enterolobii. Nematology 2019, 21, 243–251. [Google Scholar] [CrossRef] [Green Version]
- Philbrick, A.N.; Adhikari, T.B.; Louws, F.J.; Gorny, A.M. Meloidogyne enterolobii, a Major Threat to Tomato Production: Current Status and Future Prospects for Its Management. Front. Plant Sci. 2020, 11, 1773. [Google Scholar] [CrossRef]
- Hunt, D.J.; Handoo, Z.A. Taxonomy, identification and principal species. In Root-Knot Nematodes; Center for Agriculture and Bioscience International: Wallingford, UK, 2009; Volume 1, pp. 55–97. [Google Scholar]
- Karssen, G. Description of Meloidogyne fallax n. sp. (Nematoda: Heteroderidae), a root-knot nematode from The Netherlands. Fundam. Appl. Nematol. 1996, 19, 593–600. [Google Scholar]
- Holterman, M.H.; Oggenfuss, M.; Frey, J.E.; Kiewnick, S. Evaluation of High-resolution Melting Curve Analysis as a New Tool for Root-knot Nematode Diagnostics. J. Phytopathol. 2012, 160, 59–66. [Google Scholar] [CrossRef]
- Karssen, G.; Bolk, R.J.; Van Aelst, A.; van den Beld, I.; Kox, L.; Korthals, G.; Molendijk, L.; Zijlstra, C.; Van Hoof, R.; Cook, R. Description of Meloidogyne minor n. sp. (Nematoda: Meloidogynidae), a root-knot nematode associated with yellow patch disease in golf courses. Nematology 2004, 6, 59–72. [Google Scholar] [CrossRef]
- Holterman, M.; Karssen, G.; van den Elsen, S.; Van Megen, H.; Bakker, J.; Helder, J. Small subunit rDNA-based phylogeny of the Tylenchida sheds light on relationships among some high-impact plant-parasitic nematodes and the evolution of plant feeding. Phytopathology 2009, 99, 227–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, F.A.; Falloon, R.E.; Bulman, S.R. Nightshade weeds (Solanum spp.) confirmed as hosts of the potato pathogens Meloidogyne fallax and Spongospora subterranea f. sp. subterranea. Australas. Plant Pathol. 2010, 39, 492–498. [Google Scholar] [CrossRef]
- Hodgetts, J.; Ostojá-Starzewski, J.C.; Prior, T.; Lawson, R.; Hall, J.; Boonham, N. DNA barcoding for biosecurity: Case studies from the UK plant protection program. Genome 2016, 59, 1033–1048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zijlstra, C.; Van Hoof, R.A. A Multiplex Real-Time Polymerase Chain Reaction (TaqMan) Assay for the Simultaneous Detection of Meloidogyne chitwoodi and M. fallax. Phytopathology 2006, 96, 1255–1262. [Google Scholar] [CrossRef] [Green Version]
- De Haan, E.G.; Dekker, C.C.E.M.; Tameling, W.I.L.; den Nijs, L.J.M.F.D.; van den Bovenkamp, G.W.; Kooman-Gersmann, M. The MeloTuber Test: A real-time TaqMan® PCR-based assay to detect the root-knot nematodes Meloidogyne chitwoodi and M. fallax directly in potato tubers. EPPO Bull. 2014, 44, 166–175. [Google Scholar] [CrossRef]
- Reed, S.E.; Greifenhagen, S.; Yu, Q.; Hoke, A.; Burke, D.J.; Carta, L.K.; Handoo, Z.A.; Kantor, M.R.; Koch, J. Foliar nematode, Litylenchus crenatae ssp. mccannii, population dynamics in leaves and buds of beech leaf disease-affected trees in Canada and the US. For. Pathol. 2020, 50, e12599. [Google Scholar] [CrossRef]
- Ewing, C.J.; Hausman, C.E.; Pogacnik, J.; Slot, J.; Bonello, P. Beech leaf disease: An emerging forest epidemic. For. Pathol. 2019, 49, 12488. [Google Scholar] [CrossRef]
- Seinhorst, J.W. Pratylenchus fallax. In CIH-Descriptions of Plant-Parasitic Nematodes Set 7; No. 100; Center for Agriculture and Bioscience International: Slough, UK, 1977. [Google Scholar]
- Bogale, M.; Tadesse, B.; Nuaima, R.; Honermeier, B.; Hallmann, J.; DiGennaro, P. Morphometric and Molecular Diversity among Seven European Isolates of Pratylenchus penetrans. Plants 2021, 10, 674. [Google Scholar] [CrossRef]
- Ibrahim, S.K.; Perry, R.N.; Webb, R.M. Use of isoenzyme and protein phenotypes to discriminate between six Pratylenchus species from Great Britain. Ann. Appl. Biol. 1995, 126, 317–327. [Google Scholar] [CrossRef]
- Waeyenberge, L.; Viaene, N.; Moens, M. Species-specific duplex PCR for the detection of Pratylenchus penetrans. Nematology 2009, 11, 847–857. [Google Scholar] [CrossRef]
- Castillo, P.; Vovlas, N. Pratylenchus (Nematoda: Pratylenchidae): Diagnosis, Biology, Pathogenicity and Management; Brill: Boston, IL, USA, 2007. [Google Scholar]

| Character | Females | Male | Female Allolectotype | Male Hololectotype |
|---|---|---|---|---|
| n = 5 | n = 5 | n = 1 | n = 1 | |
| L | 523.0 (447.0–609.0) | 560.0 (520.0–601.0) | 544.0 | 557.0 |
| a | 42.6 (37.0–48.0) | 40.8 (35.0–45.0) | 37.0 | 38.0 |
| b | 9.6 (8.3–10.5) | 9.4 (8.4–10.5) | 9.0 | 8.5 |
| c | 27.2 (23.0–31.0) | 24.4 (21.0–29.0) | 28.0 | 23.0 |
| V% | 74.7 (72.7–77.5) | - | 73.0 | - |
| Stylet length | 12.8 (12.6–13.0) | 13.3 (12.6–13.8) | 12.6 | 13.8 |
| Spicule length | - | 21.2 (18.8–23.0) | - | 21.1 |
| Gubernaculum | - | - | - | - |
| After Thorne [30] | After Blake in Hooper [31] | After Mollov et al. [32] | After Testen et al. [33] | |||||
|---|---|---|---|---|---|---|---|---|
| Character | Female | Male | Female | Male | Female | Male | Female | Male |
| n = 48 | n = 23 | n = 6 | n = 9 | n = 16 | n = 6 | |||
| L | 1000.0–1300.0 | 1300.0 | 1300.0 | 1000.0–1300.0 | 1411.0–1636.0 | 1372.0–1558.0 | 1080.1 (972.2–1229.5) | 1589.2 (1494–1702.7) |
| a | 36.0–40.0 | 63.0 | 62.0 | 37.0–41.0 | 38.0–44.0 | 40.0–50.0 | 36.6 (33.5–41.9) | 43.0 (40.7–46.0) |
| b | 6.5–7.1 | 15.0 | 15.0 | 6.5–7.3 | 5.8–8.0 | 6.5–7.0 | 6.2 (5.3–6.8) | 6.9 (6.4–7.3) |
| c | 14.0–18.0 | 14.0 | 14.0 | 12.0–15.0 | 14.0–17.0 | 14.0–16.0 | 11.1 (9.1–12.8) | 11.7 (9.2–13) |
| V% | 80 | - | 80 | - | 79.0–81.0 | - | - | - |
| Stylet length | - | - | - | - | 11.5–12.3 | 11.5–12.3 | 10.1 (8.9–11.2) | 10.8 (10.0–12.2) |
| Tail length | - | - | - | - | 95.0–105.0 | - | - | - |
| Spicule length | - | - | - | - | - | 22.0–27.0 | - | 25.2 (23.0–26.8) |
| T | - | 72.0 | - | 65.0–72.0 | - | - | - | - |
| Gubernaculum | - | - | - | - | - | 9.0–10.0 | - | - |
| G. pallida Second Stage Juveniles (n = 80) | G. pallida Cysts (n = 80) | |||||
|---|---|---|---|---|---|---|
| Character | Range | Mean | SD | Range | Mean | SD |
| Body length | 380–533 | 452.0 | 36.0 | - | - | - |
| Stylet length | 22.5–25.0 | 23.2 | 0.7 | - | - | - |
| Tail length | 40.0–57.0 | 49.6 | 3.0 | - | - | - |
| Hyaline tail terminal length | 20.0–31.0 | 25.9 | 2.4 | - | - | - |
| Body length excluding neck a | - | - | - | 420–700 | 574 | 85 |
| Body width b | - | - | - | 400–600 | 534 | 80 |
| Distance from anus to nearest edge of fenestra (anus-vulva) | - | - | - | 30.0–80.0 | 53.5 | 11.8 |
| Fenestra length | - | - | - | 17.5–45.0 | 24.8 | 5.6 |
| Number of cuticular ridges between vulva and anus | - | - | - | 7.0–17.0 | 12.0 | 2.5 |
| Granek’s ratio | - | - | - | 1.2–3.6 | 2.2 | 0.5 |
| Species Name from Original Heterodera Description | J2 Stylet (µm) | Cyst, Number of Cuticular Ridges from Anus-Vulva | Cyst Anus-Vulva Distance (µm) | Granek’s Ratio |
|---|---|---|---|---|
| G. rostochiensis | ||||
| (Wollenweber, 1923) [44] | 19–23 (22) | 12–31 (>14) | 37–77 (>55) | 1.3–9.5 (>3) |
| (Manduric et al., 2004) [45] | 19.3–24 | 12–28 | 38–149 | 1.7–6.7 |
| Composite | 19–24 | 12–31 | 37–149 | 1.9–9.5 |
| G. pallida | ||||
| (Stone, 1973) [46] | 22–24 (23.8) | 8–20 (<14) | 22–67 (<50) | 1.2–3.5 (<3) |
| (Manduric et al., 2004) [45] | 21.1–25.5 | 9–26 | 28–88 | 1.0–6.5 |
| Composite | 21.1–25.5 | 7–26 | 22–88 | 1–6.5 |
| G.tabacum | ||||
| (Lownsbery, 1954) [47]; (Miller and Gray, 1968) [48]; (Miller and Gray, 1972) [49] | 19–28 (23–24) | 10–14 | 28–85 | 1–4.2 (<2.8) |
| G.achilliae | ||||
| (Golden and Klindic, 1973) [50] | 24–26 (25) | 4–5 | 22–34 (27) | 1.5–1.9 (1.6) |
| (Brzeski, 1988) [51] | 24–26.5 (25) | - | 12–42 (24) | 0.3–1.9 (1.2) |
| (Skantar et al.,2006) [40] | - | 5–11 (8 ± 1.7) | 25–60 (21.5 ± 2.6) | 1.2–2.1 (1.5 ± 0.3) |
| G. artemisiae | ||||
| (Eroshenko and Kazachenko, 1972) [52] | 18–29 (23) | 5 a | 25–42 (32 b) | 0.8–1.7 (1.0) |
| Cyst Measurements after Hirschmann [63] | Cyst Measurements after Burrows and Stone [64] | Cyst Measurements after Wang et al. [65] | Juvenile Measurements after Hirschmann [63] | Juvenile Measurements after Burrows and Stone [64] | Juvenile Measurements after Wang et al. [65] | |
|---|---|---|---|---|---|---|
| Character | n = 100 | - | n = 5 | n = 150 | n = 15 | |
| Body length | 580 (340–920) | 340–920 | 696 (520–866) | 440 ±6.7 (375–490) | 440 (375–490) | 438.5 (381–510) |
| Body width | 360 (200–560) | - | 399.8 (320–495) | - | - | - |
| Length/width | 1.2–2.1 | 1.19–2.05 | 1.7 (1.4–2.1) | - | - | - |
| Vulva slit | 49.7 (43–56) | 49.7 (43–56) | 48.2 (42–52) | - | - | - |
| Fenestra length | 53.7 (37–65) | 53.7 (37–65) | 58.4 (52–65) | - | - | - |
| Fenestra width | 40.5 (33–48) | 40.5 (33–48) | 37.4 (32–40) | - | - | - |
| Vulva to anus distance | 67.5 (59–77) | - | - | - | - | - |
| Underbridge | 80 (70–110) | - | - | - | ||
| Stylet length | - | - | - | 23.0 ± 0.1 (22–24) | 23.0 (22.0–24.0) | 23.8 (22.5–25) |
| Tail length | - | - | - | 50.4 ± 1.0 (42–59.4) | 50.4 (42.0–59.4) | 47.3 (42–50) |
| Hyaline tail terminal length | - | - | - | 26.6 ± 0.7 (20.0–33.0) | 26.6 (20.0–33.0) | 25.1 (22.5–27.5) |
| Character | Adult Female | Young Female | Adult Male |
|---|---|---|---|
| n = 27 | n = 10 | n = 4 | |
| L | 889 ± 119 (625–1084) | 823 ± 61 (750–947) | 548.0 ± 16.7 (534.5–566.7) |
| W | 14.2 ± 1.0 (12.1–16.1) | 11.4 ± 1.1 (9.9–13.5) | 15.1 ± 2.5 (12.1–16.7) |
| Stylet | 9.2 ± 0.5 (8.4–10.3) | 9.7 ± 0.9 (8.5–11.2) | 11.1 ± 0.5 (10.5–11.4) |
| Stylet conus | 4.6 ± 0.4 (3.6–5.2) | - | - |
| Pharynx | 193.5 ± 35.7 (126–244) | 152.6 ± 16.2 (133–186) | 113.9 ± 5.0 (108.5–118.1) |
| PUS | 34.3 ± 6.1 (22.7–45.0) | - | - |
| Tail | 54.3 ± 6.1 (39.8–64.4) | 48.3 ± 6.2 (34.5–56.4) | 35.3 ± 1.6 (33.7–37.9) |
| a | 63.0 ± 10.0 (43.8–76.8) | 72.9 ± 9.3 (61–86 | 36.1 ± 5.4 (33.3–44.1) |
| b | 4.7 ± 0.7 (3.8–6.6) | 5.4 ± 0.7 (4.5–6.6 | 4.8 ± 0.2 (4.6–4.9) |
| c | 16.4 ± 1.5 (13.3–20.1) | 17.4 ± 3.3 (13–25) | 15.5 ± 0.2 (15.3–15.9) |
| c’ | 5.7 ± 0.8 (4.3–7.9) | 6.0 ± 1.0 (4.3–7.9) | - |
| V% | 76.6 ± 1.4 (73–79) | 76.9 ± 1.2 (75–79) | - |
| PUS/VAD% | 27 ± 8 (22–50) | - | - |
| PUS/BW | 2.8 ± 0.5 (1.9–3.8) | - | - |
| Spicule length | - | - | 16.3 ± 1.4 (14.9–17.6) |
| Gubernaculum | - | - | 5.3 ± 0.8 (4.3–6.1) |
| After Seinhorst [106] | After Saikai et al. [23] | |||
|---|---|---|---|---|
| Character | Female | Male | Female | Male |
| n = 10 | n = 10 | n = 25 | n = 25 | |
| L | 420–560 | 400–500 | 529 (438–615) | 454 (381–543) |
| W | - | - | 20.1 (15.3–23.6) | - |
| a | 23–33 | 26–33 | 26.4 (24.1–30.1) | - |
| b | 5.2–6.7 | 5.0–6.2 | 54. (4.3–7.5) | - |
| c | 18–24 | 16–25 | 21.6 (16.3–28.3) | - |
| V% | 77–81 | - | 79 (77–81) | - |
| Postuterine sac | - | - | 15.0 (10.6–20.0) | - |
| Stylet length | 16–17 | 15 | 15 (13.8–16.4) | 13.4 (11.8–14.8) |
| Tail length | - | - | 29 (23.5–36.7) | - |
| Tail annules | - | - | 23 (20–30) | - |
| Spicule length | - | 14–16 | - | 16.6 (14.0–19.7) |
| Gubernaculum | - | - | - | 4.2 (3.2–6.2) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kantor, M.; Handoo, Z.; Kantor, C.; Carta, L. Top Ten Most Important U.S.-Regulated and Emerging Plant-Parasitic Nematodes. Horticulturae 2022, 8, 208. https://doi.org/10.3390/horticulturae8030208
Kantor M, Handoo Z, Kantor C, Carta L. Top Ten Most Important U.S.-Regulated and Emerging Plant-Parasitic Nematodes. Horticulturae. 2022; 8(3):208. https://doi.org/10.3390/horticulturae8030208
Chicago/Turabian StyleKantor, Mihail, Zafar Handoo, Camelia Kantor, and Lynn Carta. 2022. "Top Ten Most Important U.S.-Regulated and Emerging Plant-Parasitic Nematodes" Horticulturae 8, no. 3: 208. https://doi.org/10.3390/horticulturae8030208
APA StyleKantor, M., Handoo, Z., Kantor, C., & Carta, L. (2022). Top Ten Most Important U.S.-Regulated and Emerging Plant-Parasitic Nematodes. Horticulturae, 8(3), 208. https://doi.org/10.3390/horticulturae8030208







