Abstract
Reactive oxygen species (ROS) play an active role in plant defense. Polyphenol oxidase (PPO) and peroxidase (POD) participate in the synthesis of phytoalexins. The comparative activities of ROS, including hydrogen peroxide (H2O2), superoxide anions (O2−), and hydroxyl radicals (·OH), against the fungal pathogen Colletotrichum gloeosporioides from papaya fruit were evaluated. The effects of ROS on PPO and POD activities in papaya fruit inoculated with C. gloeosporioides and the development of natural decay in intact fruit were also investigated. ·OH was the most effective in inhibiting conidial germination and mycelial growth of C. gloeosporioides in vitro. However, 20 or 30 mM H2O2 exhibited the best control of the three ROS treatments at ameliorating the disease symptoms associated with the highest levels of PPO and POD activities in papaya fruit. Furthermore, the activities of PPO and POD negatively correlated with the disease index. Overall, H2O2 treatments can induce the resistance of papaya fruit against C. gloeosporioides owing to the enhanced activities of PPO and POD. Treatment with 20 mM H2O2 also significantly reduced the incidence of decay in intact papaya fruit in semi-commercial experiment, which could make it a potential alternative manner to control postharvest disease in papaya fruit.
1. Introduction
Numerous pathogens exist in the natural environment and result in the substantial loss of crops. However, plants have evolved specific mechanisms to counter pathogens [1,2]. The hypersensitive response (HR) is an early defense phenomenon that successfully recognizes pathogens by causing cell death and necrosis to attack pathogen growth [3]. In this phase, significant amounts of reactive oxygen species (ROS), including superoxide anions, hydrogen peroxide (H2O2), and hydroxyl radicals, are produced [4,5]. ROS have been reported to participate in the plant defense systems as antimicrobial byproducts against pathogen infection [5,6,7]. ROS, particularly H2O2, has been suggested to serve as an antimicrobial agent during the plant defense response [5,6,7]. Shettly et al. reported that treatment with 5 mM H2O2 inhibits the development of inocula from 4-day-old Septoria tritici cultures [8]. Micromolar concentrations of H2O2 markedly inhibit the germination of Peronospora tabacina, Cladosporium cucumerinum, and Colletotrichum lagenarium spores in vitro [9].
In addition to their role as simple antimicrobial byproducts, ROS also play a key role in the expression of genes for defense-responsive enzymes that encode PRs (pathogenesis-related-proteins), the generation of phytoalexins, lignification or other defense responses [6,7,10,11,12,13]. Early research showed that exogenous H2O2 or ·OH could induce the accumulation of glyceollin phytoalexins in soybean [14,15], and treatment with scavengers of ROS inhibits the accumulation of these compounds [14]. In another study, treatment with 10 mM of H2O2 initiated the expression of phenylalanine ammonia lyase (PAL) genes in Arabidopsis suspension cultures and increased the ability of Arabidopsis to resist and prevent disease [16].
Polyphenol oxidase (PPO) acts like other antioxidant enzymes and plays a vital role in plant defense mechanisms [17,18]. Thipyapong et al. [19] showed that the expression of antisense PPO in transgenic tomato plants (Solanum lycopersicum) markedly enhanced susceptibility, while the overexpression of PPO improved the resistance of the plants to Pseudomonas syringae. Transgenic plants that overexpressed PPO also exhibited increased resistance to a variety of insects [20]. Similarly, peroxidase (POD) participates in the plant defense system with multiple functions, such as the synthesis of phytoalexins [21,22] and the reinforcement of physical barriers and lignification of cellular walls [23]. The two enzymes are also considered as PRs [24]. Hameed et al. [25] reported that tomato genotypes that have higher POD activity are more resistant to infection by Alternaria alternata.
Numerous studies have shown that, upon exogenous elicitor treatment, plants exhibit enhanced activities of PPO and POD and an increased level of H2O2 that correlated with an increased resistance to diseases. Abouraicha et al. [26] reported that oligosaccharides trigger a rapid accumulation of H2O2 and an increase in the activities of PAL, PPO, and POD, as well as the levels of lignin and phenolic compounds, therefore, enhancing resistance to Penicillium expansum and Botrytis cinerea in apple (Malus domestica). Similar results have also been observed in strawberry (Fragaria × ananassa), with increased resistance to Botrytis cinerea following treatment with UV [27]. Chitosan treatment effectively enhances the activities of POD and superoxide dismutase, and levels of glutathione and H2O2, and significantly reduces the disease incidence of harvested navel oranges (Citrus × sinensis) [28]. Salicylic acid and methyl jasmonate induce the activities of PPO and POD and suppress green and blue molds in citrus fruit [29]. Ethanol activates defense responses in blueberry (Vaccinium sect. Cyanococcus) against Alternaria and Botrytis rots, by inducing the activities of defense-related enzymes, including POD, PPO, PAL, beta-1,3-glucanase, and chitinase [30]. Benzo-(1,2,3)-thiadiazole-7-carbothioic acid S-methyl ester (BTH), which can activate systemic acquired resistance in plants, increases the activities of PPO and POD, the levels of total phenolic compounds and H2O2, and disease resistance against Penicillium expansum in harvested peach (Prunus persica) fruit [31]. BTH treatment also markedly enhanced the accumulation of H2O2 and increased the activities of peroxidase while reducing the disease incidence in muskmelon (Cucumis melo) fruit [32].
Papaya (Carica papaya L.) is a popular fruit that is widely cultivated in tropical and subtropical regions [33]. However, postharvest papaya fruit are susceptible to numerous diseases after harvest [33]. Anthracnose caused by Colletotrichum gloeosporioides is one of the most important diseases of papaya fruit [33,34]. During storage and transportation, Co. gloeosporioides can rapidly spread from infected fruits to healthy fruits by direct contact. Anthracnose disease in harvested papaya fruit can be controlled by postharvest applications of fungicides and hot water treatment. However, hot water dip treatment affects the ripening process of papaya, and the use of fungicides may cause the resistance of fungal strains to these fungicides. Recently, techniques have been developed to control the occurrence of anthracnose, including hydrothermal-calcium chloride treatment [34], ozone [35], ozonized water [36], hot water in combination with chitosan coating [37], ZnO and MgO nanomaterials [38], essential oils in combination with chitosan coating [39], and plant-extracted essential oils [40]. However, low efficacies, instability, or the complexity of operation limit their use. Thus, the development of potentially safer approaches to control anthracnose on papaya fruit during storage and transportation is urgently needed.
In this study, we investigated the in vitro antimicrobial activities of H2O2, O2−, and ·OH against Co. gloeosporioides isolated from decayed papaya fruit. The roles of H2O2 in inducing resistance in harvested papaya fruit against Co. gloeosporioides and their effects on PPO and POD activities were investigated in more detail. Semi-commercial experiments were also conducted to assess the potential of H2O2 to control postharvest diseases of intact papaya fruit. This study will lead to a better understanding of the antimicrobial activities of ROS against pathogens, as well as the resistance to the pathogens, and then improve postharvest handling.
2. Materials and Methods
2.1. Fruit Material and Chemicals
Papaya (Carica papaya L. cv. Huakangyihao) fruit were harvested at a mature stage from a commercial orchard in Guangzhou, China, and then selected for uniformity of shape, color, and size. Blemished or diseased fruits were discarded during sampling.
N,N-dimethyl-4,4′-bipyridinium dichloride (Paraquat) and FeSO4/H2O2 (1:1) were used to produce O2− and ·OH as described by Takizawa [41] and Halliwell [42], respectively. Paraquat, catechol, and guaiacol were obtained from Sinopharm Chemical Reagent Co., Guangzhou, China.
2.2. Fungi and Cultures
Single spore-derived isolates of Colletotrichum gloeosporioides (P13) used in this study were provided by the plant pathologist Prof. Zhengke Zhang (Hainan University, Haikou, China). The isolate was maintained on potato dextrose agar plates (PDA) (200 mL of boiled potato extract, 20 g of dextrose and 800 mL of distilled water) at 4 °C. Conidial suspensions were obtained by flooding 14-day-old cultures of pathogens with sterile distilled water on papaya juice agar (PJA) plates (sterile papaya juice with an equal water volume that contained 3% agar).
2.3. Conidial Germination Assay
The effect of ROS on conidial germination was examined in sterile distilled water. A conidial suspension was determined by a hemocytometer (Marienfeld, Germany) and then adjusted to 105−6 conidia mL−1. An aliquot of the tested H2O2, O2−, and ·OH· solution was added to the conidial suspension with a final dilution of 1:10, and the conidial suspensions were vortexed for 5 min before inoculation on the plates. The final concentrations of ROS (H2O2, O2−, and ·OH) used in this study were 0.5, 1, 2, 3, and 4 mM. Aliquots (100 µL) of the conidial suspensions comprising ROS were placed in the center of a hollow-ground slide on the bottom of a sterile glass Petri dish (9 cm diameter). These plates were incubated at 28 °C for 48 h. The samples were examined with a light microscope (Olympus, Tokyo, Japan). Each treatment consisted of three replicates, and five visual fields (at least 100 conidia) per replicate were observed microscopically (×100) to determine the inhibition rate of conidial germination. Inhibitory rate of conidial germination = (germination rate of control − germination rate of sample)/germination rate of control × 100%.
2.4. Mycelial Growth Assay
Fungal colonies were cultured on PJA media. After 10 days of culture, agar discs (6 mm in diameter) with mycelia were excised and then transferred to the center of PJA dishes (90 mm in diameter) that contained 4–20 mM of the ROS under sterile conditions. After 4–8 days of incubation at 28 °C, conidial development was measured each day. Each assay was conducted six times. The relative inhibitory effect of the ROS on mycelial development was evaluated in comparison with that of distilled water without the ROS. Inhibitory rate of mycelial growth = (growth diameter of control − growth diameter of sample)/growth diameter of control × 100%.
2.5. Treatment of Fruit with ROS and Colletotrichum gloeosporioides
Papaya fruits were washed with tap water, surface sterilized with 1% sodium hypochlorite solution, and rinsed twice in distilled water. After drying at 25 °C, papaya fruit were dipped in different solutions of H2O2, O2−, or ·OH at 10, 20, or 30 mM for 3 min. The fruits dipped into distilled water were used as the control. The control and treated fruits were dried at 25 °C for 30 min and wounded using a sterile needle to make a 1-mm-deep and 1-mm-wide wound that was 6 ± 1 cm away from the equatorial region of the fruit surface at the two sites. Agar discs (4 mm in diameter) with mycelia were excised from a 10-day-old culture of Co. gloeosporioides on PJA and then placed on the wounded sites of papaya fruit. Each treatment included three replicates, with 12 fruit per replicate. The fruit were placed in trays sealed with 0.02 mm thick polyethylene bags to maintain high humidity and then stored at 25 °C.
The disease index was measured after 10 days of storage. The disease index was calculated using the following formula: disease index (%) = [(0 × n1 + 1 × n2 + 2 × n3 + 3 × n4 + 4 × n5 + 5 × n6 + 6 × n7 + 7 × n8 + 8 × n9 + 9 × n10)/(N × 9)] × 100. N, total number of the inoculation sites; n1, number of the inoculation sites developed no lesion; n2–n9, number of the lesion diameter between 0.5–1, 1–2, 2–3, 3–4, 4–5, 5–6, 6–7 or 7–8 cm, respectively; and n10, number of the lesion diameter of >8 cm. Fruit pericarp tissues that were 2-mm thick were placed approximately 1 cm from the lesions that were sampled to measure the activities of PPO and POD.
2.6. Determinations of PPO and POD Activities
To measure PPO and POD activities, papaya pericarp tissues (5 g) were homogenized in 25 mL of 0.2 mM phosphate buffer (pH 6.8) and 0.4 g polyvinylpyrrolidone (PVP) and then centrifuged at 15,000× g (J20-2; Beckman Coulter Life Sciences, Indianapolis, IN, USA) for 15 min at 4 °C. The clear supernatant was collected as the enzyme extracts of PPO and POD. PPO activity was assayed as described by Jiang [43] using catechol. The increase in absorbance at 398 nm was automatically recorded for 1 min using a spectrophotometer (DU-7; Beckman Coulter Life Sciences). One unit of enzyme activity was defined as the amount of the enzyme that caused a change of 0.01 in absorbance per minute. The activity of POD was measured as described by MacAdam et al. [44]. The assay mixture consisted of 2.75 mL of 0.2 mM Na phosphate buffer (pH 6.8), 0.1 mL of 0.46% H2O2, 0.1 mL of 4.0% guaiacol, and 0.05 mL of enzyme solution in a final volume of 3 mL. The increase in absorbance at 470 nm was recorded for 2 min using a spectrophotometer. One unit of enzyme activity was defined as the amount of enzyme that caused a change of 0.1 in absorbance per minute.
2.7. Treatment with ROS on Naturally Infected Papaya Fruit
The effects of ROS on the natural incidence of rot in intact papaya fruit were investigated in semi-commercial experiments. Papaya fruit were dipped into a solution of 20 mM H2O2 for 3 min. The control fruits were treated with distilled water. The fruits were dried at ambient temperature, packed in 0.02 mm polyethylene bags, and then stored at 25 °C for 20 days. The percentage of fruit decay was recorded. Each treatment included three replicates, with 20 fruits per replicate.
2.8. Statistical Analysis
The experiments were arranged in a completely randomized design, and each treatment consisted of three replicates. The data were tested by a one-way analysis of variance (ANOVA), followed by Student’s test. Statistical analysis was performed using SPSS 7.5 (SPSS, Inc., Chicago, IL, USA).
3. Results
3.1. Inhibition of Conidial Germination
Figure 1 shows the effect of ROS on the conidial germination of Co. gloeosporioides (P13). The hydroxyl radical (·OH) was the most effective in inhibiting conidial germination. Treatment with ·OH ·destroyed 100% of the spores of P13 at 2 mM, whereas only 20.2% and 36.6% were destroyed by H2O2 and O2− at the concentration, respectively.
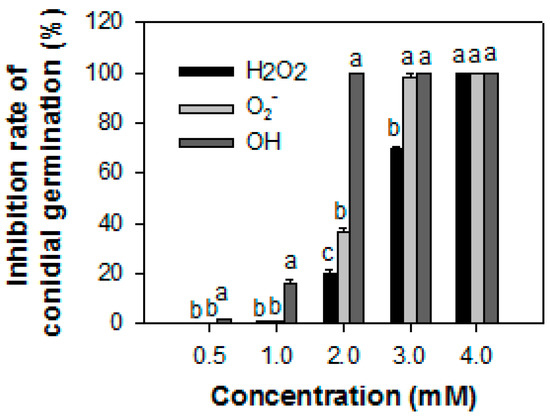
Figure 1.
The inhibitory effect (%) of reactive oxygen species on the conidial germination of Colletotrichum gloeosporioides from papaya fruit. The data were expressed as the means ± SE of three biological replicates. Different letters in the same column indicate significant differences.
3.2. Inhibition of Mycelial Growth
The antifungal activities of H2O2, O2−, and ·OH ·on the mycelial development of P13 in PJA are shown in Figure 2. Among these treatments, ·OH was the most effective at inhibiting mycelial growth. At 8 mM, treatments with ·OH, O2−, and H2O2 inhibited 84.0%, 41.1%, and 35.7% of the mycelial growth of P13, respectively.
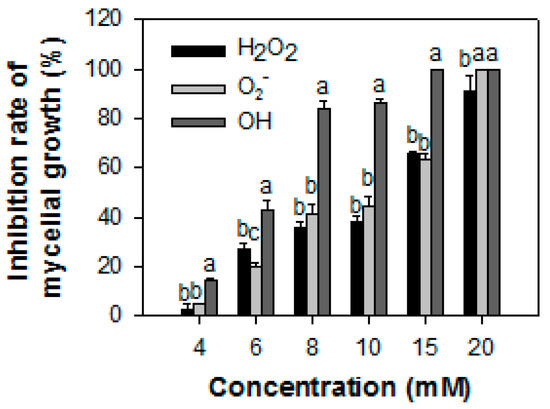
Figure 2.
The inhibitory effect (%) of reactive oxygen species on the mycelial growth of Colletotrichum gloeosporioides from papaya fruit. The data were expressed as the means ± SE of three biological replicates. Different letters in the same column indicate significant differences.
3.3. Effect of ROS on the Disease Index of Papaya Fruit Inoculated with Colletotrichum gloeosporioides
The effects of ROS on disease development caused by Co. gloeosporioides are illustrated in Figure 3. Treatment with H2O2 suppressed the development of Co. gloeosporioides on papaya fruit in a concentration-dependent manner (Figure 3A). Treatments with H2O2 at 20 or 30 mM, resulted in a disease index of 11.8% and 7.8% on papaya fruit, respectively, while that of the control fruit was 78.0% after 10 days of storage. O2− at all concentrations had no significant effect (Figure 3B). Treatment with 10 mM ·OH showed ineffective, whereas treatments with 20 and 30 mM ·OH significantly reduced the disease index (Figure 3C).
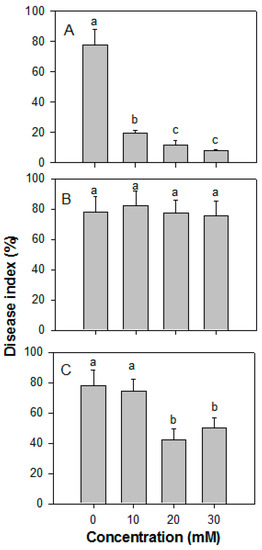
Figure 3.
Effect of different ROS at various concentrations on the disease index of papaya fruit inoculated with Colletotrichum gloeosporioides after 10 days of storage at 25 °C. The papaya fruit were treated with ROS and inoculated with C. gloeosporioides. (A) H2O2, (B) O2−, and (C) ·OH. ROS, reactive oxygen species. The data were expressed as the means ± SE of three biological replicates. Different letters indicate significant differences.
3.4. Effect of H2O2 Treatment on the Activities of PPO and POD from Papaya Fruit Inoculated with Colletotrichum gloeosporioides
Figure 4 shows that H2O2 treatment can influence the activities of PPO and POD in papaya fruit during storage. The activities of PPO and POD decreased progressively as the concentration of H2O2 increased, with a negative correlation with the disease index of papaya fruit (Figure 4). Treatments with H2O2 at 20 and 30 mM exhibited the slightest disease symptoms in association with the highest PPO and POD activities.
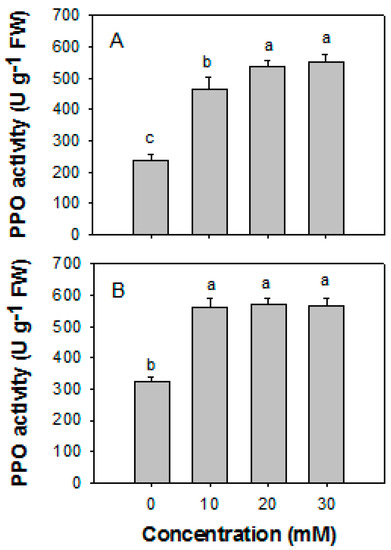
Figure 4.
Effects of H2O2 at various concentrations on the activities of PPO (A) and POD (B) of papaya fruit inoculated with Colletotrichum gloeosporioides after 10 days of storage at 25 °C. The papaya fruit were immediately inoculated with C. gloeosporioides after H2O2 treatment and dried. The pericarp tissue of papaya fruit was sampled at 0–1 cm from the lesions. POD, peroxidase; PPO, polyphenol oxidase. The data were expressed as the means ± SE of three biological replicates. Different letters indicate significant differences.
PPO and POD activities in the pericarp tissues of H2O2-treated fruit sampled at 0–1 cm from the lesions were higher than those of the control fruit (Figure 5). However, the activities of both enzymes in the pericarp tissues of H2O2-treated fruit at 2–3 cm from the lesions were lower than those of the control fruit. The activity of PPO in the pericarp tissues of H2O2-treated fruit at 4–5 cm from the lesions was higher than that of the control fruit, whereas the activities of POD in both samples were almost indistinguishable. This suggests that treatment with H2O2 increased the activities of these two enzymes, and this increase varied when the pericarp was sampled at different distances from the lesions.
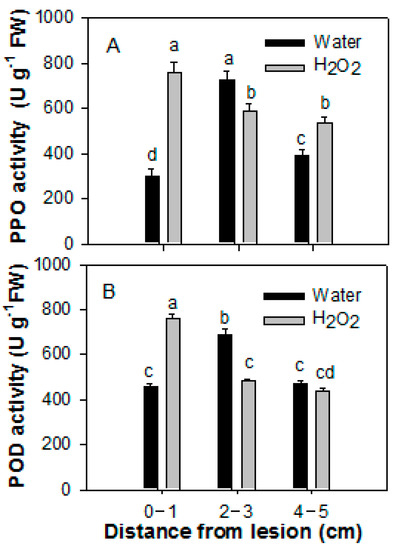
Figure 5.
Effect of 20 mM H2O2 treatment on the activities of PPO (A) and POD (B) of papaya fruit inoculated with Colletotrichum gloeosporioides. Papaya fruit were inoculated with Colletotrichum gloeosporioides immediately after H2O2 treatment. The pericarp tissue of papaya fruit inoculated with C. gloeosporioides were sampled after 8 days of incubation at 25 °C at 0–1, 2–3, and 4–5 cm from the lesions, respectively. POD, peroxidase; PPO, polyphenol oxidase. The data were expressed as the means ± SE of three biological replicates. Different letters indicate significant differences.
3.5. Effect of H2O2 on the Development of Natural Decay in Intact Papaya Fruit
The application of H2O2 significantly (p ≤ 0.05) reduced the development of decay in papaya fruit in semi-commercial experiments. After 20 days of storage at 25 °C, the incidence of decay for non-H2O2-treated (control) fruit was 35%, whereas the fruit treated with 20 mM of H2O2 had an incidence of decay of 11% (Figure 6).
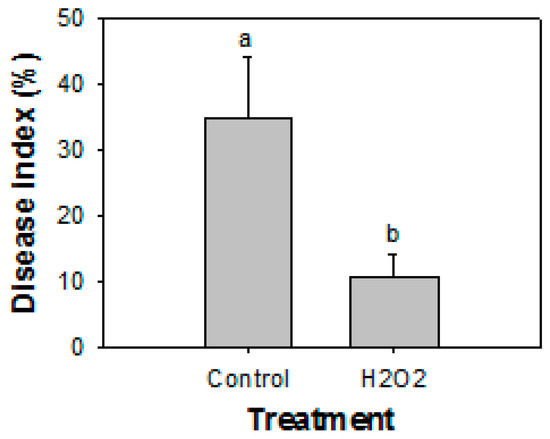
Figure 6.
Effect of treatment with 20 mM H2O2 on the development of natural decay in intact papaya fruit. The data were expressed as the means ± SE of three biological replicates. Different letters indicate significant differences.
4. Discussion
Infection with a pathogen results in the rapid production of reactive oxygen intermediates [7]. ROS are toxic to pathogens, and, therefore, they were suggested to be antimicrobial agents produced by the plant defense system [5,6,7]. The early processes of defense responses induced by pathogens are usually accompanied by the production of H2O2. Among these ROS, ·OH is generally considered to be a highly reactive free radical that can directly and indiscriminately attack lipids, proteins, and DNA [45], and, thus, inhibited the disease development of fresh-cut Chinese water chestnut (Eleocharis dulcis) [46]. In this study, ·OH was found to be the most effective at inhibiting conidial germination and mycelial growth of Co. gloeosporioides of the ROS. However, O2− was relatively less toxic to the pathogen compared with ·OH. Thus, the role of ROS against Co. gloeosporioides could be attributed to their direct toxicity to the pathogen.
H2O2 is known to be a local potentially mobile signal that mediates the induction of plant defense responses. The suppression of production of H2O2 by scavengers inhibits the transcription of defense-related genes and decreases the activity of defense-related enzymes [10]. Thus, a low amount of H2O2 may play a central role in the plant defense system, such as mediating the transcription of specific genes that encode PR proteins, inducing the generation of phytoalexins, and enhancing lignification or other defense responses [6,10,11,12]. Previous research showed that the elicitation of Arabidopsis suspension cultures with the bacterial protein harpin induced a rapid generation of H2O2 and initiated programmed cell death and the expression of defense genes [16,47]. Treatment with 10 mM of H2O2 has been reported to initiate the expression of the gene for PAL in Arabidopsis suspension cultures and reduce the occurrence of disease [16]. It has also been observed that direct exposure to 20 mM H2O2 activated the MAPK (mitogen-activated protein kinases) cascades in Arabidopsis suspension cultures [48]. The MAPK cascades are important in signaling defense responses of plants [49]. Furthermore, H2O2 has been shown to be effective in inducing disease resistance in several postharvest horticultural crops. Bayoumi et al. [50] reported that white pepper fruit (Piper nigrum) sprayed with H2O2 at 20 or 50 mM, followed by inoculation with Botrytis cinerea, exhibited a significant reduction in the rate of natural rot and an increase in shelf-life. Treatment with H2O2 at 20 mM along with cold-shock induced disease resistance of harvested tomato and increased the activities of enzymes involved in disease resistance, including PAL, chitinase, and β-1,3 glucanase [51]. This study also indicated that treatment with H2O2 at 20 or 30 mM increased the resistance of papaya fruit to Co. gloeosporioides in association with the increased activities of PPO and POD. Although knowledge of the details of plant defense responses against pathogens has been rapidly increasing, the mode of action of H2O2 remains unclear [46]. Further research toward the signaling pathways and the formation of antifungal compounds is merited to determine the contribution of H2O2 in the defense system against pathogen infection.
The induction of plant resistance to inhibit pathogen infections can be a safe and effective approach in controlling postharvest diseases of horticultural crops. Bautista-Banos et al. [52] reported that the application of 1.5% chitosan inhibited the mycelial growth and sporulation of Co. gloeosporioides in vitro and controlled 60% of the anthracnose of papaya fruit. Manenoi et al. [53] found that 1-methylcyclopropene (1-MCP) delayed the ripening of fruit and reduced the development of postharvest disease in papaya fruit. Furthermore, Peng et al. [46] observed that treatment with H2O2 effectively suppressed the activities of metabolic enzymes related to phenolics in fresh-cut Chinese water chestnut and maintained their edibility together with the taste and nutritional attributes. In this study, the application of 20 mM H2O2 effectively reduced the disease incidence of papaya fruit inoculated with Co. gloeosporioides, as well as the disease development in intact papaya fruit. This suggests that H2O2 treatments can induce the resistance of papaya fruit to Co. gloeosporioides by enhancing the activities of PPO and POD. Further study on the effects of H2O2 on the disease development of different cultivars and the maturity of harvested papaya fruits is merited for commercial applications.
Author Contributions
Conceptualization, X.D. and Y.J.; methodology, L.F.; investigation, L.F.; data curation, L.F.; writing—original draft preparation, L.F.; writing—review and editing, Y.J., X.D.; funding acquisition, X.D., J.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Guangxi Natural Science Foundation (No.2021GXNSFGA196001).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data sets supporting the results of this research are included within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nishad, R.; Ahmed, T.; Rahman, V.J.; Kareem, A. Modulation of plant defense system in response to microbial interactions. Front. Microbiol. 2020, 11, 1298. [Google Scholar] [CrossRef] [PubMed]
- Sood, M.; Kapoor, D.; Kumar, V.; Kalia, N.; Bhardwaj, R.; Sidhu, G.P.S.; Sharma, A. Mechanisms of plant defense under pathogen stress: A review. Curr. Protein Pept. Sci. 2021, 22, 376–395. [Google Scholar] [CrossRef]
- Ali, M.; Cheng, Z.; Ahmad, H.; Hayat, S. Reactive oxygen species (ROS) as defenses against a broad range of plant fungal infections and case study on ROS employed by crops against Verticillium dahliae wilts. J. Plant Interact. 2018, 13, 353–363. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, H. Reactive oxygen species and nitric oxide as mediators in plant hypersensitive response and stomatal closure. Plant Signal. Behav. 2021, 16, 1985860. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhou, X.; Huang, Y.; Liao, B.; Cheng, L.; Ren, B. Reactive oxygen species in pathogen clearance: The killing mechanisms, the adaption response, and the side effects. Front. Microbiol. 2021, 11, 3610. [Google Scholar] [CrossRef]
- Shetty, N.P.; Jorgensen, H.J.L.; Jensen, J.D.; Collinge, D.B.; Shetty, H.S. Roles of reactive oxygen species in interactions between plants and pathogens. Eur. J. Plant Pathol. 2008, 121, 267–280. [Google Scholar] [CrossRef]
- Wang, Y.; Ji, D.; Chen, T.; Li, B.; Zhang, Z.; Qin, G.; Tian, S. Production, signaling, and scavenging mechanisms of reactive oxygen species in fruit—pathogen interactions. Int. J. Mol. Sci. 2019, 20, 2994. [Google Scholar] [CrossRef]
- Shetty, N.P.; Mehrabi, R.; Lutken, H.; Haldrup, A.; Kema, G.H.J.; Collinge, D.B.; Jorgensen, H.J.L. Role of hydrogen peroxide during the interaction between the hemibiotrophic fungal pathogen Septoria tritici and wheat. New Phytol. 2007, 174, 637–647. [Google Scholar] [CrossRef]
- Peng, M.; Kuc, J. Peroxidase-generated hydrogen peroxide as a source of antifungal activity in vitro and on tobacco leaf disks. Phytopathology 1992, 82, 696–699. [Google Scholar] [CrossRef]
- Alvarez, M.E.; Pennell, R.I.; Meijer, P.J.; Ishikawa, A.; Dixon, R.A.; Lamb, C. Reactive oxygen intermediates mediate a systemic signal network in the establishment of plant immunity. Cell 1998, 92, 773–784. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed]
- Lamb, C.; Dixon, R.A. The oxidative burst in plant disease resistance. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1997, 48, 251–275. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Cristobal, J.; Garcia-Villaraco, A.; Ramos, B.; Gutierrez-Manero, J.; Lucas, J.A. Priming of pathogenesis related-proteins and enzymes related to oxidative stress by plant growth promoting rhizobacteria on rice plants upon abiotic and biotic stress challenge. J. Plant Physiol. 2015, 188, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Epperlein, M.M.; Noronhadutra, A.A.; Strange, R.N. Involvement of the hydroxyl radical in the abiotic elicitation of phytoalexins in legumes. Physiol. Mol. Plant Pathol. 1986, 28, 67–77. [Google Scholar] [CrossRef]
- Apostol, I.; Heinstein, P.F.; Low, P.S. Rapid stimulation of an oxidative burst during elicitation of cultured plant cells: Role in defense and signal transduction. Plant Physiol. 1989, 90, 109–116. [Google Scholar] [CrossRef]
- Desikan, R.; Reynolds, A.; Hancock, T.J.; Neill, J.S. Harpin and hydrogen peroxide both initiate programmed cell death but have differential effects on defence gene expression in Arabidopsis suspension cultures. Biochem. J. 1998, 330, 115–120. [Google Scholar] [CrossRef]
- Boeckx, T.; Winters, A.L.; Webb, K.J.; Kingston-Smith, A.H. Polyphenol oxidase in leaves: Is there any significance to the chloroplastic localization? J. Exp. Bot. 2015, 66, 3571–3579. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, X. Recent advances in polyphenol oxidase-mediated plant stress responses. Phytochemistry 2021, 181, 112588. [Google Scholar] [CrossRef]
- Thipyapong, P.; Stout, M.J.; Attajarusit, J. Functional analysis of polyphenol oxidases by antisense/sense technology. Molecules 2007, 12, 1569–1595. [Google Scholar] [CrossRef]
- Wang, J.H.; Constabel, C.P. Polyphenol oxidase overexpression in transgenic Populus enhances resistance to herbivory by forest tent caterpillar (Malacosoma disstria). Planta 2004, 220, 87–96. [Google Scholar] [CrossRef]
- Daudi, A.; Cheng, Z.; O’Brien, J.A.; Mammarella, N.; Khan, S.; Ausubel, F.M.; Bolwell, G.P. The apoplastic oxidative burst peroxidase in Arabidopsis is a major component of pattern-triggered immunity. Plant Cell 2012, 24, 275–287. [Google Scholar] [CrossRef]
- Mohammadi, M.A.; Zhang, Z.; Xi, Y.; Han, H.; Lan, F.; Zhang, B.; Wang-Pruski, G. Effects of potassium phosphite on biochemical contents and enzymatic activities of Chinese potatoes inoculated by Phytophthora infestans. Appl. Ecol. Environ. Res. 2019, 17, 4499–4514. [Google Scholar] [CrossRef]
- O’Brien, J.A.; Daudi, A.; Butt, V.S.; Bolwell, G.P. Reactive oxygen species and their role in plant defence and cell wall metabolism. Planta 2012, 236, 765–779. [Google Scholar] [CrossRef] [PubMed]
- van Loon, L.C.; Rep, M.; Pieterse, C.M.J. Significance of inducible defense-related proteins in infected plants. Annu. Rev. Phytopathol. 2006, 44, 135–162. [Google Scholar] [CrossRef] [PubMed]
- Hameed, A.; Akhtar, K.P.; Saleem, M.Y.; Asghar, M. Correlative evidence for peroxidase involvement in disease resistance against Alternaria leaf blight of tomato. Acta Physiol. Plant. 2010, 32, 1171–1176. [Google Scholar] [CrossRef]
- Abouraicha, E.; El Alaoui-Talibi, Z.; El Boutachfaiti, R.; Petit, E.; Courtois, B.; Courtois, J.; El Modafar, C. Induction of natural defense and protection against Penicillium expansum and Botrytis cinerea in apple fruit in response to bioelicitors isolated from green algae. Sci. Hortic. 2015, 181, 121–128. [Google Scholar] [CrossRef]
- Jin, P.; Wang, H.; Zhang, Y.; Huang, Y.; Wang, L.; Zheng, Y. UV-C enhances resistance against gray mold decay caused by Botrytis cinerea in strawberry fruit. Sci. Hortic. 2017, 225, 106–111. [Google Scholar] [CrossRef]
- Zeng, K.; Deng, Y.; Ming, J.; Deng, L. Induction of disease resistance and ROS metabolism in navel oranges by chitosan. Sci. Hortic. 2010, 126, 223–228. [Google Scholar] [CrossRef]
- Moosa, A.; Sahi, S.T.; Khan, S.A.; Malik, A.U. Salicylic acid and jasmonic acid can suppress green and blue moulds of citrus fruit and induce the activity of polyphenol oxidase and peroxidase. Folia Hortic. 2019, 31, 195–204. [Google Scholar] [CrossRef]
- Ji, Y.; Hu, W.; Liao, J.; Xiu, Z.; Jiang, A.; Yang, X.; Guan, Y.; Feng, K.; Saren, G. Effect of ethanol vapor treatment on the growth of Alternaria alternata and Botrytis cinerea and defense-related enzymes of fungi-inoculated blueberry during storage. Front. Microbiol. 2021, 12, 618252. [Google Scholar] [CrossRef]
- Liu, H.X.; Jiang, W.B.; Bi, Y.; Luo, Y.B. Postharvest BTH treatment induces resistance of peach (Prunus persica L. cv. Jiubao) fruit to infection by Penicillium expansum and enhances activity of fruit defense mechanisms. Postharvest Biol. Technol. 2005, 35, 263–269. [Google Scholar] [CrossRef]
- Ren, Y.; Wang, Y.; Bi, Y.; Ge, Y.; Wang, Y.; Fan, C.; Li, D.; Deng, H. Postharvest BTH treatment induced disease resistance and enhanced reactive oxygen species metabolism in muskmelon (Cucumis melo L.) fruit. Eur. Food Res. Technol. 2012, 234, 963–971. [Google Scholar] [CrossRef]
- Teixeira da Silva, J.A.; Rashid, Z.; Hhut, D.T.; Sivakumar, D.; Tennant, P. Papaya (Carica papaya L.) biology and biotechnology. Tree For. Sci. Biotechnol. 2007, 1, 47–73. [Google Scholar]
- Ayon-Reyna, L.E.; Gonzalez-Robles, A.; Guadalupe Rendon-Maldonado, J.; Elena Baez-Flores, M.; Edith Lopez-Lopez, M.; Odin Vega-Garcia, M. Application of a hydrothermal-calcium chloride treatment to inhibit postharvest anthracnose development in papaya. Postharvest Biol. Technol. 2017, 124, 85–90. [Google Scholar] [CrossRef]
- Ong, M.K.; Ali, A. Antifungal action of ozone against Colletotrichum gloeosporioides and control of papaya anthracnose. Postharvest Biol. Technol. 2015, 100, 113–119. [Google Scholar] [CrossRef]
- da Costa, A.R.; Faroni, L.R.D.A.; Salomao, L.C.C.; Cecon, P.R.; de Alencar, E.R. Use of ozonized water to control anthracnose in papaya (Carica papaya L.) and its effect on the quality of the fruits. Ozone Sci. Eng. 2021, 43, 384–393. [Google Scholar] [CrossRef]
- Vilaplana, R.; Chicaiza, G.; Vaca, C.; Valencia-Chamorro, S. Combination of hot water treatment and chitosan coating to control anthracnose in papaya (Carica papaya L.) during the postharvest period. Crop Prot. 2020, 128, 105007. [Google Scholar] [CrossRef]
- De la Rosa-Garcia, S.C.; Martinez-Torres, P.; Gomez-Cornelio, S.; Alberto Corral-Aguado, M.; Quintana, P.; Gomez-Ortiz, N.M. Antifungal activity of ZnO and MgO nanomaterials and their mixtures against Colletotrichum gloeosporioides strains from tropical fruit. J. Nanomater. 2018, 2018, 3498527. [Google Scholar] [CrossRef]
- Braga, S.d.P.; Lundgren, G.A.; Macedo, S.A.; Tavares, J.F.; dos Santos Vieira, W.A.; Saraiva Camara, M.P.; de Souza, E.L. Application of coatings formed by chitosan and Mentha essential oils to control anthracnose caused by Colletotrichum gloesporioides and C. brevisporum in papaya (Carica papaya L.) fruit. Int. J. Biol. Macromol. 2019, 139, 631–639. [Google Scholar] [CrossRef]
- Sarkhosh, A.; Schaffer, B.; Vargas, A.I.; Palmateer, A.J.; Lopez, P.; Soleymani, A.; Farzaneh, M. Antifungal activity of five plant-extracted essential oils against anthracnose in papaya fruit. Biol. Agric. Hortic. 2018, 34, 18–26. [Google Scholar] [CrossRef]
- Takizawa, M.; Komori, K.; Tampo, Y.; Yonaha, M. Paraquat-induced oxidative stress and dysfunction of cellular redox systems including antioxidative defense enzymes glutathione peroxidase and thioredoxin reductase. Toxicol. Vitr. 2007, 21, 355–363. [Google Scholar] [CrossRef]
- Halliwel, G. catalytic decomposition of cellulose under biological conditions. Biochem. J. 1965, 95, 35–40. [Google Scholar] [CrossRef]
- Jiang, Y.M. Role of anthocyanins, polyphenol oxidase and phenols in lychee pericarp browning. J. Sci. Food Agric. 2000, 80, 305–310. [Google Scholar] [CrossRef]
- Macadam, J.W.; Nelson, C.J.; Sharp, R.E. Peroxidase activity in the leaf elongation zone of tall fescue. 1. Spatial distribution of ionically bound peroxidase activity in genotypes differing in length of the elongation zone. Plant Physiol. 1992, 99, 872–878. [Google Scholar] [CrossRef]
- Williamson, J.D.; Scandalios, J.G. Plant antioxidant gene responses to fungal pathogens. Trends Microbiol. 1993, 1, 239–245. [Google Scholar] [CrossRef]
- Peng, L.; Yang, S.; Li, Q.; Jiang, Y.; Joyce, D.C. Hydrogen peroxide treatments inhibit the browning of fresh-cut Chinese water chestnut. Postharvest Biol. Technol. 2008, 47, 260–266. [Google Scholar] [CrossRef]
- Desikan, R.; Hancock, J.T.; Coffey, M.J.; Neill, S.J. Generation of active oxygen in elicited cells of Arabidopsis thaliana is mediated by a NADPH oxidase-like enzyme. FEBS Lett. 1996, 382, 213–217. [Google Scholar] [CrossRef]
- Desikan, R.; Clarke, A.; Hancock, J.T.; Neill, S.J. H2O2 activates a MAP kinase-like enzyme in Arabidopsis thaliana suspension cultures. J. Exp. Bot. 1999, 50, 1863–1866. [Google Scholar] [CrossRef][Green Version]
- Neill, S.J.; Desikan, R.; Clarke, A.; Hurst, R.D.; Hancock, J.T. Hydrogen peroxide and nitric oxide as signalling molecules in plants. J. Exp. Bot. 2002, 53, 1237–1247. [Google Scholar] [CrossRef] [PubMed]
- Bayoumi, Y.A. Improvement of postharvest keeping quality of white pepper fruits (Capsicum annuum L.) by hydrogen peroxide treatment under storage conditions. Acta Biol. Szeged. 2008, 52, 7–15. [Google Scholar]
- Fei, W.; Jiping, S.; Bei, F.A.N.; Mengmeng, Y.U.; Lin, S. Induced disease-resistance of postharvest tomato by cold-shock and H2O2 treatment. Food Sci. 2008, 29, 453–456. [Google Scholar]
- Bautista-Banos, S.; Hernandez-Lopez, M.; Bosquez-Molina, E.; Wilson, C.L. Effects of chitosan and plant extracts on growth of Colletotrichum gloeosporioides, anthracnose levels and quality of papaya fruit. Crop Prot. 2003, 22, 1087–1092. [Google Scholar] [CrossRef]
- Manenoi, A.; Bayogan, E.R.V.; Thumdee, S.; Paull, R.E. Utility of 1-methylcyclopropene as a papaya postharvest treatment. Postharvest Biol. Technol. 2007, 44, 55–62. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).