Sea Buckthorn Hippophae rhamnoides and Fruit Flies Rhagoletis batava: Search for Volatile Semiochemicals Involved in Pest Attraction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Sea Buckthorn Fruits
2.3. Evaluation of the Host-Plant Odour Attractiveness Using Y–Tube Olfactometer
2.4. Sampling and Analysis of Fruit-Released Volatiles
2.5. Sampling Volatiles for Olfactory Activity Evaluation
2.6. GC-EAD Recording
2.7. Identification of Olfactory Active Fruit-Released Volatiles
2.8. Chemicals
2.9. Statistical Analysis
3. Results
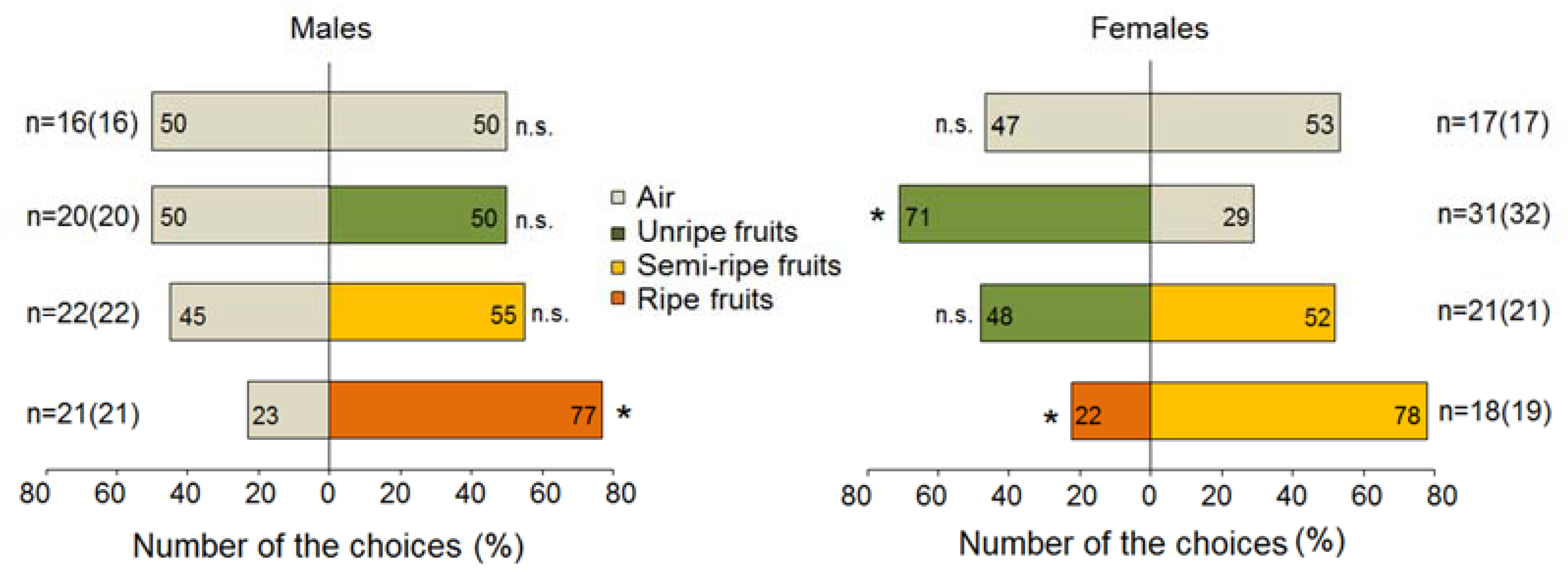
3.1. Behavioural Responses of Rhagoletis batava to Sea Buckthorn Fruits
3.2. Volatiles of Sea Buckthorn Fruits at Two Ripening Stages
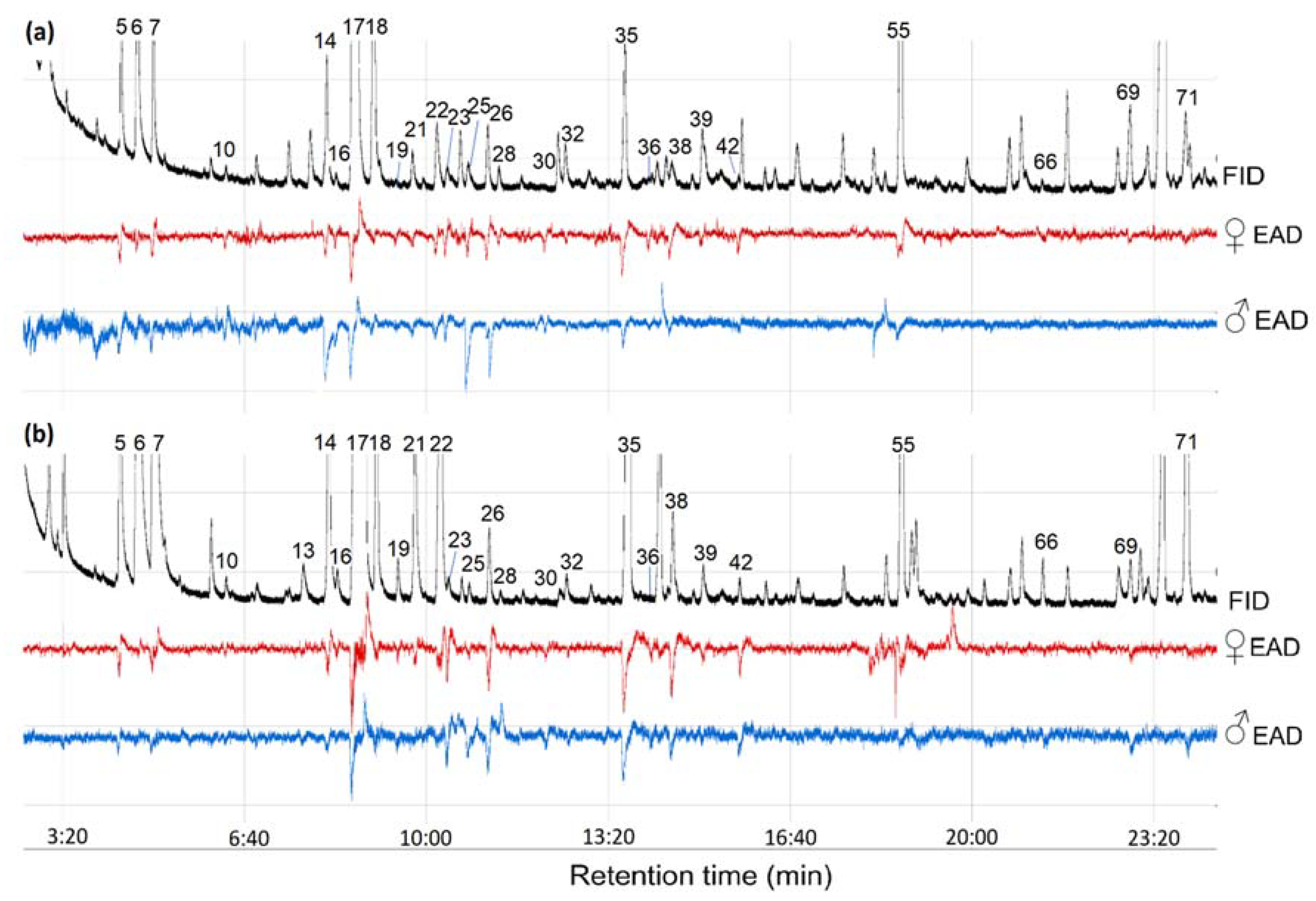
3.3. Olfactory Active Compounds of the Sea Buckthorn Fruits
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, T.S.C. Taxonomy, natural distribution and botany. In Sea Buckthorn (Hippophaė rhamnoides L.): Production and Utilization; Li, T.S.C., Beveridge, T., Eds.; NRC Research Press: Ottawa, ON, USA, 2003; pp. 7–11. [Google Scholar]
- Li, T.S.C.; Beveridge, T. Sea Buckthorn (Hippophaė rhamnoides L.): Production and Utilization; NRC Research Press: Ottawa, ON, USA, 2003. [Google Scholar]
- Kalia, R.K.; Singh, R.; Rai, M.K.; Mishra, G.P.; Singh, S.R.; Dhawan, A.K. Biotechnological interventions in sea buckthorn (Hippophae L.): Current status and future prospects. Trees 2011, 25, 559–575. [Google Scholar] [CrossRef]
- Li, T.S.C.; Schroeder, W.R. Sea buckthorn (Hippophae rhamnoides L.): A multipurpose plant. Horttechnology 1996, 6, 370–380. [Google Scholar] [CrossRef]
- Gâtlan, A.M.; Gutt, G. Sea buckthorn in plant based diets. An analytical approach of sea buckthorn fruits composition: Nutritional value, applications, and health benefits. Int. J. Environ. Res. Public Health 2021, 18, 8986. [Google Scholar] [CrossRef] [PubMed]
- Brūvelis, A. Feasibility Study for Trapping of the Sea Buckthorn Flies and Biological Control of Wilt. In Proceedings of the EuroWorkS On Air 3 “Seabuckthorn Field Technologies, Including Pests and Diseases Control”, Eurasia, Online, 23 November 2021; Available online: https://euroworks.online/wp/projects/ (accessed on 29 December 2021).
- Höhne, F.; Gießmann, H.J. Ein neuer Schädling bedroht den Sanddornanbau massives Auftreten in Versuchen der Landesforschungsanstalt 2013! Info-Blatt 2013, 22, 280–285. [Google Scholar]
- Stalažs, A. New records of some dipterans (Diptera: Cecidomyidae, Tephritidae) in North-Eastern Lithuania. Zool. Ecol. 2014, 24, 55–57. [Google Scholar] [CrossRef]
- Stalažs, A.; Balalaikins, M. Country checklist of Rhagoletis Loew (Diptera: Tephritidae) for Europe, with focus on R. batava and its recent range expansion. Proc. Latvian Acad. Sci. Section B 2017, 71, 103–110. [Google Scholar] [CrossRef]
- Shamanskaya, L.D. Bioecology of the sea-buckthorn fly (Rhagoletis batava obscuriosa Kol.) and pest control treatment in Altai. In Proceedings of the 3rd European Workshop on Sea Buckthorn EuroWorkS2014, Natural Resources Institute Finland, Naantali, Finland, 14–16 October 2014; pp. 7–20. [Google Scholar]
- Toth, M.; Lerche, S.; Holz, U.; Kerber, A.; Henning, R.; Voigt, E.; Kelemen, D. Addition of synthetic feeding attractant increases catches of Rhagoletis batava Hering and Carpomyia schineri Loew. in fluorescent yellow sticky traps. Acta Phytopathol. Entomol. Hung. 2016, 51, 69–76. [Google Scholar] [CrossRef] [Green Version]
- Leblanc, L.; Vargas, R.I.; Rubinoff, D. A comparison of nontarget captures in BioLure and liquid protein food lures in Hawaii. Proc. Hawaii. Entomol. Soc. 2010, 42, 15–22. [Google Scholar]
- Yee, W.L. Ammonium carbonate loss rates from lures differentially affect trap captures of Rhagoletis indifferens (Diptera: Tephritidae) and non-target flies. Can. Entomol. 2017, 149, 241–250. [Google Scholar] [CrossRef]
- Būda, V.; Blažytė-Čereškienė, L.; Radžiutė, S.; Apšegaitė, V.; Schultz, S.; Stamm, P.; Aleknavičius, D.; Mozūraitis, R. Male-produced (-)-δ-heptalactone as pheromone of the fruit fly Rhagoletis batava (Diptera: Tephritidae), a pest of sea buckthorn fruits. Insects 2020, 11, 138. [Google Scholar] [CrossRef] [Green Version]
- Aldrich, J.R.; Bartelt, R.J.; Dickens, J.C.; Knight, A.L.; Light, D.M.; Tumlinson, J.H. Insect chemical ecology research in the United States Department of Agriculture-Agricultural Research Service. Pest Manag. Sci. 2003, 59, 777–787. [Google Scholar] [CrossRef] [PubMed]
- Reddy, G.V.P.; Guerrero, A. Interactions of insect pheromones and plant semiochemicals. Trends Plant Sci. 2004, 9, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Sablon, L.; Dickens, J.C.; Haubruge, É.; Verheggen, F.J. Chemical ecology of the colorado potato beetle, Leptinotarsa decemlineata (Say) (Coleoptera: Chrysomelidae), and potential for alternative control methods. Insects 2013, 4, 31–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scolari, F.; Valerio, F.; Benelli, G.; Papadopoulos, N.T.; Vaníčková, L. Tephritid fruit fly semiochemicals: Current knowledge and future perspectives. Insects 2021, 12, 408. [Google Scholar] [CrossRef] [PubMed]
- Fein, B.L.; Reissig, W.H.; Roelofs, W.L. Identification of apple volatiles attractive to the apple maggot, Rhagoletis pomonella. J. Chem. Ecol. 1982, 8, 1473–1487. [Google Scholar] [CrossRef]
- Zhang, A.J.; Linn, C.; Wright, S.; Prokopy, R.; Reissig, W.; Roelofs, W. Identification of a new blend of apple volatiles attractive to the apple maggot, Rhagoletis pomonella. J. Chem. Ecol. 1999, 25, 1221–1232. [Google Scholar] [CrossRef]
- Prokopy, R.J.; Papaj, D.R. Behavior of flies of the genera Rhagoletis, Zonosemata, and Carpomya (Trypetinae: Carpomyina). In Fruit Flies (Tephritidae): Phylogeny and Evolution of Behavior; Aluja, M., Norrbom, A.L., Eds.; CRC Press: Boca Raton, FL, USA, 2001; pp. 219–252. [Google Scholar]
- Cha, D.H.; Yee, W.L.; Goughnour, R.B.; Sim, S.B.; Powell, T.H.Q.; Feder, J.L.; Linn, C.E., Jr. Identification of host fruit volatiles from domestic apple (Malus domestica), native black hawthorn (Crataegus douglasii) and introduced ornamental hawthorn (C. monogyna) attractive to Rhagoletis pomonella flies from the western United States. J. Chem. Ecol. 2012, 38, 319–329. [Google Scholar] [CrossRef] [Green Version]
- Cha, D.H.; Olsson, S.B.; Yee, W.L.; Goughnour, R.B.; Hood, G.R.; Mattsson, M.; Schwarz, D.; Feder, J.L.; Linn, C.E., Jr. Identification of host fruit volatiles from snowfruit (Symphoricarpos albus), attractive to Rhagoletis zephyria flies from the western United States. J. Chem. Ecol. 2017, 43, 188–197. [Google Scholar] [CrossRef]
- Quilici, S.; Atiama-Nurbel, T.; Brévault, T. Plant odors as fruit fly attractants. In Trapping and the Detection, Control, and Regulation of Tephritid Fruit Flies; Shelly, T., Epsky, N., Jang, E., Reyes-Flores, J., Vargas, R., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 119–144. [Google Scholar] [CrossRef]
- Tiitinen, K.; Hakala, M.; Kallio, H. Headspace volatiles from frozen fruits of sea buckthorn (Hippophae rhamnoides L.) varieties. Eur. Food Res. Technol. 2006, 223, 455–460. [Google Scholar] [CrossRef]
- Socaci, S.A.; Socaciu, C.; Tofana, M.; Rati, I.V.; Pintea, A. In-tube extraction and GC-MS analysis of volatile components from wild and cultivated sea buckthorn (Hippophae rhamnoides L. ssp Carpatica) fruit varieties and juice. Phytochem. Anal. 2013, 24, 319–328. [Google Scholar] [CrossRef]
- Leung, G.S.; Marriott, R. Year to year variation in sea buckthorn juice volatiles using headspace solid phase microextraction. Flavour Fragr. J. 2016, 31, 124–136. [Google Scholar] [CrossRef]
- Ma, X.Y.; Yang, W.; Marsol-Vall, A.; Laaksonen, O.; Yang, B.R. Analysis of flavour compounds and prediction of sensory properties in sea buckthorn (Hippophae rhamnoides L.) fruits. Int. J. Food Sci. Technol. 2020, 55, 1705–1715. [Google Scholar] [CrossRef]
- Zapalowska, A.; Matlok, N.; Zardzewialy, M.; Piechowiak, T.; Balawejder, M. Effect of ozone treatment on the quality of sea buckthorn (Hippophae rhamnoides L.). Plants 2021, 10, 847. [Google Scholar] [CrossRef] [PubMed]
- Baietto, M.; Wilson, A.D. Electronic-nose applications for fruit identification, ripeness and quality grading. Sensors 2015, 15, 899–931. [Google Scholar] [CrossRef]
- Jayanty, S.; Song, J.; Rubinstein, N.M.; Chong, A.; Beaudry, R.M. Temporal relationship between ester biosynthesis and ripening events in bananas. J. Am. Soc. Hortic. Sci. 2002, 127, 998–1005. [Google Scholar] [CrossRef] [Green Version]
- Jetti, R.R.; Yang, E.; Kurnianta, A.; Finn, C.; Qian, M.C. Quantification of selected aroma-active compounds in strawfruits by headspace solid-phase microextraction gas chromatography and correlation with sensory descriptive analysis. J. Food Sci. 2007, 72, S487–S496. [Google Scholar] [CrossRef]
- Li, G.; Jia, H.; Li, J.; Wang, Q.; Zhang, M.; Teng, Y. Emission of volatile esters and transcription of ethylene- and aroma-related genes during ripening of ‘Pingxiangli’ pear fruit (Pyrus ussuriensis Maxim). Sci. Hortic. 2014, 170, 17–23. [Google Scholar] [CrossRef]
- Wang, M.Y.; MacRae, E.; Wohlers, M.; Marsh, K. Changes in volatile production and sensory quality of kiwifruit during fruit maturation in Actinidia deliciosa Hayward’ and A. chinensis Hort16A′. Postharvest Biol. Technol. 2011, 59, 16–24. [Google Scholar] [CrossRef]
- Tait, C.; Batra, S.; Ramaswamy, S.S.; Feder, J.L.; Olsson, S.B. Sensory specificity and speciation: A potential neuronal pathway for host fruit odour discrimination in Rhagoletis pomonella. Proc. R. Soc. B 2016, 283, 20162101. [Google Scholar] [CrossRef] [Green Version]
- Lugemwa, F.N.; Lwande, W.; Bentley, M.D.; Mendel, M.J.; Alford, A.R. Volatiles of wild bluefruit, Vaccinium angustifolium: Possible attractants for the bluefruit maggot fruit fly, Rhagoletis mendax. J. Agric. Food Chem. 1989, 37, 232–233. [Google Scholar] [CrossRef]
- Hamersky, W. Electrophysiological and Behavioral Responses of Walnut Husk flies to Walnut Volatiles. Master’s Thesis, The California State University, Long Beach, CA, USA, August 1996. [Google Scholar]
- Būda, V.; Radžiutė, S.; Apšegaitė, V.; Blažytė-Čereškienė, L.; Čepulytė, R.; Bumbulytė, G.; Mozūraitis, R. Electroantennographic and behavioural responses of European cherry fruit fly, Rhagoletis cerasi, to the volatile organic compounds from sour cherry, Prunus cerasus, fruit. Insects 2022, 13, 114. [Google Scholar] [CrossRef]
- Mozūraitis, R.; Aleknavičius, D.; Vepštaitė-Monstavičė, I.; Stanevičienė, R.; Emami, S.N.; Apšegaitė, V.; Radžiutė, S.; Blažytė-Čereškienė, L.; Servienė, E.; Būda, V. Hippophae rhamnoides fruit related Pichia kudriavzevii yeast volatiles modify behaviour of Rhagoletis batava flies. J. Adv. Res. 2020, 21, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Lukša, J.; Vepštaitė-Monstavičė, I.; Apšegaitė, V.; Blažytė-Čereškienė, L.; Stanevičienė, R.; Strazdaitė-Žielienė, Ž.; Ravoitytė, B.; Aleknavičius, D.; Būda, V.; Mozūraitis, R.; et al. Fungal microbiota of sea buckthorn fruits at two ripening stages and volatile profiling of potential biocontrol yeasts. Microorganisms 2020, 8, 456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
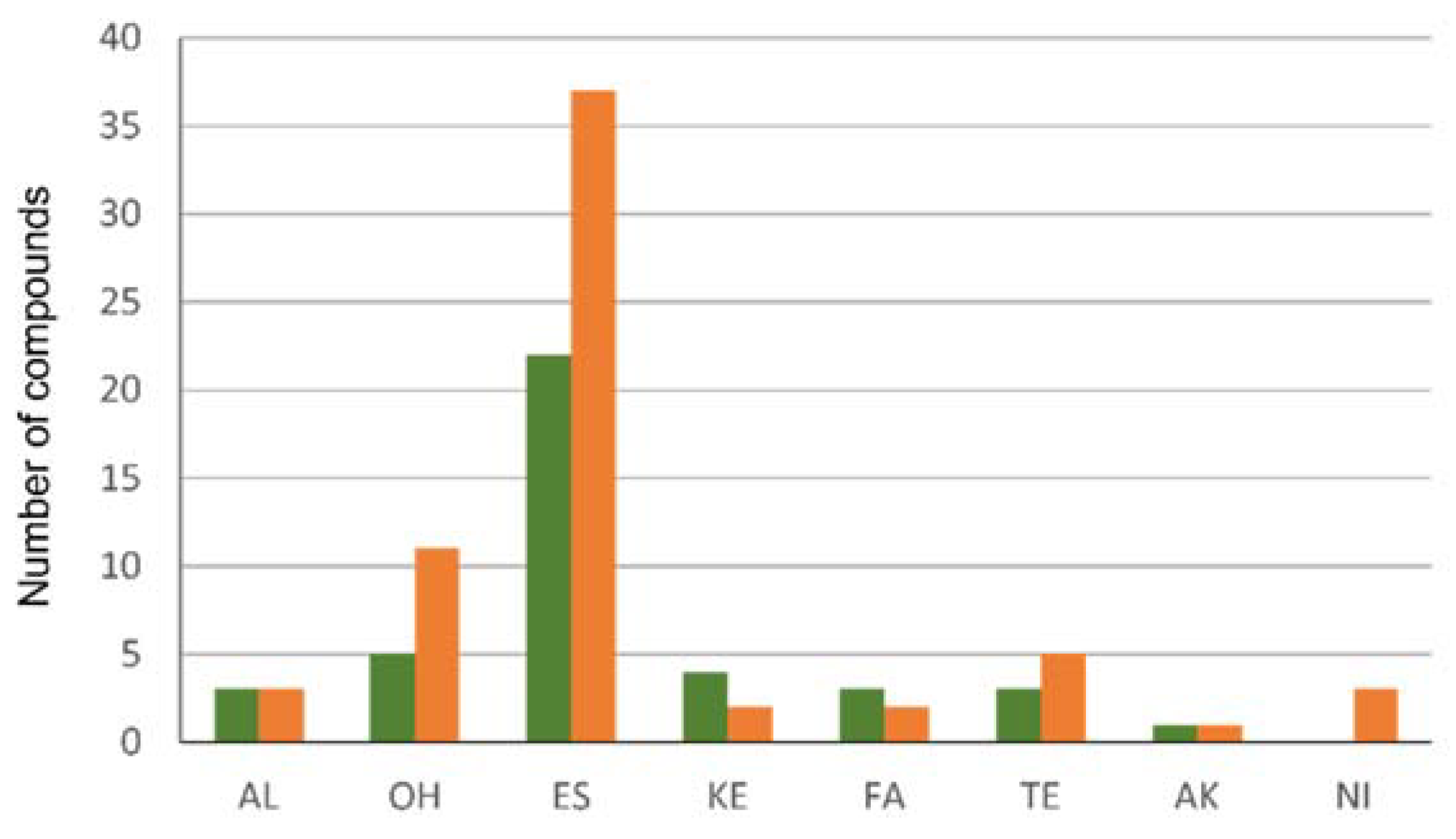
| No | Compound | Compound Group | RT | Unripe Fruits, ng/g/h ± SE 8 | Ripe Fruits, ng/g/h ± SE |
|---|---|---|---|---|---|
| 1 | 3-Methylbutanal | AL 1 | 3.46 | 1.54 ± 0.13 | |
| 2 | Ethanol | OH 2 | 3.50 | 9.49 ± 4.80 | |
| 3 | Ethyl 2-methylpropanoate | ES 3 | 3.75 | 49.38 ± 24.90 | |
| 4 | 2-Pentanone | KE 4 | 4.03 | 2.07 ± 0.31 * 9 | 12.39 ± 12.39 * |
| 5 | Ethyl butanoate | ES | 4.77 | 13.57 ± 2.96 | 114.04 ± 7.69 |
| 6 | Ethyl 2-methylbutanoate | ES | 5.05 | 14.45 ± 5.14 | 162.23 ± 63.17 |
| 7 | Ethyl 3-methylbutanoate | ES | 5.30 | 21.90 ± 3.97 | 243.23 ± 31.19 |
| 8 | 2-Methylpropan-1-ol | OH | 5.62 | 33.50 ± 8.49 | |
| 9 | 3-Methylbutyl acetate | ES | 6.25 | 4.17 ± 0.50 | 21.51 ± 3.68 |
| 10 | Ethyl pentanoate | ES | 6.47 | 3.13 ± 1.03 | |
| 11 | (Z)-3-Hexanal | AL | 6.61 | 7.63 ± 0.51 | |
| 12 | Ethyl 2-butenoate | ES | 7.02 | 1.77 ± 0.22 | |
| 13 | 3-Methylbutyl 2-methylpropionate | ES | 7.93 | 3.54 ± 2.06 | |
| 14 | 3-Methylbutan-1-ol | OH | 8.08 | 57.14 ± 4.49 | 230.21 ± 24.56 |
| 15 | (E)-2-Hexanal | AL | 8.19 | 5.18 ± 1.71 | |
| 16 | Ethyl 3-methyl-2-butenoate | ES | 8.40 | 0.81 ± 0.18 | 5.34 ± 1.46 |
| 17 | Ethyl hexanoate | ES | 8.66 | 68.17 ± 17.60 | 345.52 ± 30.96 |
| 18 | (E)-β-Ocimene | TE 5 | 9.06 | 66.45 ± 7.75 * | 90.67 ± 10.51 * |
| 19 | 3-Methylbutyl butanoate | ES | 9.48 | 13.76 ± 0.44 | |
| 20 | 3-Hydroxy-2-butanone (Acetoin) | ES | 9.75 | 37.42 ± 12.74 * | 52.97 ± 9.26 * |
| 21 | 3-Methylbutyl 2-methylbutanoate | ES | 9.79 | 66.53 ± 19.16 | |
| 22 | 3-Methylbutyl 3-methylbutanoate | ES | 10.13 | 33.27 ± 2.57 | 232.16 ± 16.42 |
| 23 | Methyl 2-hydroxy-2-methylbutanoate | ES | 10.30 | 0.02 ± 0.02 * | 3.02 ± 1.60 * |
| 24 | (Z)-3-Hexenyl acetate | ES | 10.62 | 9.90 ± 3.19 | |
| 25 | Propyl hexanoate | ES | 10.73 | 9.36 ± 1.32 | |
| 26 | Ethyl heptanoate | ES | 11.08 | 1.25 ± 0.13 | 16.67 ± 2.80 |
| 27 | 6-Methyl hept-5-en-2-one | KE | 11.08 | 0.55 ± 0.09 | |
| 28 | Ethyl (E)-2-hexenoate | ES | 11.31 | 1.09 ± 0.21 | |
| 29 | Hexan-1-ol | OH | 11.60 | 3.75 ± 1.71 | 14.66 ± 2.22 |
| 30 | 3-Methyl-3-butyl 3-methylbutanoate | ES | 11.96 | 1.36 ± 0.26 | |
| 31 | (Z)-3-Hexen-1-ol | OH | 12.33 | 8.94 ± 1.86 | 3.74 ± 0.25 |
| 32 | Methyl octanoate | ES | 12.46 | 0.78 ± 0.02 | |
| 33 | Ethyl 3-hydroxy-3-methylbutanoate | ES | 12.95 | 15.28 ± 2.48 | |
| 34 | Ethyl 2-hydroxy-3-methylbutanoate | ES | 13.30 | 1.61 ± 1.61 | |
| 35 | Ethyl octanoate | ES | 13.60 | 10.72 ± 0.85 | 114.54 ± 10.25 |
| 36 | 1-Octen-3-ol | OH | 14.00 | 1.08 ± 0.17 | |
| 37 | 3-Methylbutyl hexanoate | ES | 14.20 | 5.63 ± 0.73 | 78.48 ± 9.96 |
| 38 | Ethyl (Z)-4-octenoate | ES | 14.51 | 0.22 ± 0.22 | 13.41 ± 0.80 |
| 39 | 2-Ethylhexan-1-ol | OH | 14.98 | 0.91 ± 0.91 | 73.36 ± 6.36 |
| 40 | Dodecanal | AL | 15.20 | 3.05 ± 1.41 | |
| 41 | Benzaldehyde | AL | 15.48 | 4.93 ± 0.43 | |
| 42 | Ethyl 3-hydroxybutanoate | ES | 15.55 | 0.74 ± 0.74 | |
| 43 | Propanoic acid | FA 6 | 15.96 | 5.64 ± 1.33 * | 7.50 ± 0.90 * |
| 44 | Ethyl 2-hydroxypropanoate | ES | 16.19 | 44.48 ± 7.50 | |
| 45 | 1-Octanol | OH | 16.67 | 0.70 ± 0.70 | 14.53 ± 15.01 |
| 46 | Octyl acetate | ES | 16.68 | 9.11 ± 9.11 | |
| 47 | 2-Methylpropanoic acid | FA | 16.70 | 1.62 ± 1.62 | |
| 48 | Aristolene | TE | 16.95 | 3.08 ± 1.58 | 40.70 ± 6.53 |
| 49 | Methyl benzoate | ES | 17.87 | 8.09 ± 1.24 * | 10.69 ± 5.95 * |
| 50 | 6-Methylheptan-1-ol | OH | 18.02 | 22.30 ± 3.11 | |
| 51 | Butanoic acid | FA | 18.10 | 0.97 ± 0.97 | |
| 52 | 6-Methyloctan-1-ol | OH | 18.32 | 9.80 ± 5.01 | |
| 53 | Acetophenone | KE | 18.51 | 1.17 ± 1.17 | |
| 54 | Ethyl decanoate | ES | 18.54 | 16.93 ± 1.93 | |
| 55 | Ethyl benzoate | ES | 18.94 | 17.39 ± 3.15 | 79.04 ± 9.30 |
| 56 | Methylbutyl benzoate | ES | 18.97 | 1.02 ± 1.02 | |
| 57 | Ethyl (Z)-4-decenoate | ES | 19.03 | 2.44 ± 2.44 | 13.58 ± 2.21 |
| 58 | 3-Methylbutyl octanoate | ES | 19.04 | 28.66 ± 14.74 | |
| 59 | Unknown | 19.38 | 2.36 ± 1.32 | ||
| 60 | Unknown | 19.78 | 3.26 ± 2.21 | ||
| 61 | Germacrene D | TE | 19.98 | 0.86 ± 0.51 | |
| 62 | Heptadecane | AK 7 | 20.17 | 2.32 ± 2.32 | |
| 63 | Unknown | 20.22 | 10.61 ± 5.57 | ||
| 64 | (E, E)-α-Farnesene | TE | 20.94 | 4.80 ± 1.07 | 10.83 ± 0.82 |
| 65 | Methyl salicylate | ES | 21.29 | 8.47 ± 0.28 | 25.05 ± 2.57 |
| 66 | Ethyl phenylacetate | ES | 21.60 | 4.26 ± 0.47 | |
| 67 | 2-Methylpropyl benzoate | ES | 21.71 | 0.34 ± 0.34 | |
| 68 | Octadecane | AK | 22.47 | 2.06 ± 1.06 | |
| 69 | (E)-Geranyl acetone | KE | 23.16 | 0.4 ± 0.03 | 1.75 ± 0.96 |
| 70 | 2-Phenylethanol | OH | 24.32 | 23.75 ± 11.93 | |
| 71 | 3-Methylbutyl benzoate | ES | 24.31 | 48.83 ± 3.69 | 142.55 ± 16.00 |
| 72 | Heptanoic acid | FA | 25.13 | 13.13 ± 4.24 | |
| 73 | 3-Methylbutyl salicylate | ES | 26.93 | 1.65 ± 1.12 | |
| 74 | Eugenol | TE | 32.35 | 0.82 ± 0.52 | |
| 75 | Hydroxymethylfurfural | AL | 34.90 | 0.69 ± 0.36 | |
| 76 | Benzyl benzoate | ES | 36.94 | 0.75 ± 0.38 | |
| Total | 485.1 ± 93.85 | 2588.07 ± 440.78 |
| No 1 | Compound | CG 2 | RI 6 | RT 7 | Unripe Fruits | Ripe Fruits | EAG Activity | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Peak Area 8 | Peak Area % | Peak Area | Peak Area % | Females | Males | |||||
| 5 | Ethyl butanoate | ES 3 | 1015 | 4.31 | 8.64 ± 1.26 | 3.06 ± 0.14 | 26.59 ± 2.61 | 2.66 ± 0,04 | 5 9(5) 10 | 4(4) |
| 6 | Ethyl 2-methylbutanoate | ES | 1037 | 4.66 | 28.89 ± 4.62 | 10.18 ± 0.43 | 129.75 ± 13.20 | 12.98 ± 0.17 | 5(5) | 4(4) |
| 7 | Ethyl 3-methylbutanoate | ES | 1056 | 4.98 | 11.09 ± 1.33 | 3.95 ± 0.09 | 147.06 ± 14.85 | 14.71 ± 0.16 | 5(5) | 4(4) |
| 10 | Ethyl pentanoate | ES | 1122 | 6.27 | 0.46 ± 0.09 | 0.16 ± 0.01 | 1.02 ± 0.15 | 0.10 ± 0.01 | 4(5) | 4(4) |
| 13 | 3-Methylbutyl 2-methylpropionate | ES | 1185 | 7.69 | 0 | 0 | 2.88 ± 0.27 | 0.29 ± 0.001 | 3(5) | 2(4) |
| 14 | 3-Methylbutan-1-ol | OH 4 | 1204 | 8.16 | 8.97 ± 0.72 | 3.24 ± 0.20 | 26.49 ± 2.55 | 2.65 ± 0.25 | 5(5) | 4(4) |
| 16 | Ethyl 3-methyl-2-butenoate | ES | 1211 | 8.32 | 0.71 ± 0.09 | 0.25 ± 0.01 | 2.93 ± 0.37 | 0.29 ± 0.03 | 5(5) | 4(4) |
| 17 | Ethyl hexanoate | ES | 1232 | 8.80 | 118.03 ± 17.34 | 41.75 ± 0.52 | 384.05 ± 38.10 | 38.42 ± 0.14 | 5(5) | 4(4) |
| 18 | (E)-β-ocimene | TE 5 | 1242 | 9.03 | 28.67 ± 4.29 | 10.14 ± 0.34 | 35.23 ± 3.47 | 3.52 ± 0.02 | 5(5) | 4(4) |
| 19 | 3-Methylbutyl butanoate | ES | 1258 | 9.43 | 0.23 ± 0.05 | 0.08 ± 0.01 | 2.20 ± 0.23 | 0.22 ± 0.02 | 4(5) | 4(4) |
| 21 | 3-Methylbutyl 2-methylbutanoate | ES | 1271 | 9.75 | 2.17 ± 0.37 | 0.76 ± 0.02 | 17.76 ± 1.76 | 1.78 ± 0.01 | 5(5) | 4(4) |
| 22 | 3-Methylbutyl 3-methylbutanoate | ES | 1289 | 10.22 | 5.40 ± 0.65 | 1.97 ± 0.33 | 45.83 ± 4.54 | 4.59 ± 0.05 | 5(5) | 4(4) |
| 23 | Methyl 2-hydroxy-2-methylbutanoate | ES | 1294 | 10.36 | 0.90 ± 0.14 | 0.32 ± 0.01 | 1.62 ± 0.24 | 0.16 ± 0.01 | 5(5) | 4(4) |
| 25 | Propyl hexanoate | ES | 1309 | 10.74 | 1.77 ± 0.54 | 0.60 ± 0.13 | 0.97 ± 0.10 | 0.10 ± 0.001 | 4(5) | 4(4) |
| 26 | Ethyl heptanoate | ES | 1325 | 11.11 | 3.79 ± 0.57 | 1.34 ± 0.01 | 4.86 ± 0.50 | 0.49 ± 0.004 | 5(5) | 4(4) |
| 28 | Ethyl (E)-2-hexenoate | ES | 1342 | 11.52 | 1.12 ± 0.21 | 0.39 ± 0.02 | 0.54 ± 0.06 | 0.05 ± 0.001 | 4(5) | 4(4) |
| 30 | 3-Methyl-3-butenyl 3-methylbutanoate | ES | 1368 | 12.16 | 0.02 ± 0.004 | 0.01 ± 0.001 | 0.05 ± 0.02 | 0.01 ± 0.002 | 4(5) | 3(4) |
| 32 | Methyl octanoate | ES | 1383 | 12.54 | 3.20 ± 0.68 | 1.11 ± 0.08 | 1.77 ± 0.14 | 0.18 ± 0.02 | 5(5) | 4(4) |
| 35 | Ethyl octanoate | ES | 1428 | 13.65 | 9.73 ± 1.57 | 3.45 ± 0.25 | 67.61 ± 6.79 | 6.76 ± 0.11 | 5(5) | 4(4) |
| 36 | 1-Octen-3-ol | OH | 1446 | 14.08 | 0.12 ± 0.05 | 0.04 ± 0.01 | 0.07 ± 0.01 | 0.01 ± 0.001 | 5(5) | 4(4) |
| 38 | Ethyl (Z)-4-octenoate | ES | 1462 | 14.48 | 1.61 ± 0.24 | 0.58 ± 0.05 | 5.63 ± 0.33 | 0.58 ± 0.08 | 5(5) | 4(4) |
| 39 | 2-Ethylhexan-1-ol | OH | 1484 | 15.05 | 2.67 ± 0.80 | 0.99 ± 0.32 | 2.65 ± 0.29 | 0.26 ± 0.004 | 5(5) | 3(4) |
| 42 | Ethyl 3-hydroxybutanoate | ES | 1512 | 15.72 | 0.39 ± 0.06 | 0.14 ± 0.002 | 1.24 ± 0.13 | 1.24 ± 0.13 | 5(5) | 4(4) |
| 55 | Ethyl benzoate | ES | 1644 | 18.7 | 32.44 ± 5.50 | 11.45 ± 0.57 | 48.52 ± 4.74 | 4.85 ± 0.03 | 5(5) | 4(4) |
| 66 | Ethyl phenylacetate | ES | 1763 | 21.28 | 0.38 ± 0.07 | 0.14 ± 0.03 | 2.35 ± 0.27 | 0.23 ± 0.01 | 5(5) | 2(4) |
| 69 | (E)-Geranyl acetone | TE | 1849 | 23.08 | 5.40 ± 1.00 | 1.91 ± 0.20 | 3.19 ± 0.47 | 0.32 ± 0.03 | 4(5) | 2(4) |
| 71 | 3-Methylbutyl benzoate | ES | 1890 | 23.93 | 5.50 ± 0.88 | 1.99 ± 0.33 | 36.72 ± 3.60 | 3.67 ± 0.05 | 3(5) | 2(4) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blažytė-Čereškienė, L.; Būda, V.; Apšegaitė, V.; Radžiutė, S.; Būdienė, J.; Aleknavičius, D.; Mozūraitis, R. Sea Buckthorn Hippophae rhamnoides and Fruit Flies Rhagoletis batava: Search for Volatile Semiochemicals Involved in Pest Attraction. Horticulturae 2022, 8, 179. https://doi.org/10.3390/horticulturae8020179
Blažytė-Čereškienė L, Būda V, Apšegaitė V, Radžiutė S, Būdienė J, Aleknavičius D, Mozūraitis R. Sea Buckthorn Hippophae rhamnoides and Fruit Flies Rhagoletis batava: Search for Volatile Semiochemicals Involved in Pest Attraction. Horticulturae. 2022; 8(2):179. https://doi.org/10.3390/horticulturae8020179
Chicago/Turabian StyleBlažytė-Čereškienė, Laima, Vincas Būda, Violeta Apšegaitė, Sandra Radžiutė, Jurga Būdienė, Dominykas Aleknavičius, and Raimondas Mozūraitis. 2022. "Sea Buckthorn Hippophae rhamnoides and Fruit Flies Rhagoletis batava: Search for Volatile Semiochemicals Involved in Pest Attraction" Horticulturae 8, no. 2: 179. https://doi.org/10.3390/horticulturae8020179
APA StyleBlažytė-Čereškienė, L., Būda, V., Apšegaitė, V., Radžiutė, S., Būdienė, J., Aleknavičius, D., & Mozūraitis, R. (2022). Sea Buckthorn Hippophae rhamnoides and Fruit Flies Rhagoletis batava: Search for Volatile Semiochemicals Involved in Pest Attraction. Horticulturae, 8(2), 179. https://doi.org/10.3390/horticulturae8020179







