Root-Knot Nematode Species Associated with Horticultural Crops in the Island of Azores, Portugal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling and Nematode Isolates
2.2. Morphological Characterisation
2.3. Biochemical Characterisation
3. Results and Discussion
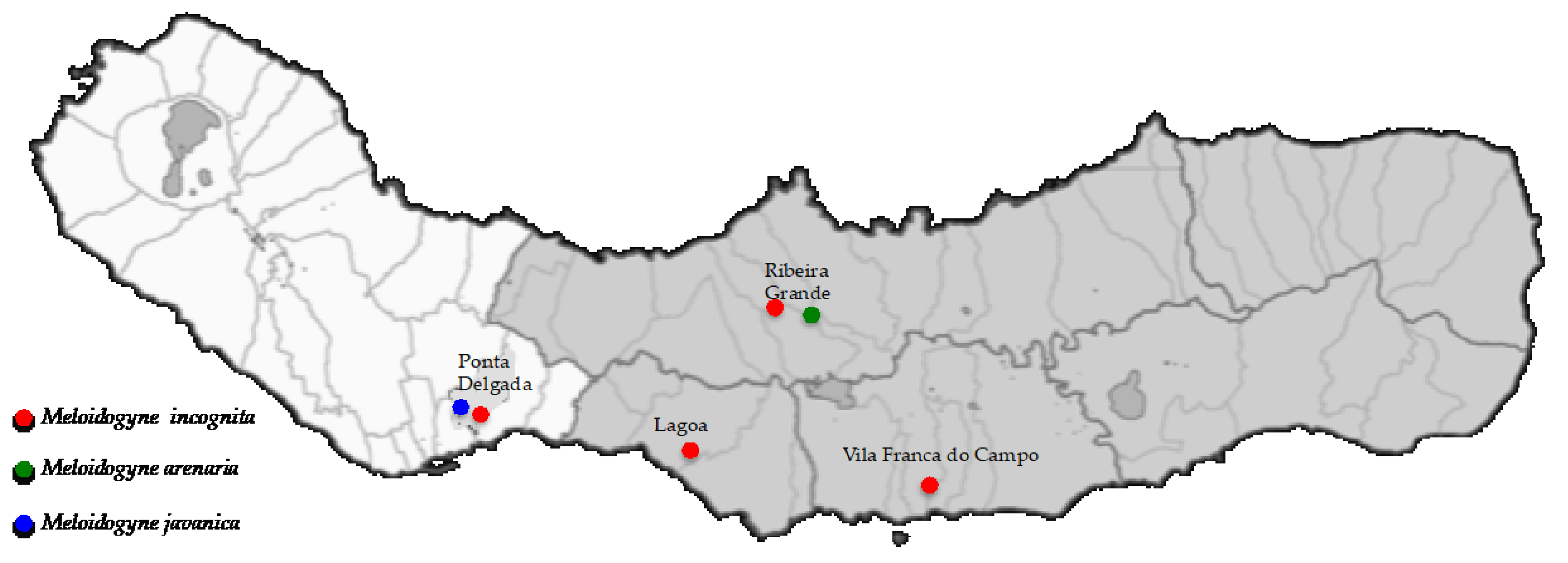
3.1. Distribution
3.2. Morphological Characterisation
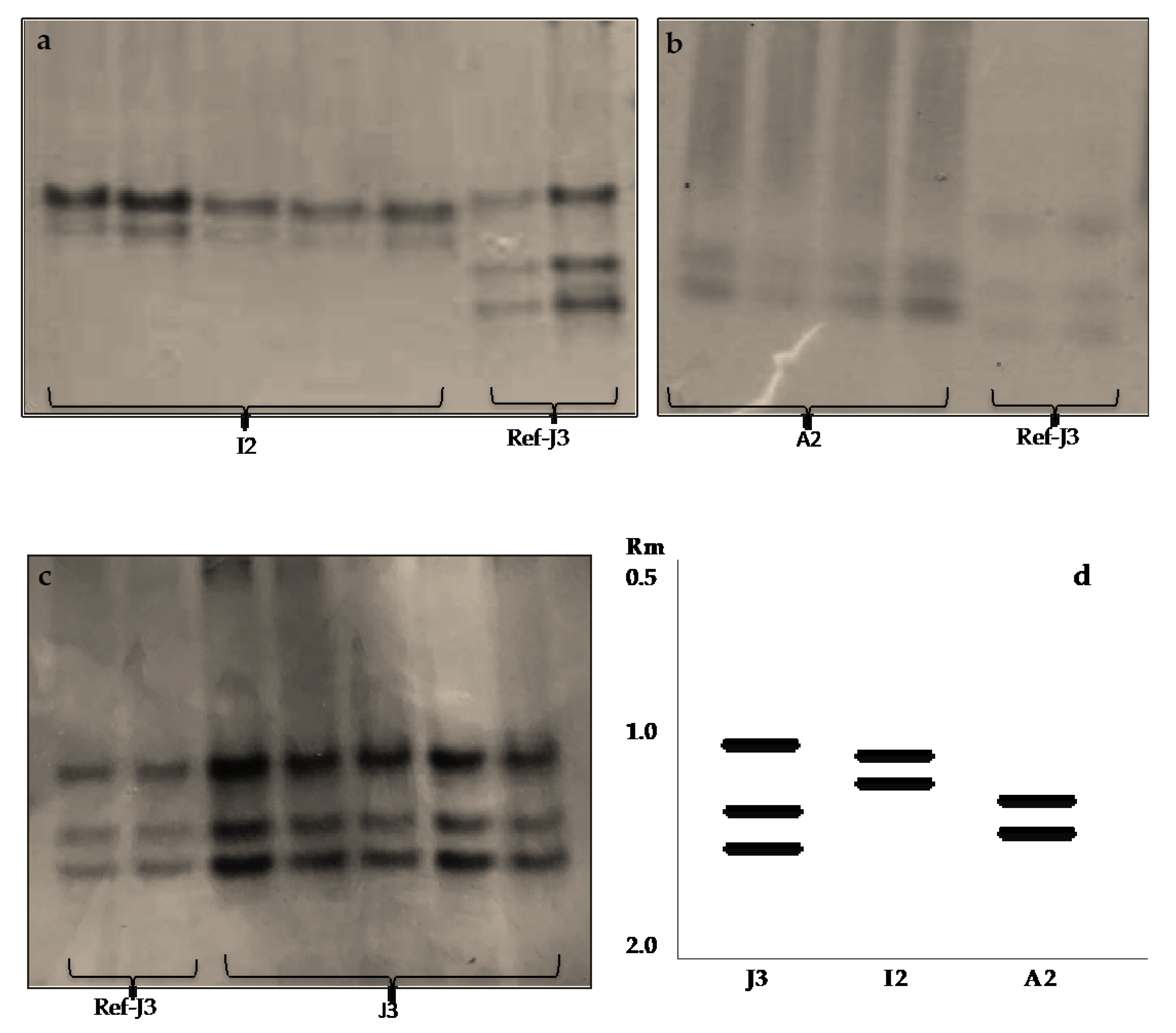
3.3. Biochemical Characterisation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Massot, A. A Agricultura do Arquipélago dos Açores-Estudo, Direcção-Geral de Políticas Internas, Departamento Temático B: Políticas Estruturais e de Coesão, União Europeia. 2015. Available online: https://www.europarl.europa.eu/RegData/etudes/STUD/2015/567667/IPOL_STU(2015)567667_PT.pdf (accessed on 17 November 2021).
- Direção Regional do Ambiente—Divisão do Ordenamento do Território. COS. A, Carta de Ocupação do Solo da Região Autónoma dos Açores. 2018. Available online: http://ot.azores.gov.pt/store/inc/cosa2018/relatorio/Relatorio_COS.A_2018.pdf (accessed on 17 November 2021).
- De Almeida, A.M.; Alvarenga, P.; Fangueiro, D. The dairy sector in the Azores Islands: Possibilities and main constraints towards increased added value. Trop. Anim. Health Prod. 2021, 53, 40. [Google Scholar] [CrossRef] [PubMed]
- Lima, F.S.O.; Mattos, S.V.; Silva, S.E.; Carvalho, M.A.S.; Teixeira, R.A.; Silva, J.C.; Correa, V.R. Nematodes Affecting Potato and Sustainable Practices for Their Management. In Potato—From Incas to All Over the World, 1st ed.; Yildiz, M., Ed.; IntechOpen: London, UK, 2018; Volume 1, pp. 107–121. [Google Scholar]
- Elling, A.A. Major emerging problems with minor Meloidogyne species. Phytopathology 2013, 103, 1092–1102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, J.T.; Haegeman, A.; Danchin, E.G.J.; Gaur, H.S.; Helder, J.; Jones, M.G.K.; Kikuchi, T.; Manzanilla-López, R.; Palomares-Rius, J.E.; Wesemael, W.M.L. Top 10 plant-parasitic nematodes in molecular plant pathology. Mol. Plant. Pathol. 2013, 14, 946–961. [Google Scholar] [CrossRef] [PubMed]
- Pais, C.S.; de Abrantes, I.M.O.; Fernandes, M.F.M.; de Santos, M.S.N.A. Tecnica de electroforese aplicada ao estudo das enzimas dos nematodes-das-galhas-radiculares, Meloidogyne spp. Ciência Biol. Ecol. Syst. 1986, 6, 19–34. [Google Scholar]
- Trudgill, D.L.; Blok, V.C. Apomictic, polyphagous root-knot nematodes: Exceptionally successful and damaging biotrophic root pathogens. An. Rev. Phytopathol. 2001, 39, 53–77. [Google Scholar] [CrossRef] [PubMed]
- Ghaderi, R.; Karssen, G. An updated checklist of Meloidogyne Göldi, 1887 species, with a diagnostic compendium for second-stage juveniles and males. J. Crop. Prot. 2020, 9, 183–193. [Google Scholar]
- Hunt, D.; Handoo, Z. Taxonomy, identification and principal species. In Root-Knot Nematodes; Perry, R.N., Moens, M., Starr, J.L., Eds.; CABI: London, UK, 2009; pp. 55–88. [Google Scholar]
- Abrantes, I.M.O.; dos Santos, M.C.V.; da Conceição, I.L.P.M.; Santos, M.S.N.; Vovlas, N. Root-knot and other plant-parasitic nematodes associated with fig trees in Portugal. Nema. Mediterr. 2008, 36, 131–136. [Google Scholar]
- Viera dos Santos, M.; Almeida, M.T.M.; Costa, S.R. First report of Meloidogyne naasi parasitizing turfgrass in Portugal. J. Nematol. 2020, 52, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Maleita, C.; Esteves, I.; Cardoso, J.M.S.; Cunha, M.J.; Carneiro, R.M.D.G.; Abrantes, I. Meloidogyne luci, a new root-knot nematode parasitizing potato in Portugal. Plant Pathol. 2018, 67, 366–376. [Google Scholar] [CrossRef] [Green Version]
- Rusinque, L.; Nóbrega, F.; Cordeiro, L.; Serra, C.; Inácio, M.L. First Detection of Meloidogyne luci (Nematoda: Meloidogynidae) Parasitizing Potato in the Azores, Portugal. Plants 2021, 10, 99. [Google Scholar] [CrossRef] [PubMed]
- EPPO. Alert List: Addition of Meloidogyne luci Together with M. ethiopica. Reporting Service 11. 2017. Available online: https://www.eppo.int/ACTIVITIES/plant_quarantine/alert_list (accessed on 30 November 2021).
- EPPO A2 List of Pests Recommended for Regulation as Quarantine Pests. Version 2021-09. Available online: https://www.eppo.int/ACTIVITIES/plant_quarantine/A2_list (accessed on 28 November 2021).
- Standard Protocol PM 7/119 (1); Nematode Extraction; EPPO Bulletin 43:471-95; EPPO: Paris, France, 2013.
- Eisenback, J.D. Detailed morphology and anatomy of second-stage juveniles, males, and females of the genus Meloidogyne (root-knot nematodes). In An Advanced Treatise on Meloidogyne; Sasser, J.N., Carter, C.C., Eds.; State University Graphics Raleigh: Raleigh, NC, USA, 1985; Volume I, pp. 47–77. [Google Scholar]
- Jepson, S.B. Identification of Root-Knot Nematodes (Meloidogyne Species), 1st ed.; CAB International: Wallingford, UK, 1987. [Google Scholar]
- Esbenshade, P.R.; Triantaphyllou, A.C. Use of enzyme phenotypes for identification of Meloidogyne species. J. Nematol. 1985, 17, 6–20. [Google Scholar] [PubMed]
- Carneiro, R.M.D.G.; Almeida, M.R.A.; Quénéhervé, P. Enzyme phenotypes of Meloidogyne spp. isolates. Nematology 2000, 2, 645–654. [Google Scholar] [CrossRef]

| District | Locality | Coordinates | Production Type | |||
|---|---|---|---|---|---|---|
| Field | Meloidogyne Species | Greenhouse | Meloidogyne Species | |||
| Ponta Delgada (39 samples = 48.75%) | São Roque (14 Samples) | 37°45′17.906″ N 25°37′43.507″ W | Leek (Allium porrum) | ND | Broccoli (Brassica oleraceae cv. italica) | M. inognita |
| Chard (Beta vulgaris subsp. vulgaris) | M. incognita | Pepper (Capsicum annuum) | M. incognita | |||
| Lettuce (Lactuca sativa) | M. incognita | Pea (Pisum sativum) | M. incognita | |||
| 37°45′23.306″ N 25°37′58.962″ W | Carrot (Daucus carota subsp. sativus) | M. incognita | Cucumber (Cucumis sativus) | M. incognita | ||
| Potato (Solanum tuberosum) | M. incognita | Tomato (Solanum lycopersicum) | M. javanica | |||
| 37°46′21.846″ N 25°37′25.018″ W | Sweet-potato (Ipomoea batatas) | ND | Green beans (Phaseolus vulgaris) | M. incognita | ||
| Carrot (Daucus carota subsp. NDsativus) | ND | Lettuce (Lactuca sativa) | M. incognita | |||
| Livramento (6 Samples) | 37°45′53.100″ N 25°35′27.100″ W | Lettuce (Lactuca sativa) | M. incognita | Lettuce | M. incognita | |
| Cabbage (Brassica oleracea cv. capitata) | M. javanica | (Lactuca sativa) | ||||
| Spinach (Spinacia oleracea) | M. incognita | |||||
| Courgette (Cucurbita pepo) | M. incognita | Tomato (Solanum lycopersicum) (2) | M. incognita | |||
| Arrifes (6 Samples) | 37°45′26.647″ N 25°41′47.965″ W | Parsley (Petroselnum cripsum) | ND | Courgette (Cucurbita pepo) | M. incognita | |
| 37°45′42.534″ N 25°40′45.588″ W | Lettuce (Lactuca sativa) | M. incognita | Lettuce (Lactuca sativa) | M.incognita | ||
| 37°45′31.730″ N 25°40′34.838″ W | Watercress (Nasturtium officinalis) | ND | Spinach (Spinacia oleracea) | M. javanica | ||
| São Pedro (2 Samples) | 37°45′12.658″ N 25°39′54.464″ W | Onion (Allium cepa) | ND | Tomato (Solanum lycopersicum) | M. javanica | |
| Ginetes (10 Samples) | 37°50′24.565″ N 25°49′58.649″ W | Cabbage (Brassica oleracea cv. capitata) | M. incognita | Cucumber (Cucumis sativus) Lettuce (Lactuca sativa) Green beans (Phaseolus vulgaris) | M. incognita M. incognita M. incognita | |
| 37°50′38.130″ N 25°50′45.420″ W | Potato (Solanum tuberosum) (6) | M. incognita | ||||
| Mosteiros (1 Sample) | 37°53′18.427″ N 25°48′49.597″ W | Potato (Solanum tuberosum cv. Rudolph) | M. incognita | |||
| Ribeira Grande (30 samples = 37.5%) | Rabo de peixe (12 Samples) | 37°47′45.038″ N 25°35′44.624″ W | Cabbage (Brassica oleracea cv. capitata) Onion (Allium cepa) | M. arenaria ND | ||
| 37°48′16.643″ N 25°35′31.848″ W | Potato (Solanum tuberosum) (4) Rocket (Eruca sativa) | M. incognita ND | Passion fruit (Passiflora edulis) Tomato (Solanum lycopersicum) Cucumber (Cucumis sativus) Green beans (Phaseolus vulgaris) Courgette (Cucurbita pepo) | ND M. incognita M. incognita M. arenaria ND | ||
| Pico da pedra (4 Samples) | 37°48′28.440″ N 25°37′26.627″ W | Strawberry (Fragaria X Ananassa) Potato (Solanum tuberosum) Broccoli (Brassica oleraceae cv. italica) Cabbage (Brassica oleracea cv. capitata) | ND M. incognita M. incognita ND | |||
| Calhetas (4 Samples) | 37°49′1.074″ N 25°35′57.127″ W | Strawberry (Fragaria X Ananassa) Parsley (Petroselnum cripsum) | ND ND | Tomato (Solanum lycopersicum) Cucumber (Cucumis sativus) | M. incognita ND | |
| Ribeira seca (10 Samples) | 37°48′27.552″ N 25°32′43.628″ W | Potato (Solanum tuberosum cv. Rudolph) (3) | M. incognita | |||
| 37°48′26.508″ N 25°33′9.157″ W | cv. Tonmedo | ND | ||||
| 37°48′43.950″ N 25°33′6.360″ W | cv. Red Scarlett | M. incognita | ||||
| 37°48′13.677″ N 25°32′23.734″ W | cv. Yona | M. incognita | ||||
| 37°48′19.435″ N 25°32′11.068″ W | cv. Picasso (2) | M. incognita | ||||
| 37°48′30.272″ N 25°33′1.325″ W | cv. Perdiz | M. incognita | ||||
| 37°48′43.950″ N 25°33′6.360″ W | cv. Agria | M. incognita | ||||
| Lagoa (9 samples = 11.25%) | Água de Pau (2 Samples) | 37°43′4.717″ N 25°29′35.569″ W | Potato (Solanum tuberosum cv. Agria) cv. Rudolph | M. incognita ND | ||
| Santa Cruz (7 Samples) | 37°44′34.365″ N 25°33′17.830″ W | Cabbage (Brassica oleracea cv. capitata) Parsley (Petroselnum cripsum) | M. incognita ND | Lettuce (Lactuca sativa) Tomato (Solanum lycopersicum) | ND M. incognita | |
| 37°44′15.399″ N 25°33′13.514″ W | Potato (Solanum tuberosum cv. picasso) (3) | M. incognita | ||||
| Vila Franca (2 samples = 2.5%) | Ribeira seca (2 samples) | 37°43′25.704″ N 25°25′11.182″ W | Potato (Solanum tuberosum cv. picasso) cv. Red scarlett | M. incognita ND | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rusinque, L.; Nóbrega, F.; Cordeiro, L.; Lima, A.; Andrade, S.; Inácio, M.L. Root-Knot Nematode Species Associated with Horticultural Crops in the Island of Azores, Portugal. Horticulturae 2022, 8, 101. https://doi.org/10.3390/horticulturae8020101
Rusinque L, Nóbrega F, Cordeiro L, Lima A, Andrade S, Inácio ML. Root-Knot Nematode Species Associated with Horticultural Crops in the Island of Azores, Portugal. Horticulturae. 2022; 8(2):101. https://doi.org/10.3390/horticulturae8020101
Chicago/Turabian StyleRusinque, Leidy, Filomena Nóbrega, Laura Cordeiro, Arlindo Lima, Samuel Andrade, and Maria Lurdes Inácio. 2022. "Root-Knot Nematode Species Associated with Horticultural Crops in the Island of Azores, Portugal" Horticulturae 8, no. 2: 101. https://doi.org/10.3390/horticulturae8020101
APA StyleRusinque, L., Nóbrega, F., Cordeiro, L., Lima, A., Andrade, S., & Inácio, M. L. (2022). Root-Knot Nematode Species Associated with Horticultural Crops in the Island of Azores, Portugal. Horticulturae, 8(2), 101. https://doi.org/10.3390/horticulturae8020101







